Abstract
A posteriori evidence suggests that radiotherapy to a targeted tumor can elicit an immune-mediated abscopal (ab-scopus, away from the target) effect in non-targeted tumors, when combined with an anti-cytotoxic T-lymphocyte antigen-4 monoclonal (CTLA-4) antibody. Concurrent radiotherapy and ipilimumab (a human monoclonal anti-CTLA-4 antibody) induced immune-mediated abscopal effects in poorly immunogenic pre-clinical tumor models and metastatic melanoma patients. However, no such reports exist for patients with metastatic lung adenocarcinoma. We report the first abscopal response in a treatment-refractory lung cancer patient treated with radiotherapy and ipilimumab. A post-treatment increase in tumor-infiltrating cytotoxic lymphocytes, tumor regression, and normalization of tumor markers was observed. One year after treatment with concurrent radiotherapy and ipilimumab the patient is without evidence of disease.
Introduction
The abscopal (ab-scopus, away from the target) effect is a term used to describe radiotherapy (RT)-induced tumor regression in lesions distant from a targeted site, and has been known for six decades as a rare unexplained phenomenon in patients receiving local RT [1]. We hypothesized that the abscopal effect may result from an RT-induced immunogenic type of cancer cell death capable of generating an in situ vaccine [2-4]. In support of this notion, interventions that promote the functionality of dendritic cells or improve T cell activation are required to produce the abscopal effect [5-8]. This strongly suggests that, while RT alone may be efficient at exposing cryptic tumor antigens, tumor cell-induced immune-suppression and immune-tolerance hamper the development of therapeutically effective antitumor immune responses [3, 4].
Immunotherapeutic strategies aimed at overcoming immune-tolerance and improving the activation of antitumor T cells represent a new promising therapeutic approach [9]. Among them, the human anti-cytotoxic T-lymphocyte antigen-4 (CTLA-4) antibody, ipilimumab, has demonstrated activity in metastatic melanoma treatment and for which it has FDA approval [10, 11]. Yet, the role of ipilimumab in other malignancies and in combination with RT still remains investigational.
In non-small cell lung cancer (NSLC), ipilimumab has been tested in combination with chemotherapy (paclitaxel [175 mg/m2 body surface area] and carboplatin [area under the curve, 6], infused every-3-weeks) in a phase II trial, including 204 patients with stage IIIB/IV or recurrent disease [12]. Induction ipilimumab was administered every-3-weeks for 4 doses at 10 mg/Kg body weight, either concurrently with chemotherapy (concurrent regimen) or after two doses of chemotherapy (phased regimen). Patients without disease progression or adverse effects to ipilimumab continued with maintenance therapy once every-12-weeks. The study met its primary endpoint of improved immune-related progression free survival (irPFS, takes into account tumor regression in the presence of new lesions) and the endpoint of progression-free survival (PFS) for the phased regimen, but not the concurrent regimen, when compared to chemotherapy alone (control regimen) [12, 13].
A difference was observed in the immune-related best overall response rates (irBORR) between the control regimen and the phased regimen, 18% versus 32%. In addition, a difference was observed in the median progression free survival (PFS) between the control regimen and the phased regimen, 4.2 months versus 5.1 months. However, no difference was observed in the irBORR between the control regimen and the concurrent regimen, 18% versus 21%. Also, no difference was observed in the median PFS between the control regimen and the concurrent regimen, 4.2 months versus 4.1 months. Of note, on subset analysis, the non-squamous histology group, including adenocarcinomas, treated with the phased regimen demonstrated a trends towards a worsened HR for overall survival, when compared with chemotherapy alone (HR, 1.17 [95% CI, 0.74 to 1.86]). Because of these results, patients with squamous cell histology are currently being recruited for a phase III trial comparing the phased regimen with the control regimen for first-line treatment [14].
The improved efficacy of the phased approach, as opposed to the concurrent regimen, suggests that additional factors (other than CTLA-4 blockade) influence tumor-specific T cell responses in advanced stage NSCLC patients. The observed differences may have been the result of the quality of tumor cell death (immunogenic vs. non-immunogenic) or the immune-modifying effects (inhibitory vs. stimulatory) of chemotherapy at the time of ipilimumab administration [4, 12] These are some of issues that underscore the challenges that remain in designing optimal combination therapies with ipilimumab.
Interestingly, when given as a monotherapy in NSCLC patients CTLA-4 blockade demonstrated no difference in PFS as compared to best supportive care (BSC). In a phase II trial, 87 NSCLC patients (locally advanced or metastatic) treated with ≥4 cycles of first-line platinum based therapy (resulting in either stable disease or response per RECIST criteria) were randomized to tremelimumab (a CTLA-4 blocking immunoglobulin G2 monoclonal antibody) as maintenance therapy (N=43) or BSC (N=43) [15]. Tremelimumab did not improve PFS; however, 2 (4.8%) partial responses (out of 9 patients without disease progression) were seen in the tremelimumab arm, whereas no partial responses (out of 6 patients without disease progression) were seen in the BSC arm. Based on these results as a single agent in NSCLC, future development of tremelimumab has not been pursued [14].
We previously demonstrated in pre-clinical models of poorly immunogenic carcinomas not responsive to anti-CTLA-4 monotherapy that local RT synergizes with anti-CTLA-4 antibody to induce anti-tumor T cell responses that inhibit the growth of locally irradiated tumors as well as their non-irradiated metastatic counterparts (abscopal effect) [5, 8, 16]. Consistent with these findings, an abscopal effect was recently reported in two treatment-refractory melanoma patients receiving RT with ipilimumab [17, 18]. However, it is unknown whether RT can potentiate the response to CTLA-4 blockade in tumor types that have previously shown little-to-no clinical response. Herein, we report the first case of an abscopal response in a patient diagnosed with metastatic NSCLC treated with RT and ipilimumab. Remarkably, the patient demonstrated regression, not only at the RT-targeted site but also in multiple abscopal sites of disease, including visceral and skeletal metastases.
Case Report
In March 2010, a 64-year-old Caucasian male with a 70 cigarette-pack-year history presented with a palpable left supraclavicular nodule. An excisional biopsy of the mass demonstrated metastatic adenocarcinoma with an immunohistochemical profile consistent with a lung primary (CK7 and TTF-1 positive and CK20 and CDX2 negative). The patient's initial PET/CT demonstrated 2 right upper lobe nodules, a left lower lobe nodule, and right supraclavicular and bilateral hilar/mediastinal adenopathy. He was staged T1bN3M1a (stage IV), according to the American Joint Commission on Cancer 7th edition cancer staging manual, with a predicted median survival of 7 months [19]. The patient was initiated on pemetrexed 500 mg/m2 body surface area (BSA) and carboplatin (area under the curve, 5) given every-3-weeks for 6 cycles. After the 6th cycle, a surveillance PET/CT demonstrated a decrease in size and metabolic activity of both the right supraclavicular adenopathy (from 2.8 × 1.7 cm and SUV of 10.2 to 2.0 × 1.2 cm and SUV of 3.8) and the left lower lobe nodule (from 6 mm and SUV of 2.3 to 3 mm and undetectable SUV). The 2 right upper lobe nodules and hilar/mediastinal nodal disease remained stable in size and metabolic activity.
The patient, thereafter, continued maintenance therapy with pemetrexed 500 mg/m2 BSA alone, given every-3-weeks. However, after 3 cycles, he developed severe lower extremity cellulitis, at which point the pemetrexed was temporarily discontinued. After antibiotic treatment and resolution of the cellulitis, the patient received an additional 3 cycles of pemetrexed and subsequently underwent a repeat PET/CT. The PET/CT revealed stable disease in the right upper lobe and left lower lobe nodules and improvement in the size and metabolic activity in the right supraclavicular and hilar/mediastinal adenopathy.
From February 2011 to April 2011, systemic chemotherapy was interrupted to start RT to the metabolically active right lung nodules and the right supraclavicular, right hilar, and mediastinal adenopathy to a total dose of 59.4 Gy delivered over 33 fractions. The patient's subsequent chest CTs in May and July 2011, in comparison to the CT before radiotherapy, demonstrated a decrease in size of the irradiated pulmonary nodules and adenopathy. However, in September 2011, a surveillance PET/CT revealed increased metabolic activity and size of the right upper lobe nodule and the previously seen left lower lobe nodule. The patient resumed treatment with pemetrexed 500 mg/m2 BSA alone, given every-3-weeks for an additional 10 cycles.
In June 2012, a repeat PET/CT revealed disease progression with new FDG-avid hepatic lesions, new periaortic adenopathy, and a new bony lesion in the sacrum. Additionally, the right upper lobe and left lower lobe nodules and hilar/mediastinal adenopathy demonstrated an increase in metabolic activity. The patient was then treated with gemcitabine 750 mg/m2 BSA and vinorelbine 30 mg/m2 BSA given every-2-weeks. After the 4th cycle, in August 2012, a PET-CT demonstrated further disease progression in the liver and growth of new lytic lesions in the bony pelvis, thoracolumbar spine, and right humerus (Figures 1 and 2).
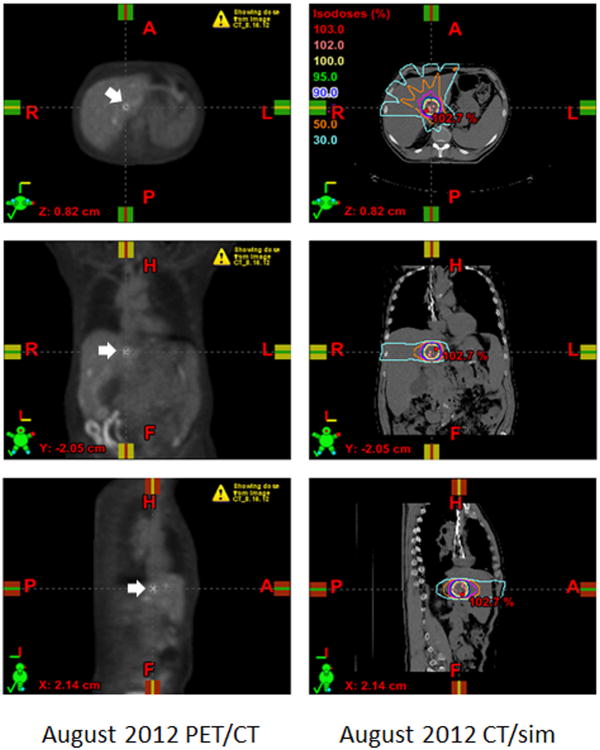
Figure 1. Registration of PET/CT and CT/simulation to target intrahepatic lesion.
The PET/CT from August 2012 (left panels) was imported into the Eclipse planning software (Varian Medical Systems, Inc., Palo Alto, California) and registered to the CT/simulation (right panels) that was acquired prior to treatment. Select axial (top panels), coronal (middle panels), and sagittal (bottom panels) images are displayed. The most hypermetabolic liver lesion was selected as the GTV (white arrows, left panels). The treatment plan was designed with 6-MV photons by means of a coplanar 5-field intensity-modulated technique to encompass the GTV with a 1 cm margin (right panels). The treatment was prescribed to the 100% isodose line to a total dose of 30 Gy distributed over 5 fractions. The isodose lines represent total doses of 30 Gy (yellow), 15 Gy (orange), and 9 Gy (light blue) (right panels).
Figure 2. Ipilimumab and local RT result in an abscopal response.
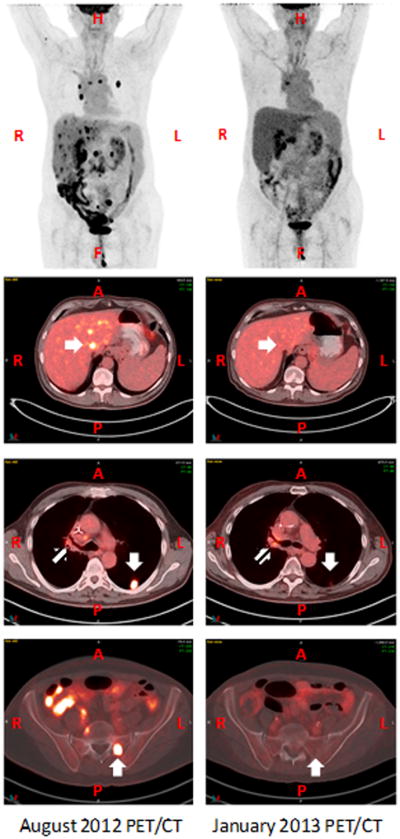
PET imaging and select fused PET/CT axial images from August 2012 (left panels) and January 2013 (right panels) are displayed. The axial images in the second row demonstrate the hypermetabolic liver lesion that was targeted and responded to RT (white arrows, second row). An abscopal response was seen in a left lower lobe lung lesion (white arrows, third row) and a left sacral lesion (white arrows, bottom row). A mixed response was seen in the hilar/mediastinal lymph nodes (striped arrows, third row).
The patient was thereafter initiated on ipilimumab (received as a compassionate exemption) and local RT to one of the hepatic metastases with the intent to generate an abscopal response. The experimental nature of this approach was extensively discussed with the patient, who was informed of the only two available reports in melanoma and the lack of available evidence for NSCLC patients.
The patient was simulated in the supine position and his CT/simulation was registered to the August 2012 PET/CT for treatment planning purposes (Figure 1). The most metabolically active liver mass, located in the caudate lobe, was selected as the RT target and contoured as the gross tumor volume (GTV). An additional 0.5 cm margin was added to create a clinical target volume (CTV) and another 0.5 cm margin was added to the CTV to create a planning target volume (PTV) (Figure 1). RT to a total dose of 30 Gy distributed over 5 fractions was delivered over a period of 10 days with 6-MV photons and a coplanar 5-field intensity-modulated technique (Figure 1). The day after the first RT fraction, the patient was infused with ipilimumab, 3 mg/Kg body weight. Thereafter, the patient completed 3 more cycles of ipilimumab, 3 mg/Kg body weight, infused at 3-week intervals. The patient tolerated RT and ipilimumab without any treatment-related adverse events. Maintenance infusions of ipilimumab were not given afterwards.
Results
After treatment with RT in combination with ipilimumab, a post-treatment chest CT (November 2012) and PET/CT (January 2013) demonstrated a dramatic treatment response of the patient's known disease. Not only was an objective response detected in the RT field, but striking responses were also observed at distant sites (Figure 2). Metabolic activity seen previously at the irradiated site in the liver has resolved. Additionally, there was resolution of multiple non-irradiated foci within the liver and the osseous metastases. There was significant decrease in the metabolic activity and size of the left lower lobe nodule and the previously irradiated right upper lobe nodule. Though, a mixed metabolic response was seen in the right hilar adenopathy (increased SUV uptake from 4 to 5.4).
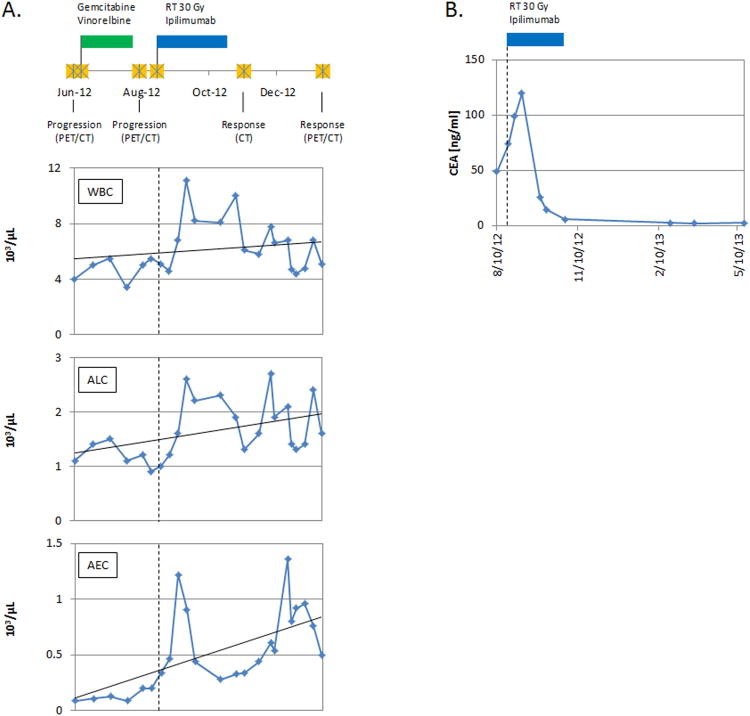
After treatment with ipilimumab and RT, there was an increase in absolute lymphocyte counts (ALCs) and absolute eosinophil counts (AECs), two biomarkers associated with improved survival rates in ipilimumab-treated melanoma patients (Figure 3A) [20-22]. An ALC ≥ 1000/μl of whole blood (vs. ALC < 1000/μl of whole blood) prior to the 3rd infusion of ipilimumab, 10 mg/Kg body weight, in chemotherapy-refractory melanoma patients predicted for higher 6 month (75% vs. 0%) and 12 month (47% vs. 0%) survival rates [20-22]. Also, an AEC increase >100/μl of whole blood between the 1st two ipilimumab infusions was associated with a longer median survival (11.3 months vs. 6.8 months) [22]. We observed that the ALC increased after RT and ipilimumab treatment (1100/μl of whole blood at baseline, 2700/μl of whole blood at peak levels, and 2200/μl of whole blood prior to the 3rd infusion of ipilimumab) and that the AEC increased between the 1st two infusions of ipilimumab (200/μl of whole blood prior to the 1st infusion and 470/μl of whole blood prior to the 2nd infusion of ipilimumab) (Figure 3A). Additionally, the post-treatment CEA levels (a non-specific tumor marker) demonstrated a dramatic drop to normal levels after a peak of 119.6 ng/ml (Figure 3B).
Figure 3. Treatment timeline and the absolute peripheral blood cell counts.
A detailed clinical timeline is displayed (A, top panel). A PET/CT on June 8, 2012 demonstrated disease progression prompting a change in the patient's chemotherapy regimen. On June 15, 2012, the patient was started on a chemotherapy regimen containing gemcitabine and vinorelbine. The green marker demarcates the treatment timeline for gemcitabine and vinorelbine (A, top panel). A repeat PET/CT on August 6, 2012, demonstrated continued disease progression. From August 22, 2012 to August 31, 2012 the patient was treated with concurrent RT and ipilimumab. Afterward, he received 3 additional cycles of ipilimumab alone. The blue marker demarcates the treatment timeline for RT and ipilimumab (A [top panel] and B) and data plotted to the right of the vertical dashed line in each graph represents post initiation of RT and ipilimumab treatment (A [bottom 3 panels] and B). The final dose of ipilimumab was given on October 26, 2012. Imaging on November 8, 2012 (CT of the chest abdomen and pelvis) and January 17, 2013 (PET/CT) demonstrated significant treatment responses. During the course of treatment, the patient had serial blood draws. The results of the peripheral absolute blood cell counts (white blood cells [WBCs], ALCs, and AECs) are displayed as number of cells [×103] per μL of whole blood (A, bottom 3 panels), in accordance with the aforementioned treatment timeline (A, top panel). A dramatic drop in CEA levels (a non-specific tumor marker) was observed after treatment with RT and ipilimumab (B). The marker peaked at 119.6 ng/ml (normal levels 0-5 ng/ml) on September 7, 2012, demonstrated a dramatic drop to 5.8 ng/ml on October 26, 2012, and thereafter was maintained at normal levels.
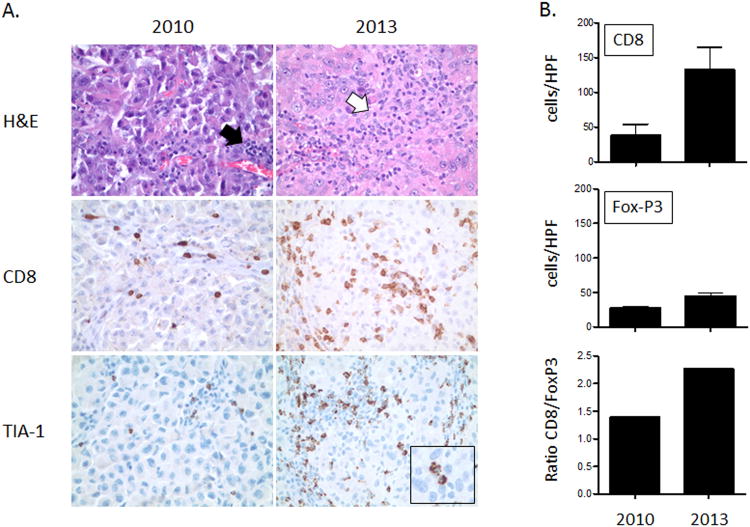
Although the tumor markers remained at normal levels, a PET/CT on April 2013 demonstrated an isolated increase in metabolic activity of a new non-irradiated left supraclavicular lymph node. The lymph node was excised and was seen to have recurrent disease. However, to our surprise, this specimen exhibited distinct immunologic differences in comparison to the previously excised left supraclavicular lymph node from 2010. Upon staining with hematoxylin and eosin (H&E), lymphocytic infiltration was largely confined to perivascular areas in the 2010 biopsy, whereas the biopsy from 2013 demonstrated lymphocyte-infiltration into the tumor cell nests (Figure 4A). On further immunohistochemical analysis with CD8 (a marker for cytotoxic T cells) and TIA-1 (a marker for cytotoxic granules) stains, the specimen from 2013 demonstrated a marked increase in CD8+ and TIA+ cells (Figure 4A-B). Additionally, FoxP3+ (a marker for regulatory T cells) cells were also increased in the 2013 specimen, although the ratio of CD8+/FoxP3+ cells was much higher in the 2013 specimen (Figure 4B).
Figure 4. Enhanced tumor-infiltrating lymphocytes in an abscopal lesion.
A treatment-naive left supraclavicular nodal metastasis was excised in 2010 and an adjacent non-irradiated nodal metastasis was excised in 2013 (post-treatment with ipilimumab and local RT). The immunologic characteristics of the two specimens were compared (A). An H&E stain demonstrated lymphocytic infiltration largely confined to perivascular areas in the 2010 biopsy specimen (A, top left panel–black arrow), whereas lymphocytes-infiltrated tumor cell nests in the 2013 biopsy specimen (A, top right panel–white arrow). Rare CD8+ cells were present in the 2010 specimen (A, middle left panel). However, a marked increase in CD8+ and TIA+ (a marker for cytotoxic granules) cells was present in the 2013 specimen (A, middle right panel). The inset demonstrates TIA-1+ cells directly interacting with tumor cells (A, bottom right panel). Cells were counted in 10 randomly selected high power fields (HPFs) (B). CD8+ cells were significantly increased (p<0.0001) in the biopsy from 2013. FoxP3+ cells also increased (p<0.05), but the ratio of CD8+/FoxP3+ cells was higher in the 2013 specimen.
An increased ratio of effector to regulatory T cells following anti-CTLA-4 treatment is a hallmark of successful tumor rejection [23]. The persistence of tumor cells in this lesion suggests the possibility for the development of adaptive resistance mediated by tumor cell upregulation of ligands inhibitory to T cells [14]. Importantly, the detected PET/CT signal in the excised lesion, although non-specific, could be caused by both heightened antitumoral inflammation and the proliferation of tumor cells, suggesting caution in the interpretation of such signals in patients following immunotherapy [13].
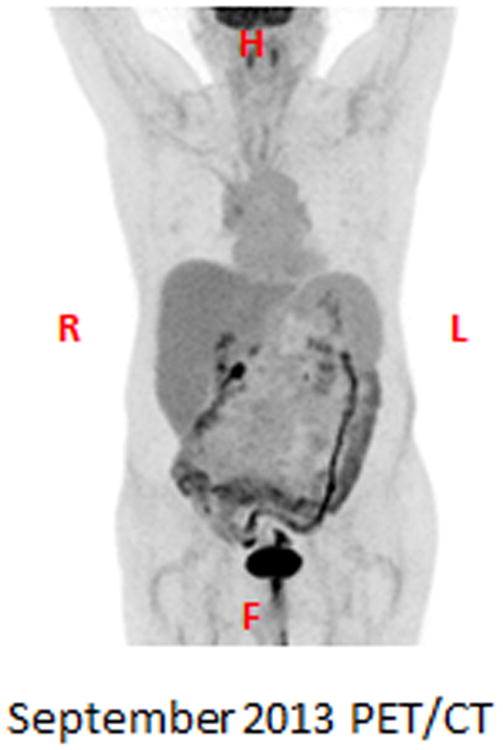
After excision of the newly hypermetabolic left supraclavicular lymph node, the patient underwent further systemic treatment with ipilimumab alone. From June 2013 to August 2013 the patient received an additional 4 cycles of ipilimumab 3mg/Kg given every 3 weeks. In September 2013, one year after treatment with concurrent radiotherapy and ipilimumab, the patient's new PET/CT demonstrated no evidence of disease (Figure 5).
Figure 5. PET image one year after treatment with concurrent radiotherapy and ipilimumab.
A PET/CT was completed September 2013, one year after treatment with concurrent radiotherapy and ipilimumab. The PET image is displayed and demonstrates no evidence of disease.
Discussion
Historically, abscopal responses are of rare occurrence. Few cases have been reported in several tumor types, including melanoma, renal cell carcinoma, and lymphoma [17, 18, 24-26]. The remarkable abscopal response seen in this patient after treatment with local RT and ipilimumab is consistent with data in preclinical models that this combination can induce therapeutically effective antitumor immune responses to poorly immunogenic carcinomas [5, 8, 16]. In the 4T1 mouse model, tumor-specific CD8 T cells were responsible for tumor regression. The synergy between RT and anti-CTLA-4 antibody was at least in part due to improved recruitment of these effector T cells to the tumor and their enhanced interaction with tumor cells via NKG2D receptor engagement [16, 27]. In addition, tumor-specific CD8 T cells were expanded in mice treated with RT and anti-CTLA-4 antibody, consistent with the ability of RT to generate an in situ tumor vaccine [28].
Recently, an abscopal response was reported in a patient with NY-ESO-1 (cancer-testis antigen) positive melanoma treated with local RT in combination with ipilimumab [17]. The patient received ipilimumab at a dose of 10 mg/Kg body weight every-3-weeks, for a total of four doses, as part of her induction therapy. Her follow-up CT scan showed overall stable disease. Nevertheless, while on maintenance ipilimumab (given every-12-weeks), the patient demonstrated radiographic evidence of disease progression. Particularly, there was growth of a paraspinal mass, causing her right-sided back pain. However, when local RT (28.5 Gy in 3 fractions over a period of 7 days) was administered to the paraspinal metastasis, concurrently with maintenance ipilimumab, an abscopal response was detected 4 months later. Concurrent treatment led to the regression of distant disease in the spleen and mediastinal lymph nodes. Interestingly, the therapeutic response temporarily correlated with an increase in antibody titers targeting epitopes in the central portion of NY-ESO-1 and other tumor-associated antigens, an increase in CD4+ T-cell and myeloid lineage activation, and a decline in the quantity of myeloid-derived suppressor cells.
Herein, we report the first abscopal response seen 2 ½ months after the start of treatment with ipilimumab and fractionated RT in a patient with chemotherapy-refractory metastatic adenocarcinoma of the lung. We treated this patient with an RT dose and fractionation schedule similar to that used to convert tumor cells into an in situ vaccine and generate an abscopal response in our preclinical model [5]. Although, CTLA-4 blockade as a monotherapy or given as a concurrent regimen with chemotherapy did not show a benefit in PFS, the possibility that ipilimumab alone may be responsible for this patient's response cannot be ruled out [12, 15]. Nevertheless, this case report supports the belief that a combination of RT and immunotherapy might prove to be a useful strategy to improve the outcomes of some cancer patients with metastases that are historically known to have dismal prognoses [3]. In conclusion, this approach in NSCLC and other types of cancer may represent a new paradigm worthy of establishing clinical trials for patients with advanced disease.
References
- 1.Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol. 1953;26:234–241. doi: 10.1259/0007-1285-26-305-234. [DOI] [PubMed] [Google Scholar]
- 2.Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10:718–726. doi: 10.1016/S1470-2045(09)70082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105:256–265. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma Y, Kepp O, Ghiringhelli F, Apetoh L, Aymeric L, Locher C, Tesniere A, Martins I, Ly A, Haynes NM, et al. Chemotherapy and radiotherapy: cryptic anticancer vaccines. Semin Immunol. 2010;22:113–124. doi: 10.1016/j.smim.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, Formenti SC. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Chakravarty PK, Alfieri A, Thomas EK, Beri V, Tanaka KE, Vikram B, Guha C. Flt3-ligand administration after radiation therapy prolongs survival in a murine model of metastatic lung cancer. Cancer Res. 1999;59:6028–6032. [PubMed] [Google Scholar]
- 8.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, Formenti SC. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–734. [PubMed] [Google Scholar]
- 9.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robert C, Thomas L, Bondarenko I, O'Day S, M DJ, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 12.Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, Sebastian M, Neal J, Lu H, Cuillerot JM, Reck M. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30:2046–2054. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 13.Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 14.Brahmer JR, Pardoll DM. Immune checkpoint inhibitors: making immunotherapy a reality for the treatment of lung cancer. Cancer Immunol Res. 2013;1:85–91. doi: 10.1158/2326-6066.CIR-13-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zatloukal P, Heo DS, Park K, Kang J, Butts C, Bradford D, Graziano S, Huang B, Healy D. Randomized phase II clinical trial comparing tremelimumab (CP-675,206) with best supportive care (BSC) following first-line platinum-based therapy in patients (pts) with advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2009;27:15s. suppl; abstr 8071. [Google Scholar]
- 16.Ruocco MG, Pilones KA, Kawashima N, Cammer M, Huang J, Babb JS, Liu M, Formenti SC, Dustin ML, Demaria S. Suppressing T cell motility induced by anti-CTLA-4 monotherapy improves antitumor effects. J Clin Invest. 2012;122:3718–3730. doi: 10.1172/JCI61931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The Abscopal Effect Associated With a Systemic Anti-melanoma Immune Response. Int J Radiat Oncol Biol Phys. 2013;85:293–295. doi: 10.1016/j.ijrobp.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edge SB. American Joint Committee on Cancer: AJCC cancer staging manual. 7th. New York; London: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 20.Ku GY, Yuan J, Page DB, Schroeder SE, Panageas KS, Carvajal RD, Chapman PB, Schwartz GK, Allison JP, Wolchok JD. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116:1767–1775. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Callahan MK, Wolchok JD, Allison JP. Anti-CTLA-4 antibody therapy: immune monitoring during clinical development of a novel immunotherapy. Semin Oncol. 2010;37:473–484. doi: 10.1053/j.seminoncol.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delyon J, Mateus C, Lefeuvre D, Lanoy E, Zitvogel L, Chaput N, Roy S, Eggermont AM, Routier E, Robert C. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann Oncol. 2013;24:1697–1703. doi: 10.1093/annonc/mdt027. [DOI] [PubMed] [Google Scholar]
- 23.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robin HI, AuBuchon J, Varanasi VR, Weinstein AB. The abscopal effect: demonstration in lymphomatous involvement of kidneys. Med Pediatr Oncol. 1981;9:473–476. doi: 10.1002/mpo.2950090510. [DOI] [PubMed] [Google Scholar]
- 25.Wersall PJ, Blomgren H, Pisa P, Lax I, Kalkner KM, Svedman C. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncol. 2006;45:493–497. doi: 10.1080/02841860600604611. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan RJ, Lawrence DP, Wargo JA, Oh KS, Gonzalez RG, Piris A. Case records of the Massachusetts General Hospital. Case 21-2013. A 68-year-old man with metastatic melanoma. N Engl J Med. 2013;369:173–183. doi: 10.1056/NEJMcpc1302332. [DOI] [PubMed] [Google Scholar]
- 27.Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, Babb JS, Schneider RJ, Formenti SC, Dustin ML, Demaria S. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181:3099–3107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pilones KA, Kawashima N, Yang AM, Babb JS, Formenti SC, Demaria S. Invariant natural killer T cells regulate breast cancer response to radiation and CTLA-4 blockade. Clin Cancer Res. 2009;15:597–606. doi: 10.1158/1078-0432.CCR-08-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]