Abstract
Experimental salmon thrombin/fibrinogen dressings have been shown to provide effective hemostasis in severe hemorrhage situations. The hypothesis for this study was that swine would still remain healthy without coagulopathy six months after exposure to salmon thrombin/fibrinogen dressings. Initial exposure was by insertion of the salmon dressing into the peritoneal cavity. Three months after the initial exposure, the same animals were subjected to two full thickness dermal wounds on the dorsal surface. One wound was bandaged with the salmon thrombin/fibrinogen bandage and the other wound was dressed with a standard bandage. The animals were monitored for an additional three months. Blood was drawn every 14 days over the six months for immunological and coagulation function analysis. All of the animals (8 pigs) remained healthy during the six month period and the dermal wounds healed without incidence. Lymph nodes and spleen showed signs of normal immune response and Western blots showed development of antibodies against salmon fibrinogen, but none of the animals made antibodies that recognized any species of thrombin. Coagulation parameters (fibrinogen concentration, thrombin time, PT and aPTT) and hematological parameters remained normal over the course of the study when compared to initial values of the subject swine.
Keywords: Salmon, Hemostasis, Coagulation, Antibodies, Thrombin, Fibrinogen, Bandage
1. Introduction
Hemorrhage is a major cause of death in trauma patients [1]. Thirty years ago, almost a third of the casualties of the Vietnam War were caused by exsanguinations [2]. Evidence coming out of Iraq today still shows that hemostasis continues to be a pressing issue [3–6]. In the study by Holcomb et al. [3], most deaths were classified as either non-survivable due to extreme trauma or complications of hemorrhage. Although extreme trauma may not have a solution at this time, the problems of severe hemorrhage and non-compressible wounds may be solvable through the use of better hemostatic devices and bandages. Therefore, control of hemorrhage remains the initial step in first aid and field trauma care and a great deal of attention and effort has been focused on developing effective dressings that can replace the standard gauze dressing.
In a study conducted by Sondeen et al. [7] in 2003 only surgical suturing or fibrinogen-based dressings were effective treatments for a severe (4 mm) injury to the abdominal aorta. The leading contender in the fibrinogen-based dressing field at the time was a product developed at the American Red Cross. However, being a device based on components extracted from human blood, it was still subject to the same limitations as the general blood supply, such as availability, cost and possible transmission of pathogens. This product was never commercially produced. In contrast, one of the other bandages tested in that trial, a hemostatic patch produced by Nycomed (Zurich, Switzerland), has continued to undergo development. Whereas early formats of the device contained mammalian coagulation components and aprotinin, the latest version, TachoSil, employs human fibrinogen and thrombin without the addition of aprotinin [8]. Recent reports of the use of TachoSil in various surgical settings have documented its success in controlling bleeding in kidney and arterial procedures [9–11]. In addition to the use of fibrinogen and thrombin in the TachoSil product, multiple commercially available fibrinogen preparations are available as fibrin glues and sealants for use in surgical procedures (for reviews see Spotnitz [12] and Perkins [13]). The ready availability of recombinant coagulation proteins also has the possibility to dramatically change the field of fibrinogen-based hemostatic devices. Profibrix (Leiden, The Netherlands) has announced the successful completion of Phase II trials (2010) of its recombinant fibrinogen product. However, even human recombinant proteins can elicit an antibody response as demonstrated by Ballard et al. [14].
Other approaches to hemostasis have focused on mineral and chitosan-based bandages. However, in a recent comparison of ten new bandages and hemostatic materials by Arnaud et al. [15], there was mixed efficacy in stopping bleeding in a femoral artery injury model depending on the nature of the dressings. Furthermore, Kheirabadi et al. [16] demonstrated that granular applications also pose as yet undefined risk of embolism when small granular elements enter the circulation. Although further study is warranted, Kheirabadi et al. speculated that induced coagulation centered on the granules may result in unwanted intravascular clotting. This hazard has prompted the suspension of the use of this agent.
A concern with the use of natural blood proteins has been the transmission of disease from either mammalian or human blood derived products. Measures to detect and inactivate the responsible agents have been vigorously pursued. Pathogen inactivation of human blood components has taken great strides since the early days of the human immunodeficiency virus epidemic and there are no reports of HIV, hepatitis B or hepatitis C virus transmission by plasma derived products since 1985[17]. Nonetheless as the spectrum of viruses that are screened for in the blood supply has increased, so too has the realization that there are still many pathogenic organisms that are associated with blood products that may have the potential to transmit disease for which there is no screen. For example, there are over 35 varieties of arboviruses such as the Dengue viruses, St Louis and eastern equine encephalitis viruses [17]. Pasteurization and solvent-detergent treatments have reduced the threat of many viruses but solvent-detergent treatment is not usually effective against non-enveloped viruses. Additionally, each protein used as a treatment must be evaluated for its ability to withstand the pathogen inactivation treatment selected.
To avoid some of the possible disadvantages associated with natural human blood components, yet still reap the benefits of protein-based coagulation, we have developed a fibrinogen dressing that utilizes thrombin and fibrinogen that are isolated from the blood of the Atlantic salmon (Salmo salar). Genetics, diet, and environment of farmed salmon are controlled and monitored to insure a uniform product. In addition, these fish receive scheduled vaccines, and health inspections for fish pathogens. There are no known salmon pathogens (with the exception of two worms) that are infective to humans. This is most likely the result of 450 million years of evolutionary divergence, and differences in body temperature in salmon and mammals. Salmon body temperature (like that of the water where they are reared) ranges from 0 °C to 17 °C, and few salmon pathogens can survive at 25 °C and above. Even prions, the putative causative agents for scrapie, Creutzfeldt-Jacob disease and other transmissible spongiform encephalopathies [18] do not seem to cross over between mammals and fish [19].
The action of the salmon thrombin and fibrinogen are very similar to that of human thrombin and fibrinogen [20,21], but without many of the disadvantages. In experiments using the swine aorta bleeding model, dressings made from these proteins stopped bleeding as well as fibrinogen dressings containing human thrombin and fibrinogen [22].
Despite the favorable aspects of a dressing based on salmon coagulation proteins, there are known concerns associated with products based on animal-derived coagulation proteins [23]. Application of the salmon thrombin/fibrinogen bandage to an open wound is almost certain to introduce salmon products to blood circulation. Once in contact with the elements of the host immune system, an immune response can be initiated that could lead to adverse effects.
Short term studies of the immune response of swine exposed to salmon thrombin/fibrinogen bandages over a 4 week period have been very encouraging [24], but longer duration studies are required to answer the question if adverse effects may not appear until after a longer period of time. It is possible that inhibitory products at low levels could be undetectable by our assays in the short periods of time that the animals were studied following their exposure to the salmon bandages, but the cumulative effects over time could lead to serious coagulopathy. Coagulopathy could be caused by different mechanisms of action. For example, antibodies could bind to coagulation proteins and directly inhibit their function or form antigen–antibody complexes that would then be removed by the macrophage system of cells in the liver and spleen[25,26]. This could cause decreasing levels of coagulation factors that would not be detected immediately, but over a period of time could cause decreased factor levels.
It is our hypothesis that swine treated with the salmon thrombin/fibrinogen bandage do not suffer adverse reactions long periods after treatment. To test this proposition, pigs were maintained in an examination period for 6 months following initial exposure to the bandage by placement of the bandage into the abdominal cavity. Three months after the initial exposure, the animals were re-exposed a second time to the salmon material when it was used to treat a dermal wound. The results of this trial demonstrated that repeated exposure to the salmon fibrinogen bandage over a six month period had no adverse effects on the coagulation response, the immune system activity or the ability of the skin to heal normally.
2. Methods
2.1. Biochemical and immunological assays
2.1.1. Purification of salmon fibrinogen and thrombin
Salmon proteins were purified from salmon blood as previously described [24]. Briefly, fibrinogen was salt precipitated twice with ammonium sulfate in a method modified from Mosher and Blout [14]. Salmon thrombin was purified from precipitates formed after addition of BaCl2 to plasma by the method of Michaud et al. [15]. Salmon fibrinogen (2000 mg) was electrospun with dextran and mixed with lyophilized salmon thrombin (5000–6000 U) to form a bandage that was approximately 10 × 10 cm. Bandages were fabricated by electro-spinning. Dextran was suspended in ddH20 overnight at a concentration of 1mg/ml and mixed on a clinical rotator (3–4 RPM/min to avoid bubble formation). For each bandage, 10ml of dextran solution was placed into a syringe capped with a blunt tipped needle and mounted into a syringe driver. The dextran solution was chargedto+22 kV using a high voltage, low amperage power supply. The syringe driver was set to deliver the dextran at a rate of 2ml/h from a position that was vertical to the ground target. A circular metal plate that was charged to −22 kV using a second power supply was used as a target to collect the fibers of electrospun dextran. For bandage fabrication, lyophilized fibrinogen (2 gm)was mixed with 5000–6000 U of thrombin. This dry mixture of proteins was salted directly onto the forming dextran fibers during the electrospinning process. These methods were adopted because we found that mixing thrombin or fibrinogen with the dextran in solution and directly electrospinning the resulting material compromised the biological activity of the coagulation proteins. Bandages were sterilized by gamma irradiation (7kGy exposure) using a J.L. Shepard Model 109 Cobalt Gamma Irradiator. Studies conducted by Sea Run Holdings, Inc. showed that lyophilized salmon protein treated with 10kGy irradiation (5 lots tested) retained 80–90% of fibrinogen activity and 65–80% of thrombin activity is retained. Qualitative in vitro studies on the bandage after electrospinning showed that immersion of the bandage into plasma induced clotting. Colorimetric analysis of the thrombin activity (Chromozym TH, Roche, Indianapolis, IN) demonstrated that thrombin component was still active.
2.1.2. Electrophoresis, Western blotting and cytokine measurement
Immunological reactivity was determined by Western blotting. For electrophoresis, fibrinogen and thrombin isolated from salmon, swine and human plasma were used as the samples. Blots generated from these gels were probed with sera of the swine exposed to the salmon fibrinogen/thrombin bandages. If the sera contained reactive antibodies, then the antibodies should bind to the protein bands on the blots. Proteins were separated under reducing conditions on Invitrogen NuPAGE 4–12% Tris-Bis gels and transferred to PVDF, followed by blocking with TTS (Tris/Saline pH 7.4 with 0.01% Tween 20) containing 5% BSA. Human fibrinogen and thrombin standards were purchased from Sigma–Aldrich, Inc (St. Louis, MO). Swine fibrinogen was purified from swine plasma using methodology similar to salmon fibrinogen. Swine thrombin was obtained from Enzyme Research Laboratories (South Bend, IN). Because the proteins were obtained by different methods from different sources there were differences in the polypeptide profiles visualized on the gels. The fluorescent visualization system used in this study (Sypro Ruby, Invitrogen/Molecular probes, Eugene, OR) is very sensitive and detected protein bands at very low concentrations and gave rise to the different gel patterns.
The primary antibody solution used for the protein detection was the swine plasma itself. This was diluted 1/500 in TTS with 4% Carnation dried milk (Nestle USA, Solon, OH). Antibodies were visualized with secondary anti-swine horseradish peroxide-conjugated antibody (HRP-swAB, Fitzgerald Industries International, Concord, MA) and treatment with Millipore chemiluminescence reagent kit (Millipore Corp., Billerica MA). Antibodies against human thrombin and fibrinogen were used as controls to identify authentic thrombin and fibrinogen (Green Mountain Antibodies, Burlington, VT)
2.1.3. Coagulation studies
Coagulation functions (prothrombin time, activated partial thromboplastin time, thrombin time and fibrinogen concentration) were assayed on a STA4 Compac (Seksui Medical Co., Ltd, Jp.). Complete blood counts were run on a Cell Dyne 1700 (Abbott Diagnostics, Abbott Park IL).
2.2. Surgical preparation of animals
All animal procedures were conducted according to a Walter Reed Army Institute of Research IACUC approved protocol. Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adhered to principles stated in the Guide for the Care and Use of Laboratory Animals, NRC Publication, 1996 edition.
Female Göttingen swine (Sus scrofa, Marshall BioResources, NY) (one year old, 25–28 kg) were prepared for surgery and monitored during the procedure as described previously [24]. Exposure to the salmon proteins was performed by two separate methods on a total of eight animals. The first exposure was accomplished by inserting a salmon thrombin/fibrinogen dressing that had been folded twice to forma square approximately 4 cm/side through a midline abdominal incision into the abdominal cavity. The bandage square was placed against the peritoneal membrane 10 cm off center from the incision on the right side of the animal. The natural adhesion of the dressing and the pressure of the intestines held the dressing in place and the incision was sutured by layers to close the wound. Skin sutures were removed at seven days post-surgery. In addition to the first method, three months into the study, paired identical full thickness dermal wounds (2 × 2 cm) were surgically created on the right and left dorsal skin surface, paramedial to the spinal column in all eight pigs. The thrombin/fibrinogen bandage was cut into quarters containing approximately 500 mg fibrinogen and 1400 IU thrombin. A Telfa dressing (Tyco Healthcare Group, LP, Gosport, UK) was applied to the left side and the thrombin/fibrinogen bandage was applied to the wound on the right side immediately after the wounds were made. The dressings were held in place by elastic wraps and remained in place for 24 h. The bandages were then removed but no attempt was made to remove pieces of the thrombin/fibrinogen bandage that may have incorporated into the clot. The animals were then monitored for the duration of the study period for infection or immune response. Bloodwas collected at two week intervals and prepared for serum for antibodies or plasma (blood collected into sodium citrate) for coagulation testing.
At the end of the time period, the animals in each group were euthanized by administration of a lethal dose of B-Euthanasia (Schering-Plough Animal Health, The Netherlands) injected intravenously in accordance with 2007 American Veterinary Medical Assoc. Guidelines on Euthanasia and the carcass presented for necropsy.
2.3. Tissue preparation for histological examination
At necropsy, tissue from the salmon fibrinogen/thrombin treated lesions and non-hemostatic bandage treated lesions, the pre-femoral lymph nodes, mesenteric lymph nodes and spleen, as well as lung, liver and small and large intestines were harvested for histopathology. Evaluation parameters on the skin sections included examination of the wound edge and monitoring for signs of granulation tissue, re-epithelialization, fibrosis, crust formation, inflammation and necrosis. Semi-quantitative scoring of the skin samples for superficial and deep inflammation was performed.
2.3.1. Statistical analysis
Differences between groups of quantifiable parameters were analyzed using a two tailed T-test assuming equal variances. Values are expressed as means ± standard error. N values and p values are included with each measurement.
3. Results
3.1. Reaction to the placement of the bandages
Bandages were placed in two different types of locations: 1) inside the abdominal cavity wedged between the visceral and parietal peritoneal membranes at the initiation of the study and 2) external to the body but in contact with the deep fascia of the muscle at the dermal wound site. This second procedure was performed three months into the six month study period. At the end of study period, physical evaluation of the animals showed that all of the eight animals were healthy with normal activity and behavioral patterns. The animals were now fully mature with normal estrus cycles. At the conclusion of the experimental period, the animals were sacrificed and a complete necropsy was performed by a certified veterinary pathologist. Examination of the major organ systems revealed no problems with lungs, gastrointestinal tract or heart. However, this does not rule out the possibility of subtle effects of the salmon proteins that were not detected by routine histology.
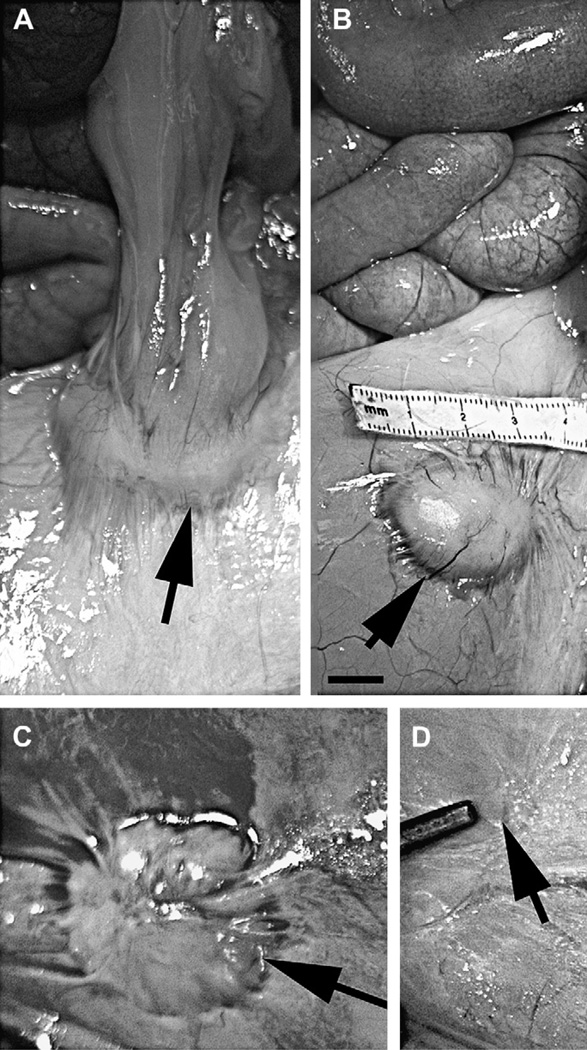
Gross examination of the abdominal implant site revealed that 7/8 animals demonstrated residual presence of the salmon/fibrinogen bandage as shown in Fig.1A. Generally, reactions were noted on the serosal surface of abdominal cavity in the form of single or multiple raised, firm yellow nodules with the largest 30 mm in diameter (granuloma). Size of these granulomas varied from 3 cm to only a vague roughened serosal surface. Microscopic examination demonstrated that the site displayed the same histology as the larger nodules, just at a fraction of the size of the other sites. Location of the nodules varied in animals somewhat, indicating that the surgical placement of the bandages was not precisely identical or there may have been some movement of the bandages which were not sutured to the body wall. Fig. 1 shows the variation of the granulomas remaining at the abdominal placement site. In one pig, abdominal omentum was attached to the nodule (Fig. 1A).
Fig. 1.
Residual bandage material remaining at the abdominal placement is encapsulated in a granuloma. Images of the viscera of four animals photographed at the end of the study at the time of necropsy are shown, depicting the size range of the granulomas (indicated by the arrows) localized in the serosa of the parietal peritoneum. The largest granuloma was approximately 4 cm while the smallest was almost undetectable. Contact between the peritoneum and the small intestine induced the formation of an adhesive bridge between the two sites. Scale bar = 1 cm.
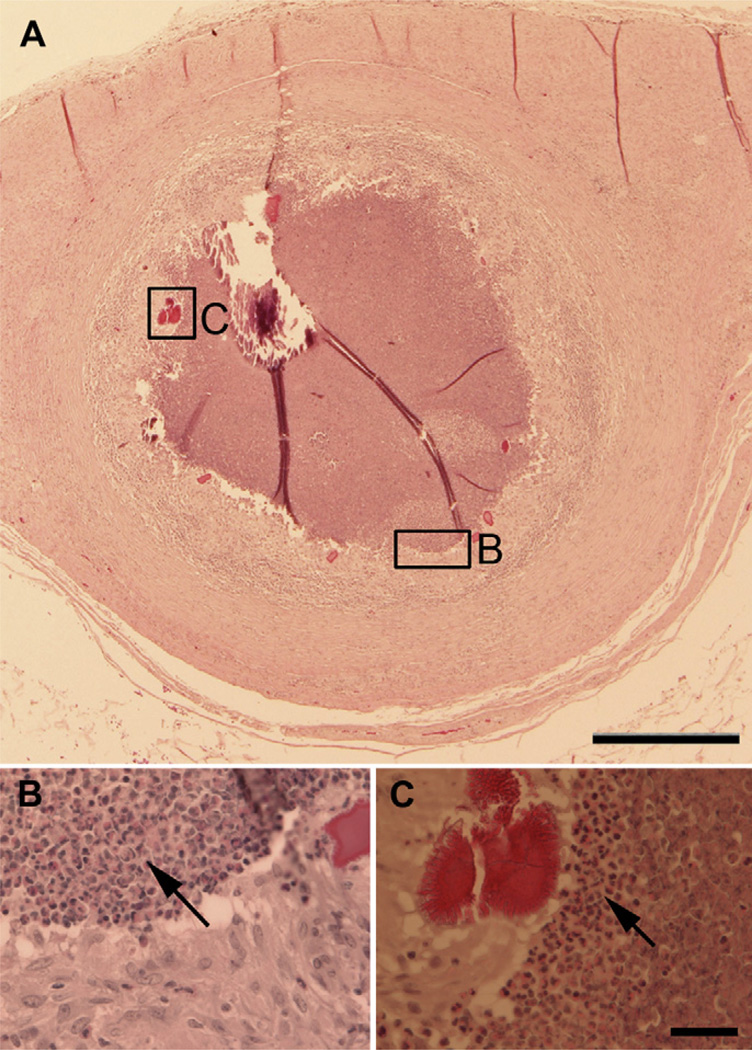
Histological examination of the implant site demonstrated granulomas within the serosa of the abdomen. The granulomas were characterized by central necrosis and mineralization bound by degenerate neutrophils, epitheloid macrophages, and multi-nucleated giant cells. In one animal, moderate Splendore–Hoeppli material was noted multifocally throughout the granuloma as shown in Fig. 2B and C. Splendore–Hoeppli material is thought to represent a localized antigen–antibody reaction and increased numbers of eosinophils could be detected in this area. The area surrounding the granulomas was further bounded by fibrosis with interspersed lymphocytes. There were moderate numbers of lymphocytes surrounding tissue associated blood vessels in all sections examined.
Fig. 2.
Placement of the thrombin/fibrinogen bandage in the abdominal cavity induces an immune response. The granuloma, indicated by arrows, is encapsulated in a layer of fibroblasts, epitheloid macrophages and multi-nucleated giant cells. Areas labeled B and C contain higher concentrations of degenerate neutrophils and eosinophils (shown at higher magnification in Panels B and C). Panel C also shows eosinophilic-staining Splendore–Hoeppli material characteristic of an antigen–antibody response (asterisk). Scale bar = 500 um Panel A, scale bar = 50 um Panels B and C.
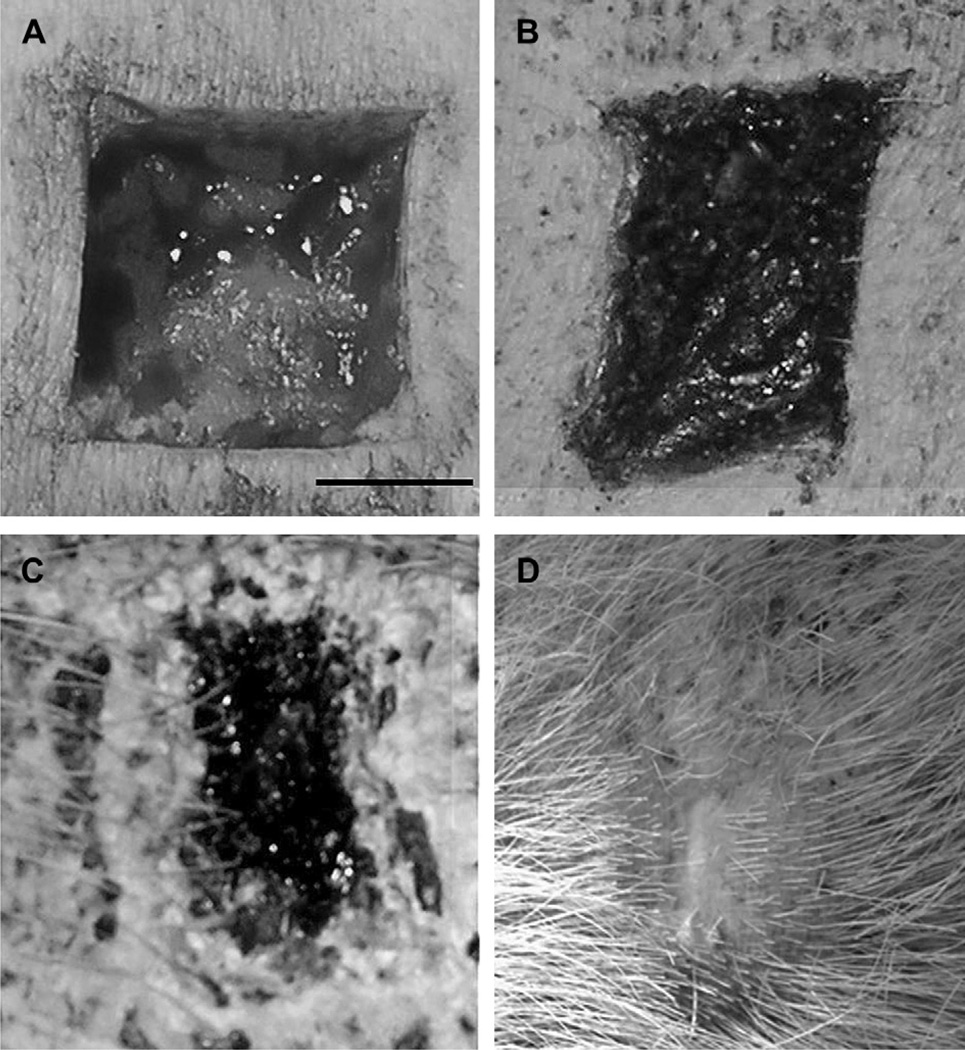
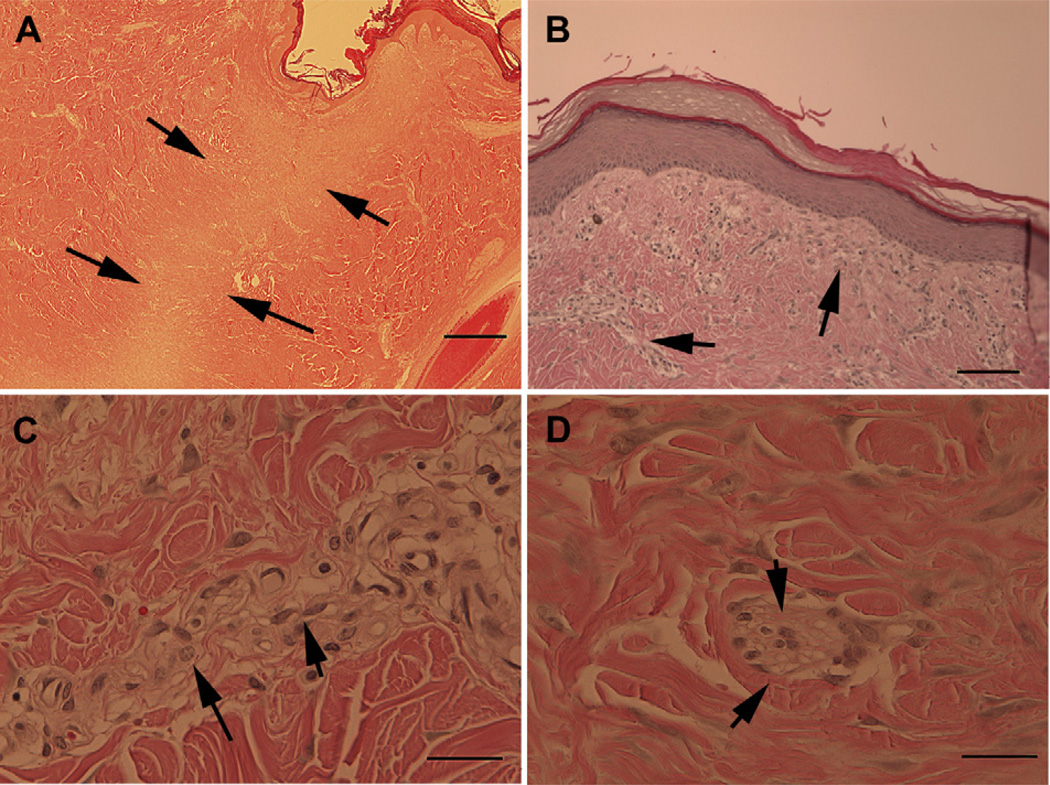
The dermal lesions had healed by secondary granulation without complication and left little trace of the original wound except for a slight depression in the area where the tissue had been initially removed. Lesions treated with both the control bandage and the salmon thrombin/fibrinogen healed in the same manner. Progression of the healing process is shown in Fig. 3. The images show the wound site at the time of surgery (Fig. 3A), at one week post-surgery (Fig. 3B), at 3 weeks (Fig. 3C) and at the completion of the study (Fig. 3D). Histological examination of sections prepared from the lesion site (Fig. 4) showed that an area of disorganization could still be discerned after the site had healed (Fig. 4A). Higher magnification examination revealed mild focal lymphohistiocytic dermatitis with a mixture of lymphocytes and macrophages (Fig. 4B and C), epidermal hyperplasia and rare multi-nucleated giant cells with birefringent material that is suspected to be residual bandage material (Fig. 4D). Overall, the observed features were characteristic of recovery from a deep cutaneous injury.
Fig. 3.
Dermal lesion heals without incident after treatment with the thrombin/fibrinogen bandage. Panel A shows the wound site at the time of surgery before treatment, at one week post-surgery after removal of the salmon thrombin/fibrinogen bandage (Panel B), at 3 weeks (Panel C) and at the completion of the study (Panel D). Scale bar ¼ 1.25 cm.
Fig. 4.
Dermal lesions heal normally with minor residual fibrosis evident at six months. Skin re-epithelialized and hair follicles had reformed by the conclusion of the six month study (Panel A) although a band of disorganization could still be discerned where the lesion was created. Higher magnification revealed bands of fibrotic tissue that could still be detected underlying the epidermis and in other areas of the regenerated dermis (panel B, arrows). The cells comprising the fibrotic tissue were mainly fibroblasts (Panel C, short arrows) with some macrophages (Panel C, long arrow), lymphocytes and rare giant multi-nuclear cells (Panel D, arrows). Scale bars = 1000 um (Panel A), 200 um (Panel B), and 50 um (Panels C and D).
Sections were prepared from lymphoid organs to assess the level of immune response and from other major organs to determine if adverse inflammatory responses were occurring. Examination of axillary, mammary and mesentery lymph nodes showed mild diffuse hyperplasia and some increased numbers of lymphocytes and macrophages reflected as interstitial lymphohistocystosis (Fig. 5A, arrow). At higher magnification examination, evidence of lymphocyte activation was evident and some hemorrhage, possibly as a result of tissue collection, was present (Fig. 5B). The spleens appeared normal with mild congestion (Fig. 5C). The kidney, colon and lung were also examined for adverse responses. The colon (Fig. 5E) and the lung (Fig. 5F) appeared healthy. Minor areas of lymphocytic infiltration could be detected in the kidney (Fig. 5D, arrow) and the lung (Fig. 5G, asterisks) but the degree or amount tissue involved was small and did not seem to be more than the normal isolated inflammatory responses encountered in animals never exposed to the salmon bandage. Overall, the necropsy findings of the treated swine were consistent with the expected responses of animals recovering from surgery.
Fig. 5.
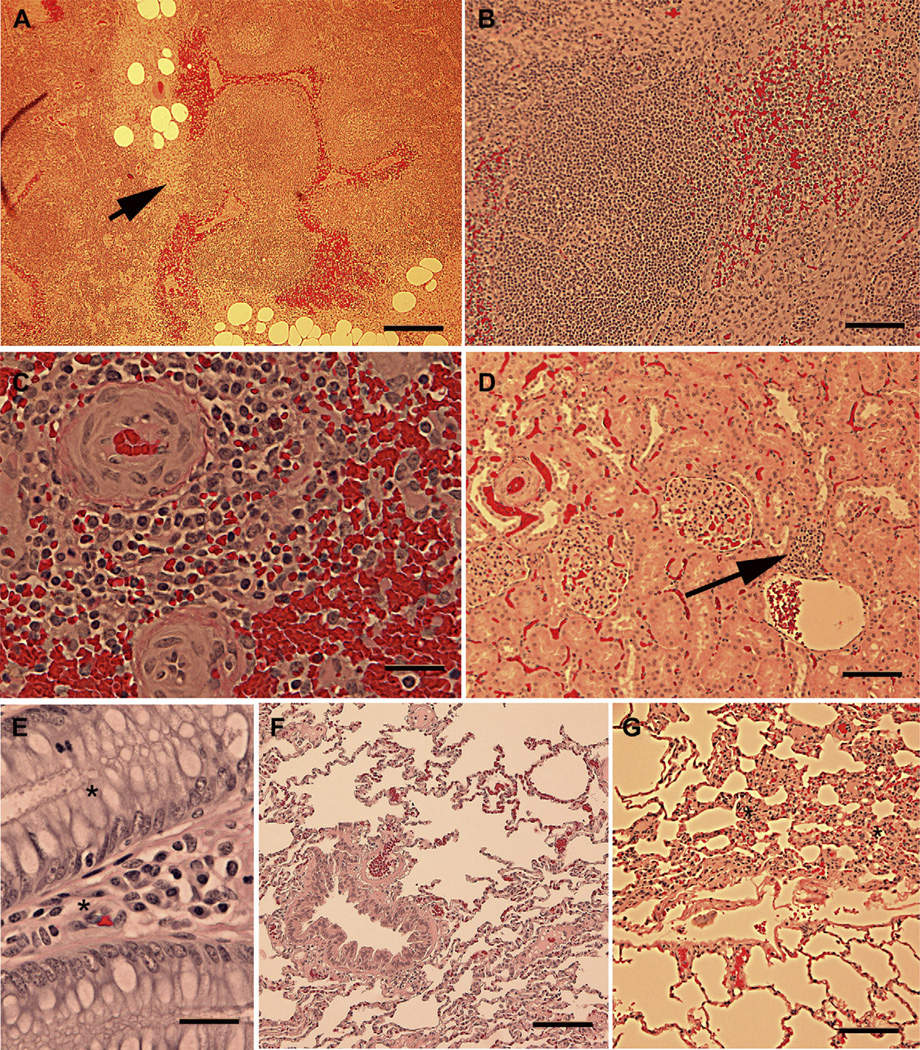
Immune organs show signs of activation and mild fibrosis following exposure to the salmon thrombin/fibrinogen bandage. Examination of the lymph nodes close to the bandage site revealed the presence of mild fibrosis (Panel A, arrow) and active lymph nodules with germinal centers (Panel B). Sections of the spleen appeared normal with signs of some activation of lymphocytes in the periarteriolar regions (Panel C). Sections of the kidney appeared with infrequent lymphocytic clusters as depicted by the arrow in Panel D. Sections of the colon (panel E) and the lung (Panel F) were normal. Some areas of fibrosis could be found near the air passages (asterisks in Panel G), but they did not appear to be more prevalent than those observed in tissue from non-surgical animals used in other protocols in the research institute. Scale bars = 500 um (Panel A), 200 um (Panel B), 50 um (Panel C), 100 um (panel D), 50 um (Panel E) and 200 um (Panel F and G).
3.2. Antibody production following exposure to the salmon thrombin/fibrinogen bandage
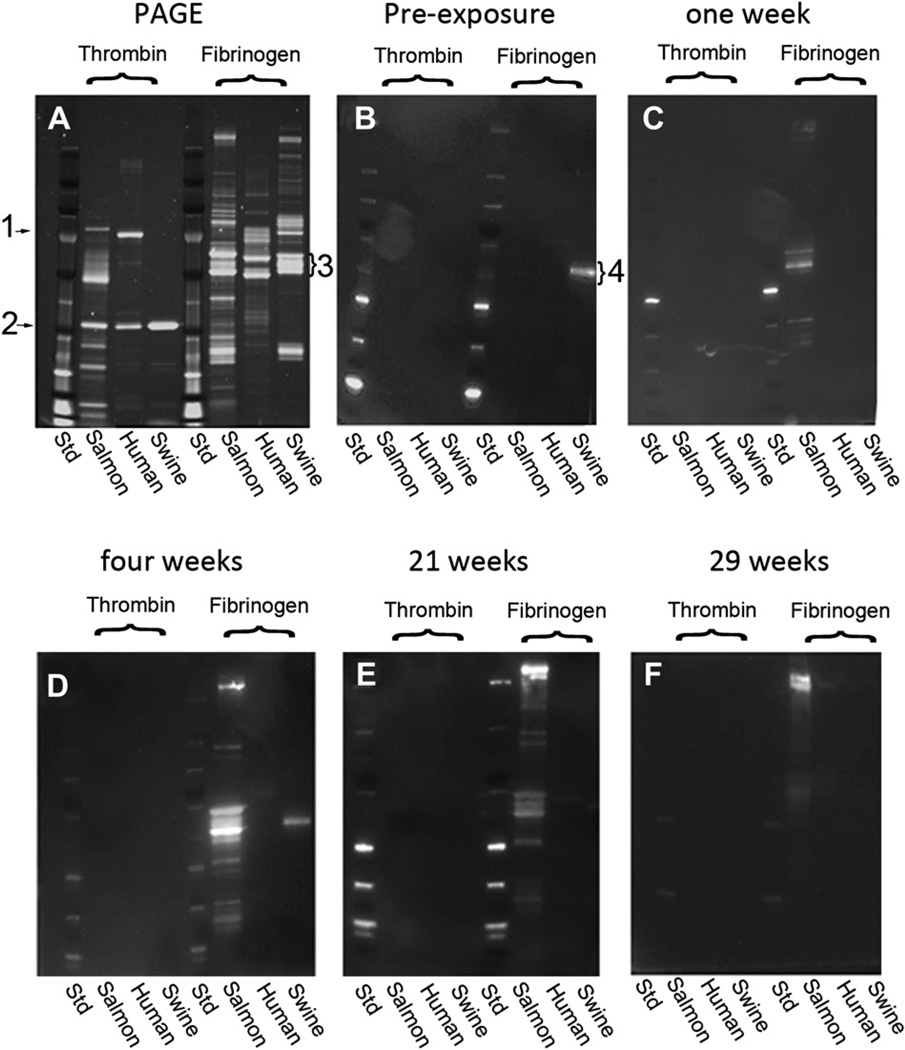
In our previous studies [24], swine exposed to the salmon thrombin/fibrinogen bandage produced antibodies that recognized salmon proteins, principally fibrinogen. In this study, the levels of antibodies that were produced were tracked over the course of six months. Fig. 6 shows the SDS-PAGE image of the different thrombin and fibrinogen species used as the samples (Fig. 6A) and the Western blots performed with the sera of one animal taken before exposure to the salmon material (Fig. 6B), at one week post-exposure (Fig. 6C), four weeks post-exposure (Fig. 6D), 21 weeks post-exposure (Fig. 6E) and 29 weeks post-exposure (Fig. 6F). Blots from the other seven animals were similar. Antibody reactions to the thrombin samples were not detected. Reactions to the salmon fibrinogen (all three subunits) and additional polypeptides could be detected out to 21 weeks. There was a strong antibody reaction to a high molecular weight doublet that was evident by the four week time point and remained detectable throughout the course of the study. In all animals, the immune reaction remained confined to interactions with the salmon proteins.
Fig. 6.
Western blots reveal antibodies generated against salmon fibrinogen. Proteins in the salmon, human and swine thrombin and fibrinogen preparations were separated by electrophoresis (Panel A, PAGE). Prothrombin (Panel A, “1”) could be detected in the salmon and human preparations but not in the swine thrombin preparations. Thrombin Panel A, “2” and the fibrinogen subunits (Panel A, “3”) were prominent bands. All three fibrinogen bands contain polypeptides in addition to the fibrinogen subunits. Western blots were performed with the sera of one animal obtained from bleeds taken before exposure to the salmon material (Panel B), at one week post-exposure (Panel C), four weeks post-exposure (Panel D), 21 weeks post-exposure (Panel E) and 29 weeks post-exposure (Panel F). The blots show the development of antibodies reactive for salmon fibrinogen and a high molecular weight component in the fibrinogen sample. The band labeled “4” is the reaction of the anti-swine secondary antibody with the residual swine immunoglobulin in some of the swine fibrinogen preparations and is evident before exposure to the fish proteins and in the absence of the swine serum (not shown).
3.3. Coagulation and hematological parameters following exposure to the salmon thrombin/fibrinogen
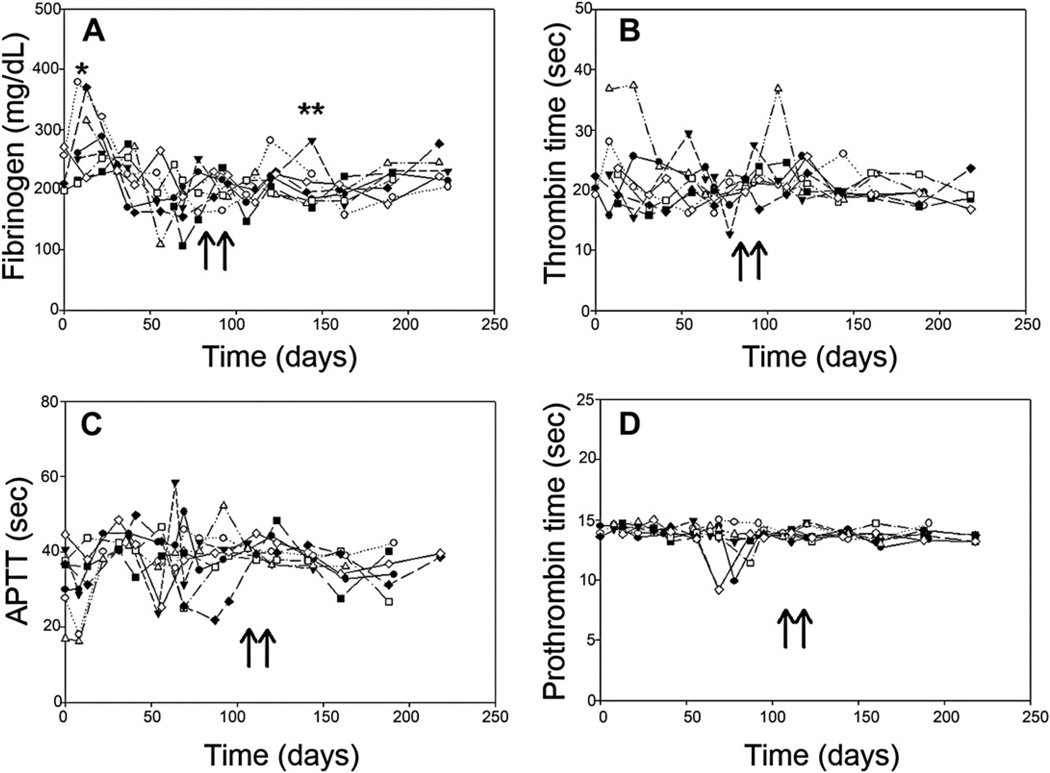
A major concern was that the coagulation parameters would gradually decline over time due to cryptic factors that were not detected in short term studies. To address this issue, blood samples were collected every two weeks over the course of the study and analyzed for clotting times and fibrinogen levels. Fig. 7 shows the progression of fibrinogen levels (Fig. 7A), thrombin time (Fig. 7B), activated partial thromboplastin time, aPTT, (Fig. 7C) and prothrombin time (Fig. 7D) with the values for each animal plotted individually. Fibrinogen levels spiked in the week following the initial surgery and in one animal that developed an infected cyst at approximately day 150. This brief increase in fibrinogen concentration in that animal was interpreted as a normal hepatic acute phase response to inflammation. Although there were individual variations that were increased or decreased over the six months, none of the four parameters displayed a systemic trend either upward or downward from the baseline. Hematological parameters, as measured by complete blood cell counts, were not changed significantly from the pre-exposure values. Day 0 and day 180 values for the Göttingen pigs whereas follows: leukocytes: 7.6 ± 1.8 v. 8.5 ± 0.9 (x109/L); erythrocytes: 5.5 ± 0.4 v. 5.7 ± 0.2 (x1012/L); hematocrit: 28.9 ± 1.2 v. 28.7 ± 2.8 and platelets: 416.0 ± 40.1 v. 392 ± 42.6 (x109/L). Historical controls taken from 34 Yorkshire pigs were similar: erythrocytes levels 5.79 ± 0.43 (x1012/L); hematocrit: 29.0 ± 3.2 and platelet counts: 444.0 ± 89.0, but differed from the Göttingen pigs in that the Yorkshire pigs averaged leukocyte counts of 19.9 ± 3.9 (x109/L).
Fig. 7.
Coagulation parameters are not altered over the course of the study. Blood was taken from each animal every two weeks and the coagulation times and fibrinogen concentration was assessed. The time of the bandage application to the dermal injury is indicated by the arrows. Fibrinogen concentration increased during the week following the initial surgery in 3 animals (Panel A, “*”). It was also seen to increase in one animal with a known infected cyst (Panel A, “**”). Thrombin time (Panel B), aPTT (Panel C) and prothrombin time (Panel D) did not show an increasing or decreasing trend over the study period.
4. Discussion
4.1. Possible concerns with the use of mammalian coagulation proteins as hemostatic agents
Interaction of the human host defense system with foreign proteins can be rapid and robust. The most common modes by which foreign antigens are encountered are by dermal contact and ingestion as food. Tens of thousands of Americans suffer from food allergies. Even discounting allergies to plant-associated allergens such as peanuts and wheat gliad which can cause violent and chronic immune responses, responses to mammalian and fish protein components are still a source of concern to investigators hoping to develop xenogeneic biologics for surgical and regeneration applications. Fortunately, while the immune responses can be vigorous, they are also specific and the epitopes responsible for the responses are becoming better characterized and understood. For example, porcine organs and tissues are being investigated as organ replacements and biological scaffolds, but the presence of the “Gal epitope” (Galα 1,3–Galβ1-4GlcNAc-R) on cell surfaces can lead to animmune response such that up1%of circulating IgG can be anti-Gal antibody [27,28]. The strategy now is to enzymatically remove the carbohydrate moiety. Food allergies to finned fish are also common, but the causative epitopes are parvalbumin (Protein M) found in the muscle of the fish and lipovitellin and vitellogenin, yolk protein components of the fish roe [29]. In contrast, the major proteins of interest in coagulation products and the proteins investigated in this study are thrombin and fibrinogen that were extracted from fresh blood collected from the fish.
There were initial concerns that exposure to the salmon proteins in the bandage could elicit an inhibitory antibody response because there have been reports that bovine coagulation products could induce adverse reactions [30–35]. In one study, cardiac surgery patients treated with fibrin glue containing bovine thrombin subsequently developed inhibitors of bovine thrombin and factor V activity [30]. Development of inhibitors to human factor V was also observed in several other cases even when human thrombin was used [31,32,36]. In several patients, this inhibitory response resulted in cases of clinical bleeding. It is suggested that the factor V inhibition arises due to an immune reaction to the contaminating presence of factor V that triggers an immune response and produces inhibitory antibodies [32].
Another possible mechanism for immune activation by coagulation factors has been recently postulated based on work by Skupsky et al. [37]. In this study, activation of thrombin by injection of exogenous Factor VIII to mice increased the immune response to Factor VIII and ovalbumin, a normally non-immunogenic molecule in this strain of mice. The signal generated by thrombin to activate the immune response is unknown and the thrombin may interact directly with antigen presenting cells to stimulate a more robust response or the thrombin may act by a more circuitous pathway that may involve platelet and macrophage activation.
However, these responses occurred when the hemostatic material was delivered as an infused reagent injected either intravenously or intraperitoneally. In a comprehensive study of the effects of a bovine thrombin/fibrinogen sealant by Bouvy et al. [38], dogs were pretreated by dermal exposure four times at nine day intervals before an intrathoracic graft consisting of a bovine-derived dressing was placed. Cellular and humoral immune responses were monitored for three weeks. A mild inflammatory reaction was observed with a low lymphocyte and immunoglobulin G (IgG) responses. There was no evidence of IgE or delayed hypersensitivity reaction after four applications of the dressings. This is similar to the responses that were observed in both this study and the previous shorter study with the salmon protein bandages.
4.2. Known response to salmon thrombin and fibrinogen in animal trials and anecdotal human exposure
The immune response of humans to salmon thrombin or fibrinogen is not well characterized. A screening in Estonia, where fish consumption is high, of patients with liver disease or systemic lupus showed that 8 out 120 patients had antibody reactivity to salmon thrombin without signs of coagulopathy [20]. In a 1991 study of nine men maintained on a diet enriched with salmon [39], platelet size was increased slightly over the 40 days but this was attributed to changes in the fatty acid content of the blood. Several animal studies have investigated the response to salmon thrombin and fibrinogen. Studies of surgical incisions in rats that were treated with salmon fibrin solutions showed no evidence of an acute immune response [21]. None of the six rats that were studied after treatment with salmon fibrin showed an inflammatory response or developed antibodies to the fibrin. When the protein was injected intramuscularly with Freund’s adjuvant, four of five rats developed antibodies against the fibrinogen. Early tests injecting hamsters, mice and rabbits have not produced adverse effects [20].
In the present study, the major responses to the placement of the bandages were the formation of granulomas at the site in the abdomen and the formation of anti-salmon fibrinogen antibodies. Granulomas form as focal points of inflammation characterized by aggregates of macrophages surrounded by lymphocytes and sometimes plasma cells. The macrophages will frequently develop epitheloid morphology and fuse to form giant multi-nucleated syncytial cells. The classic granuloma is induced by the presence of foreign material that is poorly phagocytosed by the macrophages. Examples that have been well-reported are suture reactions, reactions to cosmetic surgery fillers, especially silicone [40], and even some hemostatic sponges [41]. Immune granulomas canalso develop which will induce cell-mediated immune response. Eosinophilic material called asteroid bodies or Splendore–Hoeppli reaction can arise at the granuloma sites following the immune response. Although this reaction is often associated with fungus or parasitic infections, it can also be noted in non-infectious immune reactions [42]. The eosinophilia results from the deposition of eosinophilic basic protein from eosinophil granules and aggregates of antigen–antibody complexes. For these reasons, current surgical practice is to leave only the minimum amount of a topical hemostat behind at the completion of surgery. The bandages used in this study were composed of the salmon protein electrospun in a dextran matrix. In the arterial bleeding model, the dextran carrier dissolved within less than a minute in the blood flow, releasing the proteins. However, when placed in the abdomen, the bandage did not dissolve rapidly and appeared to remain behind for an extended time.
Preliminary studies that monitored immune responses for 28 days in 32 swine exposed to salmon thrombin/fibrinogen dressings showed that the salmon proteins can react with the swine immune system. Antibodies were formed that recognize salmon and human fibrinogen. In the previous study, it was also observed that the incidence of anti-salmon thrombin antibodies was rare and titers were very low. Froma clinical viewpoint, the generation of thrombin antibodies is considered a serious complication. Thrombin is a critical enzyme in the initiation of the clotting cascade and plays a principal role in the cleavage of fibrinogen to fibrin as well as many regulatory steps in the clotting process. Antibody inhibition or modulation of thrombin activity could have potentially critical results. In this study, we were unable to detect thrombin antibodies even after two exposures and 6 months of monitoring. In addition, none of the animals developed antibodies that recognized self proteins. Coagulation assays confirmed that all of the animals continued to display normal functions.
In conclusion, long term monitoring of swine subjected to two exposures to a salmon thrombin/fibrinogen bandage under two different modalities demonstrated that the pigs survived without adverse effects. The animals mounted an effective and expected immune response to the proteins, but this response did not interfere with normal functioning of their coagulation system and did not interfere with the ability of the bandage to act as an effective coagulant device or disrupt normal healing. This study reinforces our observation that salmon thrombin and fibrinogen may form the basis for a safe and effective hemostatic agent.
Acknowledgements
The authors are grateful to the staff of the Surgery Department, Division of Veterinary Medicine, Walter Reed Army Institute of Medicine for their expert care of the animals in all phases of the experiments.
This research project was supported by funding from the Office of Naval Research, Arlington, VA (Project No.: N0001406MP20010), The Uniformed Services University of the Health Sciences, Bethesda, MD (R070TF) and by the US Army, Military Research and Materiel Command, Ft. Detrick, MD.
Footnotes
Disclaimer
The opinions or assertions contained herein are the private ones of the authors and are not to be construed as official or reflecting the views of the Department of Defense or the Uniformed Services University of the Health Sciences.
References
- 1.Holcomb JB, Pusateri AE, Hess JR, Hetz SP, Harris RA, Tock BB, et al. Implications of new dry fibrin sealant technology for trauma surgery. Surg Clin North Am. 1997;77:943–952. doi: 10.1016/s0039-6109(05)70596-x. [DOI] [PubMed] [Google Scholar]
- 2.Larson MJ, Bowersox JC, Lim RC, Jr, Hess JR. Efficacy of a fibrin hemostatic bandage in controlling hemorrhage from experimental arterial injuries. Arch Surg. 1995;130:420–422. doi: 10.1001/archsurg.1995.01430040082018. [DOI] [PubMed] [Google Scholar]
- 3.Holcomb JB, McMullin NR, Pearse L, Caruso J, Wade CE, Oetjen-Gerdes L, et al. Causes of death in U.S. special operations forces in the global war on terrorism: 2001–2004. Ann Surg. 2007;245:986–991. doi: 10.1097/01.sla.0000259433.03754.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holcomb JB, Stansbury LG, Champion HR, Wade C, Bellamy RF. Understanding combat casualty care statistics. J Trauma. 2006;60:397–401. doi: 10.1097/01.ta.0000203581.75241.f1. [DOI] [PubMed] [Google Scholar]
- 5.Champion HR, Bellamy RF, Roberts CP, Leppaniemi A. A profile of combat injury. J Trauma. 2003;54:S13–S19. doi: 10.1097/01.TA.0000057151.02906.27. [DOI] [PubMed] [Google Scholar]
- 6.Beekley AC, Starnes BW, Sebesta JA. Lessons learned from modern military surgery. Surg Clin North Am. 2007;87:157–184. [vii]. doi: 10.1016/j.suc.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Pusateri AE, Modrow HE, Harris RA, Holcomb JB, Hess JR, Mosebar RH, et al. Advanced hemostatic dressing development program: animal model selection criteria and results of a study of nine hemostatic dressings in a model of severe large venous hemorrhage and hepatic injury in Swine. J Trauma. 2003;55:518–526. doi: 10.1097/01.TA.0000075336.92129.27. [DOI] [PubMed] [Google Scholar]
- 8.Erdogan D, vanGulik TM. Evolution of fibrinogen-coated collagen patch for use as a topical hemostatic agent. J BiomedMater Res B Appl Biomater. 2008;85:272–278. doi: 10.1002/jbm.b.30916. [DOI] [PubMed] [Google Scholar]
- 9.Celiento M, Scioti G, Pratali S, Bortolotti U. Repair of coronary artery perforation following angioplasty using TachoSil patches. Interact Cardiovasc Thorac Surg. 2010;10:328–330. doi: 10.1510/icvts.2009.225334. [DOI] [PubMed] [Google Scholar]
- 10.Grottke O, Braunschweig T, Daheim N, Coburn M, Grieb G, Rossaint R, et al. Effect of TachoSil in a coagulopathic pig model with blunt liver injuries. J Surg Res. 2010 doi: 10.1016/j.jss.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Rickenbacher A, Breitenstein S, Lesurtel M, Frilling A. Efficacy of TachoSil a fibrin-based haemostat in different fields of surgery–a systematic review. Expert Opin Biol Ther. 2009;9:897–907. doi: 10.1517/14712590903029172. [DOI] [PubMed] [Google Scholar]
- 12.Spotnitz WD. Active and mechanical hemostatic agents. Surgery. 2007;142:S34–S38. doi: 10.1016/j.surg.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Perkins JG, Cap AP, Weiss BM, Reid TJ, Bolan CD. Massive transfusion and nonsurgical hemostatic agents. Crit Care Med. 2008;36:S325–S339. doi: 10.1097/CCM.0b013e31817e2ec5. [DOI] [PubMed] [Google Scholar]
- 14.Ballard JL, Weaver FA, Singla NK, Chapman WC, Alexander WA. Safety and immunogenicity observations pooled from eight clinical trials of recombinant human thrombin. J Am Coll Surg. 2010;210:199–204. doi: 10.1016/j.jamcollsurg.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 15.Arnaud F, Teranishi K, Tomori T, Carr W, McCarron R. Comparison of 10 hemostatic dressings in a groin puncture model in swine. J Vasc Surg. 2009;50:632–639. 639 e1. doi: 10.1016/j.jvs.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Kheirabadi BS, Edens JW, Terrazas IB, Estep JS, Klemcke HG, Dubick MA, et al. Comparison of new hemostatic granules/powders with currently deployed hemostatic products in a lethal model of extremity arterial hemorrhage in swine. J Trauma. 2009;66:316–326. doi: 10.1097/TA.0b013e31819634a1. [discussion 327–8]. [DOI] [PubMed] [Google Scholar]
- 17.Bryant BJ, Klein HG. Pathogen inactivation: the definitive safeguard for the blood supply. Arch Pathol Lab Med. 2007;131:719–733. doi: 10.5858/2007-131-719-PITDSF. [DOI] [PubMed] [Google Scholar]
- 18.Aguzzi A, Heikenwalder M. Pathogenesis of prion diseases: current status and future outlook. Nat Rev Microbiol. 2006;4:765–775. doi: 10.1038/nrmicro1492. [DOI] [PubMed] [Google Scholar]
- 19.Ingrosso L, Novoa B, Valle AZ, Cardone F, Aranguren R, Sbriccoli M, et al. Scrapie infectivity is quickly cleared in tissues of orally-infected farmed fish. BMC Vet Res. 2006;2:21. doi: 10.1186/1746-6148-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michaud SE, Wang LZ, Korde N, Bucki R, Randhawa PK, Pastore JJ, et al. Purification of salmon thrombin and its potential as an alternative to mammalian thrombins in fibrin sealants. Thromb Res. 2002;107:245–254. doi: 10.1016/s0049-3848(02)00333-x. [DOI] [PubMed] [Google Scholar]
- 21.Luyendyk JP, Sullivan BP, Guo GL, Wang R. Tissue factor-deficiency and protease activated receptor-1-deficiency reduce inflammation elicited by diet-induced steatohepatitis in mice. Am J Pathol. 2010;176:177–186. doi: 10.2353/ajpath.2010.090672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothwell SW, Reid TJ, Dorsey J, Flournoy WS, Bodo M, Janmey PA, et al. A salmon thrombin-fibrin bandage controls arterial bleeding in a swine aortotomy model. J Trauma. 2005;59:143–149. doi: 10.1097/01.ta.0000171528.43746.53. [DOI] [PubMed] [Google Scholar]
- 23.Gross PL, Weitz JI. New antithrombotic drugs. Clin Pharmacol Ther. 2009;86:139–146. doi: 10.1038/clpt.2009.98. [DOI] [PubMed] [Google Scholar]
- 24.Rothwell SW, Sawyer E, Dorsey J, Flournoy WS, Settle T, Simpson D, et al. Wound healing and the immune response in swine treated with a hemostatic bandage composed of salmon thrombin and fibrinogen. J Mater Sci Mater Med. 2009;20:2155–2166. doi: 10.1007/s10856-009-3769-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 26.Kraal G. Cells in the marginal zone of the spleen. Int Rev Cytol. 1992;132:31–74. doi: 10.1016/s0074-7696(08)62453-5. [DOI] [PubMed] [Google Scholar]
- 27.Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. 2008;20:109–116. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oriol R, Ye Y, Koren E, Cooper DK. Carbohydrate antigens of pig tissues reacting with human natural antibodies as potential targets for hyperacute vascular rejection in pig-to-man organ xenotransplantation. Transplantation. 1993;56:1433–1442. doi: 10.1097/00007890-199312000-00031. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu Y, Nakamura A, Kishimura H, Hara A, Watanabe K, Saeki H. Major allergen and its IgE cross-reactivity among salmonid fish roe allergy. J Agric Food Chem. 2009;57:2314–2319. doi: 10.1021/jf8031759. [DOI] [PubMed] [Google Scholar]
- 30.Banninger H, Hardegger T, Tobler A, Barth A, Schupbach P, Reinhart W, et al. Fibrin glue in surgery: frequent development of inhibitors of bovine thrombin and human factor V. Br J Haematol. 1993;85:528–532. doi: 10.1111/j.1365-2141.1993.tb03343.x. [DOI] [PubMed] [Google Scholar]
- 31.Berruyer M, Amiral J, Ffrench P, Belleville J, Bastien O, Clerc J, et al. Immunization by bovine thrombin used with fibrin glue during cardiovascular operations. Development of thrombin and factor V inhibitors. J Thorac Cardiovasc Surg. 1993;105:892–897. [PubMed] [Google Scholar]
- 32.Israels SJ, Israels ED. Development of antibodies to bovine and human factor V in two children after exposure to topical bovine thrombin. Am J Pediatr Hematol Oncol. 1994;16:249–254. doi: 10.1097/00043426-199408000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Lawson JH, Lynn KA, Vanmatre RM, Domzalski T, Klemp KF, Ortel TL, et al. Antihuman factor V antibodies after use of relatively pure bovine thrombin. Ann Thorac Surg. 2005;79:1037–1038. doi: 10.1016/j.athoracsur.2003.09.110. [DOI] [PubMed] [Google Scholar]
- 34.Ofosu FA, Crean S, Reynolds MW. A safety review of topical bovine thrombin-induced generation of antibodies to bovine proteins. Clin Ther. 2009;31:679–691. doi: 10.1016/j.clinthera.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 35.Wai Y, Tsui V, Peng Z, Richardson R, Oreopoulos D, Tarlo SM. Anaphylaxis from topical bovine thrombin (thrombostat) during haemodialysis and evaluation of sensitization among a dialysis population. Clin Exp Allergy. 2003;33:1730–1734. doi: 10.1111/j.1365-2222.2003.01806.x. [DOI] [PubMed] [Google Scholar]
- 36.Caers J, Reekmans A, Jochmans K, Naegels S, Mana F, Urbain D, et al. Factor V inhibitor after injection of human thrombin (tissucol) into a bleeding peptic ulcer. Endoscopy. 2003;35:542–544. doi: 10.1055/s-2003-39678. [DOI] [PubMed] [Google Scholar]
- 37.Skupsky J, Zhang AH, Su Y, Scott DW. A role for thrombin in the initiation of the immune response to therapeutic factor VIII. Blood. 2009;114:4741–4748. doi: 10.1182/blood-2008-10-186452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouvy BM, Rosin E, Frishmeyer KJ, Dubielzig RR, Schultz RD. Evaluation of bovine fibrin sealant in the dog. J Invest Surg. 1993;6:241–250. doi: 10.3109/08941939309141615. [DOI] [PubMed] [Google Scholar]
- 39.Nelson GJ, Schmidt PC, Corash L. The effect of a salmon diet on blood clotting, platelet aggregation and fatty acids in normal adult men. Lipids. 1991;26:87–96. doi: 10.1007/BF02544000. [DOI] [PubMed] [Google Scholar]
- 40.Jham BC, Nikitakis NG, Scheper MA, Papadimitriou JC, Levy BA, Rivera H. Granulomatous foreign-body reaction involving oral and perioral tissues after injection of biomaterials: a series of 7 cases and review of the literature. J Oral Maxillofac Surg. 2009;67:280–285. doi: 10.1016/j.joms.2008.01.052. [DOI] [PubMed] [Google Scholar]
- 41.Tomizawa Y. Clinical benefits and risk analysis of topical hemostats: a review. J Artif Organs. 2005;8:137–142. doi: 10.1007/s10047-005-0296-x. [DOI] [PubMed] [Google Scholar]
- 42.Hussein MR. Mucocutaneous Splendore–Hoeppli phenomenon. J Cutan Pathol. 2008;35:979–988. doi: 10.1111/j.1600-0560.2008.01045.x. [DOI] [PubMed] [Google Scholar]