Abstract
Objective
To assess outcomes after endovascular abdominal aortic aneurysm repair (EVAR) in an integrated health care system.
Methods
Between 2000 and 2010, 1736 patients underwent EVAR at 17 centers. Demographic data, comorbidities, and outcomes of interest were collected. EVAR in patients presenting with ruptured or symptomatic aneurysms was categorized as urgent; otherwise, it was considered elective. Primary outcomes were mortality and aneurysm-related mortality (ARM). Secondary outcomes were change in aneurysm sac size, endoleak status, major adverse events, and reintervention.
Results
Overall, the median age was 76 years (interquartile range, 70–81 years), 86% were male, and 82% were Caucasian. Most cases (93.8%) were elective, but urgent use of EVAR increased from 4% in the first 5 years to 7.3% in the last 5 years of the study period. Mean aneurysm size was 5.8 cm. Patients were followed for an average of 3 years (range, 1–11 years); 8% were lost to follow-up. Intraoperatively, 4.5% of patients required adjunctive maneuvers for endoleak, fixation, or flow-limiting issues. The 30-day mortality rate was 1.2%, and the perioperative morbidity rate was 6.6%. Intraoperative type I and II endoleaks were uncommon (2.3% and 9.3%, respectively). Life-table analysis at 5 years demonstrated excellent overall survival (66%) and freedom from ARM (97%). Postoperative endoleak was seen in 30% of patients and was associated with an increase in sac size over time. Finally, the total reintervention rate was 15%, including 91 instances (5%) of revisional EVAR. The overall major adverse event rate was 7.9% and decreased significantly from 12.3% in the first 5 years to 5.6% in the second 5 years of the study period (P < .001). Overall ARM was worse in patients with postoperative endoleak (4.1% vs 1.8%; P < .01) or in those who underwent reintervention (7.6% vs 1.6%; P < .001).
Conclusions
Results from a contemporary EVAR registry in an integrated health care system demonstrate favorable perioperative outcomes and excellent clinical efficacy. However, postoperative endoleak and the need for reintervention continue to be challenging problems for patients after EVAR. (J Vasc Surg 2013;58:324–32.)
Since the first endovascular repair of an abdominal aortic aneurysm (AAA) in 1991,1 endovascular abdominal aortic aneurysm repair (EVAR) has become the preferred choice for AAA repair worldwide.2 Several randomized clinical trials have demonstrated decreased early morbidity and mortality with EVAR compared with traditional open repair.3–6 However, long-term survival advantage and durability with EVAR have not been well established in comparison to open surgical repair. Given the constant refinement of devices and technology, results from large trials often describe an obsolete device or outdated practice pattern and are thus difficult to compare to contemporary practice.7
Retrospective registries offer an opportunity to track outcomes that may be more generalizable. However, most large series arise from clinical trial registries8 or come from institutional databases.9–11 With the exception of several large series from high-volume centers,12–14 there have been few large reported non-industry-affiliated registries in the U.S. This study examines utilization and outcomes after EVAR in an integrated health care system.
METHODS
Kaiser Permanente is an integrated health care delivery system that offers multispecialty care for more than 3 million members in Northern California. Since 2004, implementation of digital health records has allowed access to all arenas of clinical information. This study was a retrospective review of prospectively collected outcomes data for EVAR performed in 17 medical centers in Northern California from 2000 to 2010. This study protocol was approved by the Kaiser Permanente Northern California (KPNC) Institutional Review Board and was funded by the KPNC Community Benefit Research Grant Program.
Beginning in 2000, relevant clinical information for patients undergoing EVAR was collected by trained research nurses. Decision making regarding indications, device selection, clinical success, and need for secondary procedures was at the discretion of the operating surgeon. Postoperative follow-up varied across medical centers but generally involved a 1-month postoperative computed tomography (CT) scan followed by serial CT imaging at regular intervals accompanied by clinical follow-up.
Baseline demographic data were obtained from electronic data sources within KPNC. Device type was collected from operative reports and device entry forms. EVAR in patients presenting with ruptured or symptomatic aneurysms at the preoperative CT scan was categorized as “urgent.” All other EVAR was categorized as “elective.”
The primary outcome variables were all-cause mortality and aneurysm-related mortality (ARM). ARM was defined as death within 30 days of the index EVAR or related secondary procedure that was related to aneurysm rupture or a major adverse event from the index EVAR.
Secondary outcome variables examined were occurrence of endoleak, need for reintervention, major adverse events, and change in aneurysm sac size over time. Endo-leak was classified according to established reporting standards.15 The presence of endoleak was ascertained from clinical documentation and, when possible, the etiology of the leak was listed. Aneurysm sac size was obtained from imaging studies and clinical documentation. The largest reported diameter was used as a single determinant of aneurysm size.
Major adverse events were defined as conversion to open repair, aneurysm rupture, major embolic event, graft infection requiring explantation, device migration, loss of device patency requiring reintervention, and other miscellaneous complications that significantly impacted clinical outcome. Device migration was reported if it required intervention or if adequate seal was lost, usually when reduced to less than 10 mm of the circumferential apposition length.
Statistical methods
All analyses were performed using SAS 9.13 (SAS Institute Inc, Cary, NC). Rates of various demographic and clinical characteristics were evaluated with χ2 tests or Fisher exact tests as appropriate for categorical variables. Neither age at the index EVAR nor preoperative AAA sac diameter were normally distributed; therefore, nonparametric Wilcoxon-Mann-Whitney tests were used to determine if any statistically significant difference existed between the elective and urgent groups. Survival analysis was performed using the Kaplan-Meier method to estimate the survival function with and without stratification. The log-rank test was used to compare survival functions stratified by subgroups such as case setting, gender, age, and aneurysm size. Cox proportional hazards models were used to identify factors predictive of death from any cause, death from aneurysm-related causes, and need for reintervention, with the threshold of significance set at P < .05.
RESULTS
Demographics
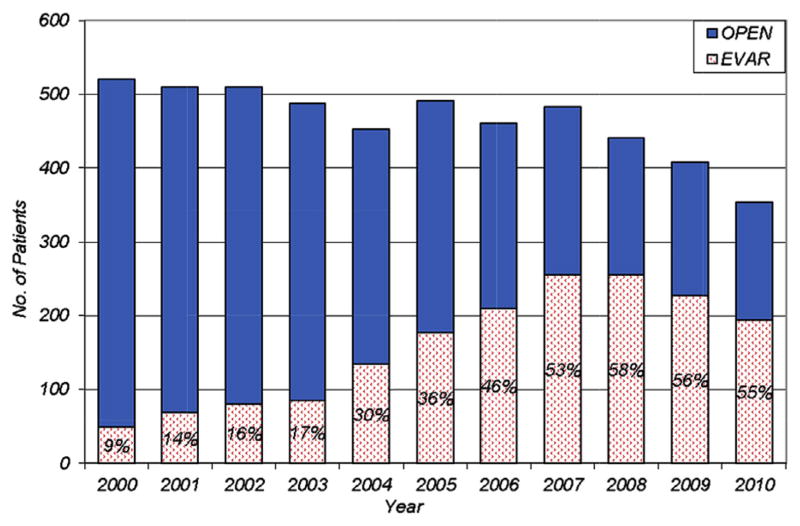
From 2000 to 2010, 1736 patients underwent primary EVAR at 17 medical centers within the health care system; during this time, there were 3382 open AAA repairs. The adoption of EVAR increased over time compared with open AAA repair (Fig 1). Interestingly, the total number of AAA repairs declined in the last 4 years of the study from over 500 in 2000 to just over 350 in 2010.
Fig 1.
Evolving pattern of abdominal aortic aneurysm repair type between 2000 and 2010 within Kaiser Permanente Northern California. EVAR, Endovascular abdominal aortic aneurysm repair.
Baseline demographics and clinical characteristics are listed in Table I. The majority of the EVAR patients were male (86.5%), and 82.1% of patients were Caucasian. The median age was 76 years (interquartile range [IQR], 70–81 years), including 502 (28.9%) patients 80 to 89 years old and 27 (1.6%) patients age 90 years or older. Nearly 94% of cases were done electively; 6% were performed urgently (ruptured AAA, n = 50; symptomatic AAA, n = 57). The proportion of urgent cases performed in the latter part of the study period (2006–2010) increased significantly compared with the proportion in the early time period (2000–2005; 7% vs 4%; P < .01). Mean aneurysm size was 5.8 cm. Among the study cohort, 116 (6.7%) patients underwent preoperative hypogastric artery embolization. Selection of device type was at the discretion of the operating surgeon. The Cook Zenith (Cook Inc, Bloomington, Ind) was used in close to half of all cases (n = 841, 48.4%), the Gore Excluder low permeability (W. L. Gore & Associates, Inc, Flagstaff, Ariz) was used in 25.2%, Medtronic AneuRx (Medtronic Vascular, Santa Rosa, Calif) in 19.5%, and other approved or investigational devices in 6.8%. Bifurcated devices were used in 85% of patients, with the remainder receiving aorto-uni-iliac devices with femoral-femoral bypass as medically indicated.
Table I.
Baseline preoperative demographic and clinical characteristics of 1736 EVAR patients, stratified by operative indication
| Characteristics a | Total, No. (%) | Elective (n = 1629), No. (%) | Urgent (n = 107), No. (%) | P valueb |
|---|---|---|---|---|
| EVAR year | ||||
| 2000–2005 | 595 (34.3) | 571 (35.0) | 24 (22) | <.01 |
| 2006–2010 | 1141 (65.7) | 1058 (65.0) | 83 (78) | |
| Males | 1501 (86.5) | 1415 (86.9) | 86 (80) | .06 |
| Age, years | .26c | |||
| Mean ± SD | 75.0 ± 7.9 | 74.9 ± 7.7 | 75.3 ± 10.2 | |
| Median [IQR] | 76.0 [70.0–81.0] | 76.0 [70.0–80.0] | 77.0 [69.0–82.0] | |
| Age group, years | ||||
| ≤79 | 1207 (69.5) | 1146 (70.4) | 61 (57) | <.01 |
| 80–89 | 502 (28.9) | 461 (28.3) | 41 (38) | |
| ≥90 | 27 (1.6) | 22 (1.4) | 5 (5) | |
| Race/ethnicity group | .13 | |||
| Asian | 122 (7.0) | 113 (6.9) | 9 (8) | |
| Black | 86 (5.0) | 76 (4.7) | 10 (9) | |
| Hispanic | 53 (3.1) | 52 (3.2) | 1 (1) | |
| Caucasian | 1426 (82.1) | 1343 (82.4) | 83 (78) | |
| Unknown | 49 (2.8) | 45 (2.8) | 4 (4) | |
| Preoperative AAA size, cm | <.001c | |||
| Mean ± SD | 5.8 ± 1.1 | 5.7 ± 1.0 | 6.6 ± 1.7 | |
| Median [IQR] | 5.6 [5.2–6.2] | 5.6 [5.2–6.2] | 6.3 [5.5–7.9] | |
| Preoperative AAA size ≥5.5 cm | 1027 (59.2) | 944 (57.9) | 83 (78) | <.001 |
| Coronary artery disease | 810 (46.7) | 769 (47.2) | 41 (38) | .07 |
| Diabetes | 442 (25.5) | 415 (25.5) | 27 (25) | .96 |
| Preoperative embolization | 116 (6.7) | 112 (6.9) | 4 (4) | .21 |
| Hyperlipidemia | 1324 (76.3) | 1251 (76.8) | 73 (68) | .04 |
| Hypertension | 1455 (83.8) | 1365 (83.8) | 90 (84) | .93 |
| Peripheral vascular disease | 367 (21.1) | 345 (21.2) | 22 (21) | .88 |
| Smoking | ||||
| Never | 1080 (62.2) | 1007 (61.8) | 73 (68) | .26 |
| Former | 347 (20.0) | 332 (20.4) | 15 (14) | |
| Current | 309 (17.8) | 290 (17.8) | 19 (18) | |
| Treated with statin | 1156 (66.6) | 1094 (67.2) | 62 (58) | .05 |
| Bifurcated graft | 1485 (85.5) | 1391 (85.4) | 94 (88) | .48 |
AAA, Abdominal aortic aneurysm; EVAR, endovascular abdominal aortic aneurysm repair; IQR, interquartile range; SD, standard deviation.
Due to rounding, group percentages may not total 100.
For comparisons between the elective and urgent groups.
Comparison result using Wilcoxon-Mann-Whitney test.
Compared with patients who received surgery in an elective manner, patients who received surgery in an urgent setting tended to have larger aneurysms (6.3 cm [IQR, 5.5–7.9 cm] vs 5.6 cm [IQR, 5.2–6.2 cm]; P < .001), more immediate complications (13% vs 6.2%; P < .01), more major adverse events (16% vs 7.4%; P < .01), and higher ARM (8 vs 2.1; P < .001). In addition, cases performed urgently had a higher proportion of post-EVAR rupture (8% vs 0.9%; P < .01).
The median length of hospital stay among EVAR cases was 2 days (IQR, 1–3 days). Documented follow-up was available for 91.9% of all patients throughout the study period. The remaining 8.1% of patients lost to follow-up declined surveillance, moved away, or left the health care system. Overall, the median follow-up was 2.7 years (IQR, 1.2–4.4 years). During this time, patients underwent a median of four postoperative CT scans (IQR, 2–6 scans).
Perioperative outcomes
Overall, 4.5% of patients required intraoperative adjunctive maneuvers for endoleak, fixation, or flow-limiting issues, including three open conversions (Table II). Reported intraoperative type I and II endoleaks occurred in 2.3% and 9.3% of cases, respectively. Of the 40 intraoperative type I leaks reported, all but three were addressed by either standard or adjunctive maneuver at the time of index EVAR. The immediate complication rate was 6.6 % (Table III).
Table II.
Unplanned intraoperative adjunctive maneuvers during EVAR
| Type | Indication | No. (%) |
|---|---|---|
| Aortic cuff/stent | Proximal seal/flxation | 24 (1.4) |
| Iliac stent/PTA | Stenosis/occlusion | 34 (2.0) |
| Exploratory laparotomy/conversion | Bleeding/rupture | 3 (0.2) |
| Renal stenting/snorkel/branch | Branch vessel patency | 14 (0.8) |
| Femoral-femoral bypass | Limb compromise | 3 (0.2) |
EVAR, Endovascular abdominal aortic aneurysm repair; PTA, percutaneous transluminal angioplasty.
Table III.
Perioperative complications after EVAR
| Complications (overall 6.6%) | No. (%) |
|---|---|
| Bleeding | 18 (1.1) |
| Bowel | 3 (0.2) |
| Cardiac | 12 (0.7) |
| Incisional | 4 (0.2) |
| Limb thrombosis | 9 (0.5) |
| Renal | 7 (0.4) |
| Stroke | 2 (0.1) |
| Unintended vessel occlusion | 3 (0.2) |
| Vessel injury | 23 (1.3) |
| Other | 34 (2.0) |
EVAR, Endovascular abdominal aortic aneurysm repair.
Major adverse events
Major adverse outcomes were seen in 7.9% of patients and decreased significantly from 12.3% in 2000 to 2005 to 5.6% in 2006 to 2010 (P < .001). There were 22 open conversions, 9 graft infections requiring graft removal, 7 major embolic events, 36 graft occlusions requiring treatment, 22 ruptures, and 40 cases of graft migration requiring intervention (Table IV).
Table IV.
Rates of adverse events and reinterventions after EVAR
| Events/interventions/outcomes | No. (%) |
|---|---|
| Major adverse events (overall) | 137 (7.9) |
| Open conversion | 22 (1.3) |
| Major embolic event | 7 (0.4) |
| Graft infection requiring explantation | 9 (0.5) |
| Graft occlusion requiring treatment | 36 (2.1) |
| Rupture | 22 (1.3) |
| Graft migration requiring treatment | 40 (2.3) |
| Other | 28 (1.6) |
| Any reintervention (overall) | 251 (14.5) |
| Endovascular-related (including revisional EVAR) | 218 (12.6) |
| Revisional EVAR | 91 (5.2) |
| Endoleak-related major | 73 (4.2) |
| Angiogram-related | 172 (9.9) |
EVAR, Endovascular abdominal aortic aneurysm repair.
Postoperative endoleak and reintervention
During the study period, postoperatively detected endoleak was seen in 29.9% of patients. The postoperative endoleak rate was significantly higher in patients with an initial intraoperative endoleak (treated or not) compared with patients with no initial intraoperative endoleak (43.8% vs 28.1%; P < .001). A total of 60 (3.5%) patients experienced a postoperative type I leak, 474 patients (27.2%) had a type II endoleak, and 16 patients had a type III leak.
Treatment strategies and outcomes differed by endoleak type. Operative treatment of type I leaks resulted in five open conversions, with the remaining treatments consisting of additional stent placement or conversion to an aorto-uni-iliac device; one patient declined treatment. Of the 474 patients with type II leaks, only 180 patients had resolution by the second postoperative scan, whereas the remaining patients had persistent leaks at 1 year or longer. Five patients with type II endoleaks were treated operatively with four open conversions and one hypogastric ligation and stent extension. Of the 16 patients with a presumed type III leak, there were three aorto-uni-iliac conversions, three open conversions, and 13 cases of additional component placement (one patient had two such procedures). Two patients with presumed type IV endo-leaks underwent endograft relining.
The overall reintervention rate was 14.5% (n = 251). Of these, 103 patients had a single intervention, with the remainder undergoing two or more interventions; 27 patients underwent 5 or more interventions. Most interventions were minimally invasive: 86.9% (218/251) of patients underwent interventions that were endovascular in nature, including 91 revisional EVARs. However, 73 patients required major operative leak-related interventions. Reinterventions related to percutaneous angiography numbered 172, including embolization of branch arteries (n = 71) and direct sac injection (n = 25). Independent predictors for reintervention included urgent indication, postoperative leak (all types), pre-existing peripheral vascular disease, and having undergone an intraoperative adjunctive maneuver at the initial EVAR (Table V).
Table V.
Multivariable predictors of all-cause mortality, aneurysm-related mortality, and need for reintervention after EVAR
| All-cause mortality (n = 440)
|
Aneurysm-related mortality (n = 43)
|
Reinterventions (n = 251)
|
||||
|---|---|---|---|---|---|---|
| Predictors | HR (95% CL) | P value | HR (95% CL) | P value | HR (95% CL) | P value |
| Females | 1.6 (1.2, 2.0) | <.001 | 2.4 (1.2, 4.8) | .02 | 1.3 (0.9, 1.9) | .10 |
| Age groups, years | ||||||
| ≤79a | 1.0 | 1.0 | 1.0 | |||
| 80–89 | 2.2 (1.8, 2.6) | <.001 | 2.6 (1.4, 5.1) | <.01 | 1.2 (0.9, 1.5) | .30 |
| ≥90 | 3.8 (2.2, 6.7) | <.001 | 3.9 (0.8, 19.2) | .10 | 1.3 (0.5, 3.3) | .53 |
| Preoperative aneurysm size ≥5.5 cm | 1.7 (1.4, 2.2) | <.001 | 4.4 (1.7, 11.3) | <.01 | 1.3 (1.0, 1.7) | .10 |
| Not on statin prior to EVAR | 1.1 (0.9, 1.4) | .40 | 0.9 (0.4, 2.0) | .84 | 1.1 (0.8, 1.5) | .56 |
| Urgent EVAR | 1.5 (1.0, 2.1) | .03 | 2.7 (1.2, 6.2) | .02 | 1.9 (1.2, 3.0) | <.01 |
| EVAR device | ||||||
| Zenitha | 1.0 | 1.0 | 1.0 | |||
| Gore | 0.8 (0.6, 1.1) | .13 | 1.5 (0.7, 3.4) | .34 | 0.7 (0.5, 1.1) | .11 |
| Medtronic | 1.0 (0.8, 1.3) | .95 | 1.2 (0.5, 2.6) | .71 | 1.2 (0.9, 1.6) | .26 |
| Others | 1.2 (0.8, 1.7) | .47 | 1.9 (0.6, 6.0) | .27 | 1.6 (1.0, 2.6) | .049 |
| Coronary artery disease | 1.2 (1.0, 1.5) | .12 | 1.2 (0.6, 2.4) | .57 | 1.0 (0.7, 1.3) | .81 |
| Diabetes | 1.3 (1.0, 1.6) | .05 | 1.3 (0.7, 2.7) | .42 | 0.9 (0.7, 1.2) | .51 |
| Hyperlipidemia | 0.7 (0.5, 0.9) | <.01 | 1.0 (0.4, 2.3) | .97 | 1.1 (0.7, 1.5) | .74 |
| Hypertension | 1.2 (0.9, 1.6) | .23 | 1.2 (0.4, 3.2) | .78 | 1.0 (0.7, 1.5) | .88 |
| Peripheral vascular disease | 1.3 (1.0, 1.6) | .03 | 1.1 (0.5, 2.3) | .82 | 1.5 (1.1, 2.1) | <.01 |
| Preoperative embolization | 1.6 (1.1, 2.2) | .01 | 2.3 (0.8, 6.6) | .13 | 1.4 (0., 2.2) | .18 |
| Smoking | ||||||
| Nevera | 1.0 | 1.0 | 1.0 | |||
| Current | 1.1 (0.8, 1.4) | .62 | 1.2 (0.5, 3.1) | .69 | 1.2 (0.8, 1.7) | .40 |
| Former | 1.0 (0.8, 1.3) | .92 | 0.9 (0.4, 2.1) | .72 | 1.0 (0.7, 1.3) | .77 |
| Intraoperative endoleak | ||||||
| Nonea,b | 1.0 | 1.0 | 1.0 | |||
| Type I | 0.8 (0.4, 1.5) | .43 | 0.7 (0.1, 2.9) | .58 | 0.9 (0.4, 1.9) | .74 |
| Type II | 0.8 (0.6, 1.2) | .36 | 1.1 (0.4, 3.2) | .85 | 1.0 (0.6, 1.5) | .84 |
| Operative adjunctive maneuver | 1.4 (0.9, 2.2) | .18 | 1.4 (0.4, 4.8) | .60 | 1.9 (1.1, 3.2) | .02 |
| Reinterventionb | 1.5 (1.2, 2.1) | <.01 | 0.4 (0.2, 0.8) | .01 | NA | |
| Postoperative leak types I, III | 1.2 (0.8, 1.9) | .43 | 2.8 (1.2, 6.6) | .02 | 4.2 (3.0, 5.9) | <.001 |
| Postoperative leak type II | 1.1 (0.8, 1.3) | .66 | 0.8 (0.4, 1.7) | .63 | 3.4 (2.6, 4.5) | <.001 |
CL, Confidence limit; EVAR, endovascular abdominal aortic aneurysm repair; HR, hazard ratio.
Referent group.
Used in the multivariable regression models for all-cause and aneurysm-related mortality only.
AAA diameter change over time
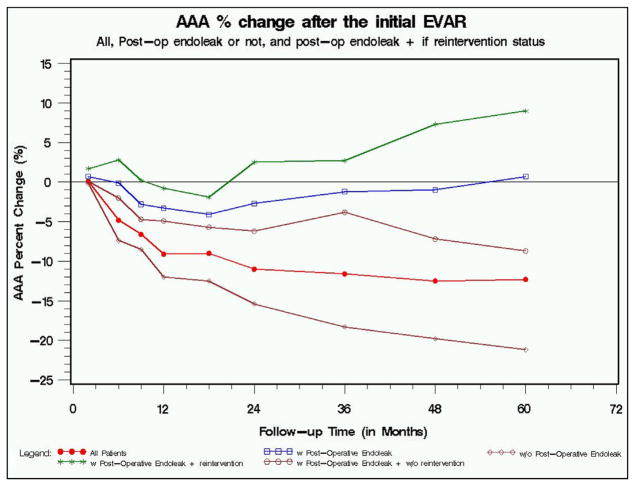
Overall, AAA sac diameter was noted to decrease during the follow-up period after EVAR. However, when stratified by endoleak status and reintervention, variation was observed in AAA sac size over time. Sac size began to increase in the second year after EVAR in those patients who experienced endoleaks and underwent reintervention. In the absence of endoleak, AAA sac size continued to decrease over time (Fig 2).
Fig 2.
Abdominal aortic aneurysm (AAA) sac size percent change among 1736 endovascular abdominal aortic aneurysm repair (EVAR) patients from 2000 to 2010.
Survival
The overall 30-day mortality was 1.2%. Octogenarians and nonagenarians had 30-day mortality rates of 2.6% and 3.7%, respectively. Early mortality rates were higher in patients operated on urgently (3.3% urgent vs 1.0% elective; P < .05), females (2.1% vs 1.1% males; P < .05), patients with large aneurysms (2.0% for AAAs ≥5.5 cm vs 0.1% for AAAs <5.5 cm; P < .001), and patients with a major adverse event (6.6% vs 0.8%; P < .0001).
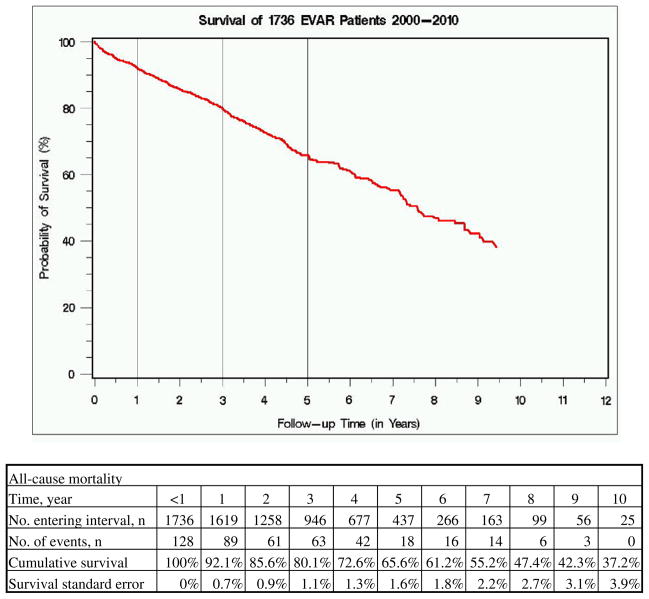
By life-table analysis, cumulative 5-year survival was 65.6% (Fig 3) and freedom from ARM was 96.6%. ARM was worse in patients with postoperative endoleak (4.1% vs 1.8%; P < .01) and in those undergoing reintervention (7.6% vs 1.6%; P < .001).
Fig 3.
All-cause Kaplan-Meier survival curve among 1736 endovascular abdominal aortic aneurysm repair (EVAR) patients from 2000 to 2010.
Using multivariable analysis, we identified urgent indication, female gender, advanced age, and AAA size as independent predictors of significantly increased risk of both all-cause mortality and ARM. The presence of any postoperative type I or III leak independently predicted increased risk for ARM but not for all-cause mortality. Type II leaks, while predictive of reintervention, did not increase the risk of ARM. Conversely, those patients who underwent AAA treatment without the need for subsequent reintervention were protected with regard to ARM (Table V).
DISCUSSION
This study described long-term outcomes after EVAR in a community-based setting during the first decade of experience with EVAR within our health care system. The results demonstrated excellent 30-day mortality and favorable rates of reintervention and freedom from ARM. Time-dependent treatment trends showed a decline in open AAA repair along with a concomitant rise in EVAR. In addition, the use of EVAR for urgent indications increased throughout the study period, likely as a result of the gain in operator experience and comfort. We were interested to observe that the overall rate of AAA repair declined throughout the study period. Pinpointing causative factors for this result was beyond the scope of this study, but possible explanations include socioeconomic factors (eg, less elective surgery because of the recent recession) as well as increased overall penetrance of statins and smoking cessation efforts.
The strengths of this report lie in the excellent documented follow-up among EVAR patients and the contemporary nature of the results. Because a very small number of these cases were performed within the context of a trial, the results reflect a rich and varied practice pattern over a broad geographic region. Unlike society-based registries, our integrated health care system leverages existing systems to automate and facilitate follow-up evaluation for patients undergoing vascular reconstruction. For more than 10 years, KPNC has employed computerized tracking to ensure that EVAR surveillance (along with several other chronic conditions) is not overlooked, resulting in a near-complete data set. As many who perform quality and auditing activities will attest, achieving near-complete long-term follow-up in patient registries is challenging. For example, in a study looking at the impact of suboptimal surveillance on outcome, Jones et al found a 32.8% incidence of incomplete follow-up after EVAR.16 This report presumed that in these “missing” patients, adverse events were more likely to have occurred.
In our study, the incidence of primary end points, such as perioperative mortality and ARM, was comparable to that seen in other cohorts. The cohort in the US OVER trial, with recruitment occurring between 2002 and 2008, more closely resembles our current cohort than those in the older, European-based EVAR I, EVAR II, and DREAM trials.4,17 However, the 30-day mortality rate in the OVER trial was reported as 0.5%, and our results are more similar to those reported in the EVAR I trial (1.8%), Lifeline registry (1.7%), and a recent meta-analysis (3.3%).4,8,18 The disparity in short-term mortality rate between the OVER trial and our results may reflect the nonexclusionary nature of our treated population.
Arguably the most important end point of EVAR treatment, freedom from ARM, was achieved by nearly 97% of patients at 5 years, which is comparable to the rate in several series that included this end point. The Eurostar registry reported 3% ARM (follow-up out to 8 years), the EVAR arm of the DREAM trial (2 years after randomization) reported 2.1%, and the OVER trial (mean follow-up of 1.8 years) reported 1.4%.4,6,19 The improved ARM in the OVER trial may reflect the later time period of recruitment, physician experience, or improved device design as compared with the earlier trial participants. In the latter 5 years of our study period, we observed an improvement in ARM that may similarly reflect overall improved treatment. As expected, ARM was worse in patients with any postoperative endoleak and in those who went on to reintervention, so the decrease in ARM corroborates the observed decrease in major adverse events in the latter half of the study period.
Large retrospective registries incorporating multiple facilities have allowed clinicians to examine contemporaneous factors that may affect clinical outcome and represent an important contribution to the EVAR knowledge base. The largest EVAR registry in the U.S. described a combined cohort of 2664 patients from four multicenter Investigational Device Exemption trials and compared them with open surgical controls.8 This study led to significant statements about EVAR’s impact on AAA-related mortality compared with open surgery in these trial patients. Importantly, this analysis did not include the Cook Zenith graft and also included the AnCure device (Guidant, Indianapolis, IN), which did not attain significant long-term clinical utilization. Similarly, the initial reports from the Eurostar registry, which began in 1996, described several early devices that were never approved in the U.S.,20 which limits the generalizability of these results.
In this study, reintervention was reported in 14.5% of patients, which is similar to multiple reports during the same time period. We did not perform specific analyses of the methods and indications for intervention, acknowledging that the threshold for intervention varies by clinician, surveillance regimen, and current practice trends. Mehta et al reported a 19% reintervention rate at 2.8 years in 1738 patients, which partially reflected their aggressive approach to type II endoleak treatment.12 Other groups have reported reintervention rates between 12% and 27%.21–24 In larger series, reintervention rates were also comparable: Lifeline, 18% at 5 years; OVER, 14% at 1.8 years; and EVAR1, 23% at 5 years.4,8,25 The increased ARM seen in the reintervention subgroup and the delayed nature of many of these procedures serves to underscore the need for long-term surveillance.
We observed worse outcomes in those patients treated with EVAR for ruptured or symptomatic aneurysms than in those who had elective treatment. Increases in all-cause mortality, ARM, and need for reintervention all logically followed the increased acuity of these patients. Further research is needed to compare these outcomes to those in patients undergoing open repair and to investigate predictors of procedural success in these patients in order to shed light on the utility of EVAR in this regard.
The variables present in our integrated dataset allow insight into the effect of patient risk factors on outcome after EVAR. For example, our study demonstrated higher 30-day mortality for women compared with men. Other authors have reported gender as an independent risk factor for morbidity and mortality.26 Additional investigation into this gender disparity will focus on anatomic and procedural differences that might explain the mortality difference. Further, in our multivariable analysis, advanced age, gender, and larger aneurysms were independently found to predict ARM. This is consistent with prior reports and reinforces the need for continued study in these areas. Interestingly, although presence of a postoperative type II leak was found to predict intervention, it did not independently predict increased ARM. This may suggest that while not all type II leaks are benign, the majority of them will not go on to cause significant mortality. Statin use, although well documented in the health record, was not shown to have an effect on mortality outcome. This is in contradistinction to data from 5892 patients in the Eurostar registry, which suggested that statin use was independently associated with reduced all-cause mortality after EVAR.27 While the overall statin rate was 66.6%, the greater incidence of hyperlipidemia suggests underutilization of statins, especially early on in the study period.
One of the limitations of our study is the incomplete collection of anatomic data or information regarding adherence to Instructions for Use guidelines in our dataset, which limited the ability to analyze outcomes across device types. Varying practice patterns specific to a surgeon or group of surgeons also limited our ability to comment on rates of reintervention and patterns that might suggest improved outcome. In addition, although outcome data were collected prospectively, major events were self-reported and were not adjudicated, thus raising the possibility of an underreporting of adverse events. Future work to more distinctly categorize these patients in our dataset will allow better insight in future analyses.
In summary, as in many other health care delivery systems, AAA repair in KPNC has changed significantly in recent years, with rapid adoption of EVAR and subsequent excellent freedom from ARM. Further, our report highlights the advantages of our integrated health care delivery system, in terms of facilitating long-term evaluation of the effectiveness of new and evolving treatments. In the future, we aim to expand our efforts toward national EVAR surveillance within Kaiser Permanente, leveraging the successful implementation of other national chronic disease registries as a guide in these initiatives. Along with registry efforts from the national vascular surgery societies, this will allow clinicians to track important outcomes and provide optimal care for patients undergoing AAA repair.
Footnotes
Author conflict of interest: none.
Presented at the Twenty-seventh Annual Meeting of the Western Vascular Society, Park City, Utah, September 22-25, 2012.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
AUTHOR CONTRIBUTIONS
Conception and design: RC, BH
Analysis and interpretation: RC, PG, BH, LT
Data collection: AR, NS, HH, SO
Writing the article: NS, HH
Critical revision of the article: LT, SO, BH, AR
Final approval of the article: RC, LT, PG
Statistical analysis: LT, PG, RC
Obtained funding: RC
Overall responsibility: RC
References
- 1.Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5:491–9. doi: 10.1007/BF02015271. [DOI] [PubMed] [Google Scholar]
- 2.Levin DC, Rao VM, Parker L, Frangos AJ. Endovascular repair vs open surgical repair of abdominal aortic aneurysms: comparative utilization trends from 2001 to 2006. J Am Coll Radiol. 2009;6:506–9. doi: 10.1016/j.jacr.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 3.EVAR Trial Participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet. 2005;365:2179–86. doi: 10.1016/S0140-6736(05)66627-5. [DOI] [PubMed] [Google Scholar]
- 4.Lederle FA, Freischlag JA, Kyriakides TC, Padberg FT, Jr, Matsumura JS, Kohler TR, et al. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial. JAMA. 2009;302:1535–42. doi: 10.1001/jama.2009.1426. [DOI] [PubMed] [Google Scholar]
- 5.Prinssen M, Verhoeven EL, Buth J, Cuypers PW, van Sambeek MR, Balm R, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004;351:1607–18. doi: 10.1056/NEJMoa042002. [DOI] [PubMed] [Google Scholar]
- 6.Blankensteijn JD, de Jong SE, Prinssen M, van der Ham AC, Buth J, van Sterkenburg SM, et al. Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2005;352:2398–405. doi: 10.1056/NEJMoa051255. [DOI] [PubMed] [Google Scholar]
- 7.Hopkins R, Bowen J, Campbell K, Blackhouse G, De Rose G, Novick T, et al. Effects of study design and trends for EVAR versus OSR. Vasc Health Risk Manag. 2008;4:1011–22. doi: 10.2147/vhrm.s3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lifeline Registry of EVAR Publications Committee. Lifeline Registry of endovascular aneurysm repair: long-term primary outcome measures. J Vasc Surg. 2005;42:1–10. doi: 10.1016/j.jvs.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Egorova N, Giacovelli J, Greco G, Gelijns A, Kent CK, McKinsey JF. National outcomes for the treatment of ruptured abdominal aortic aneurysm: comparison of open versus endovascular repairs. J Vasc Surg. 2008;48:1092–100. 1100.e1–2. doi: 10.1016/j.jvs.2008.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giles KA, Landon BE, Cotterill P, O’Malley AJ, Pomposelli FB, Schermerhorn ML. Thirty-day mortality and late survival with reinterventions and readmissions after open and endovascular aortic aneurysm repair in Medicare beneficiaries. J Vasc Surg. 2011;53:6–12. 13e11. doi: 10.1016/j.jvs.2010.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schermerhorn ML, Giles KA, Sachs T, Bensley RP, O’Malley AJ, Cotterill P, et al. Defining perioperative mortality after open and endovascular aortic aneurysm repair in the US Medicare population. J Am Coll Surg. 2011;212:349–55. doi: 10.1016/j.jamcollsurg.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta M, Sternbach Y, Taggert JB, Kreienberg PB, Roddy SP, Paty PSK, et al. Long-term outcomes of secondary procedures after endovascular aneurysm repair. J Vasc Surg. 2010;52:1442–9. doi: 10.1016/j.jvs.2010.06.110. [DOI] [PubMed] [Google Scholar]
- 13.Abbruzzese TA, Kwolek CJ, Brewster DC, Chung TK, Kang J, Conrad MF, et al. Outcomes following endovascular abdominal aortic aneurysm repair (EVAR): an anatomic and device-specific analysis. J Vasc Surg. 2008;48:19–28. doi: 10.1016/j.jvs.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Brewster DC, Jones JE, Chung TK, Lamuraglia GM, Kwolek CJ, Watkins MT, et al. Long-term outcomes after endovascular abdominal aortic aneurysm repair: the first decade. Ann Surg. 2006;244:426–38. doi: 10.1097/01.sla.0000234893.88045.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaikof EL, Blankensteijn JD, Harris PL, White GH, Zarins CK, Bernhard VM, et al. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1048–60. doi: 10.1067/mva.2002.123763. [DOI] [PubMed] [Google Scholar]
- 16.Jones WB, Taylor SM, Kalbaugh CA, Joels CS, Blackhurst DW, Langan EM, 3rd, et al. Lost to follow-up: a potential under-appreciated limitation of endovascular aneurysm repair. J Vasc Surg. 2007;46:434–41. doi: 10.1016/j.jvs.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Malas MB, Freischlag JA. Interpretation of the results of OVER in the context of EVAR trial, DREAM, and the EUROSTAR registry. Semin Vasc Surg. 2010;23:165–9. doi: 10.1053/j.semvascsurg.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Franks SC, Sutton AJ, Bown MJ, Sayers RD. Systematic review and meta-analysis of 12 years of endovascular abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2007;33:154–71. doi: 10.1016/j.ejvs.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Leurs L, Buth J, Laheij R for the EUROSTAR Collaborators. Long-term results of endovascular abdominal aortic aneurysm treatment with the first generation of commercially available stent grafts. Arch Surg. 2007;142:33–41. doi: 10.1001/archsurg.142.1.33. [DOI] [PubMed] [Google Scholar]
- 20.van Marrewijk CJ, Leurs LJ, Vallabhaneni SR, Harris PL, Buth J, Laheij RJ. Risk-adjusted outcome analysis of endovascular abdominal aortic aneurysm repair in a large population: how do stentgrafts compare? J Endovasc Ther. 2005;12:417–29. doi: 10.1583/05-1530R.1. [DOI] [PubMed] [Google Scholar]
- 21.Pitolias GA, Schulte S, Donas KP, Horsch S. Secondary endovascular and conversion procedures for failed endovascular abdominal aortic aneurysm repair: can we still be optimistic? Vascular. 2009;17:15–22. doi: 10.2310/6670.2009.00004. [DOI] [PubMed] [Google Scholar]
- 22.Verhoeven EL, Tielliu IF, Prins TR, Zeebregts CJ, van Andringa de Kempenaer MG, Cina CS, et al. Frequency and outcome of re-interventions after endovascular repair for abdominal aortic aneurysm: a prospective cohort study. Eur J Vasc Endovasc Surg. 2004;28:357–64. doi: 10.1016/j.ejvs.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Becquemin JP, Kelley L, Zubilewicz T, Desgranges P, Lapeyre M, Kobeiter H. Outcomes of secondary interventions after abdominal aortic aneurysm endovascular repair. J Vasc Surg. 2004;39:298–305. doi: 10.1016/j.jvs.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 24.Quinney BE, Parmar GM, Nagre SB, Patterson M, Passman MA, Taylor S, et al. Long-term single institution comparison of endovascular aneurysm repair and open aortic aneurysm repair. J Vasc Surg. 2011;54:1592–7. doi: 10.1016/j.jvs.2011.06.114. discussion: 1597–8. [DOI] [PubMed] [Google Scholar]
- 25.Brown LC, Powell JT, Thompson SG, Epstein DM, Sculpher MJ, Greenhalgh RM. The UK EndoVascular Aneurysm Repair (EVAR) trials: randomised trials of EVAR versus standard therapy. Health Technol Assess. 2012;16:1–218. doi: 10.3310/hta16090. [DOI] [PubMed] [Google Scholar]
- 26.Abedi NN, Davenport DL, Xenos E, Sorial E, Minion DJ, Endean ED. Gender and 30-day outcome in patients undergoing endovascular aneurysm repair (EVAR): an analysis using the ACS NSQIP dataset. J Vasc Surg. 2009;50:486–91. 491.e1–4. doi: 10.1016/j.jvs.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 27.Leurs LJ, Visser P, Laheij RJ, Buth J, Harris PL, Blankensteijn JD. Statin use is associated with reduced all-cause mortality after endovascular abdominal aortic aneurysm repair. Vascular. 2006;14:1–8. doi: 10.2310/6670.2006.00010. [DOI] [PubMed] [Google Scholar]