Abstract
Objective
Although carotid endarterectomy (CEA) is performed to prevent stroke, long-term survival is essential to ensure benefit, especially in asymptomatic patients. We examined factors associated with 5-year survival following CEA in patients with asymptomatic internal carotid artery (ICA) stenosis.
Methods
Prospectively collected data from 4114 isolated CEAs performed for asymptomatic stenosis across 24 centers in the Vascular Study Group of New England between 2003 and 2011 were used for this analysis. Late survival was determined with the Social Security Death Index. Cox proportional hazard models were used to identify risk factors for mortality within the first 5 years after CEA and to calculate a risk score for predicting 5-year survival.
Results
Overall 3- and 5-year survival after CEA in asymptomatic patients were 90% (95% CI 89%-91%) and 82% (95% CI 81%-84%), respectively. By multivariate analysis, increasing age, diabetes, smoking history, congestive heart failure, chronic obstructive pulmonary disease, poor renal function (estimated glomerular filtration rate <60 or dialysis dependence), absence of statin use, and worse contralateral ICA stenosis were all associated with worse survival. Patients classified as low (27%), medium (68%), and high risk (5%) based on number of risk factors had 5-year survival rates of 96%, 80%, and 51%, respectively (P < .001).
Conclusions
More than four out of five asymptomatic patients selected for CEA in the Vascular Study Group of New England achieved 5-year survival, demonstrating that, overall, surgeons in our region selected appropriate patients for carotid revascularization. However, there were patients selected for surgery with high risk profiles, and our models suggest that the highest risk patients (such as those with multiple major risk factors including age ≥80, insulin-dependent diabetes, dialysis dependence, and severe contralateral ICA stenosis) are unlikely to survive long enough to realize a benefit of prophylactic CEA for asymptomatic stenosis. Predicting survival is important for decision making in these patients.
Carotid endarterectomy (CEA) is commonly performed for primary and secondary stroke prevention, with nearly 120,000 CEAs performed annually in the United States.1 Evidence supporting CEA for the treatment of cerebrovascular disease is well-established and demonstrates a substantial reduction in the risk of stroke for patients with symptomatic disease.2 However, in the face of improving medical therapy, many have begun to call into question the appropriateness of CEA for asymptomatic patients,3,4 where the net benefit from preventative surgery is significantly less than that for patients with symptomatic stenosis.5,6
In terms of establishing “appropriateness” for asymptomatic CEA, several professional societies, including the Society for Vascular Surgery7 and the American Heart Association,8 offer guidelines stating that asymptomatic patients with at least a 60% carotid artery stenosis should be considered for CEA only if the patient has a predicted risk of perioperative stroke/death that is ≤3% and a minimum life expectancy of 3-5 years. Although many models exist to predict patients’ risk of perioperative stroke/death,9,10 similar data do not exist to help surgeons select patients whose life expectancy is at least 3-5 years from the time of surgery. In fact, our prior work indicates that as many as 20% of asymptomatic CEAs are performed in patients with life limiting conditions, one-half of whom are unlikely to survive 5 years from the time of CEA.11 Therefore, the purpose of this study was to describe long-term survival in asymptomatic patients undergoing CEA using data from the Vascular Study Group of New England (VSGNE). We used these data to develop a model for predicting a patient's risk of death within 5 years, to aid surgeons in their clinical decision making.
METHODS
Subjects and databases
For this report, we analyzed data collected as part of the VSGNE, a regional cooperative quality improvement initiative developed in 2002. Further details on the registry have been published previously12 and are available at www.vsgne.org.
Data were examined from 8021 patients undergoing primary, unilateral CEA performed by 114 participating surgeons, across 24 study hospitals between January 1, 2003 and January 1, 2011. Of these, we excluded 187 that were combined with coronary artery bypass graft, leaving 7834 isolated, primary CEAs for our analysis. Trained nurses, physicians, or clinical data abstractors entered data prospectively on over 100 clinical and demographic variables. Mortality was determined by matching patients with the Social Security Death Index.
Establishing a cohort of asymptomatic patients
Because the indications for revascularization of symptomatic carotid stenosis vary dramatically from those for asymptomatic disease, all patients with any prior symptoms were excluded from our analysis. Symptomatic status was assigned to 3656 patients with a documented history of one or more of the following: ipsilateral or contralateral ocular or cortical cerebrovascular accident or transient ischemic attack (TIA); vertebrobasilar cerebrovascular accident or TIA; or “non-specific” symptoms. An additional 64 patients underwent CEAs classified as urgent or emergent and were assumed to be symptomatic. The remaining 4114 patients were designated as asymptomatic (elective surgery, no history of any cerebrovascular symptoms) and were included in our cohort for analysis. All stenosis criteria were determined by duplex ultrasonography.
Predicting 5-year mortality following CEA
Survival analysis was performed in two ways. First, Kaplan-Meier survival analysis with log-rank test (for categorical variables) and Cox proportional hazard regression (for continuous variables) were used to examine univariate associations between mortality and a variety of patient, operative, and hospital characteristics. Second, all variables that were associated with mortality with P < .2 were entered into a multivariate model and backwards stepwise Cox proportional hazard regression with nested likelihood ratios was performed to generate a final model for predicting mortality at 5 years.
Assignment of low, medium, or high risk classification to each patient in the cohort
Next, we created three strata of risk for mortality: low, medium, and high. To establish these risk strata, each covariate in our prediction model was classified as either a major or minor risk factor for mortality based on the relative contribution of the variable to the final regression equation. Relative contribution was determined by examining the β-coefficient for each covariate, indexed to the lowest β-coefficient in our model (.22). Weighted point-values ranged from 1 (for estimated glomerular filtration rate <60 and contralateral internal carotid artery [ICA] stenosis 50%-80%) to 6 (for age ≥80). Those variables with a point-value less than 3 were designated as minor risk factors for mortality, and those with weighted points greater than 3 were deemed major risk factors for mortality. In total, we identified four major risk factors including age ≥80, insulin-dependent diabetes, dialysis dependence, and contralateral ICA stenosis 80%-99%. The remaining covariates in the model were deemed minor risk factors and included age 70-80 years, noninsulin-dependent diabetes, smoking history, congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), estimated glomerular filtration rate <60, absence of statin, contralateral ICA stenosis 50%-80%, or contralateral ICA occlusion. Next, categories of risk were defined based on discrete cut points in the number of major and minor risk factors present. Each patient in the cohort was designated as high, medium, or low risk based on these cut points.
Calculating risk scores to predict 5-year survival
Because not every patient who presents for consideration of surgery will fit perfectly into one of our three classifications of risk, we also generated a more precise risk scoring system. Risk scores were calculated by summing the indexed beta coefficients for each covariate in our model for each individual. Cut points for defining patients as low, medium, or high risk were selected based on the distribution of risk scores. Predicted 5-year survival was then calculated for a range of risk scores. All analyses were performed using Microsoft Excel 2010 and StataIC 12 (College Station, Tex).
RESULTS
Patient characteristics and univariate analysis of 5-year survival
Overall, 4114 patients underwent CEA for asymptomatic stenosis. Fifty-eight percent of patients were male, and mean age was 70 years. The most prevalent comorbidities included hypertension (89%), coronary artery disease (CAD, 33%) and smoking history (80%). Patient characteristics associated with a significant reduction in 5-year survival on univariate analysis included age ≥80 years, insulin-dependent diabetes, smoking history, CAD, CHF, COPD, dialysis dependence, lack of statin use, and contralateral ICA stenosis ≥50%. (Rates and significance levels are shown in Table I).
Table I.
Characteristics of patients undergoing CEA for asymptomatic stenosis and associated univariate relationship with 5-year survival
|
Percent of the 4114 patients who achieved 5-year survival following CEA
|
||||
|---|---|---|---|---|
| Percent of total cohort | If variable NOT present | If variable IS present | P valuea | |
| Age, years | ||||
| <70 | 47 | 84 | 92 | <.001 |
| 70-79 | 39 | 89 | 85 | .001 |
| ≥80 | 14 | 88 | 79 | <.001 |
| Male sex | 58 | 88 | 87 | .36 |
| Diabetes | ||||
| Nondiabetic | 68 | 85 | 89 | <.001 |
| Diet or oral medication controlled | 23 | 88 | 86 | .07 |
| Insulin dependent | 9 | 88 | 83 | <.001 |
| Past or current smoking history | ||||
| Never smoker | 20 | 87 | 91 | <.001 |
| Past (>1 year prior) | 52 | 89 | 87 | .006 |
| Current smoker | 28 | 88 | 87 | .96 |
| Hypertension | 89 | 87 | 88 | .54 |
| Coronary artery disease | 33 | 90 | 84 | <.001 |
| CHF | 8 | 89 | 78 | <.001 |
| COPD | 21 | 90 | 81 | <.001 |
| Renal function | ||||
| eGFRb ≥ 60 | 60 | 83 | 91 | <.001 |
| eGFRb < 60 | 40 | 91 | 84 | <.001 |
| Dialysis dependent | <1 | 88 | 50 | <.001 |
| Aspirin | 88 | 84 | 88 | .44 |
| Clopidogrel | 12 | 87 | 90 | .80 |
| Statin | 80 | 83 | 89 | .08 |
| Degree of ipsilateral ICA stenosis | ||||
| <50% | <1 | 88 | 95 | .36 |
| 50%-59% | 1 | 88 | 90 | .76 |
| 60%-69% | 2 | 88 | 86 | .96 |
| 70%-79% | 22 | 88 | 89 | .78 |
| ≥80%-99% | 74 | 89 | 87 | .94 |
| Occluded | <1 | 88 | 95 | .33 |
| Degree of contralateral ICA stenosis | ||||
| <50% | 57 | 86 | 89 | <.001 |
| 50%-79% | 33 | 88 | 87 | .07 |
| ≥80%-99% | 4 | 88 | 82 | .023 |
| Occluded | 6 | 88 | 82 | .046 |
| Shunt | ||||
| None | 57 | 89 | 87 | .60 |
| Routine | 39 | 87 | 89 | .20 |
| Selective | 4 | 88 | 83 | .07 |
CEA, Carotid endarterectomy; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; ICA, internal carotid artery.
P value reported for log-rank test.
eGFR in mL/min.
Main outcome measure: 5-year survival
Overall, 5-year survival for all patients in the cohort was 82%. By multivariate analysis, increasing age, diabetes, smoking history, CHF, COPD, poor renal function, degree of contralateral ICA stenosis, and lack of statin therapy were all associated with worse 5-year survival (hazard ratios are shown in Table II). Of note, perioperative mortality in our region was very low for patients undergoing CEA for asymptomatic stenosis, with only 0.4% of patients experiencing death within 30 days. Similarly, the incidence of perioperative stroke was also very low at 0.6%.
Table II.
Multivariate model for reduced 5-year survival following CEA for asymptomatic stenosis
| Covariate | HR | 95% CI | P value |
|---|---|---|---|
| Age, years | |||
| <70 | Referent | - | - |
| 70-79 | 1.8 | 1.4-2.2 | <.001 |
| ≥80 | 3.94 | 3.0-5.1 | <.001 |
| Diabetes | |||
| Nondiabetic | Referent | - | - |
| Diet or oral medication controlled | 1.34 | 1.1-1.7 | .008 |
| Insulin dependent | 1.98 | 1.5-2.7 | <.001 |
| Past or current smoking history | 1.68 | 1.3-2.2 | <.001 |
| CHF | 1.78 | 1.4-2.3 | <.001 |
| COPD | 1.66 | 1.4-2.0 | <.001 |
| Renal function | |||
| eGFRa ≥ 60 | Referent | - | - |
| eGFRa < 60 | 1.30 | 1.1-1.6 | .007 |
| Dialysis dependent | 3.41 | 1.6-7.2 | .001 |
| Not on statin | 1.27 | 1.1-1.6 | .021 |
| Degree of contralateral ICA stenosis | |||
| <50% | Referent | - | - |
| 50%-80% | 1.25 | 1.1-1.5 | .032 |
| >80%-99% | 1.95 | 1.3-2.9 | .001 |
| Occluded | 1.69 | 1.2-2.4 | .002 |
CEA, Carotid endarterectomy; CHF, congestive heart failure; CI, confidence interval; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio; ICA, internal carotid artery.
eGFR in mL/min.
Identification of low, medium, and high risk patients by number of risk factors
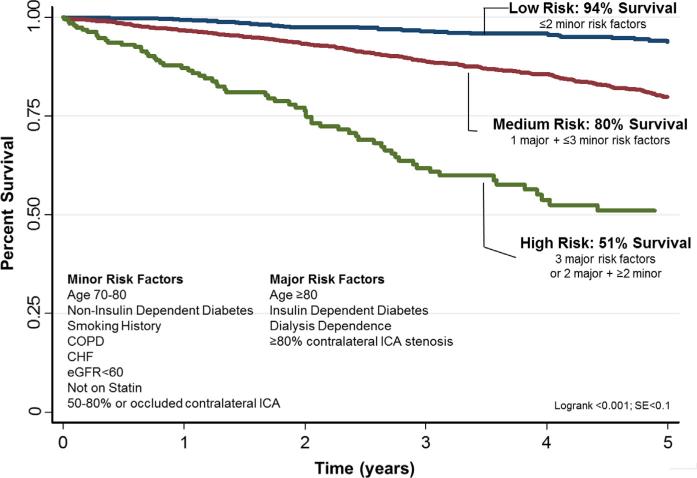
As outlined in the Methods section, we divided our cohort into three risk strata: high (three major or two major plus ≥two minor risk factors), medium (one major + ≤three minor risk factors), and low (<two minor risk factors). In total, 27% of patients in our region were low risk, defined as having one or two minor risk factors. An additional 65% were medium risk, defined as having one major or up to three minor risk factors, and the remaining 5% were high risk, defined as having three major or two major plus two minor risk factors. Five-year survival for low, medium, and high risk patients was 94%, 80%, and 51%, respectively (Fig 1).
Fig 1.
Five-year survival following carotid endarterectomy (CEA) for asymptomatic stenosis in low, medium, and high risk patients. CHF, Congestive heart failure; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; ICA, internal carotid artery.
Predicted 5-year survival based on risk score
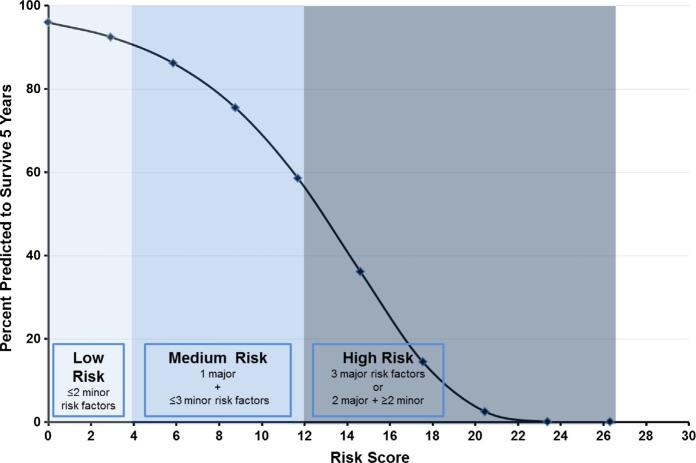
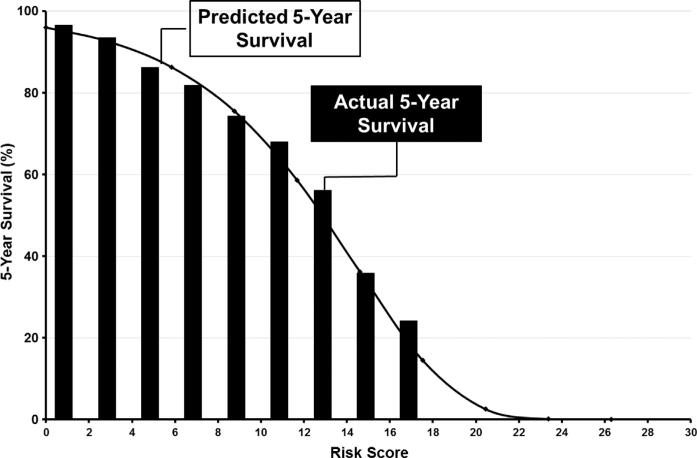
The highest possible risk score was 26 representing a patient with all risk factors, and the lowest was 0, representing patients with no risk factors. Predicted 5-year survival ranged from <0.1% for the highest risk score of 26, to 96% for a risk score of 0 (Fig 2). In VSGNE, all patients had risk scores less than 20 (ie, no asymptomatic patient with a risk score higher than 20 was selected for surgery). Fig 3 shows a comparison of predicted 5-year survival based on risk score and actual 5-year survival for patients in our cohort based on ranges of risk score. This figure demonstrates that our prediction model fits well across a wide range of risk scores.
Fig 2.
Predicted 5-year survival following carotid endarterectomy (CEA) for asymptomatic stenosis based on calculated risk score.
Fig 3.
Comparison of predicted 5-year survival (line) to actual 5-year survival (columns) based on calculated risk score for asymptomatic patients undergoing carotid endarterectomy (CEA).
DISCUSSION
The Society of Vascular Surgery guidelines state that asymptomatic patients selected for CEA should have a minimum life expectancy of 3 years and a risk of perioperative stroke/death that is <3%. In our study of over 4000 patients undergoing CEA for asymptomatic carotid stenosis, we utilized data from our regional registry, linked with the Social Security Death Index, to determine that 90% of patients selected for surgery in the VSGNE survived at least 3 years following their procedure and more than four out of five patients achieved 5-year survival. Our region also has low rates of perioperative stroke (0.6%) and death (0.4%). The clinical detail available in our registry allowed us to identify several major risk factors (such as age ≥80 and dialysis dependence) and minor risk factors (such as CHF and COPD) associated with death prior to 5 years after surgery. Several prior population-based studies have described prevalence and risk factors for cerebrovascular disease.13,14 Similar studies have examined the impact of cerebrovascular disease on survival.15-18 As with our study, this literature highlights the important role risk factors such as age, smoking, and diabetes play in determining outcomes in patients with cerebrovascular disease. However, our study adds to the current literature in that we examine long-term survival among patients whose carotid disease was deemed advanced enough to warrant revascularization. Further, our study offers a tool surgeons can use to predict survival for individual patients being offered CEA based on actual survival data from similar patients.
Appropriateness of CEA for asymptomatic stenosis
In the 1990s, several large prospective trials including the Asymptomatic Carotid Atherosclerosis Study (ACAS),5 the Asymptomatic Carotid Surgery Trial,6 and the Veterans Cooperative Affairs Trial19 established the small but measurable benefit of CEA over medical therapy alone for the prevention of stroke in patients with asymptomatic ICA stenosis. Many professional societies used these trials to define appropriateness criteria for surgery in asymptomatic patients.8,20,21 However, despite evidence from landmark trials, many have called into question the appropriateness of CEA for asymptomatic patients in real-world clinical practice. For example, in 1999 Gould et al performed a study comparing the efficacy (determined from results of ACAS) and effectiveness (based on real-world clinical data) of CEA in asymptomatic patients with severe ICA stenosis. This study found that trial data suggests the number needed to treat to prevent one major stroke or death in asymptomatic patients is 38. However, estimated complication rates from real-world practice, where a higher proportion of patients undergoing CEA are older and have more comorbid conditions than those enrolled in ACAS, is nearly twice as high, with 63 patients needed to treat to prevent one major stroke or death 63.22 Wennberg et al23 also questioned how well the results from randomized trials had translated into clinical practice, by demonstrating that patients undergoing CEA outside of randomized clinical trials had significantly higher rates of perioperative mortality than their presumed healthier counterparts enrolled in ACAS and North American Symptomatic Carotid Endarterectomy Trial (NASCET).
These questions regarding CEA, initially posed in the late 1990s, were followed by substantial changes in medical therapy, such as more widespread use of antiplatelet agents and statins.24 As a result, more have begun to question whether CEA continues to offer benefit over medical management in asymptomatic patients. For example, a recent meta-analysis, performed by Abbott in 2009, examined trends over time in the incidence of stroke in asymptomatic patients managed with medical therapy alone who were enrolled in clinical trials. This study demonstrated a 7% decrease the rate of ipsilateral stroke/TIA between 1985 and 2005, with a corresponding increase in the use of aspirin at baseline.4 Unfortunately, data pertaining to baseline use of statins over this time period were largely unreported, a “testament to the unavailability of many now commonly used drugs and the growing appreciation of the significant impact they have on vascular risk,” noted by Abbott.4 A similar study reporting the effect of medical therapy on stroke risk in patients with asymptomatic ICA stenosis was performed by Spence et al. This study compared rates of spontaneous microemboli detection by transcranial Doppler, as well as incidence of stroke/death/myocardial infarction between patients seen before 2003 and those seen after 2003. The findings demonstrated a significant reduction in microemboli (12.6% vs 3.7%), a significant reduction in cardiovascular events (17.6% vs 5.6%), and a corresponding reduction in total plasma cholesterol (179 mg/dL pre-2003 vs 166 mg/dL post-2003).25
Although controversy over what constitutes appropriate indication for CEA in asymptomatic patients persists, there are patients identified as high risk for death from other causes, in whom CEA is not the preferred management strategy. For example, our recent work examined the incidence and outcomes of CEA in asymptomatic patients with life-limiting conditions, such as severe COPD, symptomatic CHF, and metastatic cancer. We found that 20% of asymptomatic CEAs are performed in patients with poor predicted long-term survival. Further, these patients fare worse in the postoperative period, experiencing significantly higher rates of postoperative stroke and death than their unafflicted counterparts.11 Similarly, Cronenwett et al demonstrated that CEA is not cost-effective when performed in patients over the age of 7026; Ascher et al highlighted worse outcomes among asymptomatic patients with chronic renal insufficiency undergoing CEA27; and Chaturvedi et al highlighted increased risk following CEA among African Americans.28 The current study adds to this body of literature, indicating that there are high risk patients, specifically those with multiple major risk factors such as age ≥80, insulin-dependent diabetes, dialysis dependence, and severe contralateral stenosis, who are unlikely to benefit from prophylactic CEA based on long-term survival. Further, our study offers several user-friendly prediction rules to assist surgeons in identifying these high risk patients.
Limitations
Our study has several limitations. First, our long-term outcome measure is survival, rather than stroke-free survival. Cause of death was not available for patients in our registry nor could we determine if they remained stroke-free between the date of their procedure and the date of their long-term follow-up. Second, our procedure-based registry only contains those patients who underwent a procedure, and thus, we have no medical treatment arm for comparison. Third, several of the risk factors we defined as major predictors of mortality, such as dialysis dependence and contralateral ICA stenosis 80%-99%, were only present in a small subset of our cohort. However, because the dataset examined was so large, the absolute numbers provided were substantial enough to generate sound prediction rules. Finally, the data used to derive our prediction models are from a regional registry and therefore may not be generalizable to regions where quality improvement programs are not widespread. Our future work will aim to externally validate these prediction rules using a national database, which will serve to test the statistical strength of our predictive model on a larger, nonregionalized population.
Summary
More than four out of five asymptomatic patients selected for CEA in New England achieved 5-year survival with 30-day stroke/death rates less than 1%. However, a small proportion of patients selected for surgery had high risk profiles, such as those with multiple major risk factors including age ≥80, insulin-dependent diabetes, dialysis dependence, and severe contralateral ICA stenosis. Our study demonstrates that these patients are unlikely to survive long enough to realize a benefit of CEA for asymptomatic stenosis. In the current healthcare environment, where vascular surgeons strive to improve the value and appropriateness of carotid revascularization, especially in asymptomatic patients,29 predicting survival is especially important for decision making in patients with asymptomatic carotid atherosclerosis. Our prediction models appear to help with these decisions, as they discriminate well between patients who will and will not survive long-term following CEA, and will be made available online for public use to help surgeons appropriately select patients for carotid revascularization.
Acknowledgments
Dr Goodney was supported by a K08 Award (K08 HL05676) from the NHLBI and a Society for Vascular Surgery Foundation Lifeline Award.
DISCUSSION
Dr R. Clement Darling (Albany, NY). One of the parts of the data set which is incomplete is on those patients who did not get operated on who are at high risk. Do you have any idea of exactly what the stroke rate was in that population?
Dr Jessica B. Wallaert. We don't. The VSGNE registry only has data for patients who underwent surgery. We agree that one of the biggest limitations to our study is this lack of a control group. It could be that patients who are at high risk for mortality are also at highest risk for experiencing stroke if they don't undergo endarterectomy. Unfortunately, we don't have the data needed to compare rates of stroke-free survival in medically managed patients to long-term survival for patients in our registry.
Dr Richard Cambria (Boston, Mass). You wrote a paper in Stroke on the same topic using a different database—I believe it was Medicare—so could you give us an insight into the comparative data between the two, if they're similar or not?
My second question is how did you do the late survival data in this study? Our database doesn't really capture that survival data, so did you just use Social Security Death Index or what?
And, my last question is really a comment that the limitations of the database are such that there may be mitigating factors that cause a surgeon to make a decision in an asymptomatic patient, such as demonstrated progression with sequential follow-up, that aren't captured in the database that might affect clinical decision making.
Dr Wallaert. Our recent article in Stroke described the incidence and outcomes of carotid endarterectomy in asymptomatic patients defined as having life-limiting conditions using the American College of Surgeons National Surgical Quality Improvement Program Registry (ACS-NSQIP). The results were strikingly similar to those presented today, using our regional registry. In ACS-NSQIP, we found that 20% of patients are unlikely to survive 5 years following endarterectomy. These results were based on predicted long-term mortality. The current study uses actual mortality and found that 18% of patients die within 5 years following endarterectomy. In both studies, incidence of postoperative stroke, death, and myocardial infarction were also higher in patients defined as high risk. Overall, the results from these two separate databases gave quite similar results.
Regarding your second question about late survival, all of the data from VSGNE were linked to Social Security Death Index to determine mortality for this study.
Dr Jacob Schneiderman (Ramat Gan, Israel). In your presentation, you don't consider carotid plaque composition as a risk factor. If you were given the information that a 60% stenosing internal carotid artery plaque in an asymptomatic patient has a giant vulnerable component, namely a sizable necrotic core with a thin fibrous cap, would you consider this rupture-prone plaque as risk-full, thus necessitating carotid endarterectomy?
Dr Wallaert. Yes, I probably would consider that a high-risk patient and would certainly factor those details into my decision to operate or not for that individual. Unfortunately, this study uses registry data that do not include measures such as the plaque characteristics you described. Therefore, we were unable to account for such variables in our analysis.
Dr William Jordan (Birmingham, Ala). I actually have some disagreement with your conclusions specifically relative to your high-risk cohort. I think you're looking at the glass as half empty instead of half full. Specifically, while you've identified this high-risk group after the operation, could their survival be improved if we directed more efforts on treating their medical disease? Stated differently, I don't believe the operation itself is the incident that causes the decreased survival; potentially, they might still benefit from the carotid repair if we can improve their medical therapy. Can you comment?
Dr Wallaert. I agree. I don't believe that operation itself is responsible for reduced long-term survival in these high-risk patients. I do believe, however, that it is our responsibility as surgeons to take into consideration patient characteristics that may make an individual less likely to benefit from an operation because he or she has medical comorbidities that will reduce his or her lifespan and thus their opportunity to benefit from stroke prevention provided by an endarterectomy.
We recognize that our definition of poor long-term survival or “inappropriate” is somewhat arbitrary, and many would argue that a 50% 5-year survival is perfectly reasonable. I think that definition of appropriateness is going to vary based on the individual patient and his or her surgeon. Ultimately, it's going to come down to you, your patient, and a discussion about that individual's risk of postoperative mortality or stroke, as well as their long-term chance of surviving to benefit from a durable repair.
Dr Jordan. Can your group then improve on the medical therapy afterwards? Potentially, if we did a better job of taking care of heart disease or lung disease, then they might have better long-term survival.
Dr Wallaert. That's certainly a consideration and I agree that optimizing medical management of patients’ comorbidities, preoperatively and postoperatively, is critical. Unfortunately, we don't have data to suggest whether we are doing a good job of this or not, so I can't comment on the effect improved medical management on our result. I suspect it would have little impact.
Footnotes
Author conflict of interest: none.
Presented as a plenary presentation at the 2012 Vascular Annual Meeting of the Society for Vascular Surgery, National Harbor, Md, June 9, 2012.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
AUTHOR CONTRIBUTIONS
Conception and design: JW, PG
Analysis and interpretation: JW, JC, DB, BN, JJ, RD, PG
Data collection: JC, DB, BN, JJ, RD, PG
Writing the article: JW
Critical revision of the article: JW, JC, DB, BN, JJ, RD, PG
Final approval of the article: JW, JC, DB, BN, JJ, RD, PG
Statistical analysis: JW, PG
Obtained funding: Not applicable
Overall responsibility: JW, PG
REFERENCES
- 1.Agency for Healthcare Research and Quality [November 2011]; Available at: http://www.ahrq.gov/data/hcup.
- 2.Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1991;325:445–53. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi S. Public health impact of carotid endarterectomy. Neuroepidemiology. 1999;18:15–21. doi: 10.1159/000026191. [DOI] [PubMed] [Google Scholar]
- 4.Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke. 2009;40:e573–83. doi: 10.1161/STROKEAHA.109.556068. [DOI] [PubMed] [Google Scholar]
- 5.Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995;273:1421–8. [PubMed] [Google Scholar]
- 6.Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363:1491–502. doi: 10.1016/S0140-6736(04)16146-1. [DOI] [PubMed] [Google Scholar]
- 7.Ricotta JJ, Aburahma A, Ascher E, Eskandari M, Faries P, Lal BK. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1–31. doi: 10.1016/j.jvs.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 8.Biller J, Feinberg WM, Castaldo JE, Whittemore AD, Harbaugh RE, Dempsey RJ, et al. Guidelines for carotid endarterectomy: a statement for healthcare professionals from a Special Writing Group of the Stroke Council, American Heart Association. Circulation. 1998;97:501–9. doi: 10.1161/01.cir.97.5.501. [DOI] [PubMed] [Google Scholar]
- 9.Calvillo-King L, Xuan L, Zhang S, Tuhrim S, Halm EA. Predicting risk of perioperative death and stroke after carotid endarterectomy in asymptomatic patients: derivation and validation of a clinical risk score. Stroke. 2010;41:2786–94. doi: 10.1161/STROKEAHA.110.599019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodney PP, Likosky DS, Cronenwett JL. Factors associated with stroke or death after carotid endarterectomy in Northern New England. J Vasc Surg. 2008;48:1139–45. doi: 10.1016/j.jvs.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Wallaert JB, De Martino RR, Finlayson SR, Walsh DB, Corriere MA, Stone DH, et al. Carotid endarterectomy in asymptomatic patients with limited life expectancy. Stroke. 2012;43:1781–7. doi: 10.1161/STROKEAHA.112.650903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cronenwett JL, Russell MT, Likosky DS, Eldrup-Jorgenson J, Stanley AC, Nolan BW. A Regional Registry for Quality Assurance and Improvement: the Vascular Study Group of Northern New England (VSG-NNE). J Vasc Surg. 2007;46:1093–101. doi: 10.1016/j.jvs.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Mathiesen EB, Joakimsen O, Bonaa KH. Prevalence of and risk factors associated with carotid artery stenosis: the Tromso Study. Cerebrovasc Dis. 2001;12:44–51. doi: 10.1159/000047680. [DOI] [PubMed] [Google Scholar]
- 14.Ghilardi G. [Carotid stenotic-obliterative lesions. Distribution in 16,379 subjects 45-75 years of age]. Minerva Cardioangiol. 1994;42:345–50. [PubMed] [Google Scholar]
- 15.Terent A. Survival after stroke and transient ischemic attacks during the 1970s and 1980s. Stroke. 1989;20:1320–6. doi: 10.1161/01.str.20.10.1320. [DOI] [PubMed] [Google Scholar]
- 16.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. A prospective study of acute cerebrovascular disease in the community: the Oxford-shire Community Stroke Project–1981-1986. 2. Incidence, case fatality rates and overall outcome at one year of cerebral infarction, primary intracerebral and subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 1990;53:16–22. doi: 10.1136/jnnp.53.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dennis MS, Burn JP, Sandercock PA, Bamford JM, Wade DT, Warlow CP. Long-term survival after first-ever stroke: the Oxfordshire Community Stroke Project. Stroke. 1993;24:796–800. doi: 10.1161/01.str.24.6.796. [DOI] [PubMed] [Google Scholar]
- 18.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, et al. Heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 19.Hobson RW, III, Weiss DG, Fields WS, Goldstone J, Moore WS, Towne JB, et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med. 1993;328:221–7. doi: 10.1056/NEJM199301283280401. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:517–84. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- 21.Chaturvedi S, Bruno A, Feasby T, Holloway R, Benavente O, Cohen SN, et al. Carotid endarterectomy–an evidence-based review: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2005;65:794–801. doi: 10.1212/01.wnl.0000176036.07558.82. [DOI] [PubMed] [Google Scholar]
- 22.Gould DA, Birkmeyer JD. Efficacy versus effectiveness of carotid endarterectomy. Eff Clin Pract. 1999;2:30–6. [PubMed] [Google Scholar]
- 23.Wennberg DE, Lucas FL, Birkmeyer JD, Bredenberg CE, Fisher ES. Variation in carotid endarterectomy mortality in the Medicare population: trial hospitals, volume, and patient characteristics. JAMA. 1998;279:1278–81. doi: 10.1001/jama.279.16.1278. [DOI] [PubMed] [Google Scholar]
- 24.Spence JD. Asymptomatic carotid stenosis: mainly a medical condition. Vascular. 2010;18:123–6. doi: 10.2310/6670.2010.00023. discussion: 127-9. [DOI] [PubMed] [Google Scholar]
- 25.Spence JD, Coates V, Li H, Tamayo A, Munoz C, Hackam DG, et al. Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol. 2010;67:180–6. doi: 10.1001/archneurol.2009.289. [DOI] [PubMed] [Google Scholar]
- 26.Cronenwett JL, Birkmeyer JD, Nackman GB, Fillinger MF, Bech FR, Zwolak RM, et al. Cost-effectiveness of carotid endarterectomy in asymptomatic patients. J Vasc Surg. 1997;25:298–309. doi: 10.1016/s0741-5214(97)70351-3. discussion: 310-1. [DOI] [PubMed] [Google Scholar]
- 27.Ascher E, Marks NA, Schutzer RW, Hingorani AP. Carotid endarterectomy in patients with chronic renal insufficiency: a recent series of 184 cases. J Vasc Surg. 2005;41:24–9. doi: 10.1016/j.jvs.2004.10.047. [DOI] [PubMed] [Google Scholar]
- 28.Chaturvedi S, Madhavan R, Santhakumar S, Mehri-Basha M, Raje N. Higher risk factor burden and worse outcomes in urban carotid endarterectomy patients. Stroke. 2008;39:2966–8. doi: 10.1161/STROKEAHA.108.516062. [DOI] [PubMed] [Google Scholar]
- 29.Stoner M, Davies M, Forbes T, LoGerfo F, McDaniel H, Meissner M. Society for Vascular Surgery position statement: comparative effectiveness research in vascular disease management. J Vasc Surg. 2009;49:1592–3. doi: 10.1016/j.jvs.2009.04.024. [DOI] [PubMed] [Google Scholar]