Abstract
Melanocortin receptors are critical modulators of energy balance and glucose homeostasis. Companion studies published in Science (Asai et al., 2013; Sebag et al., 2013) establish a role for melanocortin receptor accessory protein 2 (Mrap2) in regulating melanocortin receptor activity and in the development of obesity in zebrafish, rodents, and humans.
The rates of obesity and diabetes are rising around the world. Although environmental conditions have been implicated in these diseases, accumulating evidence suggests genetics also contributes to early-onset and severe obesity. The melanocortin system provides one example (Balthasar et al., 2005; Cone, 2006; Greenfield et al., 2009; Sohn et al., 2013). It is comprised of ligands and a family of receptors (MC1–5 receptors) that directly link to a myriad of biophysiological systems regulating food intake, energy expenditure, body length, and glucose metabolism (Cone, 2006). Notably, disruptions of MC4 receptors in rodents result in severe obesity and diabetes (Cone, 2006). Moreover, mutations of MC4 receptors in humans lead to analogous deficits of metabolism and are the most common monogenic form of human obesity (Cone, 2006; Greenfield et al., 2009). Thus, understanding the molecular mechanisms responsible for regulating MC4 signaling may lead to better therapies targeting body weight and glucose homeostasis. Now companion articles (Asai et al., 2013; Sebag et al., 2013) in the journal Science add a new layer of complexity to the regulation of MC4 receptors. They report that melanocortin receptor accessory protein 2 (Mrap2) coordinates signaling by MC4 receptors, and demonstrate that Mrap2 is required for growth and development as well as metabolism across species including humans (Asai et al., 2013; Sebag et al., 2013).
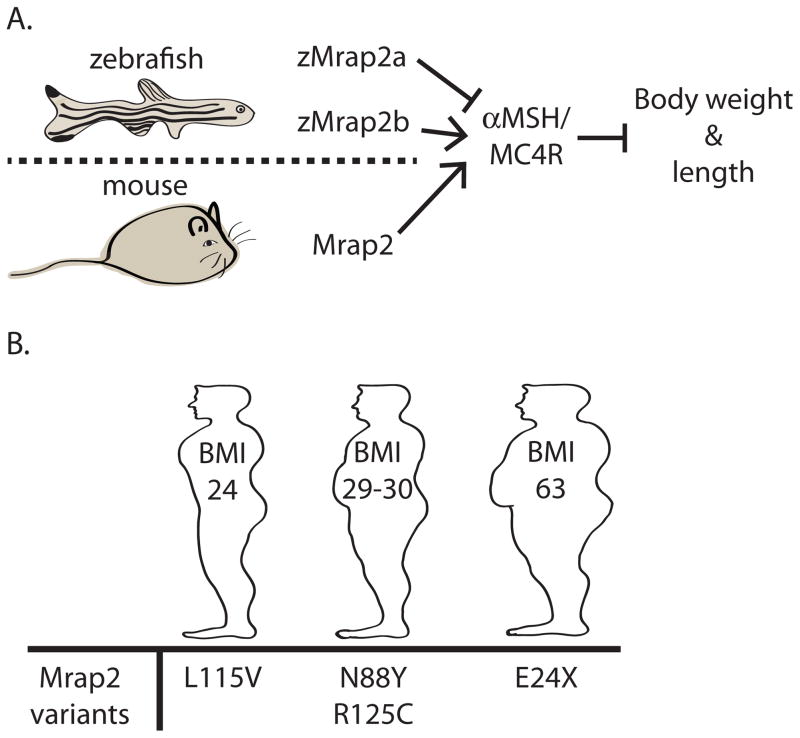
MC4 receptors are G protein coupled receptors (GPCRs) that stimulate classical signaling cascades, which ultimately regulate metabolism at least in part via expression in sites involved in autonomic and endocrine function, including the paraventricular nucleus of the hypothalamus (PVH) (Balthasar et al., 2005; Cone, 2006). However, recent work suggests that melanocortin signaling is more complicated than previously anticipated. In particular, melanocortin receptor accessory proteins (Mrap1 and Mrap2) regulate transport of melanocortin receptors to the cell membrane and also abrogate or facilitate ligand-induced activation of intracellular signaling(Ramachandrappa et al., 2013). Asai et. al. now demonstrate that Mrap2 is highly expressed in the PVH including cells expressing the MC4 receptor (Asai et al., 2013; Cone, 2006). In agreement with Mrap2 playing a role in MC4 receptor signaling (Figure 1), they also find that rodents deficient for Mrap2 are obese even in the absence of excess food. Moreover, selective loss of Mrap2 in neurons expressing the transcription factor single-minded 1 (Sim1) recapitulates the obese phenotype observed in global Mrap2 deficiency, consistent with the notion that Sim1 neurons expressing MC4 receptors are key regulators of energy balance (Balthasar et al., 2005; Holder et al., 2000). Notably, Asai and colleagues found four rare heterozygous variants of Mrap2 in human subjects (Figure 1) (Asai et al., 2013). The subject carrying the E24X variant exhibited severe obesity, while two of the remaining subjects (N88Y and R125C variants) were modestly obese compared to the subject carrying the normal BMI variant (L115V) (Figure 1).
Figure 1. Role of Mrap2 in the regulation of central melanocortin receptors in zebrafish, mice and humans.
(a) In zebrafish, Mrap2a inhibits, whereas Mrap2b stimulates, the activity of MC4 receptors (upper panel). In rodents, Mrap2 stimulates the activity of MC4 receptors (lower panel).
(b) In humans, four rare heterozygous Mrap2 variants have been identified. Subjects carrying the E24X variant exhibit severe obesity, subjects with the two variants N88Y and R125C are moderately obese whereas subjects with the L115V variant display normal BMI.
In the companion report, Sebag et al. explore the role of Mrap2 in a zebrafish model (Figure 1) (Sebag et al., 2013). The authors demonstrated that the paralog zMrap2a is restricted to larval development whereas zMrap2b is expressed in the adult. Using knockdown and expression assays, Sebag et. al show that zMrap2a abrogates MC4 receptor signaling, thereby stimulating larval growth, whereas zMrap2b mimicks mammalian Mrap2 by increasing the receptors sensitivity to ligand (Figure 1). The authors predict that the differential expression and regulation of zMrap2a and zMrap2b is necessary to properly regulate energy needs throughout lifespan. It is intriguing to also think of these findings within the context of metabolic alterations in the pre- or peri-natal environment that may be linked to long lasting metabolic effects in the offspring.
To date, very few compounds have been successfully developed to treat obesity. This is due, at least in part, to the inherent difficulty in developing agents that regulate food intake and energy expenditure. In particular, compounds that increase energy expenditure can result in generalized increased sympathetic nerve activity which is frequently associated with deleterious side effects. Given their key role in regulating metabolism, MC4 receptors are compelling candidates in the search for therapeutic targets for the treatment of obesity (Wikberg and Mutulis, 2008). However, evidence for melanocortin receptor-induced changes in blood pressure have raised concerns about MC4 receptors in the treatment of obesity (Greenfield et al., 2009; Sohn et al., 2013; Wikberg and Mutulis, 2008). Furthermore, MC4 receptors have been implicated in anorectic conditions including cachexia (Marks et al., 2001). The companion reports demonstrate that Mrap2 accessory proteins differentially regulate the activity of MC4 receptors - by activating or inhibiting MC4 dependent signaling. Thus, it may be possible to develop novel strategies for altering specific activities of MC4 receptors, which may provide avenues for promoting the beneficial effects of MC4 receptors on metabolism while minimizing the adverse side effects.
The current studies raise many questions for future investigation. For instance, the functional relationship between Mrap2 and MC4 is not fully understood. Mice deficient for Mrap2 do not display all of the metabolic hallmarks of MC4 receptor deficiency, suggesting that Mrap2 may regulate other melanocortin receptors. In addition, it remains possible that Mrap2 expression in peripheral tissues also contributes to metabolic regulation outside the CNS. Furthermore, Sebag et. al. predict that the Mrap proteins likely regulate the activity of other GPCRs as well, which would extend the complexity of the physiology of this family of proteins. In terms of their role in obesity, it remains to be determined whether additional Mrap2 variants are involved in the pathogenesis of the more common forms of obesity. Finally, in order to develop pharmacotherapy for the treatment of various metabolic disorders, future work will need to clarify how many factors are involved in the regulation of melanocortin receptors, and their specific molecular mechanisms.
The companion papers are excellent examples of the power of combining studies in parallel model systems (zebrafish, mice, and humans) using multiple strategies. In recent years, there has been an exponential increase in the use of targeted molecular strategies in various animal models, allowing detailed examination of feeding behavior as well as energy and glucose homeostasis. However, some of these studies have generated conflicting data on the roles of receptors, enzymes, neuropeptides, and other factors affecting metabolism. Moreover, studies of metabolism in model organisms have not always translated well into human metabolic (patho)physiology. Asai et al. and Sebag et al. nicely demonstrate that a tractable system in model organisms (i.e., zebrafish and rodents) may accelerate metabolic research and potentially drug discovery in humans. As the field moves forward, it will be critical to integrate basic science in animal models with human physiology to develop tools to combat the growing epidemics of obesity and diabetes.
This is a commentary on article Imamura Y, Fujiwara T, Margolis R, Spaide RF. Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science. 2013;341(6143):275-8.
This is a commentary on article Sebag JA, Zhang C, Hinkle PM, Bradshaw AM, Cone RD. Developmental control of the melanocortin-4 receptor by MRAP2 proteins in zebrafish. Science. 2013;341(6143):278-81.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asai M, Ramachandrappa S, Joachim M, Shen Y, Zhang R, Nuthalapati N, Ramanathan V, Strochlic DE, Ferket P, Linhart K, et al. Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science. 2013;341:275–278. doi: 10.1126/science.1233000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, et al. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med. 2009;360:44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- Holder JL, Jr, Butte NF, Zinn AR. Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum Mol Genet. 2000;9:101–108. doi: 10.1093/hmg/9.1.101. [DOI] [PubMed] [Google Scholar]
- Marks DL, Ling N, Cone RD. Role of the central melanocortin system in cachexia. Cancer Res. 2001;61:1432–1438. [PubMed] [Google Scholar]
- Ramachandrappa S, Gorrigan RJ, Clark AJ, Chan LF. The melanocortin receptors and their accessory proteins. Front Endocrinol (Lausanne) 2013;4:9. doi: 10.3389/fendo.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebag JA, Zhang C, Hinkle PM, Bradshaw AM, Cone RD. Developmental control of the melanocortin-4 receptor by MRAP2 proteins in zebrafish. Science. 2013;341:278–281. doi: 10.1126/science.1232995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn JW, Harris LE, Berglund ED, Liu T, Vong L, Lowell BB, Balthasar N, Williams KW, Elmquist JK. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell. 2013;152:612–619. doi: 10.1016/j.cell.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikberg JE, Mutulis F. Targeting melanocortin receptors: an approach to treat weight disorders and sexual dysfunction. Nat Rev Drug Discov. 2008;7:307–323. doi: 10.1038/nrd2331. [DOI] [PubMed] [Google Scholar]