Abstract
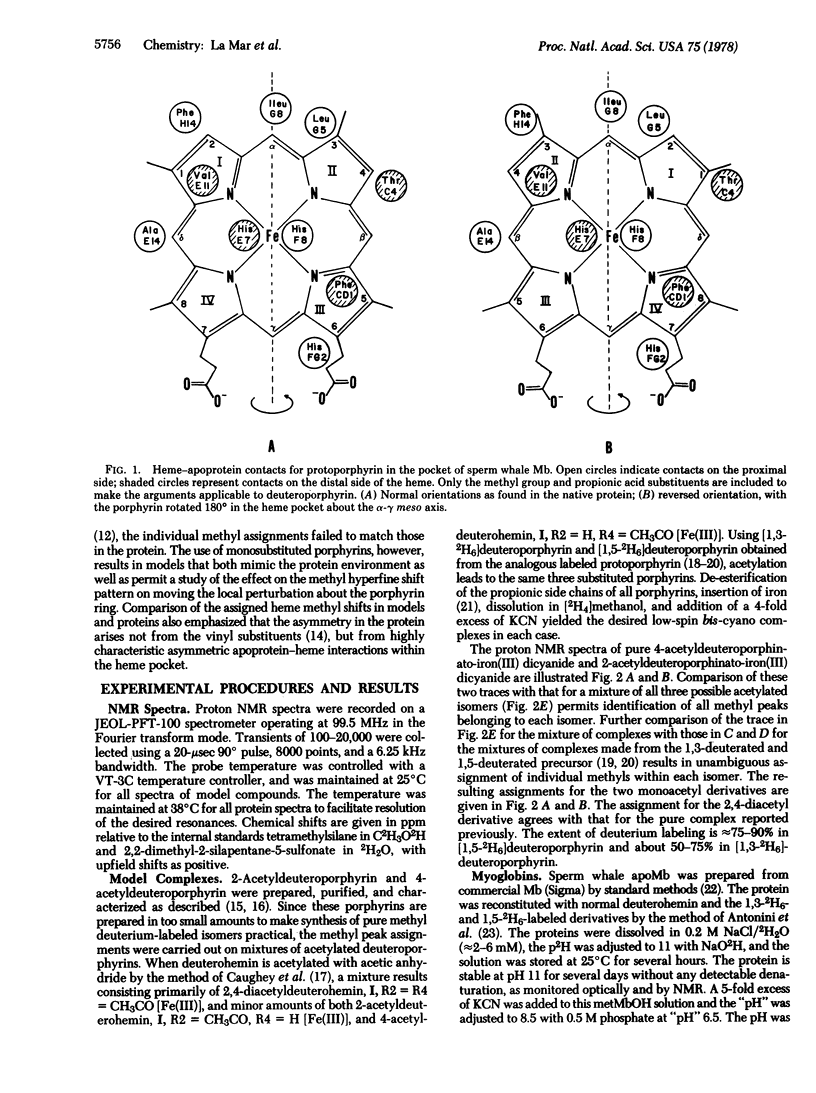
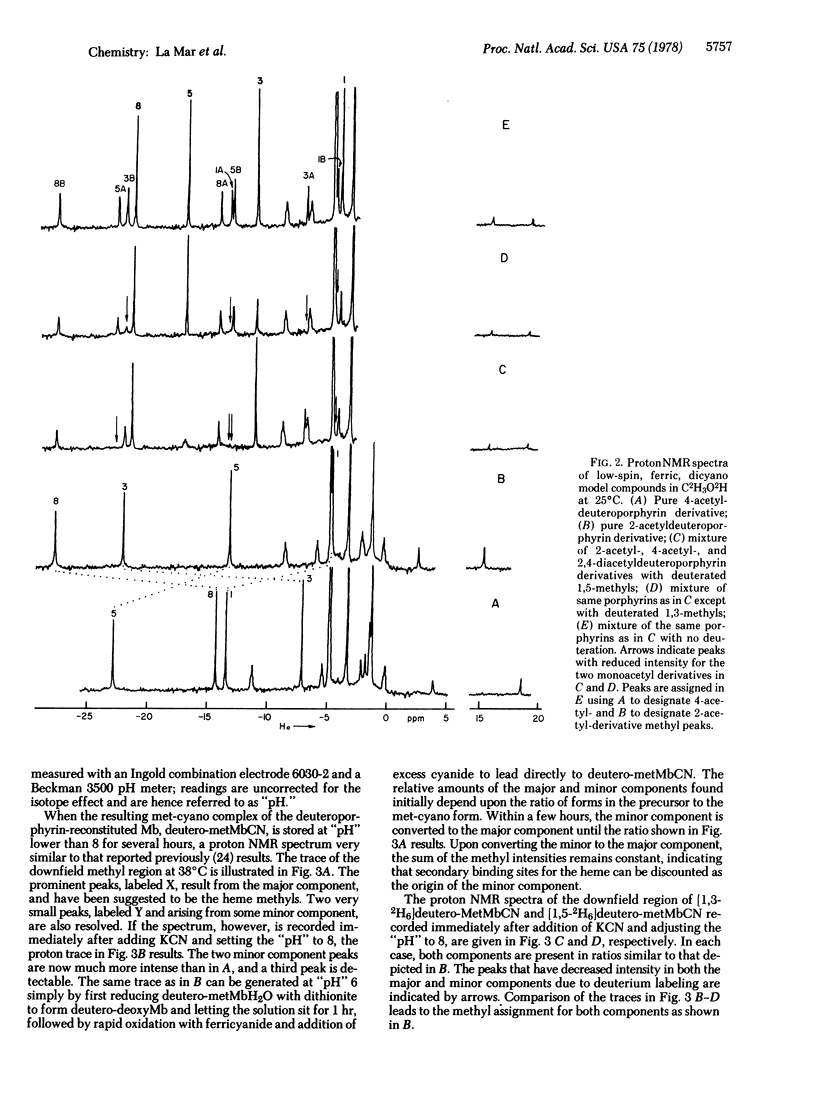
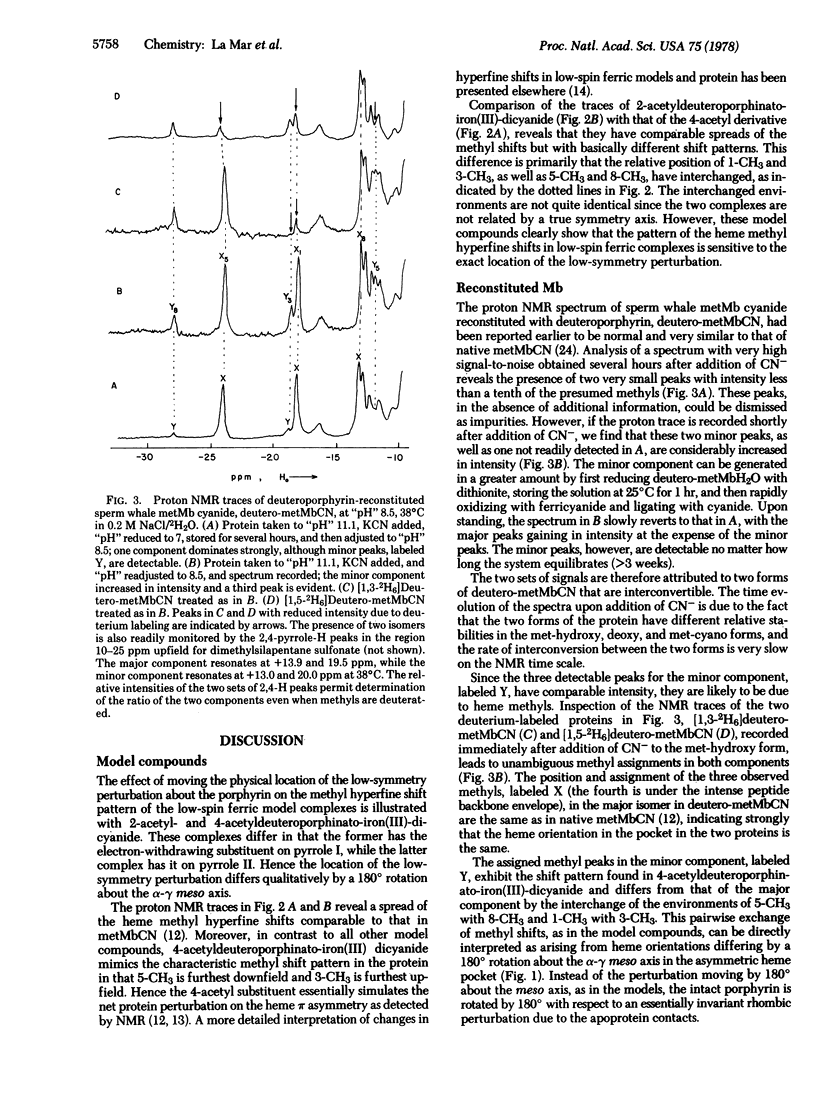
A proton NMR method is described for determining the orientation of a porphyrin within the heme pocket of a hemoprotein. The pattern of the hyperfine-shifted heme methyl resonances in low-spin ferric model compounds is demonstrated to characteristically reflect the position of a localized low-symmetry perturbation on the pi system. The specific assignments via deuteration of the two interconvertible sets of methyl resonances observed for deuteroporphyrin-reconstituted sperm whale metmyoglobin cyanide lead to the conclusion that the low-symmetry perturbations on the heme due to the apo-protein contacts differ for the two protein components by a 180 degrees rotation about the alpha-gamma meso axis. Hence the heme in the reconstituted myoglobin is "disordered" in solution, and the altered functional properties of the reconstituted protein cannot be simply attributed to the local effect of the heme substituent. This NMR technique has applicability for determining the relative heme orientation in related hemoproteins, and may clarify the origin of doubling of heme resonances observed in several native hemoproteins.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTONINI E., BRUNORI M., CAPUTO A., CHIANCONE E., FANELLI A. R., WYMAN J. STUDIES ON THE STRUCTURE OF HEMOGLOBIN. III. PHYSICOCHEMICAL PROPERTIES OF RECONSTITUTED HEMOGLOBINS. Biochim Biophys Acta. 1964 Mar 30;79:284–292. doi: 10.1016/0926-6577(64)90009-9. [DOI] [PubMed] [Google Scholar]
- Argos P., Mathews F. S. The structure of ferrocytochrome b5 at 2.8 A resolution. J Biol Chem. 1975 Jan 25;250(2):747–751. [PubMed] [Google Scholar]
- Brockmann H., Jr, Bliesener K. M., Inhoffen H. H. Formyl- und Acetyl-substituierte Deuteroporphyrine. Justus Liebigs Ann Chem. 1968;718:148–161. doi: 10.1002/jlac.19687180116. [DOI] [PubMed] [Google Scholar]
- Caughey W. S., Alben J. O., Fujimoto W. Y., York J. L. Substituted deuteroporphyrins. I. Reactions at the periphery of the porphyrin ring. J Org Chem. 1966 Aug;31(8):2631–2640. doi: 10.1021/jo01346a042. [DOI] [PubMed] [Google Scholar]
- Evans B., Smith K. M., La Mar G. N., Visco D. B. Regioselective base-catalyzed exchange of ring methyl protons in protoporphyrin IX. A new facet of porphyrin chemistry. J Am Chem Soc. 1977 Oct 12;99(21):7070–7072. doi: 10.1021/ja00463a055. [DOI] [PubMed] [Google Scholar]
- Fermi G. Three-dimensional fourier synthesis of human deoxyhaemoglobin at 2-5 A resolution: refinement of the atomic model. J Mol Biol. 1975 Sep 15;97(2):237–256. doi: 10.1016/s0022-2836(75)80037-4. [DOI] [PubMed] [Google Scholar]
- Huber R., Epp O., Steigemann W., Formanek H. The atomic structure of erythrocruorin in the light of the chemical sequence and its comparison with myoglobin. Eur J Biochem. 1971 Mar 1;19(1):42–50. doi: 10.1111/j.1432-1033.1971.tb01285.x. [DOI] [PubMed] [Google Scholar]
- Keller R., Groudinsky O., Wüthrich K. Contact-shifted resonances in the 1H NMR spectra of cytochrome b5. Resonance identification and spin density distribution in the heme group. Biochim Biophys Acta. 1976 Apr 14;427(2):497–511. doi: 10.1016/0005-2795(76)90192-6. [DOI] [PubMed] [Google Scholar]
- La Mar G. N., Overkamp M., Sick H., Gersonde K. Proton nuclear magnetic resonance hyperfine shifts as indicators of tertiary structural changes accompanying the Bohr effect in monomeric insect hemoglobins. Biochemistry. 1978 Jan 24;17(2):352–361. doi: 10.1021/bi00595a025. [DOI] [PubMed] [Google Scholar]
- Mayer A., Ogawa S., Shulman R. G., Yamane T., Cavaleiro J. A., Rocha Gonsalves A. M., Kenner G. W., Smith K. M. Assignments of the paramagnetically shifted heme methyl nuclear magnetic resonance peaks of cyanometmyoglobin by selective deuteration. J Mol Biol. 1974 Jul 15;86(4):749–756. doi: 10.1016/0022-2836(74)90351-9. [DOI] [PubMed] [Google Scholar]
- Padlan E. A., Eaton W. A. A crystallographic study of deoxy cobalt (II) mesoporphyrin IX myoglobin. J Biol Chem. 1975 Sep 10;250(17):7069–7073. [PubMed] [Google Scholar]
- Padlan E. A., Love W. E. Three-dimensional structure of hemoglobin from the polychaete annelid, Glycera dibranchiata, at 2.5 A resolution. J Biol Chem. 1974 Jul 10;249(13):4067–4078. [PubMed] [Google Scholar]
- Perutz M. F. Structure and mechanism of haemoglobin. Br Med Bull. 1976 Sep;32(3):195–208. doi: 10.1093/oxfordjournals.bmb.a071363. [DOI] [PubMed] [Google Scholar]
- Seybert D. W., Moffat K. The structure of hemoglobin reconstituted with deuteroheme. J Mol Biol. 1976 Sep 25;106(3):895–902. doi: 10.1016/0022-2836(76)90272-2. [DOI] [PubMed] [Google Scholar]
- Shulman R. G., Wüthrich K., Yamane T., Antonini E., Brunori M. Nuclear magnetic resonances of reconstituted myoglobins. Proc Natl Acad Sci U S A. 1969 Jul;63(3):623–628. doi: 10.1073/pnas.63.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. M., Kamen M. D. Proton magnetic resonance spectra of Rhodospirillum rubrum cytochrome c2. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4303–4306. doi: 10.1073/pnas.71.11.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita Y., Yoneyama Y. Oxygen equilibrium of hemoglobins containing unnatural hemes. Effect of modification of heme carboxyl groups and side chains at positions 2 and 4. J Biol Chem. 1971 Jan 25;246(2):389–394. [PubMed] [Google Scholar]
- TEALE F. W. Cleavage of the haem-protein link by acid methylethylketone. Biochim Biophys Acta. 1959 Oct;35:543–543. doi: 10.1016/0006-3002(59)90407-x. [DOI] [PubMed] [Google Scholar]
- Takano T. Structure of myoglobin refined at 2-0 A resolution. II. Structure of deoxymyoglobin from sperm whale. J Mol Biol. 1977 Mar 5;110(3):569–584. doi: 10.1016/s0022-2836(77)80112-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Yonetani T. Studies on modified hemoglobins. IV. Hybrid hemoglobins containing proto- and mesohemes and their oxygenation characteristics. J Biol Chem. 1974 Dec 25;249(24):7964–7968. [PubMed] [Google Scholar]



