Abstract
In plants, the shoot apical meristem (SAM) serves as a reservoir of pluripotent stem cells from which all above ground organs originate. To sustain proper growth, the SAM must maintain homeostasis between the self-renewal of pluripotent stem cells and cell recruitment for lateral organ formation. At the core of the network that regulates this homeostasis in Arabidopsis are the WUSCHEL (WUS) transcription factor specifying stem cell fate and the CLAVATA (CLV) ligand-receptor system limiting WUS expression. In this study, we identified the ERECTA (ER) pathway as a second receptor kinase signaling pathway that regulates WUS expression, and therefore shoot apical and floral meristem size, independently of the CLV pathway. We demonstrate that reduction in class III HD-ZIP and ER function together leads to a significant increase in WUS expression, resulting in extremely enlarged shoot meristems and a switch from spiral to whorled vegetative phyllotaxy. We further show that strong upregulation of WUS in the inflorescence meristem leads to ectopic expression of the AGAMOUS homeotic gene to a level that switches cell fate from floral meristem founder cell to carpel founder cell, suggesting an indirect role for ER in regulating floral meristem identity. This work illustrates the delicate balance between stem cell specification and differentiation in the meristem and shows that a shift in this balance leads to abnormal phyllotaxy and to altered reproductive cell fate.
Keywords: AGAMOUS (AG), Cell fate, WUSCHEL (WUS)
INTRODUCTION
In higher plants, all the aerial organs are produced post-embryonically through the activity of a pluripotent stem cell reservoir that resides in the apex of the meristem at the shoot tip (Carles and Fletcher, 2003; Sablowski, 2004). These cells divide slowly to renew themselves and to supply cells to the interior or to the periphery of the shoot apical meristem (SAM), where their progeny divide more rapidly to provide cells for the stem and for lateral organ primordia (Williams and Fletcher, 2005). The ability of the SAM to produce lateral organs while maintaining the appropriate size requires tightly controlled balance between cell proliferation in the central zone (CZ) and cell recruitment for primordium initiation in the peripheral zone (PZ) (Clark et al., 1997; Golz and Hudson, 2002; Wahl et al., 2010). Therefore, plants have evolved a complex genetic network that controls SAM size. At the core of this network is the WUSCHEL (WUS) transcription factor, which specifies the pluripotent state in the CZ, and the CLAVATA (CLV) ligand-receptor system that limits WUS expression to maintain a stable stem cell population size (Brand et al., 2000; Carles and Fletcher, 2003; Schoof et al., 2000). This negative-feedback loop is further fine-tuned by many other signaling pathways and molecular mechanisms such as epigenetic factors and other receptor systems (Perales and Reddy, 2012). Extensive genetic screens have identified numerous mutants with abnormal shoot and/or floral meristem size, uncovering many genes that participate in the regulation of meristem size and function (DeYoung et al., 2006; Fletcher, 2001; Furner and Pumfrey, 1992; Kaya et al., 2001; Laux et al., 1996; Long and Barton, 1998; Vidaurre et al., 2007; Wu et al., 2005). Nevertheless, some genes may act in partially redundant parallel pathways and therefore have not yet been uncovered.
Redundancy in SAM regulation is illustrated by the three members of the Arabidopsis ERECTA (ER) gene family that encode leucine-rich-repeat receptor kinases (Torii et al., 1996), which are broadly expressed and play redundant roles in diverse aspects of plant development (Shpak et al., 2004; Uchida et al., 2012; Yokoyama et al., 1998). Mutations in the ER gene enhance the SAM phenotypes of other mutants (Durbak and Tax, 2011; Uchida et al., 2011), and it has been suggested that the ER family regulates meristem homeostasis by buffering cytokinin responsiveness in the SAM (Uchida et al., 2013).
In the SAM peripheral zone, leaves initiate with a regular spacing that determines the phyllotaxis (Sussex, 1998; Traas, 2013). An accepted model for phyllotaxis regulation is that high auxin level accumulation at a precise position in the PZ triggers organ initiation. Local auxin maxima are achieved by auxin polar transporters that actively deplete the auxin from nearby cells (Vieten et al., 2007), resulting in low concentrations in the vicinity of the existing organ primordia. This allows a new auxin maximum, and therefore a new organ primordium, to develop only at a specific minimum distance from the pre-existing primordia (Jönsson et al., 2006; Lohmann et al., 2010; Smith et al., 2006).
Once the SAM switches from vegetative to reproductive phase, lateral organ identity changes and the inflorescence meristem (IM) produces floral meristems (FM) on its flanks. Each FM provides all the cells to form the four whorls of floral organs - sepals, petals, stamens and carpels - before ceasing its meristematic activity. The identity of each floral organ is specified by different combinations of transcription factors encoded by several classes of genes that form the basis for the ABCE[D] model of flower development (Bowman et al., 2012; Krizek and Fletcher, 2005). In Arabidopsis, the C-class floral homeotic gene AGAMOUS (AG) is expressed in the center of the FM and specifies carpel identity in whorl four, while also acting with B-class genes to specify stamen identity in whorl three (Coen and Meyerowitz, 1991). During wild-type flower development, WUS is expressed in stage 1 floral primordia (stages according to Smyth et al., 1990) and is repressed once the carpel primordia form at stage 6, which results in FM termination (Lenhard et al., 2001). WUS binds directly to the second intron of AG and together with the LEAFY transcription factor, activates AG expression at floral stage 3 (Busch et al., 1999).
We have previously reported on the role of the Arabidopsis microRNA miR166g in regulating WUS-dependent SAM activity (Williams et al., 2005). We demonstrated that in jabba-1D (jba-1D) plants, overexpression of miR166g causes a decrease in the transcript levels of several class III homeodomain-leucine-zipper (HD-ZIPIII) target genes, which leads to a dramatic increase in WUS transcript levels and expansion of its expression domain, resulting in SAM enlargement (Williams et al., 2005).
To uncover novel genes that act redundantly with the HD-ZIPIII pathway in meristem regulation, we conducted a genetic screen to identify second-site mutations that modified the jba-1D enlarged SAM phenotypes and identified a mutation in the ER gene as an enhancer. jba-1D plants carrying the mutated ER modifier exhibit extremely enlarged SAMs with altered leaf phyllotaxis and ectopic carpels forming directly from the inflorescence meristem. We demonstrate that, owing to aberrant ER function, the WUS gene is upregulated, leading to ectopic AG expression and subsequent ectopic carpel formation. Our results indicate that the ER gene regulates SAM size in a genetic pathway parallel to those of the HD-ZIPIII and CLV pathways, and that ER has a similar function in the floral meristem, where it plays a role in regulating meristem size and homeostasis.
RESULTS
A mutation in the ERECTA gene enhances the jba-1D/+ enlarged meristem phenotype
Arabidopsis jba-1D plants display pleiotropic developmental phenotypes, in which the most prominently is a dramatic enlargement of the SAM that results in extremely fasciated stems and enlarged IMs that produce many extra flowers (Williams et al., 2005) (supplementary material Fig. S1). These phenotypes are caused by dosage-dependent upregulation of miR166g. To identify other genes involved in SAM regulation, we performed a genetic modifier screen of ethyl methanesulfonate (EMS) mutagenized jba-1D/+ plants. We identified a strong modifier exhibiting meristem-related phenotypes that we termed jabba Modifier1 (jM1). The jM1 mutant was backcrossed twice into the jba-1D/+ parental background and the modifier was shown to act as a single nuclear trait.
jM1 homozygous single mutant plants display round leaves with short petioles and a compact inflorescence with flowers clustering at the top (supplementary material Fig. S2). This phenotype closely resembles those of Landsberg erecta (Ler) plants (Torii et al., 1996), although jM1 plants are in the Col-0 genetic background. This resemblance led us to investigate whether the modifier mutation in jM1 was in the ER gene. Sequencing the ER gene from jM1 individuals confirmed the presence of a missense mutation that causes an isoleucine to threonine substitution at amino acid 750 in the cytoplasmic serine/threonine protein kinase domain (Lease et al., 2001). We performed a complementation assay (supplementary material Fig. S3), demonstrating that the missense mutation in the ER gene causes the strong enhancement of the jba-1D/+ phenotype. We therefore named this recessive allele er-20.
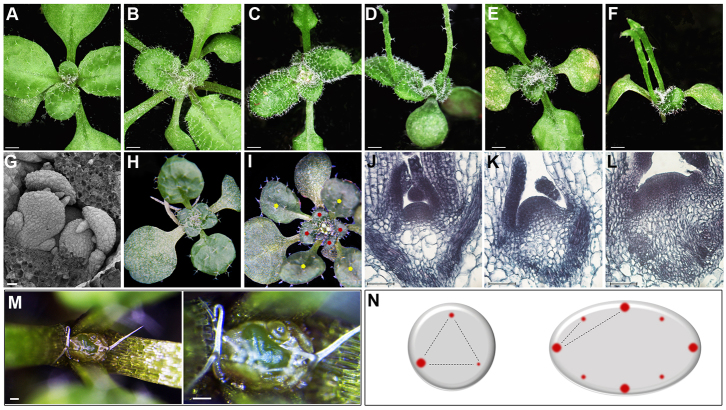
Analysis of jba-1D/+er-20 plants revealed two distinct morphological phenotypes (Figs 1, 3). At the vegetative stage, jba-1D/+er-20 seedlings lack the typical spiral phyllotaxis displayed by wild-type and jba-1D/+ seedlings (Fig. 1A-C,H,I), and the SAMs of jba-1D/+er-20 plants show additional enlargement relative to jba-1D/+ plants (Fig. 1J-L). In wild-type and jba-1D/+ seedlings (Fig. 1A-C), the first pair of true rosette leaves emerges simultaneously in a decussate pattern, and all subsequent leaves arise following a spiral phyllotaxy characterized by the ‘golden angle’ of about 137.5°. Sixteen-day-old jba-1D er-20 seedlings exhibit similar phenotype to jba-1D seedlings in their first two radial leaves and successive curled leaves, although somewhat more severe (Fig. 1D,F). By contrast, jba-1D/+er-20 seedlings exhibit a decussate pattern for the first pair of leaves followed by a transition to a whorled phyllotaxis (Fig. 1H,I). Four rosette leaves arise at the same time in an iterative manner throughout the vegetative phase, as do the cauline leaves (supplementary material Fig. S1). We examined the leaf primordia initiation by performing scanning electron microscopy (SEM) on jba-1D/+er-20 seedlings and found that the four primordia emerge simultaneously at the SAM periphery (Fig. 1G), suggesting the concurrent establishment of auxin maxima at four distinct locations around the SAM (Fig. 1N).
Fig. 1.
The jba-1D/+er-20 SAM is enlarged and shows altered phyllotactic patterning. (A-F) Sixteen-day-old seedlings. (A) Ler, (B) Col, (C) jba-1D/+, (D) jba-1D, (E) jba-1D/+er-20 and (F) jba-1D er-20. (G) SEM of jba-1D/+er-20 seedling showing four leaf primordia emerging from the SAM. (H,I) jba-1D/+er-20 seedlings with four leaves arising simultaneously in concentric whorls, the first four marked with yellow and the next four with red. (J-L) Longitudinal sections of 9-day-old seedlings. (J) Col. (K) jba-1D/+. (L) jba-1D/+er-20 and (M) jba-1D/+er-20 vegetative SAM at two different magnifications. (N) Schematic diagram of auxin maxima (red dots) at leaf primordia initiation sites in meristems of two different sizes. Scale bars: 1 mm in A-E; 200 μm in G; 50 μm in J-L; 100 μm in M.
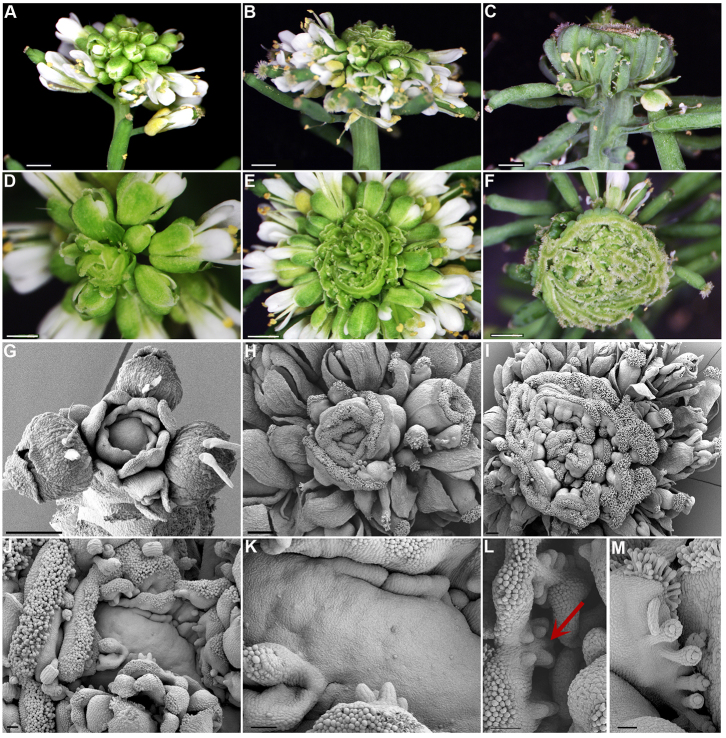
Fig. 3.
jba-1D/+er-20 IMs ectopically produce multiple fused carpels. (A-F) Side and top views of progressively older jba-1D/+er-20 inflorescence. (A,D) Young inflorescence. (B,E) Older inflorescence with whorls of ectopic fused carpels. (C,F) Severely fasciated inflorescence displaying multiple whorls of ectopic fused carpels. (G-M) Scanning electron microscopy of progressively older jba-1D/+er-20 IMs. (G) Young dome-shaped IM with carpels beginning to form around the flanks. (H,I) Older IMs with multi-fused carpels consisting of distinctive valves, replum, style and stigma. (J) Unfused multi-carpel gynoecia. (K) Extremely enlarged IM with ectopic carpels developing directly from the SAM flanks. (L) Developing ovule primordia projecting on the opposing placenta (red arrows). (M) A mature unfused ectopic gynoecium with multiple ovules. Scale bars: 2 mm in A-I; 200 μm in J-M.
Next, we examined at the histological level the SAM of wild-type, jba-1D/+ and jba-1D/+er-20 plants. Sections through 9-day-old seedlings revealed that the er-20 mutation enhances the jba-1D/+ enlarged SAM phenotype (Fig. 1K) leading to an extremely enlarged meristem (Fig. 1L). In some cases, the huge meristem can be seen easily with the naked eye (Fig. 1M). The jba-1D/+er-20 SAM enlargement and its altered phyllotaxis are consistent with reports showing that altered SAM size can lead to changes in phyllotaxis (Clark et al., 1993; Giulini et al., 2004; Laufs et al., 1998a; Medford et al., 1992; Leyser and Furner, 1992; Takahashi et al., 2002).
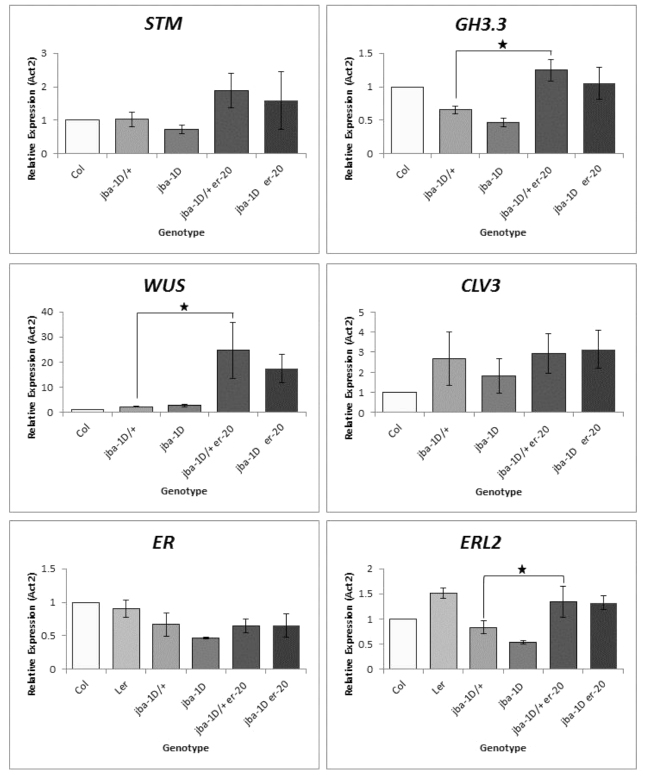
To further test the hypothesis that meristem enlargement is accompanied by simultaneous establishment of several auxin maxima (Fig. 1N), we performed RT-qPCR on 8-day-old whole seedlings using gene specific primers for SHOOT MERISTEMLESS (STM) and GH3.3 (Fig. 2). STM encodes a class I KNOX transcription factor that is expressed throughout the SAM and promotes meristem cell proliferation (Long et al., 1996). The GH3.3 gene is an early auxin-responsive gene that is expressed during primordium initiation, from the first sign of cell bulging at the SAM periphery to leaf plastochron 4 (Efroni et al., 2008; Mallory et al., 2005). The results showed an approximately twofold increase in STM expression levels in jba-1D/+er-20 compared with jba-1D/+ seedlings, consistent with the meristem enlargement phenotype (Fig. 1L). The moderate increase in STM levels reflects the small fraction of meristematic cells within the whole seedling samples. With a higher proportion of meristematic cells in jba-1D/+er-20, we would expect a reduction in GH3.3 transcript levels due to dilution of the GH3.3-expressing primordia cells within the whole seedlings. Yet RT-qPCR analysis showed an approximately twofold increase in GH3.3 expression in jba-1D/+er-20 plants (Fig. 2). This indicates that the jba-1D/+er-20 SAM contains more cells expressing the early auxin-responsive GH3.3 gene, consistent with the formation of additional auxin maxima for organ initiation. Our results therefore indicate that ER restricts SAM size during vegetative development, and that restricting SAM size may prevent the production of more than one auxin maximum at a time.
Fig. 2.
Relative transcript levels of STM, GH3.3, WUS, CLV3, ER and ERL2 in wild-type and mutant seedlings. Relative transcript levels (mean±s.e.m.) were calculated from triplicate RT-qPCR reactions of independent RNA samples prepared from three different sets of 8-day-old Arabidopsis seedlings. The transcript levels in Col are set to 1. Relative expression is normalized to ACTIN2. Asterisks indicate statistical significance (P<0.05, n=3).
It has recently been suggested that the SAM possesses a functional buffering mechanism regulated by the ER receptor kinase family to maintain CLV3 homeostasis regardless of an increase in WUS expression induced by cytokinin treatment (Uchida et al., 2013). We therefore assessed the WUS and CLV3 expression levels by RT-qPCR. We observed a dramatic increase in WUS expression in jba-1D/+er-20 compared with jba-1D/+seedlings, whereas CLV3 expression levels were not significantly different (Fig. 2). In the absence of functional ER we would expect increased levels of CLV3 transcripts because of the increase in WUS expression. Therefore, this result raises the possibility that the jba-1D/+er-20 meristem contains more cells of all types and that the ratio between the whole meristem tissue and the CLV3-expressing stem cells is maintained.
To test whether other ER family members compensate for the mutation in ER, we analyzed the expression of ER, ERECTA LIKE 1 and ERECTA LIKE 2 (ERL1 and ERL2). We found no differential expression for ER and ERL1 but a 1.7-fold increase in ERL2 expression levels in jba-1D/+er-20 relative to jba-1D/+ plants. These results are consistent with the view that ER genes redundantly regulate the vegetative SAM (Uchida et al., 2013) and imply that ERL2 upregulation compensates for the mutation in ER in maintaining CLV3 homeostasis.
jba-1D/+ er-20 inflorescence meristems ectopically produce multi-fused carpels
During reproductive development, mature jba-1D/+er-20 plants form ectopic, multi-fused carpels around the IM (Fig. 3). Like jba-1D plants, jba-1D/+er-20 form fasciated IMs that produce numerous flowers (Fig. 3A). However, 4-5 weeks after bolting, the inflorescence starts to form carpels at the periphery in place of complete flowers (Fig. 3A,D). Initially, a single or a few fused carpels emerge in a spiral phyllotaxy around the inflorescence (Fig. 3D) and the structures gradually develop as multi-fused carpels that encircle the IM almost as one piece (Fig. 3B,C,E,F). Iterations of whorls of ectopic multi-carpels form internal to one another, ultimately resulting in a massive structure of carpels within carpels at the top of the inflorescence stem (Fig. 3C,F; supplementary material Fig. S4C). The IM continues to produce carpels even 16 weeks after bolting, after the rest of the plant has senesced (supplementary material Fig. S5).
In Arabidopsis, each flower primordium initiates at the IM periphery as a small bulge of cells that divide and expand in three dimensions, generating a hemispherical primordium that becomes separated from the IM by a small groove (Kwiatkowska, 2006; Smyth et al., 1990). This primordium represents a de novo FM from which the four types of floral organs will arise in concentric rings called whorls. To determine whether the jba-1D/+er-20 ectopic carpels develop from FMs or arise directly from the IM, we performed SEM analysis (Fig. 3G-M). Initially, the jba-1D/+er-20 IM develops normal flowers, until a sharp transition to the formation of carpels as lateral organs occurs (Fig. 3G). Each carpel primordium arises directly from the meristem as a bulge on its edge that gradually becomes delineated from the IM (Fig. 3G,J,K; supplementary material Fig. S4A). We conclude that the ectopic carpels initiate directly from the jba-1D/+er-20 IM, indicating a change in cell identity from FM fate to carpel fate at the IM periphery.
The wild-type Arabidopsis gynoecium initiates as a raised rim around the center of the FM that grows and forms a hollow cylinder (Alvarez-Buylla et al., 2010). Very rarely the jba-1D/+er-20 inflorescence forms typical cylindrical gynoecium structures in the center of the huge IM, in addition to ectopic carpels on the flanks (supplementary material Fig. S4B). All the ectopic carpels on the jba-1D/+er-20 IM consist entirely of carpel-specific tissues, but they fail to form an open-ended tube that will fuse to generate the seed-bearing ovaries of a mature gynoecium. In each ectopic carpel, a valve flanked with valve margin, style bearing stigmatic papillae, replum and septum tissues are visible (Fig. 3H,I,L,M; supplementary material Fig. S4C). The adaxial side of the fused carpels shows reduced septum tissues with two rows of ovule primordia growing on opposite sides (Fig. 3L) and in the unfused mature gynoecium the ovule funiculus, inner and outer integuments are observed (Fig. 3M).
In summary, these observations indicate that miR166g overexpression and aberrant ER function in jba-1D/+er-20 plants lead to an extreme IM enlargement and to the formation of ectopic carpel structures in place of functional FMs. Therefore, miR166g and ER function are together required to restrict IM size and specify floral meristem identity.
Many gynoecium development genes are upregulated in jba-1D/+ er-20 IMs
To further explore the hypothesis that jba-1D/+er-20 IMs develop carpels on the periphery due to a switch in cell identity, we carried out an RNA-Seq analysis on jba-1D/+ and jba-1D/+er-20 IMs. We collected 30 dissected inflorescences from each genotype at 4 weeks after bolting, once the first plant began to exhibit ectopic carpel formation. All the flowers were removed, leaving either bare meristems with undetachable primordia for jba-1D/+ IMs, or bare meristems with a mixture of un-detachable floral and carpel primordia for jba-1D/+er-20 IMs. The meristems were independently pooled for mRNA isolation, sequencing and differential expression analysis (supplementary material Table S1).
As expected, WUS expression levels are fourfold higher in jba-1D/+er-20 IMs compared with jba-1D/+ IMs, even though in jba-1D/+ the remaining floral primordia on the IMs contributed to the total WUS transcript levels, whereas in jba-1D/+er-20 the source was mainly cells from the IMs themselves. This result indicates a much higher level of WUS expression in jba-1D/+er-20 IMs, and demonstrates that ER negatively regulates WUS in the IM. As in the vegetative SAM, the GH3.3 gene is upregulated in jba-1D/+er-20 IMs (Table 1), again suggesting the occurrence of additional auxin maxima around the IM. For the ER genes, although ER and ERL2 are not differentially expressed, ERL1 expression levels were threefold higher in jba-1D/+er-20 IMs.
Table 1.
Selected upregulated genes in jba-1D/+er-20 compared with jba-1D/+ IMs
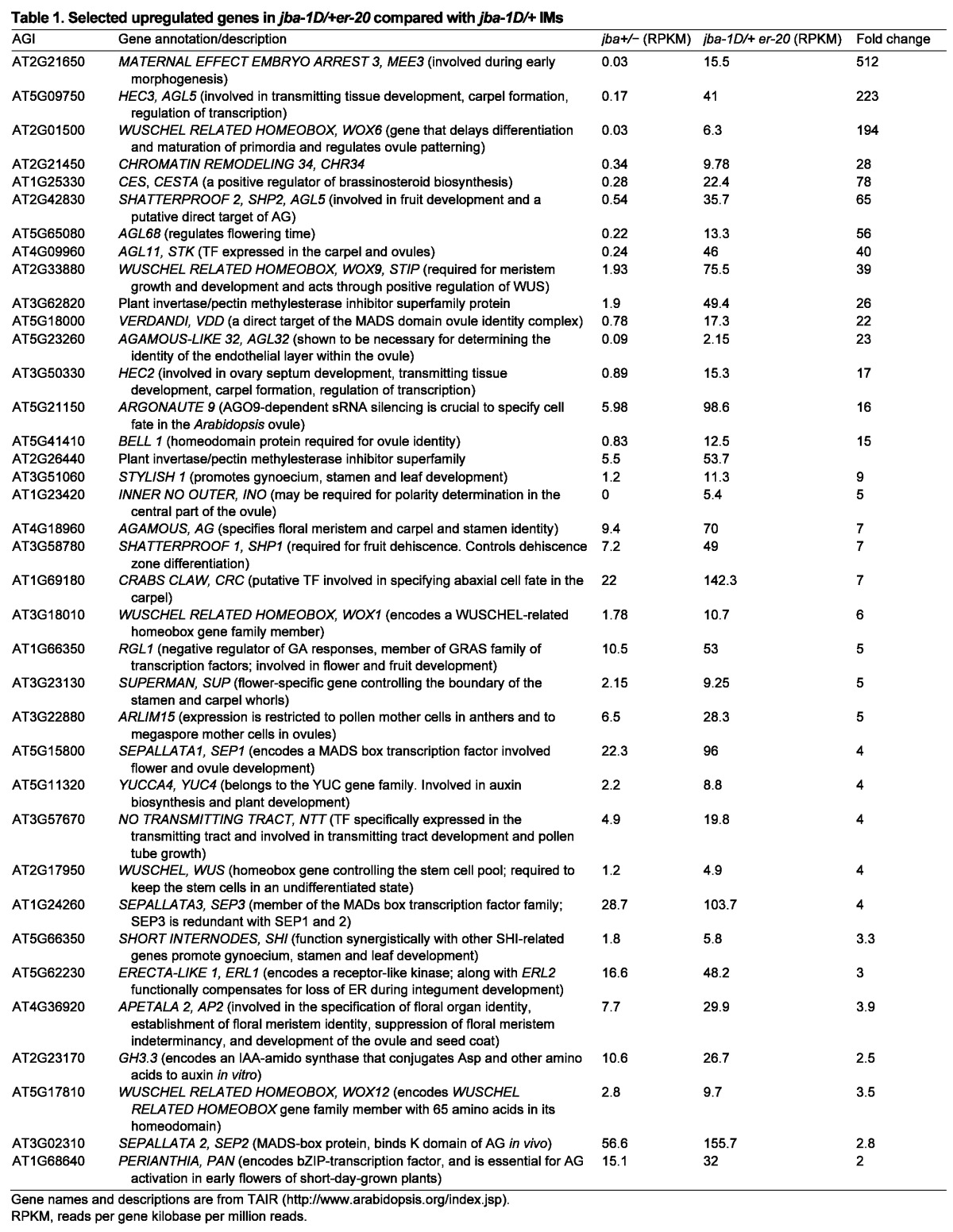
In addition, many of the CE[D] class floral organ identity genes are upregulated in jba-1D/+er-20 plants (Table 1). For example, carpels are specified by the class C MADS-box gene AG, along with the related SHATTERPROOF1 (SHP1), SHP2 and SEEDSTICK (STK) genes (Becker and Theissen, 2003; Pinyopich et al., 2003). STK also acts redundantly with SHP1 and SHP2 to promote ovule identity determination (Colombo et al., 2010). Because ectopic carpels develop directly from the jba-1D/+er-20 IMs, we expected these four genes to be highly expressed. Indeed, AG and SHP1 are both upregulated sevenfold in jba-1D/+er-20 compared with jba-1D/+ IMs, SHP2 is elevated 65-fold, and STK is elevated 40-fold (Table 1). In addition, three SEPALLATA (SEP) genes that function redundantly as class E genes are upregulated in jba-1D/+er-20 IMs: SEP1 by fourfold, SEP2 by threefold and SEP3 by 2.8-fold. The AG transcription factor orchestrates the expression of numerous downstream genes (Gómez-Mena et al., 2005; Ito et al., 2007; Ito et al., 2004). We found that many AG immediate targets and directly bound genes are upregulated in jba-1D/+er-20 IMs, including CRC, SEP3 and SHP1 (Gómez-Mena et al., 2005) (supplementary material Table S2). In summary, many early gynoecium specification genes are upregulated in jba-1D/+er-20 plants.
Together, our morphological and molecular datasets indicate that the ER gene is required to constrain WUS mRNA expression in jba-1D/+ meristems. We also find that it limits the transcription levels of floral organ identity genes, such as AG. We propose that in jba-1D/+er-20 IMs, high levels of WUS transcripts lead to ectopic activation of AG expression. At the IM periphery, where the cells are competent to develop into flowers, this ectopic AG expression activates its downstream genes, leading to a switch in cell identity and the initiation of carpel formation.
jba-1D/+ er-20 IMs show ectopic expression of WUS and AG
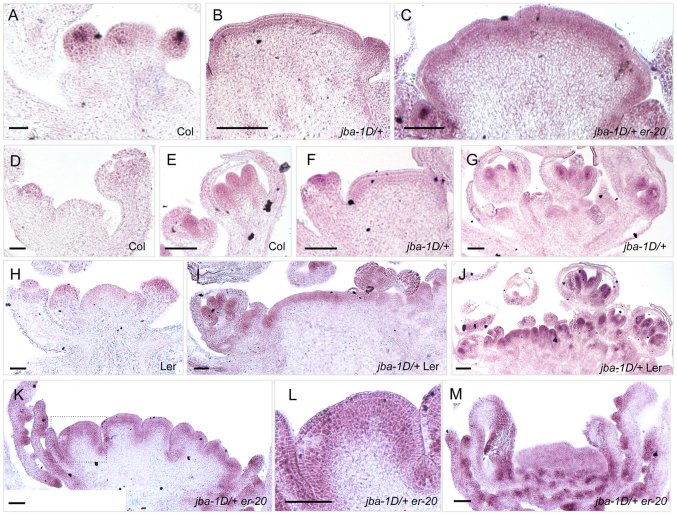
In jba-1D/+er-20 plants, the vegetative SAM and IM exhibit elevated levels of WUS expression, indicating that ER negatively regulates WUS. Yet, it is unclear whether ER tunes WUS transcription levels or limits its expression domain. To analyze this, we performed RNA in situ hybridization with a WUS probe (Fig. 4). In wild-type Col IMs, WUS is expressed in the central cells of the IM and the FM, a region termed the organizing center (OC) (Fig. 4A). In jba-1D/+ IMs, the WUS expression pattern is similar to that of clv3-2 IMs (Fig. 4B), in which the domain expands laterally and upward into the outermost two to three layers across the meristem (Brand et al., 2000). In jba-1D/+er-20 IMs, not only is the WUS signal is more intense than in jba-1D/+ IMs, it expands further into the interior cell layers (Fig. 4C). Thus, ER is a regulator that contributes both to limiting WUS transcript levels and to restricting the WUS expression domain to the OC. We have previously reported that wus-1 jba-1D meristems are indistinguishable from wus-1 meristems (Williams et al., 2005), indicating that WUS activity is absolutely required to obtain the jba SAM phenotypes. As wus-1 suppresses the jba-1D/+ phenotypes, it will also suppress the jba-1D/+ er-20 phenotypes, as er-20 single mutants do not exhibit ectopic carpel formation.
Fig. 4.
Ectopic WUS and AG expression in jba-1D/+er-20 IM. Inflorescence tissues hybridized (purple staining) with a (A-C) WUS antisense or an (D-M) AG antisense RNA probe. (A,D,E) Col IM and flower primordia. (H) Ler inflorescence. (B,C) Ectopic WUS expression in the outer layers throughout jba-1D/+ and jba-1D/+er-20 IMs. (F) Weak AG signal in the three uppermost cell files of the jba-1D/+ IM. (G) AG expression in jba-1D/+ floral primordia. (I,J) Strong AG expression in jba-1D/+ Ler floral primordia and in the three or four uppermost cell files of the IM. (K-M) Ectopic AG expression in jba-1D/+er-20 IMs. Intense AG signal in the four outer cell layers of the splitting IMs, as well as in the adaxial region of the ectopic carpels and in their ovules. (L) Magnified view of the boxed area in K. Scale bars: 50 μm.
To further investigate the hypothesis that ectopic AG expression can lead to a switch in cell identity at the IM periphery, we tested whether ectopic AG expression accompanies the initiation of ectopic carpels in jba-1D/+er-20 IMs using RNA in situ hybridization. In wild-type plants, AG mRNA is absent from the IM but is first detected in the center of stage 3 floral primordia prior to stamen and carpel initiation (Fig. 4D,E,H). AG expression is later confined to the inner two whorls of the flower. At stage 9, AG mRNA is detected at low levels in the valves of the developing carpels and at high levels in ovule primordia (Drews et al., 1991; Ito et al., 2004; Yanofsky et al., 1990). AG expression is unaltered in jba-1D/+ floral primordia, but unexpectedly we also detect a faint signal in the L1, L2 and uppermost L3 cell layers across the IM (Fig. 4F,G), indicating that AG is ectopically expressed in jba-1D/+ IMs. However, in jba-1D/+Ler and jba-1D/+er-20 IMs, AG expression is much more intense in the outer cell layers (Fig. 4I-M) than in jba-1D/+ IMs, and spreads to more layers inwards. Furthermore, jba-1D/+er-20 ectopic carpels show strong AG expression on their adaxial side and in ovule primordia (Fig. 4K,M). These results are consistent with our proposed scenario that strong ectopic AG expression at the IM periphery leads to a cell identity switch from flower founder cell identity to carpel cell identity.
Next, we tested whether AG is required for the ectopic carpel phenotype by crossing jba-1D/+er-20 to ag-1-null mutant plants. The stems of jba-1D/+er-20 ag-1 plants are extremely fasciated and the IMs develop flowers lacking stamens and carpels (Fig. 5K,L). However, the IMs never generate ectopic carpel structures on their flanks, even late in their life cycles (Fig. 5K,L), indicating that AG is required to confer the jba-1D/+er-20 ectopic carpel phenotype.
Fig. 5.

Genetic interactions between jba-1D/+, jba-1D/+er-20 and ER family mutants. (A-C) jba-1D/+ crossed to Ler exhibiting multi-fused carpels (red arrows) (A,B) or enlarged IM with concentric ring of carpels (C). (D,E) jba-1D/+ Col er-2 plants display whorls of multifused carpels within carpels. (F-H) jba-1D er-20 erl1 plant 2 weeks after bolting. (F) jba-1D er-20 inflorescence (G,H) jba-1D er-20 erl1 inflorescence exhibits ectopic carpel formation (red arrow). (I,J) jba-1D/+er-20 erl1 plant exhibits an extremely fasciated stem. (I) jba-1D/+er-20 (left) and jba-1D/+er-20 erl1 (right). (J) jba-1D/+er-20 erl1 enhanced multi-fused carpel structure. (K,L) jba-1D/+er-20 ag-1 plants display extremely fasciated stems that generate supernumerary flowers composed entirely of sepals and petals. Scale bars: 2 mm in A,F-H,J; 1 mm in L; 250 μm in C-E.
Effect of ER gene family mutations on the jba-1D/+ phenotype
To determine whether other mutations in ER could generate phenotypes like those of jba-1D/+er-20 plants, we analyzed the genetic interactions between jba-1D/+ and either the Ler accession or the Col accession carrying the er-2 allele. Ler contains a point mutation in the ER kinase domain (Torii et al., 1996) and is likely to be a null allele (Lease et al., 2001). jba-1D/+ Ler plants display two types of phenotypes. The first is a less severe version of the jba-1D/+er-20 carpel phenotype, where ectopic carpel formation ceases as the plants senesce (Fig. 5A,B). The second is the formation of a macroscopic IM with several whorls of ectopic carpels that encircle the IM but do not form reiterative multi-fused carpels structures (Fig. 5C). The er-2 allele is an X-ray-induced frameshift mutation (Hall et al., 2007). jba-1D/+er-2 IMs showed the same ectopic carpel formation phenotype as jba-1D/+er-20 IMs (Fig. 5D,E). These results indicate that loss of ER function is responsible for the jba-1D/+ modifier phenotypes.
The ER genes redundantly regulate SAM function (Shpak et al., 2004; Uchida et al., 2013). Therefore, we asked whether ERL1 upregulation in jba-1D/+er-20 IMs partially compensates for the mutation in ER. We generated triple mutants by crossing jba-1D/+er-20 to the erl1-null mutant (SALK_081669) and observed two types of enhancement according to the jba-1D background (hemi-or homozygous) (Fig. 5F-J). In jba-1D er-20 erl1 plants, the ectopic carpels are evident after only four or five normal flowers have formed (Fig. 5F-H). jba-1D/+ er-20 erl1 plants display enhanced fasciation of the stems, which appear to be flattened with more multi-fused carpels, suggesting the presence of an enlarged wider IM (Fig. 5I,J). Consistent with the expression of ER genes in the shoot apex (Shpak et al., 2004; Uchida et al., 2013; Yokoyama et al., 1998), these results implicate the ER family members in IM size regulation.
The ER gene regulates meristem development in a CLV-independent pathway
Several er mutations enhance the SAM enlargement and gynoecium phenotypes of the CLV pathway mutants (Durbak and Tax, 2011). To test whether the er-20 mutation acts in the CLV pathway, we generated jba-1D/+er-20 clv3-2 plants (Fig. 6). Compared with wild-type Col and Ler (Fig. 6A,B) inflorescences, both clv3-2-null and jba-1D/+er-20 inflorescences are enlarged and produce supernumerary flowers (Fig. 6C,E,L). jba-1D/+er-20 clv3-2 plants, however, form enormously enlarged IMs that produce few flowers before shifting to carpel formation at the periphery (Fig. 6G,M). The huge IM gradually increases in size and the multi-fused carpel structure keeps growing even 14 weeks after bolting (Fig. 6O). This synergistic interaction between clv3-2 and jba-1D/+er-20 indicates that CLV3, HD-ZIPIII and ER regulate SAM size in separate genetic pathways (supplementary material Fig. S6).
Fig. 6.
Genetic interactions between jba-1D/+er-20 and clv3 plants. (A-G) Top view of inflorescences 2 weeks after bolting. (A) Col, (B) Ler, (C) clv3-2, (D) jba-1D/+, (E) jba-1D/+er-20, (F) jba-1D/+ clv3-2 and (G) jba-1D/+er-20 clv3-2 plants display huge meristems. (H) Gynoecia from the same genotypes as A-G in the same order. (I-K) Short sphere-shaped jba-1D/+er-20 clv3-2 gynoecia (I) at an early stage containing 12 valves, (J) with an indeterminate floral meristem (red arrow) producing numerous carpels and (K) bursting due to prolonged meristem activity that continuously produces carpels. (L-O) Side views of jba-1D/+er-20 (L,N) and jba-1D/+ er-20 clv3-2 (M,O) plants at 2 (L,M) and 14 (N,O) weeks after bolting. Scale bars: 1 mm.
The jba-1D/+er-20 clv3-2 gynoecium phenotypes also show a synergistic effect. Wild-type, jba-1D/+ and jba-1D/+er-20 gynoecia consist of two fused carpels, whereas clv3-2 gynoecia are composed of four to six carpels (Fig. 6H). jba-1D/+er-20 clv3-2 gynoecia are short and consist of 10 to 14 carpels, causing the fruit to have a sphere-like appearance (Fig. 6H,I). Because carpel number is tightly associated with FM size (Clark et al., 1993), these results indicate a direct role for ER in FM size regulation. In wild-type plants, stem cell termination in the FM is tightly coupled to the formation of carpel primordia, resulting in a mature gynoecium with two fused carpels (Fig. 6H) (Lenhard et al., 2001). By contrast, jba-1D/+er-20 clv3-2 flowers not only form multi-carpel gynoecia, but their FMs fail to terminate and continue to produce numerous additional carpels, causing the gynoecia to burst (Fig. 6J,K). Thus, each jba-1D/+er-20 clv3-2 gynoecium develops into a structure that resembles the multifused carpel structure of the jba-1D/+er-20 IM. These gynoecium phenotypes are consistent with a role for ER in regulating FM size and FM termination through a separate genetic pathway from the CLV pathway.
DISCUSSION
In this study we show that interference with SAM homeostasis between cell proliferation and cell recruitment for lateral organ formation by reducing HD-ZIPIII and ER gene function results in abnormal development, characterized by phyllotaxis alteration at the vegetative stage and ectopic carpel formation at the reproductive stage.
ER regulates WUS transcription and vegetative phyllotaxis
The ER gene is expressed in the SAM and throughout the FM at stages 1-3 (Roeder and Yanofsky, 2006; Shpak et al., 2004; Uchida et al., 2013; Yokoyama et al., 1998). Here, we show that jba-1D/+ plants carrying a mutation in ER develop enormous SAMs that are wider and taller than jba-1D/+ meristems, and contain more cells. These data fit with previous work showing that mutations in ER enhance meristem-derived phenotypes (Diévart et al., 2003; Durbak and Tax, 2011; Uchida et al., 2013). An increase in meristem size can occur via several pathways: a higher rate of cell division within the meristem, an increase in the size of the stem cell reservoir due to expansion of the OC that specifies their identity, or an accumulation of meristematic cells at the SAM periphery due to reduced cell incorporation into organ primordia (Kirch et al., 2003; Laufs et al., 1998b; Seidlová, 1980). The ER genes play overlapping roles in promoting cell proliferation in stems, leaves and floral organs (Torii et al., 1996; Shpak et al., 2003). If ER has the same function in the SAM, then mutations in ER should lead to a reduction SAM cell number. However, we observe that loss of ER activity in jba-1D/+ leads to a dramatic increase in SAM size and cell number (Figs 1,3,4; supplementary material Fig. S1), suggesting that ER does not directly promote cell division in the SAM.
In jba-1D plants, a reduction in HD-ZIPIII transcript levels results in WUS upregulation and expression domain expansion (Williams et al., 2005). The er-20 mutation causes a further significant increase in WUS transcript levels, revealing a function for ER as a negative regulator of WUS expression. Furthermore, the enlarged jba-1D/+er-20 SAM produces supernumerary organ primordia, indicating that the increase in meristem size is not due to a reduced rate of primordium initiation but is the result of defective ER regulation of WUS.
It has been suggested that meristem size could have a major influence on phyllotaxis (Bainbridge et al., 2008). Our observations that jba-1D/+er-20 plants have extremely enlarged SAMs from which four primordia initiate simultaneously demonstrates the impact of meristem size on phyllotactic pattern. An accepted model for controlling phyllotaxis is that new primordia initiate at auxin maxima points on the SAM periphery (Benková et al., 2003; Heisler et al., 2005; Vanneste and Friml, 2009). Once an auxin maximum forms, it depletes auxin from the adjacent cells, resulting in the formation of a new auxin maximum, and therefore the emergence of a new primordium, only at a maximal distance from the established organs (Berleth et al., 2007; Lohmann et al., 2010; Reinhardt et al., 2003). In wild-type Arabidopsis plants, single auxin maxima form sequentially, resulting in a spiral phyllotaxis. In er-20 plants there is no alteration in phyllotaxis, indicating that ER on its own does not regulate leaf primordia initiation. We therefore propose that the increased surface of jba-1D/+er-20 vegetative meristems provides adequate distances to allow four auxin maxima to develop simultaneously (Fig. 1N), and that an increase in meristematic cells enables the SAM periphery to supply a sufficient number of founder cells to initiate extra organ primordia.
This idea is consistent with the elevated expression levels of the GH3.3 early auxin-responsive gene in jba-1D/+er-20 plants, which implies that additional auxin is present to stimulate its transcription. In conclusion, our observations indicate that ER negatively regulates WUS transcription, such that loss of ER function in the jba-1D/+ background leads to a massive increase in SAM size that affects phyllotaxis rather than to direct regulation of primordia initiation.
ER and HD-ZIPIII genes link SAM size maintenance with floral meristem identity specification
Upon bolting, the fasciated jba-1D/+er-20 IMs produce numerous normal flowers until a cell fate switch occurs at the IM periphery, leading to ectopic carpel formation. Two important questions raised are why does this cell identity switch occur, and why does it take place around 4 weeks after bolting rather than exactly at the reproductive transition? Because the phenotype occurs in the context of high levels of ectopic WUS and AG mRNA expression across the IMs, which is more dramatic in jba-1D/+er-20 than in jba-1D/+, we propose that it is a matter of reaching a threshold of AG ectopic expression during inflorescence development.
In wild-type FMs, AG transcription is activated at stage 3 by WUS and LEAFY (Hong et al., 2003). In both jba-1D/+ and jba-1D/+er-20 plants, the inflorescence stems gradually increase in width (supplementary material Fig. S1), indicating a progressively enlarging IM as a result of a gradual increase in the size of the WUS expression domain (Fig. 4). A similar gradual increase in WUS expression is detected in CLV mutants during their development (Clark et al., 1993; Würschum et al., 2006). In jba-1D/+ plants, WUS is upregulated to a level that leads to AG ectopic activation across the IM (Fig. 4), yet the amount of AG mRNA is not sufficient to induce a switch from FM to carpel identity. We propose that once jba-1D/+er-20 plants bolt, WUS mRNA levels are high enough to activate AG across the IM, but not to the level required for carpel specification. During the next 4 weeks, WUS and AG expression continues to increase throughout the jba-1D/+er-20 IMs (Figs 2,4; Table 1), with the gradual increase in WUS transcription ultimately inducing sufficient AG ectopic expression to surpass a threshold that triggers carpel formation.
Consistent with this concept, early studies indicate that AG likely functions in a dose-dependent fashion (Mizukami and Ma, 1995). In addition, in 35S::AG lines that constitutively express AG, the development of carpeloid structures correlates with high levels of AG expression, whereas lines with low levels of AG transcription show no visible phenotypes (C.C.C. and J.C.F., unpublished). This model is also consistent with evidence from jba-1D er-20 erl1 and jba-1D/+er-20 clv3-2 plants, in which the IMs form ectopic carpels much earlier. The meristems of these mutants appear larger than those of jba-1D/+er-20 plants, implying that WUS transcript levels are higher, as they are in er erl1 erl2 meristems (Uchida et al., 2013). We propose that in the triple mutant IMs, the extremely elevated WUS transcription levels leads to the activation of AG to the required threshold much earlier than in jba-1D/+er-20 IMs, and therefore to the appearance of ectopic carpels shortly after bolting.
Although AG is expressed in the upper cell layers across the jba-1D er-20 IM, ectopic carpel initiation is restricted to the periphery. One possible explanation for this is the presence of factors that specify pluripotent fate and preventing cell differentiation at the SAM center. These factors would be absent or inhibited at the periphery, allowing cell differentiation and thus permitting AG function to promote carpel formation. Supporting evidence is the phenotype of jba-1D/+er-20 clv3-2 plants, which exhibit gigantic IMs with reduced ectopic carpel formation at the periphery. Mutations in CLV3 result in WUS domain expansion and a consequent increase in stem cell accumulation in shoot and floral meristems (Brand et al., 2000). We propose that in jba-1D/+er-20 clv3-2 IMs, homeostasis is shifted towards stem cell identity, leaving fewer cells with the ability to differentiate. As a consequence, the triple mutant has a gigantic meristem with fewer carpeloid structures. Another explanation may be that other factors necessary for AG-mediated specification of carpel identity are absent from the center of the SAM. The need for additional factors is consistent with overexpression studies showing that ectopic AG activation is not sufficient to fully induce gynoecium formation outside of the flower (Lenhard et al., 2001; Mizukami and Ma, 1992), and with mutants such as clf in which ectopic AG expression in leaves does not cause ectopic gynoecium formation (Goodrich et al., 1997; Laufs et al., 1998b).
Our results indicate that ER and the miR166g-regulated HD-ZIPIII genes play an indirect role in specifying Arabidopsis FM identity. By negatively regulating WUS levels and restricting the WUS domain they prevent ectopic AG activation across the inflorescence meristem after the transition to flowering. This in turn enables the specification of floral meristem identity and precludes a switch to carpel cell identity on the IM flanks. Consistent with our data, a recent study (Bemis et al., 2013) independently confirms a function for the ER family genes in flower meristem development.
ER plays a role in meristem regulation independently from the CLV pathway
jba-1D/+ gynoecia have two carpels with no detectable fifth whorl, indicating that FM size is not significantly increased and FM termination is unaffected. This suggests that repression of WUS expression in the center of the FM is not delayed in a miR166g overexpression background. The gynoecia of jba-1D/+er-20 flowers also show no morphogenetic defects, suggesting either that ER and the HD-ZIPIII genes play a minor role in regulating FM size and determinacy or that FM size and termination are more tightly controlled by other factors, such as CLV3 (Clark et al., 1997), than the SAM size.
jba-1D/+er-20 clv3-2 plants show strong synergistic phenotypes in both the IMs and flowers. When these three pathways are non-functional, the plants exhibit severe phenotypes corresponding to four distinct morphological changes. The IMs display a macroscopic increase in size, with ectopic carpel formation around the periphery, the flowers form more carpels, and the clv3-2 FM indeterminacy defect is enhanced. None of the single mutants or double mutant combinations shows any resemblance to the jba-1D/+er-20 clv3-2 flowers; therefore, we conclude that all three pathways have an important role in FM regulation. Because all of the jba-1D/+er-20 clv3-2 flower phenotypes can be explained by altered WUS expression, we conclude that the three factors - miR166, ER and CLV3 - regulate WUS transcription in the FM via different genetic pathways.
In conclusion, our work illustrates the delicate balance between stem cell specification and differentiation in the meristem, and shows that a shift from this balance leads not only to increased meristem size but also to abnormal phyllotaxy and reproductive development. We have identified a role for a second receptor kinase signaling pathway - the ER pathway - in WUS regulation, and therefore in SAM and FM size regulation, that acts independently of the CLV signaling pathway. In addition, we have determined that negative regulation of both the WUS expression domain and its transcription levels by the ER gene family and the miR166g-regulated HD-ZIPIII genes is associated with a shift from spiral to whorled leaf phyllotaxis and is required for proper specification of FM identity. Future studies are needed to determine the intracellular elements downstream of the ER receptor kinase that regulate WUS transcription. It will also be of great interest to identify the upstream ligand controlling this cascade.
MATERIALS AND METHODS
Growth conditions and plant materials
Plants were grown under long days (16 hours light/8 hours dark) and temperatures of 18-22°C, in soil or on Murashige and Skoog (MS) plates.
EMS mutagenesis of jba-1D seeds
EMS mutagenesis was performed as described previously (Jander et al., 2003). M2 seeds from pools of 50 M1 plants were planted and screened for modified jba-1D phenotypes.
Plant materials
The plant materials used in this study were: Columbia (Col-0), Landsberg erecta (Ler), jba-1D (Williams et al., 2005), clv3-2 (Brand et al., 2000), ag-1 (SALK_014999), er-2 (CS3401) and erl1 (SALK_081669). Double mutants were generated by crossing jba-1D/+ plants to Ler, clv3-2 and er-2 plants, and by crossing jba-1D/+er-20 plants to clv3-2 and erl1 plants. Double mutant plants were identified phenotypically and by PCR genotyping the F2 progeny.
Construction of transgenic lines
A 9 kb genomic fragment corresponding to the ER promoter and gene, spanning from the 3′ end of the ER upstream gene to the 5′ UTR side of the ER downstream gene was amplified from jba-1D/+ DNA, cloned into pENTR/D-TOPO (Invitrogen) and recombined into a modified pK2GW7 binary vector (Ghent University) using the Gateway LR Clonase enzyme (Invitrogen). Transgenic lines were generated by the Agrobacterium-mediated floral dip method (Clough and Bent, 1998) into jba-1D/+er-20 and selected on MS plates containing kanamycin to select for the ER transgene and glufosinate ammonium (Fluka 45520) to select for the jba-1D allele.
Microscopy and histology
Plant images were captured using an Olympus SZX7 Stereomicroscope. Scanning electron microscopy was performed as described previously (Bowman et al., 1989) using a Hitachi 4700 scanning electron microscope. For histology analyses, 9-day-old seedlings were fixed, embedded and sectioned as described previously (Carles et al., 2004), and stained in Toluidine Blue solution.
Quantitative RT-PCR analysis
Total RNA was isolated from 8-day-old seedlings growing on MS plates using the Qiagen RNeasy Mini-kit. cDNA synthesis was performed with the Invitrogen SuperScript II Reverse Transcriptase, using 1 μg of RNA. RT-qPCR analysis was carried out using a Rotor-Gene-Q-instrument (Qiagen), with Absolute-Blue-qPCR-SYBR-Green Mix (Thermo). Three biological replicates were used for each genotype and three independent technical replicates were performed for each cDNA sample. ACTIN2 (AT3G18780) was used as control and relative expression analysis was calculated using the 2-ddCT method (Livak and Schmittgen, 2001). Statistical analysis was performed using JMP software (SAS Institute). Student’s t-test was used for comparison of means (significantly different at P<0.05). Primers used for RT-qPCR analysis are as follows: ACT2-AT3G18780, GGATCTGTACGGTAACATTGTGC (forward) and CCACCGATCCAGACACTGTAC (reverse); WUS-AT2G17950, CCAGCTTCAATAACGGGAATTTAAATCATGCA (forward) and TCATGTAGCCATTAGAAGCATTAACAACACCACAT (reverse); STM-AT1G62360, GATAGGAACAATAATGGGTCATCCG (forward) and AACCACTGTACTTGCGCAAGAG (reverse); GH3.3-AT2G23170, GTCCGGTGCTCACGAGTTAT (forward) and ATGCTTTGGGATGAGTCTGG (reverse); ER-AT2G26330, AGACGGGGAACAATGAAGTG (forward) and GGTGCATAGGAGTGCCAGTT (reverse); CLV3-AT2G27250, GTTCAAGGACTTTCCAACCGCAAGATGAT (forward) and CCTTCTCTGCTTCTCCATTTGCTCCAACC (reverse); ERL2-AT5G07180, CGTCTCCACCTCCAAAGAAG (forward) and TCACGGAACTGAACAAACCA (reverse).
Differential expression analysis by mRNA-seq
Four weeks after bolting, 30 jba-1D/+ and 30 jba-1D/+er-20 IMs were collected by immersing the IM in liquid nitrogen and detaching the FM under a stereomicroscope. Bare meristems were pooled and total RNA isolated using Qiagen RNeasy-Mini-kit. mRNA was used to prepare libraries using TruSeqTM-RNA and single-end sequencing was performed by multiplexing on Illumina Hiseq-2000 at the Technion Genome Center. Data analysis was carried out at the Bioinformatics-Core-Facility in Ben-Gurion University. Raw sequence reads (Fastq files) were quality assessed using the FastQC software, and then aligned to the Arabidopsis genome using TopHat (70M for jba-1D/+ and 50M for jba-1D/+ er-20). TAIR10 Genome reference sequence and gene model annotations for TopHat analysis were obtained from Illumina iGenomes website (http://tophat.cbcb.umd.edu/igenomes.html). Estimation of transcript and gene abundances in each sample as FPKM values (fragments per kilobase of exon per million fragments mapped) and differential expression analysis were carried out using CuffDiff (parameters -c 5 -b -u -M). Genes with P<0.01 and [fold of change]>2 (total of 3524 genes) were considered differentially expressed. Genes are annotated with TAIR AGI locus names (‘TAIR genes’).
In situ RNA hybridization
Inflorescences were harvested 4 weeks after bolting. Tissue fixation and in situ hybridization were performed as described previously (Seidlová, 1980). Probes were transcribed using a digoxigenin-labeling mix (Roche). WUS antisense probe was generated as described previously (Mayer et al., 1998). AG antisense probe was generated by T7 RNA polymerase activity from a 1 kb insert cloned into the pBS KS+ vector (Seidlová, 1980).
Supplementary Material
Acknowledgments
We thank Hanita Zemach (Volcani center) for technical assistance and the Arabidopsis Biological Resource Center for insertion lines.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
L.E.W. developed the concepts, T.M., F.M. and Y.K. performed experiments, L.E.W. wrote the manuscript, C.C.C. and J.C.F. edited and prepared the manuscript prior to submission.
Funding
This work was supported by the Israel Science Foundation [1351/10 to T.M.], by Vaadia-BARD [IS-4336-10R to Y.K.], by the US Department of Agriculture [5335-21000-029-00D to J.C.F], by the Centre National de la Recherche Scientifique (CNRS Higher Education chair [position 0428-64 to C.C.C.] and by Alpes county [ADR Cluster 7, 12-01293101 to F.M.]. Deposited in PMC for immediate release.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.104687/-/DC1
References
- Alvarez-Buylla E. R., Benitez M., Corvera-Poire A., Chaos Cador A., de Folter S., Gamboa de Buen A., Garay-Arroyo A., Garcia-Ponce B., Jaimes-Miranda F., Perez-Ruiz R. V., et al. (2010). Flower development. Arabidopsis Book 8, e0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge K., Guyomarc’h S., Bayer E., Swarup R., Bennett M., Mandel T., Kuhlemeier C. (2008). Auxin influx carriers stabilize phyllotactic patterning. Genes Dev. 22, 810–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker A., Theissen G. (2003). The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Mol. Phylogenet. Evol. 29, 464–489 [DOI] [PubMed] [Google Scholar]
- Bemis S. M., Lee J. S., Shpak E. D., Torii K. U. (2013). Regulation of floral patterning and organ identity by Arabidopsis ERECTA-family receptor kinase genes. J. Exp. Bot. [Epub ahead of print] doi:10.1093/jxb/ert270 [DOI] [PubMed]
- Benková E., Michniewicz M., Sauer M., Teichmann T., Seifertová D., Jürgens G., Friml J. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115, 591–602 [DOI] [PubMed] [Google Scholar]
- Berleth T., Scarpella E., Prusinkiewicz P. (2007). Towards the systems biology of auxin-transport-mediated patterning. Trends Plant Sci. 12, 151–159 [DOI] [PubMed] [Google Scholar]
- Bowman J. L., Smyth D. R., Meyerowitz E. M. (1989). Genes directing flower development in Arabidopsis. Plant Cell 1, 37–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman J. L., Smyth D. R., Meyerowitz E. M. (2012). The ABC model of flower development: then and now. Development 139, 4095–4098 [DOI] [PubMed] [Google Scholar]
- Brand U., Fletcher J. C., Hobe M., Meyerowitz E. M., Simon R. (2000). Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science 289, 617–619 [DOI] [PubMed] [Google Scholar]
- Busch M. A., Bomblies K., Weigel D. (1999). Activation of a floral homeotic gene in Arabidopsis. Science 285, 585–587 [DOI] [PubMed] [Google Scholar]
- Carles C. C., Fletcher J. C. (2003). Shoot apical meristem maintenance: the art of a dynamic balance. Trends Plant Sci. 8, 394–401 [DOI] [PubMed] [Google Scholar]
- Carles C. C., Lertpiriyapong K., Reville K., Fletcher J. C. (2004). The ULTRAPETALA1 gene functions early in Arabidopsis development to restrict shoot apical meristem activity and acts through WUSCHEL to regulate floral meristem determinacy. Genetics 167, 1893–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. E., Running M. P., Meyerowitz E. M. (1993). CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development 119, 397–418 [DOI] [PubMed] [Google Scholar]
- Clark S. E., Williams R. W., Meyerowitz E. M. (1997). The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89, 575–585 [DOI] [PubMed] [Google Scholar]
- Clough S. J., Bent A. F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 [DOI] [PubMed] [Google Scholar]
- Coen E. S., Meyerowitz E. M. (1991). The war of the whorls: genetic interactions controlling flower development. Nature 353, 31–37 [DOI] [PubMed] [Google Scholar]
- Colombo M., Brambilla V., Marcheselli R., Caporali E., Kater M. M., Colombo L. (2010). A new role for the SHATTERPROOF genes during Arabidopsis gynoecium development. Dev. Biol. 337, 294–302 [DOI] [PubMed] [Google Scholar]
- DeYoung B. J., Bickle K. L., Schrage K. J., Muskett P., Patel K., Clark S. E. (2006). The CLAVATA1-related BAM1, BAM2 and BAM3 receptor kinase-like proteins are required for meristem function in Arabidopsis. Plant J. 45, 1–16 [DOI] [PubMed] [Google Scholar]
- Diévart A., Dalal M., Tax F. E., Lacey A. D., Huttly A., Li J., Clark S. E. (2003). CLAVATA1 dominant-negative alleles reveal functional overlap between multiple receptor kinases that regulate meristem and organ development. Plant Cell 15, 1198–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews G. N., Bowman J. L., Meyerowitz E. M. (1991). Negative regulation of the Arabidopsis homeotic gene AGAMOUS by the APETALA2 product. Cell 65, 991–1002 [DOI] [PubMed] [Google Scholar]
- Durbak A. R., Tax F. E. (2011). CLAVATA signaling pathway receptors of Arabidopsis regulate cell proliferation in fruit organ formation as well as in meristems. Genetics 189, 177–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efroni I., Blum E., Goldshmidt A., Eshed Y. (2008). A protracted and dynamic maturation schedule underlies Arabidopsis leaf development. Plant Cell 20, 2293–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J. C. (2001). The ULTRAPETALA gene controls shoot and floral meristem size in Arabidopsis. Development 128, 1323–1333 [DOI] [PubMed] [Google Scholar]
- Furner I. J., Pumfrey J. E. (1992). Cell fate in the shoot apical meristem of Arabidopsis-thaliana. Development 115, 755–764 [Google Scholar]
- Giulini A., Wang J., Jackson D. (2004). Control of phyllotaxy by the cytokinin-inducible response regulator homologue ABPHYL1. Nature 430, 1031–1034 [DOI] [PubMed] [Google Scholar]
- Golz J. F., Hudson A. (2002). Signalling in plant lateral organ development. Plant Cell 14 Suppl., S277–S288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Mena C., de Folter S., Costa M. M., Angenent G. C., Sablowski R. (2005). Transcriptional program controlled by the floral homeotic gene AGAMOUS during early organogenesis. Development 132, 429–438 [DOI] [PubMed] [Google Scholar]
- Goodrich J., Puangsomlee P., Martin M., Long D., Meyerowitz E. M., Coupland G. (1997). A Polycomb-group gene regulates homeotic gene expression in Arabidopsis. Nature 386, 44–51 [DOI] [PubMed] [Google Scholar]
- Hall M. C., Dworkin I., Ungerer M. C., Purugganan M. (2007). Genetics of microenvironmental canalization in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 104, 13717–13722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler M. G., Ohno C., Das P., Sieber P., Reddy G. V., Long J. A., Meyerowitz E. M. (2005). Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr. Biol. 15, 1899–1911 [DOI] [PubMed] [Google Scholar]
- Hong R. L., Hamaguchi L., Busch M. A., Weigel D. (2003). Regulatory elements of the floral homeotic gene AGAMOUS identified by phylogenetic footprinting and shadowing. Plant Cell 15, 1296–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Wellmer F., Yu H., Das P., Ito N., Alves-Ferreira M., Riechmann J. L., Meyerowitz E. M. (2004). The homeotic protein AGAMOUS controls microsporogenesis by regulation of SPOROCYTELESS. Nature 430, 356–360 [DOI] [PubMed] [Google Scholar]
- Ito T., Ng K. H., Lim T. S., Yu H., Meyerowitz E. M. (2007). The homeotic protein AGAMOUS controls late stamen development by regulating a jasmonate biosynthetic gene in Arabidopsis. Plant Cell 19, 3516–3529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jander G., Baerson S. R., Hudak J. A., Gonzalez K. A., Gruys K. J., Last R. L. (2003). Ethylmethanesulfonate saturation mutagenesis in Arabidopsis to determine frequency of herbicide resistance. Plant Physiol. 131, 139–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson H., Heisler M. G., Shapiro B. E., Meyerowitz E. M., Mjolsness E. (2006). An auxin-driven polarized transport model for phyllotaxis. Proc. Natl. Acad. Sci. USA 103, 1633–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya H., Shibahara K. I., Taoka K. I., Iwabuchi M., Stillman B., Araki T. (2001). FASCIATA genes for chromatin assembly factor-1 in arabidopsis maintain the cellular organization of apical meristems. Cell 104, 131–142 [DOI] [PubMed] [Google Scholar]
- Kirch T., Simon R., Grünewald M., Werr W. (2003). The DORNROSCHEN/ENHANCER OF SHOOT REGENERATION1 gene of Arabidopsis acts in the control of meristem ccll fate and lateral organ development. Plant Cell 15, 694–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizek B. A., Fletcher J. C. (2005). Molecular mechanisms of flower development: an armchair guide. Nat. Rev. Genet. 6, 688–698 [DOI] [PubMed] [Google Scholar]
- Kwiatkowska D. (2006). Flower primordium formation at the Arabidopsis shoot apex: quantitative analysis of surface geometry and growth. J. Exp. Bot. 57, 571–580 [DOI] [PubMed] [Google Scholar]
- Laufs P., Dockx J., Kronenberger J., Traas J. (1998a). MGOUN1 and MGOUN2: two genes required for primordium initiation at the shoot apical and floral meristems in Arabidopsis thaliana. Development 125, 1253–1260 [DOI] [PubMed] [Google Scholar]
- Laufs P., Grandjean O., Jonak C., Kiêu K., Traas J. (1998b). Cellular parameters of the shoot apical meristem in Arabidopsis. Plant Cell 10, 1375–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux T., Mayer K. F., Berger J., Jürgens G. (1996). The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122, 87–96 [DOI] [PubMed] [Google Scholar]
- Lease K. A., Lau N. Y., Schuster R. A., Torii K. U., Walker J. C. (2001). Receptor serine/threonine protein kinases in signalling: analysis of the erecta receptor-like kinase of Arabidopsis thaliana. New Phytolog, 151, 133–143 [DOI] [PubMed] [Google Scholar]
- Lenhard M., Bohnert A., Jürgens G., Laux T. (2001). Termination of stem cell maintenance in Arabidopsis floral meristems by interactions between WUSCHEL and AGAMOUS. Cell 105, 805–814 [DOI] [PubMed] [Google Scholar]
- Leyser H. M. O., Furner I. J. (1992). Characterisation of three shoot apical meristem mutants of Arabidopsis thaliana. Development 116, 397–403 [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- Lohmann D., Stacey N., Breuninger H., Jikumaru Y., Müller D., Sicard A., Leyser O., Yamaguchi S., Lenhard M. (2010). SLOW MOTION is required for within-plant auxin homeostasis and normal timing of lateral organ initiation at the shoot meristem in Arabidopsis. Plant Cell 22, 335–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J. A., Barton M. K. (1998). The development of apical embryonic pattern in Arabidopsis. Development 125, 3027–3035 [DOI] [PubMed] [Google Scholar]
- Long J. A., Moan E. I., Medford J. I., Barton M. K. (1996). A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379, 66–69 [DOI] [PubMed] [Google Scholar]
- Mallory A. C., Bartel D. P., Bartel B. (2005). MicroRNA-directed regulation of Arabidopsis AUXIN RESPONSE FACTOR17 is essential for proper development and modulates expression of early auxin response genes. Plant Cell 17, 1360–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer K. F., Schoof H., Haecker A., Lenhard M., Jürgens G., Laux T. (1998). Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95, 805–815 [DOI] [PubMed] [Google Scholar]
- Medford J. I., Behringer F. J., Callos J. D., Feldmann K. A. (1992). Normal and abnormal development in the arabidopsis vegetative shoot apex. Plant Cell 4, 631–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami Y., Ma H. (1992). Ectopic expression of the floral homeotic gene AGAMOUS in transgenic Arabidopsis plants alters floral organ identity. Cell 71, 119–131 [DOI] [PubMed] [Google Scholar]
- Mizukami Y., Ma H. (1995). Separation of AG function in floral meristem determinacy from that in reproductive organ identity by expressing antisense AG RNA. Plant Mol. Biol. 28, 767–784 [DOI] [PubMed] [Google Scholar]
- Perales M., Reddy G. V. (2012). Stem cell maintenance in shoot apical meristems. Curr. Opin. Plant Biol. 15, 10–16 [DOI] [PubMed] [Google Scholar]
- Pinyopich A., Ditta G. S., Savidge B., Liljegren S. J., Baumann E., Wisman E., Yanofsky M. F. (2003). Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature 424, 85–88 [DOI] [PubMed] [Google Scholar]
- Reinhardt D., Pesce E. R., Stieger P., Mandel T., Baltensperger K., Bennett M., Traas J., Friml J., Kuhlemeier C. (2003). Regulation of phyllotaxis by polar auxin transport. Nature 426, 255–260 [DOI] [PubMed] [Google Scholar]
- Roeder A. H., Yanofsky M. F. (2006). Fruit development in Arabidopsis. Arabidopsis Book 4, e0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablowski R. (2004). Plant and animal stem cells: conceptually similar, molecularly distinct? Trends Cell Biol. 14, 605–611 [DOI] [PubMed] [Google Scholar]
- Schoof H., Lenhard M., Haecker A., Mayer K. F., Jürgens G., Laux T. (2000). The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100, 635–644 [DOI] [PubMed] [Google Scholar]
- Seidlová F. (1980). The rate of cell-division in the shoot apical meristem during photoperiodic induction and transition to flowering. Biol. Plant. 22, 428–433 [Google Scholar]
- Shpak E. D., Lakeman M. B., Torii K. U. (2003). Dominant-negative receptor uncovers redundancy in the Arabidopsis ERECTA Leucine-rich repeat receptor-like kinase signaling pathway that regulates organ shape. Plant Cell 15, 1095–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpak E. D., Berthiaume C. T., Hill E. J., Torii K. U. (2004). Synergistic interaction of three ERECTA-family receptor-like kinases controls Arabidopsis organ growth and flower development by promoting cell proliferation. Development 131, 1491–1501 [DOI] [PubMed] [Google Scholar]
- Smith R. S., Guyomarc’h S., Mandel T., Reinhardt D., Kuhlemeier C., Prusinkiewicz P. (2006). A plausible model of phyllotaxis. Proc. Natl. Acad. Sci. USA 103, 1301–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth D. R., Bowman J. L., Meyerowitz E. M. (1990). Early flower development in Arabidopsis. Plant Cell 2, 755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussex I. (1998). Themes in plant development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, xiii–xxii [DOI] [PubMed] [Google Scholar]
- Takahashi T., Matsuhara S., Abe M., Komeda Y. (2002). Disruption of a DNA topoisomerase I gene affects morphogenesis in Arabidopsis. Plant Cell 14, 2085–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii K. U., Mitsukawa N., Oosumi T., Matsuura Y., Yokoyama R., Whittier R. F., Komeda Y. (1996). The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular leucine-rich repeats. Plant Cell 8, 735–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traas J. (2013). Phyllotaxis. Development 140, 249–253 [DOI] [PubMed] [Google Scholar]
- Uchida N., Igari K., Bogenschutz N. L., Torii K. U., Tasaka M. (2011). Arabidopsis ERECTA-family receptor kinases mediate morphological alterations stimulated by activation of NB-LRR-type UNI proteins. Plant Cell Physiol. 52, 804–814 [DOI] [PubMed] [Google Scholar]
- Uchida N., Shimada M., Tasaka M. (2012). Modulation of the balance between stem cell proliferation and consumption by ERECTA-family genes. Plant Signal. Behav. 7, 1506–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N., Shimada M., Tasaka M. (2013). ERECTA-family receptor kinases regulate stem cell homeostasis via buffering its cytokinin responsiveness in the shoot apical meristem. Plant Cell Physiol. 54, 343–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S., Friml J. (2009). Auxin: a trigger for change in plant development. Cell 136, 1005–1016 [DOI] [PubMed] [Google Scholar]
- Vidaurre D. P., Ploense S., Krogan N. T., Berleth T. (2007). AMP1 and MP antagonistically regulate embryo and meristem development in Arabidopsis. Development 134, 2561–2567 [DOI] [PubMed] [Google Scholar]
- Vieten A., Sauer M., Brewer P. B., Friml J. (2007). Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci. 12, 160–168 [DOI] [PubMed] [Google Scholar]
- Wahl V., Brand L. H., Guo Y. L., Schmid M. (2010). The FANTASTIC FOUR proteins influence shoot meristem size in Arabidopsis thaliana. BMC Plant Biol. 10, 285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L., Fletcher J. C. (2005). Stem cell regulation in the Arabidopsis shoot apical meristem. Curr. Opin. Plant Biol. 8, 582–586 [DOI] [PubMed] [Google Scholar]
- Williams L., Grigg S. P., Xie M., Christensen S., Fletcher J. C. (2005). Regulation of Arabidopsis shoot apical meristem and lateral organ formation by microRNA miR166g and its AtHD-ZIP target genes. Development 132, 3657–3668 [DOI] [PubMed] [Google Scholar]
- Wu X., Dabi T., Weigel D. (2005). Requirement of homeobox gene STIMPY/WOX9 for Arabidopsis meristem growth and maintenance. Curr. Biol. 15, 436–440 [DOI] [PubMed] [Google Scholar]
- Würschum T., Gross-Hardt R., Laux T. (2006). APETALA2 regulates the stem cell niche in the Arabidopsis shoot meristem. Plant Cell 18, 295–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky M. F., Ma H., Bowman J. L., Drews G. N., Feldmann K. A., Meyerowitz E. M. (1990). The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature 346, 35–39 [DOI] [PubMed] [Google Scholar]
- Yokoyama R., Takahashi T., Kato A., Torii K. U., Komeda Y. (1998). The Arabidopsis ERECTA gene is expressed in the shoot apical meristem and organ primordia. Plant J. 15, 301–310 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.