Abstract
Background
Sudden gains are significant, rapid improvements in symptoms, larger than typical between-session symptom reduction.[8] Sudden gains in a large sample of individuals with PTSD have not been studied, and only one study has looked at it in pharmacotherapy, but not in PTSD. In the present study, we examined the occurrence of sudden gains in psychotherapy, specifically prolonged exposure (PE), and pharmacotherapy, specifically sertraline, for chronic PTSD.
Method
Sudden gains in PTSD symptoms (PTSD Symptom Scale-Self-Report[23]) were assessed in 200 individuals with PTSD during 10 weeks of PE or sertraline.
Results
Individuals in both PE (42.2%) and sertraline (31%) exhibited sudden gains. Individuals in PE made more gains toward the end of treatment (7.2%) than sertraline (2%, OR = 3.82). However, individuals in sertraline made larger gains during early treatment (M = 18.35, SD = 8.15) than PE (M = 12.53, SD = 5.16, d = .85). Notably, those on sertraline were more likely to exhibit a reversal of sudden gains than those in PE (OR = .23). Pointing to clinical significance, the presence of a sudden gain was associated with better reduction in symptoms from pre- to post-treatment (β = -.49).
Conclusions
Individuals in both PE and sertraline experienced gains, though sertraline was associated with earlier large but reversible gains, and PE was associated with later gains. This differential pattern of discontinuous change highlights potential differential mechanism for these therapies and marks important transition points for further detailed analyses of change mechanisms.
Keywords: PTSD, cognitive behavior therapy, pharmacotherapy, anxiety, assessment/diagnosis
Although many studies demonstrate the efficacy of treatment for PTSD,[1–6] it is still not clear how and when the changes of improvement occur across various forms of effective therapy. Hayes and colleagues[7] emphasized the importance of looking at the shape of individual symptom trajectories rather than group means pre- and post-treatment to see whether change is characterized by significant shifts of symptoms. These large, rapid improvements in symptoms have been termed “sudden gains.”[8] Sudden gains mark points of important transitions in critical sessions for detailed analyses of possible change mechanisms triggering the significant shifts in symptoms.[8] Individuals who show sudden gains have better treatment outcome and maintenance of improvements than in those who do not show sudden gains.[8–10]
Theoretically, sudden gains are thought to occur as a result of cognitive shifts occurring during cognitive psychotherapy. Tang and DeRubeis[8] proposed a three-stage model of sudden gains: a preparation stage, where the foundation for cognitive change is laid; a critical session/sudden gain stage where the client experiences significant cognitive changes that leads to a decrease in symptoms and thus, a sudden gain; and an upward spiral stage where sudden gains lead to improved therapeutic alliance and mood promoting further improvement in cognitive changes. The theoretical framework for sudden gains occurring with other forms of psychotherapy or pharmacotherapy has not been fully explicated to date.
Sudden gains have largely been examined in psychotherapy for major depression; and to date, only three studies have investigated sudden gains in clinical samples of posttraumatic stress disorder (PTSD).[11–13] In cognitive-behavioral therapy for major depression, between 39% to 46% of clients exhibited sudden gains.[8,9,14] Lower frequencies (17% to 43%) of sudden gains have been reported in other forms of psychotherapy for depression, such as brief dynamic therapy,[15] non-manualized psychotherapies in clinic settings,[10] and non-directive supportive therapy.[16] In treatment for PTSD, 39% to 52% of patients receiving exposure[11,12] or cognitive therapy[13] exhibited sudden gains. Notably, those who showed sudden gains during therapy had lower PTSD severity at post-treatment than those who did not.[11–13] Thus, sudden gains in PTSD appear stable and maintained throughout treatment.
Patterns in the trajectory of response to pharmacotherapy and placebo for depression have been examined through latent class analyses.[17,18] It was shown that early (first three weeks) and delayed (second three weeks) improvements of at least a 20% symptom reduction are common during treatment with SSRIs and tricyclic antidepressants.[18] However, these latent class analyses do not allow for a systematic, reliable way of examining individual trajectories of change, taking into account the psychometrics of the measure assessing change, as the assessment of sudden gains. To date, only one study has looked at the phenomenon of sudden gains in pharmacotherapy, specifically for atypical depression. Vittengl and colleagues[19] examined sudden gains in acute treatment of cognitive therapy, pharmacotherapy with clinical management, and pill placebo with clinical management. They reported sudden gain rates of 25% to 46.9%, with no difference between treatments in frequency or magnitude of gains; however, patients receiving pharmacotherapy and pill placebo exhibited higher rates of reversals of sudden gains. The presence of sudden gains across treatments may challenge the assumption that shifts in insight alone are responsible for the gains.[8] Instead, the authors suggested that mood lability and strong reactions to internal and external events might coincide with the sudden gains.[19]
To understand who is predisposed to experience sudden gains, possible predictors have been examined. Several studies have examined pre-treatment symptom severity, cognitive content, or social interpersonal functioning as predictors of experiencing sudden gains but found no consistent or strong associations.[8,12,16] However, higher pre-treatment self-reported dysfunctional attitudes and higher depression were associated with more sudden gains,[9] though this was not replicated in other studies.
In the present study, we examined the occurrence of sudden gains in psychotherapy, specifically prolonged exposure (PE), and pharmacotherapy, specifically sertraline. To date, no study has compared sudden gains in psychotherapy to pharmacotherapy in a large, randomized trial; and, in PTSD, none has examined sudden gains in pharmacotherapy. Consistent with previous studies in PTSD and depression,[8,9,12–14] we hypothesized that sudden gains would occur in a subset of the sample and would be associated with better treatment outcome. We expected to find higher rates of sudden gains in PE versus sertraline, based on limited comparative literature of psychotherapy and pharmacotherapy PTSD treatments.[20] We also examined possible predictors (demographics, pre-treatment symptom severity, and trauma-related beliefs) of sudden gains. Based on Hardy and colleagues'[9] findings, we hypothesized those with more severe pre-treatment severity and trauma-related beliefs would be more likely to have sudden gains.
Method
Participants
Participants were 200 treatment-seeking women (75.5%, n = 151) and men (24.5%, n = 49) with chronic PTSD.[21] Participants were recruited through community referrals and advertisements. Inclusion criteria included being between ages 18 to 65 with current primary DSM-IV chronic PTSD diagnosis.[22] Exclusion criteria were limited and included: current diagnosis of schizophrenia or delusional disorder; medically unstable bipolar disorder, depression with psychotic features, or severe depression that require immediate psychiatric treatment (e.g., actively suicidal); no clear trauma memory or trauma before age of three; current diagnosis of alcohol or substance dependence within the previous three months; ongoing intimate relationship with perpetrator (in assault cases); and unwillingness or medically not advisable to discontinue current psychotherapy or antidepressant medication based on condition assignment. Diagnostic comorbidity was allowed, including borderline personality disorder.
Participants' mean age was 37.41 years (SD = 11.30). Mean time since the target trauma was 11.91 years (SD = 12.69). Thirty-one percent reported a target trauma of adult sexual assault, 24% childhood sexual assault, 22.5% adult nonsexual assault, 13.5% accident (motor vehicle accident, natural disaster), 6.5% death or violence to a loved one, and 2.5% combat or war. Sixty-six percent (65.5%) were Caucasian; 70% were not college educated; 48.5% had an annual household income of $20,000 or less; and 67.1% had other diagnostic comorbidity.
Interview Measures
The Structured Clinical Interview for DSM-IV (SCID-IV)[23,24] was used to assess primary diagnosis of PTSD and co-occurring disorders. The Posttraumatic Symptom Scale-Interview (PSS-I)[25] was used to assess DSM-IV PTSD diagnosis and severity. Approximately 10% of the cases were rerated for diagnostic reliability. Overall, there was good SCID-IV agreement for current MDD (κ = .68, ppos = .88, pneg = .80), anxiety disorders (κ = 1.00, ppos = 1.00, pneg = 1.00), substance abuse disorders (ppos = .00, pneg = 1.00), and other diagnoses (ppos = 0.00, pneg = 1.00). The intraclass correlation coefficient for PTSD severity scores (PSS-I) was high (ICC = .985).
Self-Report Measures
Sudden gains were assessed weekly during active treatment using the PTSD Symptom Scale-Self-Report (PSS-SR)[25], assessing symptoms in the past week. The correlation between pre-treatment PSS-I and PSS-SR was r = .53, p < .001. Additional pre-treatment self-report measures included: Beck Depression Inventory (BDI);[26,27] Anxiety Sensitivity Index (ASI);[28] State-Trait Anxiety Inventory (STAI-Trait);[29] Post-Traumatic Cognitions Inventory (PTCI);[30] and the Trauma-Related Guilt Inventory (TRGI).[31]
Treatment
Clinicians who provided psychotherapy held a master's or doctorate in clinical psychology. Board-certified psychiatrists provided pharmacotherapy. All clinicians attended multiple-day initial training workshops where they received standardized clinical training. Throughout the trial clinicians received on-going clinical supervision.
Prolonged Exposure (PE)[32]
PE was delivered in 10 weekly sessions that were 90-120 minutes following the PE manual[32]. Outside raters reviewed 10% of sessions to assess treatment adherence, 90% of key components were completed.
Sertraline
Pharmacotherapy consisted of 10 weeks of sertraline, a FDA-approved medication for PTSD. Dosage started at 25 mg/day and followed a standardized titration algorithm, with the goal of 200 mg/day if indicated and tolerated. Average final dosage was 115 mg/day (SD = 78.00). Outside raters reviewed 10% of sessions to assess treatment adherence, 96% of key components were completed.
Data Analyses of Sudden Gains
We utilized Tang and DeRubeis'[8] three primary criteria for identifying sudden gains. First, sudden gains should be large in absolute terms between any session N (pre-gain session) and session N+1 (after-gain session). We identified sudden gains of PTSD symptoms where there was a reduction of at least seven points on the PSS-SR based on a reliable change index and similar to other studies,[12,33] using Jacobson and Truax's[34] guidelines for computing reliable, clinically significant change. This was calculated using the standard error difference in PTSD symptoms on the PSS-SR of 6.15,[35] based on two to three-week test-retest reliability (.83) and standard deviation (10.54).[25] Calculation of the sudden gain cutoff criterion varies.[8,33] Tang and DeRubeis[8] chose a BDI cutoff of one standard deviation from the clinical sample mean, constituting 11% of the range.[11] Comparably, our cut off value of seven on the PSS-SR constitutes 13.7% of the range. Second, the magnitude of the gain should be large compared to the pre-gain symptom severity, at least a 25% reduction of the pre-gain session PSS-SR score. Third, the magnitude of the gain should be large relative to symptom fluctuations before and after the sudden gain. This was determined by comparing the mean PSS-SR score of three pre-gain sessions (sessions N-2, N-1, and N) to the mean score of the after-gain session and mean score of two post-gain sessions (N+1, N+2, and N+3) using a two-sample t-test. We utilized critical values of t(4) = 2.78, p = .05 or t(3) = 3.19, p = .05 (when only two pre- or post-gain sessions were available). Following Tang and DeRubeis,[8] we defined reversal of sudden gains when there was an increase of reported symptoms of at least 50% of the original improvement of the sudden gain. For missing data, the previous observation was used,[11,12,36] conservatively limiting sudden gains. Thus, sudden gains are large, rapid and stable decreases in symptom severity that occurs between the beginning of one session and the beginning of the next.
Procedure
Potential study patients were initially screened through a semi-structured telephone interview. Following written informed consent, independent evaluators, blind to treatment assignment, completed the PSS-I and SCID-IV. Potential patients also received a physical exam and completed a laboratory panel. If eligible, patients completed pre-treatment self-reports and were randomized using a computer generated urn randomization sequence, with appropriate concealment of assignment prior to randomization.[21] Patients received 10 weekly sessions of either PE or sertraline. The PSS-SR was completed prior to every session. The PSS-I was again completed at post-treatment by a blind, independent evaluator.
Results
Occurrence of Sudden Gains
Following the first criterion of sudden gains, reductions of at least seven points on the PSS-SR were observed in 272 of 1800 (15.1%) between session intervals across both treatments. After the second criterion where the magnitude of symptom reductions should exceed 25% of the pre-gain score, 227 of 272 (83.5%) sudden gains remained. Following the third criterion of sudden gains, we conducted two-sample independent t-tests to examine whether sudden gains were large relative to symptom fluctuations of pre- and post-gain sessions, and 137 of 227 (60.4%) sudden gains remained. Finally, after omitting reversals of sudden gains when patients reported symptoms of at least 50% of the original sudden gain symptom improvement, 105 of 137 (76.6%) sudden gains remained. Thus, a total of 105 sudden gains were exhibited by 75 of 200 (37.5%) patients.
Sudden gains in PE versus sertraline
Comparing treatments, 49 out of 116 (42.2%) patients receiving PE and 26 out of 84 (31.0%) patients receiving sertraline experienced sudden gains. As shown in Table 1, the number of patients who experienced sudden gains did not differ by treatment, χ2(1, N = 200) = 2.65, ns. However, there was a difference in the number of sudden gains that occurred by treatment, χ2(1, N = 1800) = 4.24, p = .04. Patients receiving PE experienced 71 out of 1044 (6.8%) possible sudden gains. Seventeen patients in PE experienced multiple sudden gains (12 people exhibited two gains and five people exhibited three gains). Patients receiving sertraline experienced 34 out of 756 (4.5%) possible sudden gains. Eight patients in sertraline experienced multiple sudden gains (two gains only).
Table 1. Comparisons of Sudden Gains in PE and Sertraline.
| Variable | PE | Sertraline | χ2 | t-test | Odds Ratio |
|---|---|---|---|---|---|
| Sudden Gains | 42.2% | 31.0% | 2.65 | -- | 1.63 |
| Multiple Sudden Gains | 14.7% | 9.5% | 2.65 | -- | 1.63 |
| Median Session of Occurrence (2- 10) | 6 | 5 | 1.48 | -- | -- |
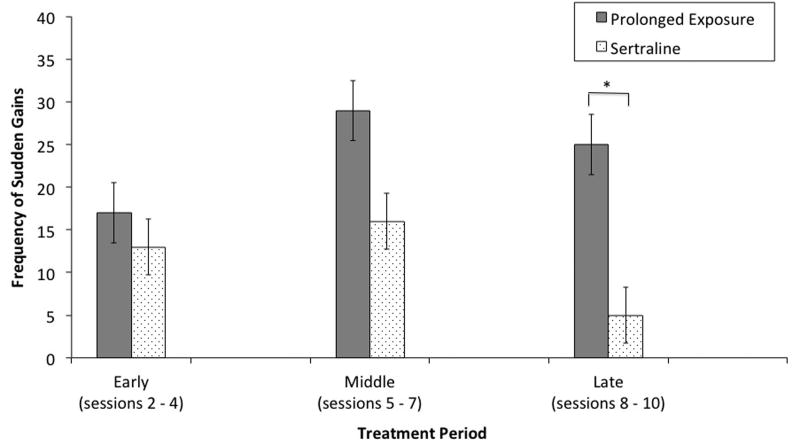
| Early Treatment Gains (Sessions 2-4) | 17 | 13 | .23 | -- | .94 |
| Middle Treatment Gains (Sessions 5-7) | 29 | 16 | .83 | -- | 1.34 |
| Late Treatment Gains (Sessions 8-10) | 25 | 5 | 8.32* | -- | 3.82 |
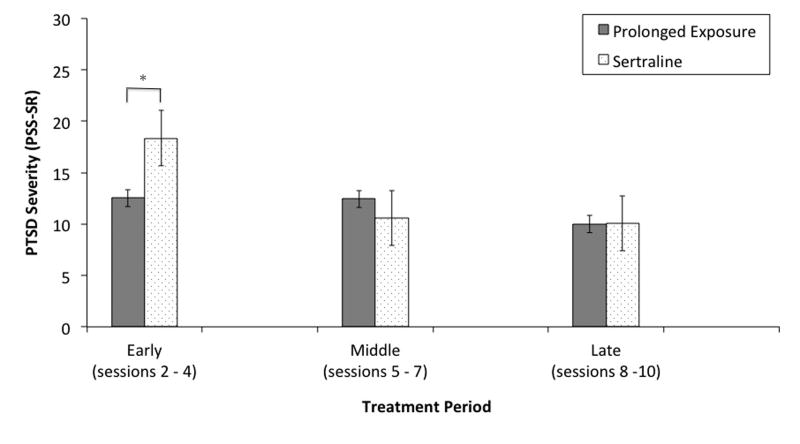
| Magnitude Early Gains (PSS-SR) | 12.53 (5.16) | 18.35 (8.15) | -- | -2.39* | -- |
| Magnitude Middle Gains (PSS-SR) | 12.43 (6.34) | 10.56 (4.55) | -- | 1.04 | -- |
| Magnitude Late Gains (PSS-SR) | 9.99 (3.88) | 10.08 (2.34) | -- | -.05 | -- |
| Sudden Gains as % of Total Improvement | 21.2% | 27.6% | -- | 1.11 | -- |
| Sudden Gains Reversed | 30.4% | 38.2% | 11.68* | -- | .23 |
Note.
p < .05.
PSS-SR = Posttraumatic Symptom Scale-Self-Report.
Patterns of frequency of sudden gains
As shown in Figure 1, during early treatment (Sessions 2-4), the number of sudden gains did not differ by PE (17 out of 348, 4.9%) and sertraline (13 out of 252, 5.2%), χ2(1, N = 600) = .23, ns. The number of sudden gains occurring during middle treatment (Sessions 5-7) also did not differ between PE (29 out of 348, 8.3%) and sertraline (16 out of 252, 6.4%), χ2(1, N = 600) = .83, ns. However, there was a significant difference in sudden gains during late treatment (Sessions 8-10) with individuals in PE experiencing more gains (25 out of 348, 7.2%) than those in sertraline (5 out of 252, 2%), χ2(1, N = 600) = 8.32, p = .004.
Figure 1.
Frequency of Sudden Gains Experienced in PE and Sertraline.
Note. * p < .05
Patterns of magnitude of sudden gains
As shown in Figure 2, during early treatment (Sessions 2-4), the magnitude of sudden gains differed by treatment with the magnitude of gains in PE being lower (M = 12.53, SD = 5.16) than sertraline (M = 18.35, SD = 8.15), t(28) = -2.39, p = .024, Cohen's d = .85. The magnitude of sudden gains during middle treatment (sessions 5-7) did not differ between PE (M = 12.43, SD = 6.34) and sertraline (M = 4.55, SD = 4.55), t(43) = 1.04, ns. The magnitude of sudden gains occurring during late treatment (session 8-10) also did not differ between PE (M = 9.99, SD = 3.88) and sertraline (M = 10.08, SD = 2.34), t(28) = -.05, ns. Finally, the overall magnitude of sudden gains were similar in PE (M = 11.59, SD = 5.36) and sertraline (M = 13.47, SD = 7.03), t(103) = -1.51, ns.
Figure 2.
Magnitude of Sudden Gains Experienced in PE and Sertraline.
Note. * p < .05
Reversal of Sudden Gains
Out of 137 sudden gains, 32 (23.4%) were reversals exhibited by 10 patients receiving PE and 18 patients receiving sertraline (one patient experienced two reversals and another three). As shown in Table 1, those receiving sertraline were more likely to exhibit a reversal of sudden gains than those in PE, χ2(1, N = 137) = 11.28, p = .001.
Sudden Gains' Relation to Treatment Outcome
Hierarchical regression analysis tested whether the experience of sudden gains was associated with better treatment outcome (post-treatment PSS-I). We entered pre-treatment PTSD symptom severity (PSS-I) in step 1 and the experience of sudden gains (0 = no sudden gains, 1 = sudden gain) in step 2. After step 1, with pre-treatment PSS-I in the equation, R2 = .07, Finc(1, 153) = 11.88, p = .001. After step 2, with the experience of sudden gains added to the prediction of pre-treatment symptom severity, R2 = .31, F(2, 152) = 34.56, p < .001. Specifically, the occurrence of sudden gains was associated with better treatment outcome (β = -.49, t = -7.29, p < .001) over and above pre-treatment symptom severity, suggesting sudden gains are stable patterns of symptom change accounting for significant portions of improvement.
Predictors of Sudden Gains
We examined predictors of sudden gains, specifically demographic variables (age, gender, ethnicity, education, income, and years since trauma), pre-treatment symptom severity (BDI, ASI, and STAI-trait), and negative beliefs (PTCI: negative cognitions of self, negative cognitions of world, and self-blame, and TRGI: guilt cognitions). Three logistic regression analyses were conducted using the demographic factors and psychopathology measures as predictor variables and the presence of sudden gains as the criterion variable.1 As shown in Table 2, younger individuals were more likely to experience sudden gains (B = -.03, Wald = 5.53, p = .019).
Table 2. Direct Logistic Regression Analyses Predicting Occurrence of Sudden Gains.
| Variable | r | B | SE B | Wald's Statistic | Odds Ratio |
|---|---|---|---|---|---|
| Demographic Predictors | |||||
| Constant | 1.01 | 1.60 | .21 | 3.60 | |
| Age | -.20 | -.03 | .01 | 5.53* | .97 |
| Gendera | .01 | -.15 | .36 | .16 | .86 |
| Ethnicityb | .07 | .17 | .33 | .25 | 1.18 |
| Educationc | -.03 | -.01 | .34 | .00 | .99 |
| Incomed | -.09 | -.39 | .31 | 1.54 | .68 |
| Years since Trauma | -.14 | -.02 | .01 | 1.70 | .98 |
| Pre-Treatment Psychopathology Predictors | |||||
| Constant | -1.64 | .94 | 3.08 | .19 | |
| Depression (BDI) | .03 | .01 | .02 | .39 | .99 |
| Anxiety Sensitivity (ASI) | .03 | .00 | .01 | .00 | 1.00 |
| Trait Anxiety (STAI) | .09 | .03 | .02 | 1.53 | 1.03 |
| Pre-Treatment Negative Belief Predictors | |||||
| Constant | -1.67 | .78 | 4.56 | .19 | |
| Self (PTCI) | .10 | .03 | .18 | .03 | 1.03 |
| World (PTCI) | .06 | .08 | .17 | .23 | 1.09 |
| Blame (PTCI) | .13 | .08 | .14 | .32 | 1.08 |
| Guilt Cognitions (TRGI) | .14 | .20 | .23 | .72 | 1.22 |
Note.
p < .05;
(1 = Male, 2 = Female);
(0 = Minority, 1 = Caucasian);
(0 = a 4 year or more college degree, 1 = Not college educated),
(0 = more than $20,000, 1 = Less than $20,000 per year). BDI = Beck Depression Inventory; ASI = Anxiety Sensitivity Index; STAI = State-Trait Anxiety Inventory; PTCI = Post-Traumatic Cognitions Inventory; TRGI = Trauma-Related Guilt Inventory.
Discussion
This is the first study to examine sudden gains in a large clinical trial for PTSD directly comparing pharmacotherapy, sertraline, and psychotherapy, PE. Consistent with our hypothesis, 37.5% of individuals with PTSD experienced a sudden gain. These gains were strongly associated with better treatment outcome. The number of patients experiencing sudden gains was similar in PE and sertraline, even after accounting for higher rates of sudden gain reversals in sertraline. Notably, the pattern of gains was different between the two. Patients in PE made more multiple gains over the course of psychotherapy than patients in sertraline. Further, patients in sertraline made larger early gains in the first two to four weeks of initiation of the medication; whereas, individuals in PE made more gains in the last two weeks of treatment. Pre-treatment symptom severity, negative beliefs, and demographic factors, with the exception of younger age, did not predict the occurrence of sudden gains. These results highlight the presence of discontinuous and differential change processes between PE and sertraline in PTSD treatment.
The similar rates of patients experiencing sudden gains in PE and sertraline suggest that sudden gains are not unique to psychotherapy. Given that Tang and DeRubeis[8] proposed an underlying cognitive mechanism, it may be that individuals on serotonergic medications are similarly gaining cognitive insight during treatment, without direct targeting of maladaptive cognitions by a therapist. This may indeed be the case, as therapists in PE also do not directly target maladaptive cognitions. A critical next step is to consider the association between changes in patients' negative, trauma-related beliefs and the experience of sudden gains. In contrast, sudden gains may occur through other mechanisms, such as mood lability and symptom drops in response to internal and external events.[19] Similarly, patient-therapist and patient-pharmacotherapist relationship shifts may underlie these gains, though not found in other studies.[8]
Different patterns of frequency and magnitude of sudden gains in PE and sertraline may suggest divergent therapeutic mechanisms. Patients in PE experienced more gains during later portions of treatment where imaginal exposure focuses on “hot spots” of the traumatic memory.[32] Increased fear activation and successful processing of distressing aspects of the traumatic memory may underlie later PTSD improvements. Alternatively, these shifts may reflect a placebo response or regression to the mean, though unlikely.[37] In contrast, those receiving sertraline experienced larger sudden gains during early treatment than those in PE. This may be characteristic of early responses often seen in serotonergic medications.[1,38] In a previous study, early response status on sertraline was associated with reduced risk for relapse.[2] These findings suggest that in PE there may be continuing, steady improvement throughout therapy, while in sertraline the bulk of therapeutic improvement may be observed through early sudden gains. Similar to Vittengl and colleagues' study,[19] patients in sertraline experienced a relatively large number (38.2%) of reversals of gains, potentially indicative of an initial placebo response being reversed.
The strong association of the experience of sudden gains with better treatment outcome in this study and the literature points to their clinical significance. Tracking individual symptom trajectories during treatment allows clinicians to assess patients' progress and make appropriate treatment adjustments.[39] Notably, clinicians can expect to see sudden gains occurring throughout treatment in clients receiving PE, with the majority occurring later in treatment. In contrast, clinicians can expect patients to exhibit an early, sharp drop in symptoms with sertraline, being mindful that in a significant minority these gains will be reversed. Particularly for sertraline, experiencing sudden gains early could have significant impact on a patient's course of treatment, strengthening their confidence in therapeutic approach, improving therapeutic alliance, and increasing motivation to be adherent with treatment.
Similar to other studies,[8,12,16] we did not find strong predictors of sudden gains. Only younger age predicted the experience of sudden gains. One potential explanation may be that a decline of cognitive flexibility with age[40] decreases the ability to quickly gain insights and rapidly modify existing beliefs. The lack of other predictors suggests people are not necessarily predisposed to experiencing a sudden gain; instead, it may be the treatment itself eliciting sudden gains. Thus, within-treatment, more proximal factors should be further studied.
There are several limitations. There was no assessment only control allowing for the examination of natural symptom fluctuations. That said, both psychotherapy waitlist[3–5] and placebo control trials[2,39] in PTSD show that spontaneous recovery is highly unlikely. Further, given that sudden gains are based on the reliability of the measure, it is unlikely that spontaneous fluctuations are being identified as sudden gains. A second limitation is we may not have examined other salient individual difference variables, such as neuroticism and executive functioning of cognitive flexibility. Third, the ten week treatment duration is shorter than other sudden gain studies,[8,9] limiting the number of opportunities to observe sudden gains or reversals. Finally, we did not examine possible mediators, such as medication and homework adherence. However, these variables, particularly for psychotherapy, are difficult to assess on the same rubric.
Ultimately, this research points to differential patterns and potentially different mechanisms, or processes, underlying sudden gains in PE and sertraline. The absence of strong, pre-treatment predictors highlights the need to research salient within-treatment factors more proximal to the sudden gains. In-session cognitive changes, emotional processing, or hope occurring in sessions prior to sudden gains should be examined to identify aspects of treatment that can be enhanced.[7,8,15] Future research should also directly examine in-session events instead of relying on self-report, retrospective accounts. Further examination of sudden gains will elucidate trajectories and mechanisms of individual change in PTSD treatment, clarifying when and how therapeutic change occurs. This will allow us to identify modifiable components of treatment that clinicians can utilize to personalize and enhance treatment to meet unique, individual needs.
Acknowledgments
This research was funded by grants from the National Institute of Mental Health, R01MH066347 (PI: Zoellner) and R01MH066348 (PI: Feeny).
Footnotes
Disclosure of Potential Conflicts of Interest: This research was funded by grants from the National Institute of Mental Health, R01MH066347 (PI: Zoellner) and R01MH066348 (PI: Feeny). Pfizer, Inc. provided medication free of charge. Pfizer, Inc. did not contribute to the design, implication, analyses, or preparation of this manuscript.
Controlling for treatment modality did not change results.
References
- 1.Brady KT, Pearlstein T, Asnis GM, et al. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: A randomized controlled trial. JAMA. 2000;283:1837–1844. doi: 10.1001/jama.283.14.1837. [DOI] [PubMed] [Google Scholar]
- 2.Davidson J, Pearlstein T, Londborg P, et al. Efficacy of sertraline in preventing relapse of posttraumatic stress disorder: Results of a 28-week double-blind, placebo-controlled study. Am J Psychiatry. 2001;158:1974–1981. doi: 10.1176/appi.ajp.158.12.1974. [DOI] [PubMed] [Google Scholar]
- 3.Foa EB, Dancu CV, Hembree EA, et al. A comparison of exposure therapy, stress inoculation training, and their combination for reducing posttraumatic stress disorder in female assault victims. J Consult Clin Psychol. 1999;67:194–200. doi: 10.1037//0022-006x.67.2.194. [DOI] [PubMed] [Google Scholar]
- 4.Foa EB, Hembree EA, Cahill SP, et al. Randomized trial of prolonged exposure for posttraumatic stress disorder with and without cognitive restructuring: Outcome at academic and community clinics. J Consult Clin Psychol. 2005;73:953–964. doi: 10.1037/0022-006X.73.5.953. [DOI] [PubMed] [Google Scholar]
- 5.Foa EB, Rothbaum BO, Riggs DS, Murdock TB. Treatment of posttraumatic stress disorder in rape victims: A comparison between cognitive-behavioral procedures and counseling. J Consult Clin Psychol. 1991;59:715–723. doi: 10.1037//0022-006x.59.5.715. [DOI] [PubMed] [Google Scholar]
- 6.Resick PA, Nishith P, Weaver TL, et al. A comparison of cognitive-processing therapy with prolonged exposure and a waiting condition for the treatment of chronic posttraumatic stress disorder in female rape victims. J Consult Clin Psychol. 2002;70:867–879. doi: 10.1037//0022-006x.70.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayes AM, Laurenceau JP, Cardaciotto L. Methods for capturing the process of change. In: Nezu AM, Nezu CM, editors. Evidence-based outcome research: A practical guide to conducting randomized controlled trials for psychosocial interventions. New York, NY: Oxford University Press; 2008. pp. 335–358. [Google Scholar]
- 8.Tang TZ, DeRubeis RJ. Sudden gains and critical sessions in cognitive-behavioral therapy for depression. J Consult Clin Psychol. 1999;67:894–904. doi: 10.1037//0022-006x.67.6.894. [DOI] [PubMed] [Google Scholar]
- 9.Hardy GE, Cahill J, Stiles WB, et al. Sudden Gains in Cognitive Therapy for Depression: A Replication and Extension. J Consult Clin Psychol. 2005;73:59–67. doi: 10.1037/0022-006X.73.1.59. [DOI] [PubMed] [Google Scholar]
- 10.Stiles WB, Leach C, Barkham M, et al. Early sudden gains in psychotherapy under routine clinic conditions: Practice-based evidence. J Consult Clin Psychol. 2003;71:14–21. [PubMed] [Google Scholar]
- 11.Aderka IM, Appelbaum-Namdar E, Shafran N, Gilboa-Schechtman E. Sudden gains in prolonged exposure for children and adolescents with posttraumatic stress disorder. J Consult Clin Psychol. 2011;79:441–446. doi: 10.1037/a0024112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doane LS, Feeny NC, Zoellner LA. A preliminary investigation of sudden gains in exposure therapy for ptsd. Behav Res Ther. 2010;48:555–560. doi: 10.1016/j.brat.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly KA, Rizvi SL, Monson CM, Resick PA. The impact of sudden gains in cognitive behavioral therapy for posttraumatic stress disorder. J Trauma Stress. 2009;22:287–293. doi: 10.1002/jts.20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Busch AM, Kanter JW, Landes SJ, Kohlenberg RJ. Sudden Gains and Outcome: A Broader Temporal Analysis of Cognitive Therapy for Depression. Behav Ther. 2006;37:61–68. doi: 10.1016/j.beth.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Tang TZ, Luborsky L, Andrusyna T. Sudden gains in recovering from depression: Are they also found in psychotherapies other than cognitive-behavioral therapy? J Consult Clin Psychol. 2002;70:444–447. [PubMed] [Google Scholar]
- 16.Gaynor ST, Weersing VR, Kolko DJ, et al. The prevalence and impact of large sudden improvements during adolescent therapy for depression: A comparison across cognitive-behavioral, family, and supportive therapy. J Consult Clin Psychol. 2003;71:386–393. doi: 10.1037/0022-006x.71.2.386. [DOI] [PubMed] [Google Scholar]
- 17.Gueorguieva R, Mallinckrodt C, Krystal JH. Trajectories of depression severity in clinical trials of duloxetine: Insights into antidepressant and placebo responses. Arch Gen Psychiatry. 2011;68:1227–1237. doi: 10.1001/archgenpsychiatry.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uher R, Mors O, Rietschel M, et al. Early and delayed onset of response to antidepressants in individual trajectories of change during treatment of major depression: A secondary analysis of data from the Genome-Based Therapeutic Drugs for Depression (GENDEP) study. J Clin Psychiatry. 2011;72:1478–1484. doi: 10.4088/JCP.10m06419. [DOI] [PubMed] [Google Scholar]
- 19.Vittengl JR, Clark LA, Jarrett RB. Validity of Sudden Gains in Acute Phase Treatment of Depression. J Consult Clin Psychol. 2005;73:173–182. doi: 10.1037/0022-006X.73.1.173. [DOI] [PubMed] [Google Scholar]
- 20.van Etten ML, Taylor S. Comparative efficacy of treatments for post-traumatic stress disorder: a meta-analysis. Clin Psychol Psychother. 1998;5:126–144. [Google Scholar]
- 21.The Optimizing PTSD Treatment (OPT) Team. A doubly randomized preference trial comparing prolonged exposure and sertraline in the treatment of PTSD. 2013 Manuscript in preparation. [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: 1994. [Google Scholar]
- 23.First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV Axis I disorders-Patient Edition. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 24.Skre I, Onstad S, Torgersen S, Kringlen E. High interrater reliability for the Structured Clinical Interview for DSM-III-R Axis I (SCID-I) Acta Psychiatr Scand. 1991;84:167–173. doi: 10.1111/j.1600-0447.1991.tb03123.x. [DOI] [PubMed] [Google Scholar]
- 25.Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. J Trauma Stress. 1993;6:459–473. [Google Scholar]
- 26.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 27.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 28.Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the predictions of fearfulness. Behav Res Ther. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- 29.Spielberger CD. Manual of state-trait anxiety inventory (STAI: Form Y) Palo Alto, CA: Consulting Psychologists; 1983. [Google Scholar]
- 30.Foa EB, Ehlers A, Clark DM, et al. The Posttraumatic Cognitions Inventory (PTCI): Development and validation. Psych Assess. 1999;11:303–314. [Google Scholar]
- 31.Kubany ES, Haynes SN, Abueg FR, et al. Development and validation of the Trauma-Related Guilt Inventory (TRGI) Psych Assess. 1996;8:428–444. [Google Scholar]
- 32.Foa EB, Hembree EA, Dancu CV. Prolonged Exposure (PE) manual: Revised version. 2002 Unpublished manuscript. [Google Scholar]
- 33.Hofmann SG, Schulz SM, Meuret AE, et al. Sudden gains during therapy of social phobia. J Consult Clin Psychol. 2006;74:687–697. doi: 10.1037/0022-006X.74.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobson NS, Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 35.Devilly GJ, Foa EB. The investigation of exposure and cognitive therapy: Comment on Tarrier et al. (1999) J Consult Clin Psychol. 2001;69:114–116. doi: 10.1037//0022-006x.69.1.114. [DOI] [PubMed] [Google Scholar]
- 36.Drymalski WM, Washburn JJ. Sudden gains in the treatment of depression in a partial hospitalization program. J Consult Clin Psychol. 2011;79:364–368. doi: 10.1037/a0022973. [DOI] [PubMed] [Google Scholar]
- 37.Foa EB, Hembree EA, Rothbaum BO. Prolonged exposure therapy for PTSD: Emotional processing of traumatic experiences: Therapist guide. New York, NY: Oxford University Press; 2007. [Google Scholar]
- 38.Davidson JR, Rothbaum BO, van der Kolk BA, et al. Multicenter, double-blind comparison of sertraline and placebo in the treatment of posttraumatic stress disorder. Arch Gen Psychiatry. 2001;58:485–492. doi: 10.1001/archpsyc.58.5.485. [DOI] [PubMed] [Google Scholar]
- 39.Laurenceau JP, Hayes AM, Feldman GC. Some methodological and statistical issues in the study of change processes in psychotherapy. Clin Psychol Rev. 2007;27:682–695. doi: 10.1016/j.cpr.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Head D, Kennedy KM, Rodrigue KM, Raz N. Age differences in perseveration: cognitive and neuroanatomical mediators of performance on the Wisconsin Card Sorting Test. Neuropsychologia. 2009;47:1200–1203. doi: 10.1016/j.neuropsychologia.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]