Abstract
Objective
Acute limb ischemia remains one of the most challenging emergencies in vascular surgery. Historically, outcomes following interventions for acute limb ischemia have been associated with high rates of morbidity and mortality. The purpose of this study was to determine contemporary outcomes following lower extremity bypass performed for acute limb ischemia.
Methods
All patients undergoing infrainguinal lower extremity bypass between 2003 and 2011 within hospitals comprising the Vascular Study Group of New England were identified. Patients were stratified according to whether or not the indication for lower extremity bypass was acute limb ischemia. Primary end points included bypass graft occlusion, major amputation, and mortality at 1 year postoperatively as determined by Kaplan-Meier life table analysis. Multivariable Cox proportional hazards models were constructed to evaluate independent predictors of mortality and major amputation at 1 year.
Results
Of 5712 lower extremity bypass procedures, 323 (5.7%) were performed for acute limb ischemia. Patients undergoing lower extremity bypass for acute limb ischemia were similar in age (66 vs 67; P = .084) and sex (68% male vs 69% male; P = .617) compared with chronic ischemia patients, but were less likely to be on aspirin (63% vs 75%; P < .0001) or a statin (55% vs 68%; P < .0001). Patients with acute limb ischemia were more likely to be current smokers (49% vs 39%; P < .0001), to have had a prior ipsilateral bypass (33% vs 24%; P = .004) or a prior ipsilateral percutaneous intervention (41% vs 29%; P = .001). Bypasses performed for acute limb ischemia were longer in duration (270 vs 244 minutes; P = .007), had greater blood loss (363 vs 272 mL; P < .0001), and more commonly utilized prosthetic conduits (41% vs 33%; P = .003). Acute limb ischemia patients experienced increased in-hospital major adverse events (20% vs 12%; P < .0001) including myocardial infarction, congestive heart failure exacerbation, deterioration in renal function, and respiratory complications. Patients who underwent lower extremity bypass for acute limb ischemia had no difference in rates of graft occlusion (18.1% vs 18.5%; P = .77), but did have significantly higher rates of limb loss (22.4% vs 9.7%; P < .0001) and mortality (20.9% vs 13.1%; P < .0001) at 1 year. On multivariable analysis, acute limb ischemia was an independent predictor of both major amputation (hazard ratio, 2.16; confidence interval, 1.38–3.40; P = .001) and mortality (hazard ratio, 1.41; confidence interval, 1.09–1.83; P = .009) at 1 year.
Conclusions
Patients who present with acute limb ischemia represent a less medically optimized subgroup within the population of patients undergoing lower extremity bypass. These patients may be expected to have more complex operations followed by increased rates of perioperative adverse events. Additionally, despite equivalent graft patency rates, patients undergoing lower extremity bypass for acute ischemia have significantly higher rates of major amputation and mortality at 1 year.
Acute lower extremity ischemia resulting from arterial embolus, in situ thrombosis, or bypass graft thrombosis remains one of the most common vascular surgery emergencies. The postprocedure rates of mortality and limb loss have traditionally been reported to be as high as 20%–40% and 12%–50%, respectively.1–6 Conventional treatment of patients with acute lower extremity ischemia has been systemic anticoagulation and emergent open surgical intervention, specifically thromboembolectomy or bypass. However, the therapeutic approach to patients presenting with acute lower extremity ischemia underwent a transformation in the mid-1990s with the advent of endovascular therapy, and more specifically, the use of catheter-directed thrombolysis. As reported in several randomized controlled trials,7–12 this endovascular approach offers a less invasive approach to conventional surgical revascularization but often requires more time to restore arterial flow, can be associated with higher rates of hemorrhage, and has not been shown definitively to improve limb salvage. Despite advances in endovascular techniques and equipment,13–15 along with improvements in preventative care and early diagnosis, a significant proportion of patients presenting with acute lower extremity ischemia still require urgent surgical bypass for limb salvage.
The characteristics of patients who present with acute lower extremity ischemia remain ill-defined, particularly in the setting of contemporary application of both elective endovascular and surgical interventions for peripheral vascular disease. Such patients may represent a subgroup of patients with peripheral vascular disease who have more advanced disease or may not be medically optimized, placing them at risk for acute events. The purpose of this study was to utilize data from the prospectively collected Vascular Study Group of New England (VSGNE) database to better characterize the acute lower extremity ischemia patient population and to determine outcomes following lower extremity bypass performed for acute lower extremity ischemia. We hypothesized that patients undergoing lower extremity bypass for acute ischemia represent a less medically optimized subgroup of patients with peripheral vascular disease who are at higher risk for peri-operative adverse events and worse outcomes at 1 year.
METHODS
Study design and database
This study was a retrospective analysis of patients undergoing infrainguinal lower extremity bypass between January 1, 2003 and December 31, 2011 at all 30 centers that participate in the VSGNE. The VSGNE is a regional cooperative quality improvement initiative developed by community and academic centers in New England in 2002 to evaluate regional outcomes in vascular surgery. Details regarding this registry have been previously published and are available at www.vsgne.org.16 All data are self-reported and sent to a central data repository where it is aggregated and reviewed.
Data are collected at the time of the initial operation, including preoperative, intraoperative, and postoperative information. Follow-up data are then collected at 1 year. One-year data collected includes ambulation status, symptom status, graft patency, ankle-brachial index, need for graft revisions, and amputations. Vital status is entered directly into the database and matched to the Social Security Death Index.
Definitions
Patient information for more than 100 clinical and demographic variables was collected (complete list available at www.vsgne.org). Specific comborbidities examined included coronary artery disease (CAD, prior myocardial infarction, or angina), chronic obstructive pulmonary disease (medication-dependent or home oxygen-dependent), congestive heart failure (CHF, by history), diabetes mellitus (insulin-dependent diabetes mellitus or noninsulin-dependent diabetes mellitus, controlled by oral medication or diet), hypertension (history of hypertension or blood pressure ≥140/90 mm Hg on the preoperative evaluation), and history of tobacco use (never, <1 year prior, or current). Renal disease was classified as normal (serum creatinine ≤1.8 mg/dL), renal insufficiency (serum creatinine >1.8 mg/dL), or dialysis-dependent. Relevant surgical history is also obtained at the time of initial operation, including prior ipsilateral and contralateral endovascular and open revascularizations along with prior ipsilateral and contralateral amputations.
Indications for bypass are classified as asymptomatic, claudication, rest pain, tissue loss, and acute ischemia. Urgency of bypass is specified as elective, urgent, or emergent. Pathology treated is identified as occlusive or aneurysm. Patients were included in the study group if the indication for bypass was acute ischemia and the urgency was either urgent or emergent. The study group as defined here was subsequently compared with all other patients undergoing infrainguinal lower extremity bypass.
Immediate postoperative data relating to major inhospital adverse events including the occurrence of myocardial infarction, dysrhythmia, CHF, change in renal function, respiratory complications, and stroke are recorded. Long-term follow-up data included vital status, graft patency, and amputation status. Graft patency is determined at 1 year by the practitioners via one of the following methods: Doppler examination, palpable graft pulse, palpable distal pulse, ankle-brachial index increase >0.15 from preoperative value, or duplex. Postoperative graft surveillance is not standardized across institutions.
Study end points
The primary study end points were bypass graft occlusion, major amputation, and mortality at 1 year postoperatively. Secondary end points included inhospital major adverse events (specifically myocardial infarction, dysrhythmia, CHF, change in renal function, and respiratory complications), along with freedom from major adverse limb events (combined end point of major amputation, new bypass graft, graft revision, or thrombectomy/thrombolysis of bypass) at 1 year and amputation-free survival at 1 year.
Statistical analysis
Baseline characteristics were compared between groups using Pearson χ2 analysis for categorical variables and the t-test for continuous variables. Kaplan-Meier life table analyses were used to calculate all time-to-event end points. Intergroup differences were evaluated using the log rank test. Cox proportional hazards models were constructed to determine independent predictors of major amputation, graft occlusion, and mortality at 1 year. Variables with P < .2 in univariate analysis were included in a backward stepwise selection multivariable analysis. Covariates with P < .05 were included in the final model. All software analyses were performed using SAS 9.1 software (SAS Institute, Cary, NC).
RESULTS
Cohort characteristics
Three hundred twenty-three (5.7%) of 5712 lower extremity bypasses were performed for the indication acute limb ischemia and classified as an emergent or urgent procedure. Specifically, 64% of these bypasses were documented as urgent and 36% as emergent. When compared with those patients undergoing lower extremity bypass for all other indications, this population was no different in age or sex distribution (Table I). Additionally, there were no differences in the rates of CAD, CHF, renal insufficiency, or hemodialysis dependence between the two groups. However, patients undergoing lower extremity bypass for acute ischemia had a higher incidence of chronic obstructive pulmonary disease but a lower incidence of diabetes and hypertension. Furthermore, patients undergoing lower extremity bypass for acute ischemia were more likely to be current smokers, and less likely to be taking a beta-blocker, an aspirin, or a statin.
Table I.
Patient demographics
| Variable | Bypass performed for acute limb ischemia (n = 323) | All other bypasses (n = 5389) | P value |
|---|---|---|---|
| Age, mean, years | 66.3 | 67.4 | .084 |
| Male sex, % | 67.8 | 69.1 | .617 |
| CAD, % | 30.3 | 35.7 | .051 |
| CHF, % | 13.9 | 15.7 | .393 |
| COPD, % | 32.2 | 26.2 | .017 |
| Diabetes, % | |||
| NIDDM | 18.0 | 25.3 | <.001 |
| IDDM | 18.9 | 25.2 | <.001 |
| Hypertension, % | 81.4 | 86.5 | .010 |
| Renal insufficiency, % | 13.9 | 13.1 | .687 |
| Hemodialysis, % | 5.3 | 6.0 | .193 |
| Current tobacco use, % | 48.5 | 39.4 | <.001 |
| Beta-blocker usage, % | 71.1 | 76.7 | .023 |
| Aspirin usage, % | 62.5 | 74.8 | <.001 |
| Statin usage, % | 54.8 | 68.1 | <.001 |
| Prior ipsilateral bypass, % | 32.8 | 23.5 | .004 |
| Prior ipsilateral percutaneous intervention, % | 41.1 | 28.8 | <.001 |
| Aneurysm as indication, % | 14.0 | 6.8 | <.001 |
CAD, Coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; IDDM, insulin-dependent diabetes mellitus; NIDDM, noninsulin-dependent diabetes mellitus.
Patients undergoing lower extremity bypass for acute ischemia were more likely to have had undergone a previous ipsilateral endovascular intervention than those undergoing bypass for all other indications (41.1% vs 28.8%; P < .001). Additionally, these patients were more likely to have undergone a prior ipsilateral bypass (32.8% vs 23.5%; P = .004). Aneurysm as the indication for bypass was more frequent for the acute ischemia group (14.0% vs 6.8%; P < .001).
Intraoperative differences were noted between the two groups. Specifically, patients undergoing lower extremity bypass for acute ischemia had longer operative times than those undergoing lower extremity bypass for all other indications (270 minutes vs 244 minutes; P = .007). These procedures were associated with higher rates of blood loss (363 vs 272 mL; P < .001) and more common use of prosthetic conduit (40.6% vs 32.6%; P = .003). There were no differences seen in the rates of tibial targets (42.4% vs 38.0%; P = .110) between the groups but the use of completion imaging studies was less frequent in the acute ischemia group (46.8% vs 53.7%; P = .039).
Perioperative major adverse events
Patients undergoing lower extremity bypass for acute ischemia experienced an increased rate of in-hospital major adverse events compared with those undergoing bypass for all other indications (19.8% vs 11.6%; P < .0001). Specifically, these patients had higher rates of postoperative myocardial infarctions (7.5% vs 3.6%; P = .001), CHF exacerbations (5.6% vs 3.3%; P = .028), deterioration in renal function (6.6% vs 4.4%; P = .001), and respiratory complications (3.7% vs 1.4%; P = .004) (Table II).
Table II.
Perioperative major adverse events
| Variable | Bypass performed for acute limb ischemia (n = 323) | All other bypasses (n = 5389) | P value |
|---|---|---|---|
| Any in-hospital major adverse event, % | 19.8 | 11.6 | <.0001 |
| Myocardial infarction, % | 7.5 | 3.6 | .001 |
| Dysrhythmia, % | 4.4 | 4.0 | .727 |
| CHF, % | 5.6 | 3.3 | .028 |
| Deterioration in renal function, % | 6.6 | 4.4 | .001 |
| Respiratory, % | 3.7 | 1.4 | .004 |
CHF, Congestive heart failure.
In addition to these medical adverse events, patients undergoing lower extremity bypass for acute ischemia were more likely to return to the operating room (including for amputation) during their hospitalization than those undergoing bypass for all other indications (23.7% vs 11.2%; P < .0001).
One-year outcomes
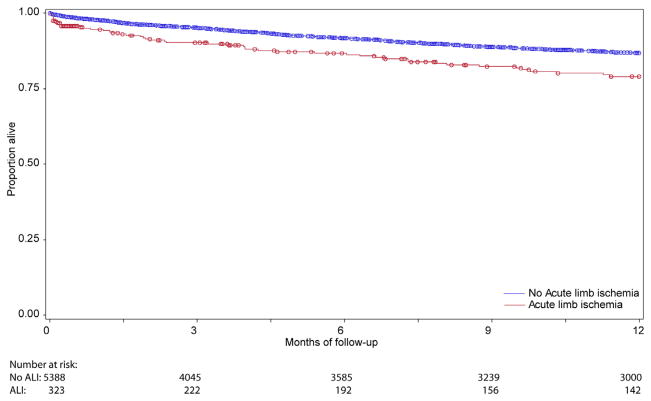
Although patients undergoing lower extremity bypass for acute ischemia experienced no difference in rates of graft occlusion at 1 year compared with patients undergoing bypass for all other indications (18.1% vs 18.5%; P = .77) (Fig 1), these patients did experience higher rates of both major amputation (22.4% vs 9.7%; P < .0001) and mortality at 1 year (20.9% vs 13.1%; P < .0001) (Figs 2 and 3). As a result, amputation-free survival at 1 year was significantly lower for patients undergoing lower extremity bypass for acute ischemia compared with patients undergoing bypass for all other indications (62.8% vs 77.4%; P < .0001) (Fig 4). Freedom from major adverse limb events was lower in patients undergoing lower extremity bypass for acute ischemia (60.4% vs 66.7%; P < .0001). Of the patients undergoing lower extremity bypass for acute ischemia with pathology classified as aneurysm, the 1-year major amputation rate was 13.6% and the 1-year mortality was 20%.
Fig. 1.
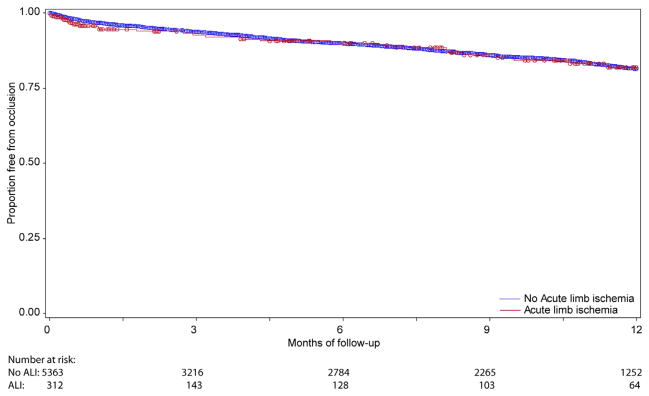
Freedom from graft occlusion.
Fig. 2.
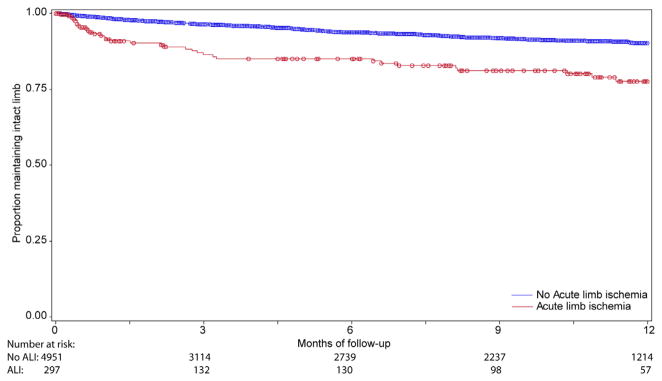
Freedom from major amputation.
Fig. 3.
Survival.
Fig. 4.
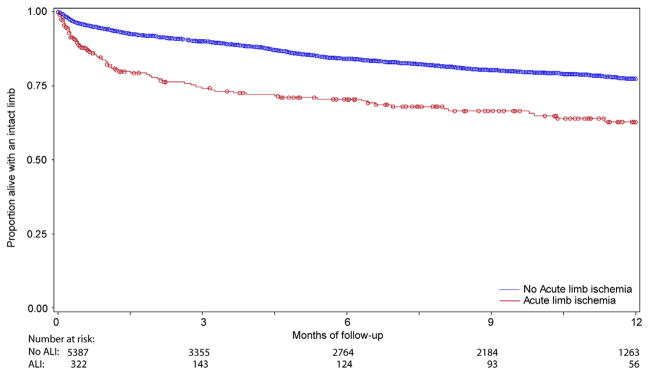
Amputation-free survival.
Multivariable analysis
Acute ischemia was an independent predictor of both amputation (hazard ratio, 2.16; confidence interval, 1.38–3.40; P < .0001) (Table III) and mortality at 1 year (hazard ratio, 1.41; confidence interval, 1.09–1.83; P = .0009) (Table IV) on multivariable analysis.
Table III.
Independent predictors of amputation at 1 year
| HR | 95% CI | P value | |
|---|---|---|---|
| NIDDM | 1.56 | 1.09–2.24 | .016 |
| IDDM | 1.98 | 1.44–2.74 | <.0001 |
| Prosthetic conduit | 2.03 | 1.51–2.72 | <.0001 |
| Prior ipsilateral percutaneous intervention | 2.08 | 1.23–3.53 | .007 |
| Acute ischemia | 2.16 | 1.38–3.40 | <.0001 |
| Tibial target vessel | 2.75 | 2.05–3.69 | <.0001 |
CI, Confidence interval; HR, hazard ratio; IDDM, insulin-dependent diabetes mellitus; NIDDM, noninsulin-dependent diabetes mellitus.
Table IV.
Independent predictors of mortality at 1 year
| HR | 95% CI | P value | |
|---|---|---|---|
| Statin therapy | 0.80 | 0.71–0.91 | .0007 |
| Tibial target | 1.15 | 1.01–1.32 | .036 |
| CAD | 1.19 | 1.05–1.36 | .009 |
| IDDM | 1.26 | 1.08–1.48 | .004 |
| NIDDM | 1.29 | 1.10–1.51 | .029 |
| Prosthetic conduit | 1.30 | 1.13–1.49 | .0002 |
| Acute ischemia | 1.41 | 1.09–1.83 | .009 |
| COPD | 1.46 | 1.28–1.67 | <.0001 |
| Renal insufficiency | 1.58 | 1.36–1.83 | <.0001 |
| CHF | 1.63 | 1.41–1.89 | <.0001 |
| Perioperative major adverse event | 1.73 | 1.48–2.02 | <.0001 |
CAD, Coronary artery disease; CHF, congestive heart failure; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; IDDM, insulin-dependent diabetes mellitus; NIDDM, noninsulin-dependent diabetes mellitus.
DISCUSSION
Despite advances in preventative care along with the increased treatment of both peripheral arterial disease and cardiac dysrhythmias, a significant number of patients continue to present with acute lower extremity ischemia. These patients most often require urgent intervention and have been traditionally thought to be at high risk for both limb loss and death. Therapeutic options include endovascular approaches, most commonly with the use of thrombolytic therapy, surgical approaches including thromboembolectomy and bypass, and primary amputation. Although clinical experience and anecdote might suggest that patients requiring urgent or emergent surgical revascularization will fare worse than their elective counterparts, these outcomes have not been rigorously compared. This study demonstrates that patients with acute lower extremity ischemia who undergo lower extremity bypass, when compared with patients undergoing elective bypass, represent a distinct group who are at higher risk for both perioperative adverse events and worse outcomes, specifically mortality and limb loss, at 1 year.
Although a variety of risk factors predisposing patients to the development of acute lower extremity ischemia have been identified, including peripheral arterial disease, atrial fibrillation, and known hypercoagulable disorders, the characteristics of those patients undergoing lower extremity bypass for acute ischemia remain ill-defined. Patients in this study who presented with acute lower extremity ischemia requiring surgical bypass represented a less medically optimized group of patients when compared with those undergoing elective bypass. While the incidence of multiple medical comorbidities including CAD, CHF, and renal insufficiency were no different between the two groups, there were significantly more active tobacco users in the acute lower extremity ischemia group along with significantly fewer patients on aspirin, beta-blockers, and statins. These lower rates of cardioprotective medication utilization likely not only placed these patients at risk for the inciting acute ischemia event but also predisposed these patients to higher rates of perioperative adverse events. The effect of statin use specifically has been studied previously for patients with lower extremity atherosclerotic disease undergoing elective surgery and has been shown to be associated with decreased perioperative cardiac complications17 along with improved graft patency and limb salvage18 and 1-year postoperative survival.19 Consistent with these findings, our multivariable analysis of predictors of 1-year mortality identified statin use as protective.
In addition to the differences in medical characteristics between patients undergoing bypass for acute lower extremity ischemia and those undergoing elective bypass, there were also significant differences in the rates of both prior ipsilateral bypasses and prior ipsilateral peripheral vascular interventions. Nearly one-third of patients in the acute lower extremity ischemia group had a prior ipsilateral bypass, and over 40% had undergone a prior ipsilateral peripheral vascular intervention. Previously VSGNE investigators, Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial study investigators, and others have demonstrated that failed percutaneous interventions lead to worse outcomes after subsequent bypass with lower rates of limb salvage.20–22 However, to date, the need for acute intervention at the time of percutaneous intervention failure has not been well reported. Although it is not possible to determine the denominator of patients undergoing prior percutaneous intervention in this dataset, it is evident that at the time of failure, a significant proportion will present acutely and require urgent intervention, including the need for surgical bypass. When compared with elective bypass, patients requiring bypass for acute lower extremity ischemia are subject to longer operations with greater blood loss and the more frequent use of prosthetic conduit. The role of previous interventions, both surgical and percutaneous, likely contributes to this increased complexity of such urgent bypasses and their subsequent inferior outcomes.
Given the patient characteristics along with the increased operative time and blood loss, it is not unexpected that patients undergoing lower extremity bypass for acute ischemia are at higher risk for perioperative major adverse events. In this study, patients undergoing lower extremity bypass for acute ischemia had a nearly 20% rate of major adverse perioperative adverse events including a twofold higher incidence of myocardial infarction compared with elective bypass patients. Additionally, these patients were twice as likely to return to the operating room during their initial admission. Unfortunately, patients with acute lower extremity ischemia most often necessitate urgent intervention and many may lack any revascularization option aside from surgical bypass. Accordingly, the ability to medically optimize these patients preoperatively is relatively limited. However, these rates of in-hospital adverse events should prompt the use of strict adherence to the best medical management in the perioperative period.
Along with inferior perioperative outcomes, patients undergoing bypass for acute lower extremity ischemia had inferior outcomes at 1 year, specifically mortality and limb loss, despite no differences in rates of graft secondary patency compared with patients who underwent bypass for all other indications. Although not all of the anatomic factors are known for these patients, specifically the status of their runoff, it is evident that a significant number of patients undergoing bypass for acute lower extremity ischemia will require a major amputation in the setting of a patent bypass. Previously, in a review of nearly 900 patients with thrombosed popliteal artery aneurysms, it has been shown that the adjunctive use of thrombolysis improves graft patency rates but does not reduce the number of amputations, demonstrating that improving patency does not equate to limb salvage in the setting of acute lower extremity ischemia.23 An additional factor that likely contributes to limb loss is the extent of irreversible muscle damage at the time of revascularization necessitating amputation despite adequate revascularization. In review of the Kaplan-Meier curves for amputation, it is evident that the majority of the amputations occur relatively early following surgery as the curves are parallel beyond the first few postoperative months.
In a previous review of factors associated with death after lower extremity bypass in the VSGNE database, emergent nature of the procedure was the strongest predictor of death at 1 year with a hazard ratio of 3.4.24 In this review, acute lower extremity ischemia was an independent predictor of 1-year mortality as well. Furthermore, the occurrence of a perioperative adverse event was also a predictor of 1-year mortality, which patients with acute lower extremity ischemia suffered from at significantly higher rates than other patients. As with the amputations, the Kaplan-Meier curves for survival are parallel beyond the early postoperative period, indicative that the worse 1-year outcomes for patients with acute limb ischemia stem from their initial postoperative courses.
Unfortunately, the VSGNE database cannot be used to make any direct comparisons between the outcomes of patients with acute limb ischemia treated with endovascular interventions vs those treated with surgical bypass. Data on peripheral interventions only began being collected by the VSGNE in 2011, and they have not yet been validated and released for analysis. Taken in aggregate, the earlier randomized trials that attempted to address this specific comparative effectiveness question demonstrated that endovascular intervention could be beneficial in certain clinical settings such as thrombosis (as opposed to embolization) in a limb without motor and sensory compromise.7–11 As these studies are now 15 years old, further work is necessary to better understand which patient subgroups might benefit more from an endovascular approach compared with an open surgical bypass. However, it remains apparent that surgical intervention in these patients continues to be associated with poor outcomes at 1 year.
The primary limitation of this study was its retrospective nature. An additional limitation is that this study is reporting only on outcomes following surgical bypass and does not include patients with acute limb ischemia who were treated with either thromboembolectomy alone or with endovascular techniques. While data in the VSGNE are collected prospectively, the database is queried in a retrospective fashion. Despite the breadth and detail of the information, which is obtained at the time of lower extremity bypass, certain patient factors are not captured. Specifically relevant to this study is the lack of information regarding the exact etiology of the acute ischemia. Although pathology is differentiated between aneurysmal disease and occlusive disease, there is no means of determining whether the occlusive disease is due to an embolic or a thrombotic event. However, in each case, the pathology did require surgical bypass. There is, however, no means of determining if patients in this cohort underwent attempts at thrombolytic therapy or surgical thromboembolectomy immediately prior to their surgical intervention. Furthermore, the presence of a documented hypercoagulable state is not captured nor is a history of atrial fibrillation, both of which would be of relevance to this patient population. Additionally, the data are self-reported, which may lead to observer bias. VSGNE data are collected from multiple institutions and multiple surgeons within those institutions, and, as such, postoperative surveillance protocols may differ, which may also contribute to disparate outcomes.
Despite these important limitations, it is important to recognize that the VSGNE has successfully collected data with very high accuracy and that these data have been validated with regularly scheduled audits.16 These data are generalizable secondary to the diversity of the practitioners and hospitals participating in this quality improvement registry. This report is the largest study to date describing the characteristics of patients undergoing lower extremity bypass for acute limb ischemia and documenting the inhospital and 1-year outcomes that can be anticipated in this setting.
CONCLUSIONS
Lower extremity bypass remains an important tool among the treatment options for patients presenting with acute ischemia despite advances in endovascular therapy. Of patients undergoing lower extremity bypass, patients who present with acute ischemia represent a less medically optimized subgroup that often have had a failed prior endovascular or surgical intervention and can be expected to require longer operations with higher associated blood losses. Postoperatively, these patients will have increased rates of in-hospital perioperative adverse events. Additionally, despite equivalent graft patency rates, patients undergoing lower extremity bypass for acute limb ischemia have significantly higher rates of amputation and mortality at 1 year. Although treatment for these patients most often cannot be delayed or altered, these outcomes should be factored into the decision making process for patients presenting with acute lower extremity ischemia.
Footnotes
Author conflict of interest: none.
Presented at the Society for Clinical Vascular Surgery Forty-first Annual Symposium, Miami, Fla, March 12–16, 2013.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
AUTHOR CONTRIBUTIONS
Conception and design: DB
Analysis and interpretation: DB, NH, AS, DJ, JM, JC
Data collection: DB, NH, AS
Writing the article: DB, VP, JC, AS
Critical revision of the article: DB, VP, DJ, PG, JM, NH, JC, AS
Final approval of the article: DB, VP, DJ, PG, JM, NH, JC, AS
Statistical analysis: NH
Obtained funding: Not applicable
Overall responsibility: DB
References
- 1.Blaisdell FW, Steele M, Allen RE. Management of acute lower extremity arterial ischemia due to embolism and thrombosis. Surgery. 1978;84:822–34. [PubMed] [Google Scholar]
- 2.Aune S, Trippestad A. Operative mortality and long-term survival of patients operated on for acute lower limb ischaemia. Eur J Vasc Endovasc Surg. 1998;15:143–6. doi: 10.1016/s1078-5884(98)80135-4. [DOI] [PubMed] [Google Scholar]
- 3.Ljungman C, Adami HO, Bergqvist D, Berglund A, Persson I. Time trends in incidence rates of acute, nontraumatic extremity ischemia: a population-based study during a 19-year period. Br J Surg. 1991;78:857–60. doi: 10.1002/bjs.1800780727. [DOI] [PubMed] [Google Scholar]
- 4.Ljungman C, Holmberg L, Bergqvist D, Bergström R, Adami HO. Amputation risk and survival after embolectomy for acute arterial ischaemia. Time trends in a defined Swedish population. Eur J Vasc Endovasc Surg. 1996;11:176–82. doi: 10.1016/s1078-5884(96)80048-7. [DOI] [PubMed] [Google Scholar]
- 5.Kuukasjärvi P, Salenius JP. Perioperative outcome of acute lower limb ischaemia on the basis of the national vascular registry. The Finnvasc Study Group. Eur J Vasc Surg. 1994;8:578–83. doi: 10.1016/s0950-821x(05)80594-8. [DOI] [PubMed] [Google Scholar]
- 6.Eliason JL, Wainess RM, Proctor MC, Dimick JB, Cowan JA, Jr, Upchurch GR, Jr, et al. A national and single institutional experience in the contemporary treatment of acute lower extremity ischemia. Ann Surg. 2003;238:382–9. doi: 10.1097/01.sla.0000086663.49670.d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ouriel K, Shortell CK, DeWeese JA, Green RM, Francis CW, Azodo MV, et al. A comparison of thrombolytic therapy with operative revascularization in the initial treatment of acute peripheral arterial ischemia. J Vasc Surg. 1994;19:1021–30. doi: 10.1016/s0741-5214(94)70214-4. [DOI] [PubMed] [Google Scholar]
- 8.Ouriel K, Veith FJ, Sasahara AA. A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. Thrombolysis or Peripheral Arterial Surgery (TOPAS) Investigators. N Engl J Med. 1998;338:1105–11. doi: 10.1056/NEJM199804163381603. [DOI] [PubMed] [Google Scholar]
- 9.Ouriel K, Veith FJ, Sasahara AA. Thrombolysis or peripheral arterial surgery: phase I results. TOPAS Investigators. J Vasc Surg. 1996;23:64–73. doi: 10.1016/s0741-5214(05)80036-9. discussion: 74–5. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson L, Albrechtsson U, Jonung T, Ribbe E, Thorvinger B, Thörne J, et al. Surgical treatment versus thrombolysis in acute arterial occlusion: a randomised controlled study. Eur J Vasc Surg. 1992;6:189–93. doi: 10.1016/s0950-821x(05)80239-7. [DOI] [PubMed] [Google Scholar]
- 11.Weaver FA, Comerota AJ, Youngblood M, Froehlich J, Hosking JD, Papanicolaou G. Surgical revascularization versus thrombolysis for nonembolic lower extremity native artery occlusions: results of a prospective randomized trial. The STILE Investigators. Surgery versus Thrombolysis for Ischemia of the Lower Extremity. J Vasc Surg. 1996;24:513–21. doi: 10.1016/s0741-5214(96)70067-8. discussion: 521–3. [DOI] [PubMed] [Google Scholar]
- 12.Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity. The STILE trial. Ann Surg. 1994;220:251–66. doi: 10.1097/00000658-199409000-00003. discussion: 266–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schrijver A, Vos J, Hoksbergen AW, Fioole B, Fritschy W, Hulsebos R, et al. Ultrasound-accelerated thrombolysis for lower extremity ischemia: multicenter experience and literature review. J Cardiovasc Surg (Torino) 2011;52:467–76. Review. [PubMed] [Google Scholar]
- 14.Sarac TP, Hilleman D, Arko FR, Zarins CK, Ouriel K. Clinical and economic evaluation of the trellis thrombectomy device for arterial occlusions: preliminary analysis. J Vasc Surg. 2004;39:556–9. doi: 10.1016/j.jvs.2003.10.061. [DOI] [PubMed] [Google Scholar]
- 15.Kasirajan K, Gray B, Beavers FP, Clair DG, Greenberg R, Mascha E, et al. Rheolytic thrombectomy in the management of acute and subacute limb-threatening ischemia. J Vasc Interv Radiol. 2001;12:413–21. doi: 10.1016/s1051-0443(07)61878-8. [DOI] [PubMed] [Google Scholar]
- 16.Cronenwett JL, Likosky DS, Russell MT, Eldrup-Jorgensen J, Stanley AC, Nolan BW, et al. A regional registry for quality assurance and improvement: the Vascular Study Group of Northern New England (VSGNNE) J Vasc Surg. 2007;46:1093–101. doi: 10.1016/j.jvs.2007.08.012. discussion: 1101–2. [DOI] [PubMed] [Google Scholar]
- 17.O’Neil-Callahan K, Katsimaglis G, Tepper MR, Ryan J, Mosby C, Ioannidis JP, et al. Statins decrease perioperative cardiac complications in patients undergoing noncardiac vascular surgery: the Statins for Risk Reduction in Surgery (StaRRS) study. J Am Coll Cardiol. 2005;45:336–42. doi: 10.1016/j.jacc.2004.10.048. [DOI] [PubMed] [Google Scholar]
- 18.Henke PK, Blackburn S, Proctor MC, Stevens J, Mukherjee D, Rajagopalin S, et al. Patients undergoing infrainguinal bypass to treat atherosclerotic vascular disease are underprescribed cardioprotective medications: effect on graft patency, limb salvage, and mortality. J Vasc Surg. 2004;39:357–65. doi: 10.1016/j.jvs.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 19.Schanzer A, Hevelone N, Owens CD, Beckman JA, Belkin M, Conte MS. Statins are independently associated with reduced mortality in patients undergoing infrainguinal bypass graft surgery for critical limb ischemia. J Vasc Surg. 2008;47:774–81. doi: 10.1016/j.jvs.2007.11.056. [DOI] [PubMed] [Google Scholar]
- 20.Nolan BW, De Martino RR, Stone DH, Schanzer A, Goodney PP, Walsh DW, et al. Prior failed ipsilateral percutaneous endovascular intervention in patients with critical limb ischemia predicts poor outcome after lower extremity bypass. J Vasc Surg. 2011;54:730–5. doi: 10.1016/j.jvs.2011.03.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: analysis of amputation free and overall survival by treatment received. J Vasc Surg. 2010;51(5 Suppl):18S–31S. doi: 10.1016/j.jvs.2010.01.074. [DOI] [PubMed] [Google Scholar]
- 22.Al-Nouri O, Krezalek M, Hershberger R, Halandras P, Gassman A, Aulivola B, et al. Failed superficial femoral artery intervention for advanced infrainguinal occlusive disease has a significant negative impact on limb salvage. J Vasc Surg. 2012;56:106–10. doi: 10.1016/j.jvs.2011.10.108. discussion: 110–1. [DOI] [PubMed] [Google Scholar]
- 23.Kropman RH, Schrijver AM, Kelder JC, Moll FL, de Vries JP. Clinical outcome of acute leg ischaemia due to thrombosed popliteal artery aneurysm: systematic review of 895 cases. Eur J Vasc Endovasc Surg. 2010;39:452–7. doi: 10.1016/j.ejvs.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Goodney PP, Nolan BW, Schanzer A, Eldrup-Jorgensen J, Stanley AC, Stone DH, et al. Factors associated with death 1 year after lower extremity bypass in Northern New England. J Vasc Surg. 2010;51:71–8. doi: 10.1016/j.jvs.2009.07.123. [DOI] [PubMed] [Google Scholar]