Abstract
Background
P63 is a gene located in chromosome 3q27–29, which has been implicated in regulation of stem cell commitment and promotion of squamous differentiation in various tissues. The aim of this study was to investigate whether there was a correlation between p63 expression, differential diagnosis of lung carcinoma, and prognosis.
Material/Methods
Immunohistochemical expression of p63 in 62 lung carcinomas was investigated and mRNA analysis using RT-PCR method was done in 6 selected cases.
Results
When cases were evaluated for p63 staining, 24 of 25 (96%) squamous cell carcinomas were strongly positive. Six of 20 adenocarcinomas (25%) and 1 (100%) large cell carcinoma (except neuroendocrine carcinoma) were mildly positive. p63 staining was statistically significant in favor of squamous cell carcinoma than other tumors (p<0.001). Forty percent of squamous cell carcinomas had squamous carcinoma in situ, whereas adenocarcinomas had none. There was a significant statistical difference between squamous cell carcinoma and adenocarcinoma (p=0.002). p63 was strongly positive in all of 12 squamous carcinoma in situ cases. In 6 cases where mRNA analysis was performed by RT-PCR method, DNp63 was strongly positive in 3 squamous cell carcinomas, mildly positive in 1 adenocarcinoma, and negative in 1 carcinoid tumor. TAp63 was strongly positive in non-tumoral lung tissue but negative in all tumors, except 1 squamous cell carcinoma.
Conclusions
Our data suggest that poorly differentiated squamous cell carcinoma had strong and widespread staining for immunohistochemical expression of p63. Therefore, p63 can be a useful marker in differentiating squamous cell carcinoma from poorly differentiated adenocarcinoma and squamous cell carcinoma from large cell neuroendocrine carcinoma.
Keywords: Carcinoma, Non-Small-Cell Lung – pathology, Diagnosis, Differential, Reverse Transcriptase Polymerase Chain Reaction – methods
Background
Non-small cell lung carcinomas are a heterogeneous group of tumors having poor prognosis. Eighty percent of lung cancer cases are at an inoperable advanced stage at the time of diagnosis [1,2]. A wide range of therapies, including targeted therapy, are being used for treatment. Generally, histological diagnosis must be done on bronchoscopic small biopsy specimens [3]. It frequently shows histological heterogeneity, varying in appearance and differentiation from one microscopic field to another and from one histological section to the next (2). Small specimens may not show differentiation when the tumor is excised. During morphologic evaluation, usually supported by immunohistochemical markers in routine work, 30–40% of tumors are accurately classified in small biopsy specimens, especially when the tumor is a poorly differentiated or is an undifferentiated carcinoma [4].
Defined for the first time in 1998, P63 is a gene located in chromosome 3q27–29, containing 15 exons and at least 6 protein isoforms [5,6]. P63 has 6 different isotypes that have been identified to date. It is suggested that the proteins coded from these isotypes have biological functions differing from one another. P63 isotypes are basically generated by 2 promoters (TA and DN) and 3 alternative splice regions (a, b, and c) that are located in the carboxy terminal region [7–10]. There is a transactivation region at the amino terminal in TAp63, which is one of the two most important isotypes, whereas there is no transactivation region at the amino terminal in DNp63, the other important isotype. In this way, the TAp63 isotype activates the targeted genes of p53 (p21waf1/cip1, mdm2 and bax) to stop the cell cycle and makes the cell turn to apoptosis. The DNp63 isoform, by contrast, acts “dominant-negative” to suppress this transcriptional activation and trigger cell proliferation [7,10–12].
Recently, p63 has been implicated in regulation of stem cell commitment and promotion of squamous differentiation in various tissues. Experimental studies have shown that in the process of embryonic development, p63 expression is needed during epidermal morphogenesis that involves formation of extremities and structures such as tooth, hair, mammary, and prostate glands, and sweat and lacrimal glands [13]. Its basic role is to form the squamous epithelial phenotype by suppressing mesenchymal cell characteristics [14]. Transformation of early stem cells into basal cells is significant for the development of the normal tracheobronchial pseudo-stratified epithelium and the squamous epithelium of the esophagus [15]. That is why it is especially expressed in areas where there is an epithelial-mesenchymal interaction. In animals with deficient p63, there are problems in structures that require a proper epithelial-mesenchymal interaction during their morphogenesis, and such animals have been shown to die a short time after birth [14].
In adults, p63 has an important role in securing stem cell population in squamous and other multi-layered epithelia. While bronchial reserve cells are intensely stained immunohistochemically in normal lung tissue, no staining is seen in ciliated cells, goblet cells, alveolar epithelial cells, or non-epithelial cells. Positive cells in terminal bronchioles have a dispersed distribution. Staining is also seen in myoepithelial cells in sub-mucosal bronchial glands. There is staining in squamous metaplasia and this staining is observed in many cell sequences [16,17]; this is an indication that p63-positive reserve cells can build the squamous epithelium in lungs when necessary [17]. In some lung cancers, p63 is expressed excessively. However, p53 positivity rates for subtypes may differ in the results of various studies, although there are some similarities among them [2,16–18].
In this project, we analyzed p63 expression in non-small cell carcinoma and preinvasive lesions (squamous carcinoma in situ and atypical adenomatous hyperplasia) of non-small cell carcinoma. Overall data was evaluated to search for any effect on the differential diagnosis of non-small cell lung carcinoma and clarification of histogenesis of lung tumors. In a group of chosen cases, the isotype-specific gene expressions of TAp63 and DNp63 at mRNA level were analyzed in lung tumor tissue. Results obtained may serve to further clarify lung tumor pathogenesis and to help differentiate diagnosis in these cases.
Material and Methods
A total of 62 archival, routinely processed, formalin-fixed and paraffin-embedded surgical pathology specimens from lobectomy and pneumonectomy specimens for primary lung carcinoma were acquired from the pathology archives of Pamukkale University Hospital, Denizli, Turkey. Information on standard identities of patients, preoperative stages of tumors, method of treatment, follow-up periods, and disease outcomes (data on disease-free survival, relapses, and cancer-related deaths) was obtained from the records. All cases of tissue samples were retrieved, reviewed, and classified according to the 2004World Health Organization classification of lung tumors [19] (Table 1).
Table 1.
Histopathological types of cases.
| Histopathological types | Number of patients (n) | % |
|---|---|---|
| Squamous cell carcinoma | 24 | 38.7 |
| Adenocarcinoma | 20 | 32.3 |
| Carcinoid tumor | 5 | 8.1 |
| Large cell neuroendocrine carcinoma | 3 | 4.8 |
| Sarcomatoid carcinoma | 3 | 4.8 |
| Atypical carcinoid tumor | 1 | 1.6 |
| Large cell carcinoma, not specified | 1 | 1.6 |
| Adenosquamous carcinoma | 1 | 1.6 |
| Mukoepidermoid carcinoma | 1 | 1.6 |
| Squamous cell carcinoma + large cell neuroendocrine carcinoma | 1 | 1.6 |
| Adenocarcinoma + carcinoid tumor | 1 | 1.6 |
| Adenocarcinoma + squamous cell carcinoma | 1 | 1.6 |
Immunohistochemistry
Expression of p63 in 62 lung carcinomas was investigated immunohistochemically. Sections expressing the most representative tumor were chosen for further immunohistochemical analysis. Five-micron sections of formalin-fixed, paraffin-embedded tissue sections were tested for the presence of immunohistochemically detectable antigens with anti-p63 antigen (Clone 4A4, BioGenex, USA). Tissue sections were processed with the standard streptavidin-biotin-immunoperoxidase method on an automatic Ventana-Nexes immunostainer (Ventana Medical Systems, Tucson, Arizona). Nuclear p63 staining was evaluated using a semi-quantitative and subjective grading system that considered both the intensity of staining and the proportion of tumor cells in a tissue section showing an unequivocal positive reaction. Staining percentage of nuclear staining area was graded as 0 (0–10 tumor cells positive), 1 (positive staining in 10–44% of the tumor cells), or 2 (positive staining in 45% to 100% of the tumor cells).
mRNA analysis of TAp63 and DNp63 using RT-PCR
Specific gene expressions of TAp63 and DNp63 isotypes were analyzed using RT-PCR in a group of 6 tumor and adjacent non-tumor lung tissues. Total RNA was isolated from paired fresh-frozen tumor and non-tumor lung tissues using Tri-Reagent (Sigma). Extracted RNA was precipitated with isopropanol, and then assessed for integrity of ribosomal RNA by agarose gel electrophoresis. Multiplex RT-PCR was performed using the OneStep RT-PCR kit (Cat # 210212, Qiagen) according to the manufacturer’s protocol. Appropriate cycles were chosen to ensure the termination of PCR amplification before reaching a stable stage in each reaction. Gene expression was presented as the yield of PCR products from target sequences relative to the yield of PCR products from the GAPDH housekeeping gene. Multiplex OneStep RT-PCR reaction parameters used for TAp63/GAPDH and DNp63/GAPDH were as follows: reverse transcription at 50°C for 30 min, initial activation of DNA polymerase enzyme at 95°C for 15 min, followed by 35 cycles of 94°C for 1 min, 62°C for 1 min, 72°C for 1 min, and a final extension of 72°C for 10 min. PCR products were subjected to electrophoresis on a 4% ethidium bromide (Et-Br)-stained MS agarose (Roche) gel and visualized under UV for the control of their specificity and integrity. PCR primers are listed in Table 2.
Table 2.
Primers used in one step RT-PCR.
| Isotype | Primers | Amplicon |
|---|---|---|
| TAp63 | 5′-GGTGCGACAAACAAGATTGA-3′ (forward) | 169bp |
| 5′-GCGCGTGGTCTGTGTTATAG-3′ (reverse) | ||
| DNp63 | 5′-GGAAAACAATGCCCAGACTC-3′ (forward) | 169bp |
| 5′-GCGCGTGGTCTGTGTTAAG-3′ (reverse) | ||
| GAPDH | 5′-CCCCACACACATGCACTTACC-3′ (forward) | 100bp |
| 5′-CCTAGTCCCAGGGCTTTGATT-3′ (reverse) |
Results
The mean disease-free survival time of the 62 patients who were included in the study was 15.8±10.2 months (minimum 1 month, maximum 38 months). The disease relapsed in 10.5% of the patients and 82.5% of the patients on follow-up were still alive at the end of the follow-up period.
According to the AJCC TNM staging system, the majority of the cases were at Stage IB (38.7%), Stage IA (17.7%), Stage IIB (17.7%), and Stage IIIA (22.6%), and only 3.2% of them were at Stage IIIB. The sizes of tumors ranged between 1.9 and 8 cm, with a mean size of 3.8±1.3 cm. Most of the cases had a tumor size of T2 (58.1%). The clinicopathologic findings of the cases are summarized in Table 3.
Table 3.
Clinicopathological factors.
| Clinicopathological factors | Number of patients (n) | % |
|---|---|---|
| Age | 4 | 6.5 |
| <40 | ||
| 40–65 | 41 | 66.1 |
| >65 | 17 | 27.4 |
| Sex | ||
| Male | 58 | 93.5 |
| Female | 4 | 6.5 |
| Localization | ||
| Right | 39 | 62.9 |
| Left | 23 | 37.1 |
| Metastasis of lymph node | ||
| Yes | 19 | 30.6 |
| No | 43 | 69.4 |
When cases were evaluated for p63 staining by immunohistochemistry, 24 of 25 (96%) squamous cell carcinomas were strongly positive (Figure 1). Six of 20 adenocarcinomas (25%) and 1 (100%) large cell carcinoma (except neuroendocrine carcinoma) were mildly positive. P63 staining was statistically significant in favor of squamous cell carcinoma as compared to other tumors (p<0.001).
Figure 1.
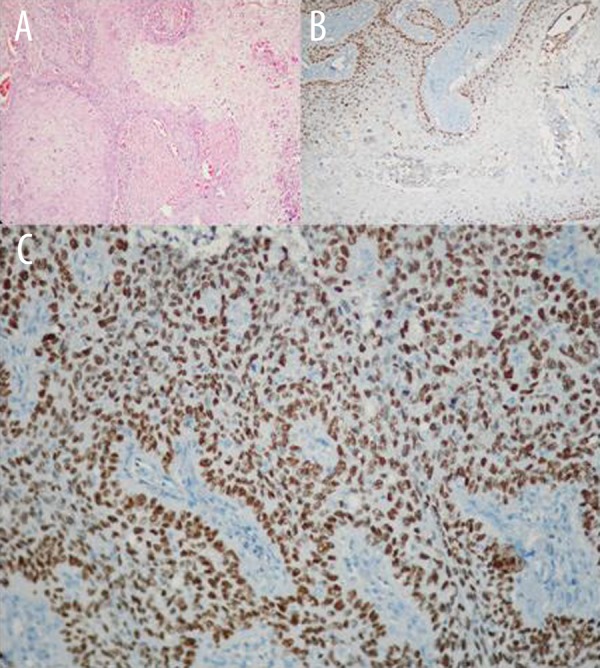
(A, B). Decreased expression pattern of p63 in well differentiated squamous cell carcinoma particularly at central of squamous island (H&E, p63 immunohistochemistry ×100). (C) Expression pattern of p63 in poorly differentiated squamous cell carcinoma (p63 immunohistochemistry ×200).
In cases with squamous cell carcinoma, we noticed that in well-differentiated areas, p63 staining was stronger on the peripheries of tumor islands, but the extent and intensity of staining decreased towards the center. In poorly differentiated areas, on the other hand, the intensity of staining in the center and on the peripheries were similar and staining was more intense and widespread than in well-differentiated areas (Figure 1). When 5 cases of adenocarcinoma that were stained with p63 were assessed in terms of different subtypes (acinar, papillary, solid, nonmucinous bronchoalveolar, and signet ring area), staining was found to be between 2–30%. In 10 cases of adenocarcinoma that had solid areas, there was mild staining in 5 of these solid areas (2% in 3 cases, 5% in 1 case, and 18% in 1 case) and there was no staining in the other 5.
Forty percent of squamous cell carcinomas had squamous carcinoma in situ (Figure 2), whereas adenocarcinomas had none. There was a significant statistical difference between squamous cell carcinoma and adenocarcinoma in p63 staining (p=0.002). P63 was strongly positive in all of 12 squamous carcinoma in situ cases, but in 83.3% of atypical adenomatous hyperplasia, p63 was negative.
Figure 2.
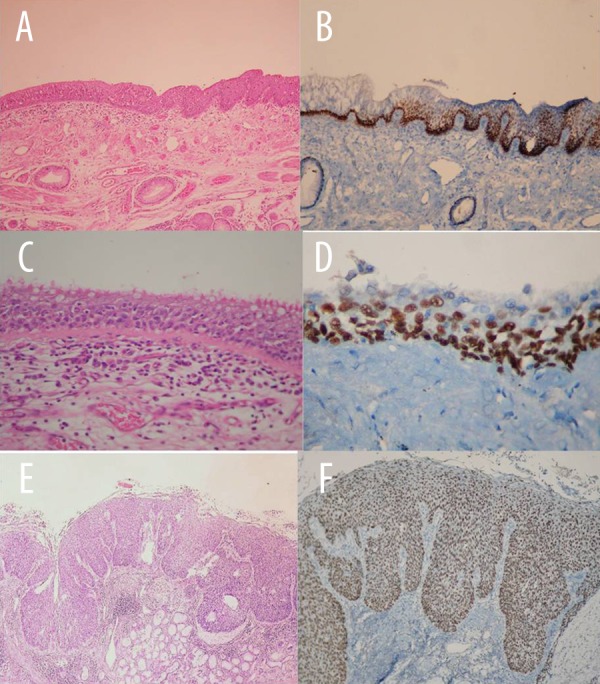
(A) Squamous metaplasia (H&E ×100). (B) Squamous metaplasia (p63 immunohistochemistry ×100). (C) Displasia (H&E ×100). (D) Displasia (p63 immunohistochemistry ×100). (E) Squamous carcinoma in situ (H&E X100). (F) Squamous carcinoma in situ (p63 immunohistochemistry ×100).
In 6 cases in which mRNA analysis was performed by the OneStep multiplex RT-PCR method, DNp63 was strongly positive in 3 (3/3) squamous cell carcinoma cases, mildly positive in 1 (1/1) adenocarcinoma case, and negative in 1 carcinoid tumor case (Figure 3A). TAp63 was strongly positive in non-tumoral lung tissue, but was negative in all tumors, except for 1 squamous cell carcinoma (Figure 3B).
Figure 3.
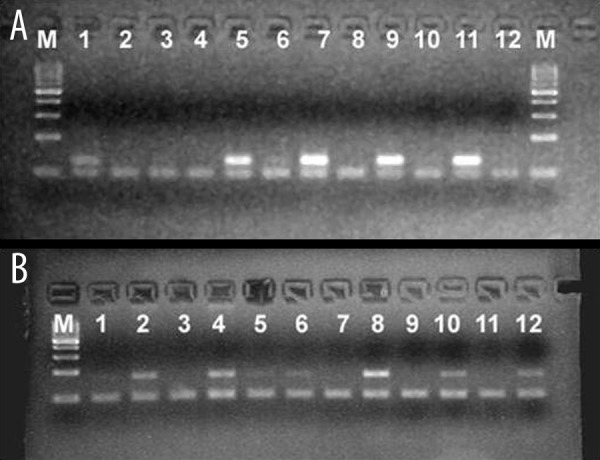
(A) DNp63 and GAPDH mRNA expression ((M: Marker, 1: AC-T, 2: AC-N, 3: Carcinoid-T, 4: Carcinoid-N 5: SCC-T, 6: SCC-N, 7:SCC-T, 8: SCC-NT, 9: MEC-T, 10: MEC-N, 11:SCC-T, 12:SCC-N, M:Marker) (AC: Adenocarcinoma, SCC: Squamous cell carcinoma, MEC: Mukoepidermoid Carcinoma, T: Tumor, N: Normal ). (B) TAp63 ve GAPDH mRNA expression (M: Marker, 1: AC-T, 2: AC-N, 3: Carcinoid-T, 4: Carcinoid-N 5: SCC-T, 6: SCC-N, 7: SCC-T, 8: SCC-NT, 9: MEC-T, 10: MEC-N, 11: SCC-T, 12: SCC-N) (AC: Adenocarcinoma, SCC: Squamous cell carcinoma, MEC: Mukoepidermoid Carcinoma, T: Tumor, N: Normal).
The mean follow-up period was 32±3 months for the 22 cases that showed no staining with p63, 16±3 months for those whose p63 index showed low staining, and 37±3 months for cases involving high staining. Survival rates were 81.82% in the group that had no staining with p63, 66.67% in the group whose p63 index showed low staining, and 88.46% in the group that showed high staining. This relationship was not found statistically significant (p>0.05).
In multi-variation analysis, the effect of age, largest tumor size, sex, TNM stage, tumor differentiation, distant metastasis, lymph node metastasis, angiolymphatic invasion, pleural invasion, neural invasion, stromal reaction type, presence of endobronchial lesion, presence of distant organ metastasis, presence of relapse, stage, tumor size, tumor localization, and p63 antibody reactivation on the rate of survival was explored and only presence of metastasis (p<0.001) was found to be an independent prognostic factor in determining the prognosis.
Discussion
Male sex and advanced age are related to poor prognosis in stage I and II lung cancer, regardless of the histological type. At stage III, the worsening of general condition and an elevated serum lactate dehydrogenase (LDH) level indicate poor prognosis [20]. No significant correlation was found in this study between survival and the clinico-pathologic parameters of age, sex, tumor localization, presence of endobronchial lesion, pleural invasion, angiolymphatic invasion, neural invasion, nature of stromal response to tumor, stage, or p63 expression. However, the relationship between tumor diameter and survival showed that the survival rate was 95.65% in patients whose tumor diameter was 3 cm and shorter, but the rate dropped to 73.53% in tumors having a diameter longer than 3 cm. As another parameter, the Ts that are involved in the TNM classification used for staging and that assess both tumor diameter and the position of the tumor in the lungs as well as its relationship with the other organs in the mediastinum, were evaluated separately and the survival rates that were 92.86% for T1 and 87.88% for T2 went down to 50% for T3 and T4, and this relationship was found to be statistically significant by Kaplan-Meier survival analysis. There are studies in the literature that stress the effect of TNM stage of a tumor on prognosis in support of the findings of the present study [21,22].
The studies in the literature that relate to p63 expression in squamous cell carcinoma are in complete agreement with each other. As in the present study, they also reported strong p63 positivity in squamous cell carcinoma. They have demonstrated that in well-differentiated tumors, staining is strong on the peripheries of the tumor mass and is weakened in the center, where differentiation increases and p63 positivity becomes stronger as tumor differentiation decreases. They have described that there is intense staining in the centers of solid tumor masses, similar to that on their peripheries [16,17,23,24]. Conforming with the literature, the present study also demonstrated that cases involving moderate and poorly differentiated squamous cell carcinoma had markedly strong p63 expression immunohistochemically. As described in the literature, in well-differentiated areas that were seen in the form of focal points in these tumors, the p63 staining in the centers of tumor masses was weaker (Figure 1).
Squamous carcinoma in situ is a lesion that develops in bronchial epithelia in the large airways; it can exist by itself or with invasive carcinoma and is a precursor to squamous cell carcinoma [25]. While squamous carcinoma in situ was found in 40% of the squamous cell carcinoma cases, it was not found in any of the adenocarcinoma cases. This finding may, in small biopsy materials, help interpret the biopsy in favor of squamous cell carcinoma by taking into consideration the presence of squamous carcinoma in situ accompanying the tumor. Atypical adenomatous hyperplasia is another lesion that is accepted as a precursor of peripheral pulmonary adenocarcinoma and is generally noticed incidentally and multifocally in materials resected due to adenocarcinoma [25,26]. In the present study, atypical adenomatous hyperplasia accompanied 20% of adenocarcinoma cases and 1% of squamous cell carcinoma cases. Since there is no significant statistical difference between these 2 groups in terms of atypical adenomatous hyperplasia, it is not possible for us to make a similar interpretation for atypical adenomatous hyperplasia.
There are just a few studies in the literature on p63 expression in preinvasive lesions. Massion et al investigated 43 preinvasive squamous lesions using the in situ hybridization (FISH) method, reporting increased p63 gene copy numbers in high-grade lesions involving severe dysplasia and carcinoma in situ and they found this amplification correlated to p63 immune staining [23]. In their study including 221 cases of stage I non-small cell carcinoma and 57 cases of stage I–IV neuroendocrine carcinoma, Pelosi et al examined p63 staining in hyperplastic, metaplastic, and dysplastic bronchial epithelia. They found increasing p63 positivity in basal cell hyperplasia, squamous metaplasia, and mild, moderate, and severe dysplasia (44.9±2.8%, 62.3±6.2%, 52.6±14.5%, 65.7±9.7%, and 76.3±4.1%, respectively) [16]. In both these studies, there was a distinct increase in p63 expression during the transition from preinvasive to invasive lesion. They reported in another study that they found full-layer p63 staining in an unstated number of areas with squamous carcinoma in situ, which accompanied 30 cases of squamous cell carcinoma [17]. In the present study, p63 was found highly positive in all (100%) of the 12 squamous carcinoma in situ lesions. An assessment of the 6 atypical adenomatous hyperplasia areas that were encountered in the study showed that there was mild staining in only 1 case (16.7%) and no staining in the other 5 cases (83.3%). This finding is similar to that of the study by Sheikh et al., who reported p63 positivity in 2 of the 5 atypical adenomatous hyperplasia areas [27].
In addition to the immunohistochemical method, Massion et al. carried out investigations using the FISH and RT-PCR methods. They obtained 88% p63 positivity in squamous cell carcinoma using the FISH method. Using the RT-PCR method, they found positivity with delta-N p63-alpha and delta-N p63-beta in all of the squamous cell carcinoma cases and they found slight positivity with TA p63 in some of the squamous cell carcinoma cases. They found negativity with both delta-N p63 and TA p63 in normal lung tissue [23]. Similar to Massion’s previous study, we also obtained negative results in our study in squamous cell carcinoma, with the exception of the positivity we obtained with RT-PCR in delta-N p63 (3/3) and the positivity we found in 1 case for TA p63. Unlike Pierre’s study, we obtained a positive result with TA p63 in normal lung tissue [23].
There are inconsistent results in the literature with respect to immunohistochemical expression of p63 in adenocarcinoma. While some studies found 12%, 11%, 15.8%, and 30% (6, 8, 76, 78, respectively) immunohistochemical p63 positivity in adenocarcinoma, p63 was negative in adenocarcinoma in some other studies [2,27]. In the study by Sheikh et al (one of the studies that reported positivity), less than 5% staining was observed in 3 of the 4/33 adenocarcinoma cases stained with p63 positive and, by contrast, more than 80% positivity was found in 1 case [27]. P63 positivity was 15.8% in the 15 adenocarcinoma cases included in the study of Pelosi et al. The proportion of positive cells in the cases accepted as positive has been reported to be 5.4±15.9% (16).
Massion et al reported 11% gene amplification using the FISH method and 18% protein expression using the immunohistochemical method in adenocarcinoma cases. They also found that a limited number of adenocarcinoma cases they worked on were negative with delta-N p63-alpha, delta-N p63-beta, and TA p63 splice variants (23). In the present study, slight delta-N p63 positivity and TA p63 negativity were found in the tumor of a single adenocarcinoma case using RT-PCR and delta-N p63 negativity and TA p63 positivity in normal lung parenchyma.
In their study involving 408 lung carcinoma cases, Au et al used in 2 different laboratories p63 antibodies of the same concentration and used different protocols. While the results in these 2 laboratories were similar for squamous cell carcinoma, they obtained different results in types outside this carcinoma. Staining was 30% in one laboratory and 8.6% in the other for adenocarcinoma [18]. They explained this difference, which occurred although they used p63 of the same concentration in the same series, by citing technical differences and differences in the other chemicals used.
In normal neuroendocrine cells, neuroepithelial bodies, and neuroendocrine tumorlets, p63 negativity shows that these cells developed from stem cells independent of p63 (p63–, SK+) or that they lost p63 expression at an early stage. Pelosi et al concluded that because p63 positivity was found in high-grade neuroendocrine carcinoma (45%) and especially in large-cell neuroendocrine carcinoma, at least some of these tumors developed from stem cells similar to those in other non-small cell lung carcinomas [6]. As p63 positivity was not found in the present study in any of the 3 large-cell neuroendocrine carcinoma cases, this suggests in a similar way that not all of even large-cell neuroendocrine carcinomas follow the same development path.
The p63 positivity, which was found to be 17.5% in 57 neuroendocrine carcinoma cases included in another study, was reported to be as follows for respective subtypes: 7/10 for large-cell neuroendocrine carcinoma, 2/10 for small-cell carcinoma, 1/28 for carcinoid tumors, and 0/9 for atypical carcinoid tumors [16]. In the study by Au et al in 2 separate laboratories using p63 of the same concentration on 408 cases, different results were obtained in carcinoma types other than squamous cell carcinoma. In 1 of the laboratories, positivity was 37% in large-cell carcinoma, 1.9% in carcinoid tumors, 30.8% in atypical carcinoid, 50% in large-cell neuroendocrine carcinoma, and 76.9% in small-cell carcinoma. These results show that p63 expression increases as differentiation decreases in neuroendocrine carcinoma. When the same cases were stained with p63 in the second laboratory, positivity was 23.6% in large-cell carcinoma, 1.7% in carcinoid tumors, 14.8% in atypical carcinoid, 0% in large-cell neuroendocrine carcinoma, and 30.8% in small-cell carcinoma [18]. They stressed that this difference, which occurred although they used p63 of the same concentration in the same series, was due to technical differences and differences in the other chemicals and that p63 positivity was not a reliable marker of p63 positivity in neuroendocrine carcinoma.
In addition to these studies reporting varying positivity in neuroendocrine carcinoma, there are also studies in which no staining was found. Zhang et al. reported that they found staining in none of the 28 small-cell neuroendocrine carcinoma cases [5]. Kargı et al. reported p63 positivity in the same number of small-cell neuroendocrine carcinoma cases [24]. There was no staining reported in the study by Wang et al. on 9 small-cell carcinoma cases and 5 carcinoid tumor cases [17].
From the 9 neuroendocrine carcinoma cases that were included in the present study, 5 were typical carcinoid tumors, 3 were large-cell neuroendocrine carcinomas, and 1 was an atypical carcinoid tumor. Staining with p63 was not found in any of these. In the carcinoid tumor case for which mRNA analysis was carried out for p63 using RT-PCR, a negative result was obtained with delta-N p63 and TA p63. However, similar to other tumor types, negativity was found with TA p63 and positivity with delta-N p63 in samples from normal lung tissues of this case. These results show that p63 negativity can be used to verify neuroendocrine carcinoma in cases where there is a differential diagnosis problem in deciding between poorly-differentiated squamous cell carcinoma and large-cell neuroendocrine carcinoma.
In a study in which 5 adenosquamous carcinomas were assessed, p63 positivity was found in all of the cases. Positivity in the squamous areas of these cases ranged between 5% and 90%, with more than 50% in most of them. There was positivity ranging between 0% and 20% in areas with adenocarcinoma [27]. Another study reported 100% p63 positivity in 5 adenosquamous carcinoma cases, but did not mention the distribution of such positivity between areas of adenocarcinoma and squamous areas [17]. In the single adenosquamous carcinoma case that appeared in the present study, there was moderate staining in squamous areas, but no staining was observed in areas of adenocarcinoma.
In their study investigating the effect of p63 on prognosis, Narahashi et al found 50% nuclear and 51% cytoplasmic p63 positivity in adenocarcinoma [27]. From the 20 adenocarcinoma cases included in the present study, 6 that were stained with p63 showed faint staining (10–40%), similar to results in the literature. An examination of solid areas, which are most frequently confused with poorly differentiated squamous cell carcinoma, revealed that there was p63 positivity in 5 of the 10 solid areas. In these 5 cases that had solid areas stained with p63 positive, there was slight staining in solid areas (2% in 3 cases, 5% in 1 case, and 18% in 1 case).
Conclusions
Our data suggest that poorly differentiated squamous cell carcinoma has strong and widespread immunohistochemical staining of p63. Therefore, p63 should be a useful marker in distinguishing poorly differentiated squamous cell carcinoma from poorly differentiated adenocarcinoma and squamous cell carcinoma from large cell neuroendocrine carcinoma. Our RT-PCR results also reveal that evaluating mRNA of p63 can be helpful alongside immunohistochemical staining when diagnosis is difficult in poorly differentiated squamous cell carcinoma, adenocarcinoma, and large-cell neuroendocrine carcinoma. However, it should be borne in mind that because p63 expression is also seen in squamous cell carcinoma of the head-neck, squamous cell carcinoma of the esophagus, transitional cell carcinoma of the urinary bladder, and in gastric carcinoma, p63 is not useful in distinguishing the metastasis of these tumors to the lungs from primary lung carcinoma.
Footnotes
Source of support: Departmental sources
References
- 1.Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. J Clin Oncol. 2013;31:992–1001. doi: 10.1200/JCO.2012.46.9270. [DOI] [PubMed] [Google Scholar]
- 2.Camilo R, Capelozzi VL, Siqueira SA, Del Carlo Bernardi F. Expression of p63, keratin 5/6, keratin 7, and surfactant-A in non-small cell lung carcinomas. Hum Pathol. 2006;37:542–46. doi: 10.1016/j.humpath.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Terry J, Leung S, Laskin J, et al. Optimal immunohistochemical markers for distinguishing lung adenocarcinomas from squamous cell carcinomas in small tumor samples. Am J Surg Pathol. 2010;34:1805–11. doi: 10.1097/PAS.0b013e3181f7dae3. [DOI] [PubMed] [Google Scholar]
- 4.Warburton D, Schwarz M, Tefft D, et al. The molecular basis of lung morphogenesis. Mech Dev. 2000;15:55–81. doi: 10.1016/s0925-4773(99)00325-1. [DOI] [PubMed] [Google Scholar]
- 5.Zhang H, Liu J, Cagle PT, et al. Distinction of pulmonary small cell carcinoma from poorly differentiated squamous cell carcinoma: an immunohistochemical approach. Mod Pathol. 2005;18(1):111–18. doi: 10.1038/modpathol.3800251. [DOI] [PubMed] [Google Scholar]
- 6.Pankiewicz W, Minarowski L, Niklińska W, et al. Immunohistochemical markers of cancerogenesis in the lung. Folia Histochem Cytobiol. 2007;45(2):65–74. [PubMed] [Google Scholar]
- 7.Osada M, Ohba M, Kawahara C, et al. Cloning and functional analysis of human p51, which structurally and functionally resembles p53. Nat Med. 1998;4(7):839–43. doi: 10.1038/nm0798-839. [DOI] [PubMed] [Google Scholar]
- 8.Senoo M, Seki N, Ohira M, et al. A second p53-related protein, p73L, with high homology to p73. Biochem Biophys Res Commun. 1998;248(3):603–7. doi: 10.1006/bbrc.1998.9013. [DOI] [PubMed] [Google Scholar]
- 9.Trink B, Okami K, Wu L, et al. A new human p53 homologue. Nat Med. 1998;4(7):747–48. doi: 10.1038/nm0798-747. [DOI] [PubMed] [Google Scholar]
- 10.Yang A, Kaghad M, Wang Y, et al. p63, a p53 Homolog at 3q27–29, Encodes Multiple Products with Transactivating, Death-Inducing, and Dominant-Negative Activities. Mol Cell. 1998;2(3):305–16. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 11.Signoretti S, Waltregny D, Dilks J, et al. p63 Is a Prostate Basal Cell Marker and Is Required for Prostate Development. Am J Pathol. 2000;157(6):1769–75. doi: 10.1016/S0002-9440(10)64814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dohn M, Zhang S, Chen X. p63a and DNp63a can induce cell cycle arrest and apoptosis and differentially regulate p53 target genes. Oncogene. 2001;20(25):3193–205. doi: 10.1038/sj.onc.1204427. [DOI] [PubMed] [Google Scholar]
- 13.Moll UM, Slade N. p63 and p73 Roles in development and tumor formation. Mol Cancer Res. 2004;2(7):371–86. [PubMed] [Google Scholar]
- 14.Barbieri CE, Pietenpol JA. p63 and epithelial biology. Exp Cell Res. 2006;312(6):695–706. doi: 10.1016/j.yexcr.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 15.Daniely Y, Liao G, Dixon D, et al. Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am J Physiol Cell Physiol. 2004;287(1):C171–81. doi: 10.1152/ajpcell.00226.2003. [DOI] [PubMed] [Google Scholar]
- 16.Pelosi G, Pasini F, Olsen Stenholm C, et al. p63 immunoreactivity in lung cancer: yet another player in the development of squamous cell carcinomas? J Pathol. 2002;198(1):100–9. doi: 10.1002/path.1166. [DOI] [PubMed] [Google Scholar]
- 17.Wang BY, Gıl J, Kaufman D, et al. p63 in pulmonary epithelium, pulmonary squamous neoplasms and other pulmonary tumors. Hum Pathol. 2002;33(9):921–26. doi: 10.1053/hupa.2002.126878. [DOI] [PubMed] [Google Scholar]
- 18.Au NH, Gown AM, Cheang M, et al. P63 expression in lung carcinoma: a tissue microarray study of 408 cases. Appl Immunohistochem Mol Morphol. 2004;12(3):240–47. doi: 10.1097/00129039-200409000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Travis WD, Brambilla E, Hermelink HKM, Haris CC. Pathology and Genetics of Tumors of the Lung, Plevra, Thymus and Heart. Lyon: IARC Press; 2004. pp. 26–76. [Google Scholar]
- 20.Friedel G, Steger V, Kyriss T, et al. Prognosis in N2 NSCLC. Lung Cancer. 2004;45(Suppl 2):S45–53. doi: 10.1016/j.lungcan.2004.07.993. [DOI] [PubMed] [Google Scholar]
- 21.Moro D, Nagy-Mignotte H, Bolla M, et al. Evaluation of survival and prognostic factors of 2,000 broncho-pulmonary cancers registered during 10 years in a multidisciplinary oncology department. Bull Cancer. 1997;84(2):155–61. [PubMed] [Google Scholar]
- 22.Zöchbauer S, Krajnik G, Huber H. Bronchial cancer – development, diagnosis, therapy, prognosis. Wien Klin Wochenschr. 1994;106(14):431–47. [PubMed] [Google Scholar]
- 23.Massion PP, Taflan PM, Jamshedur Rahman SM, et al. Significance of p63 Amplification and Overexpression in Lung Cancer Development and Prognosis. Cancer Res. 2003;(63):7113–21. [PubMed] [Google Scholar]
- 24.Kargi A, Gurel D, Tuna B. The diagnostic value of TTF-1, CK 5/6, and p63 immunostaining in classification of lung carcinomas. Appl Immunohistochem Mol Morphol. 2007;15(4):415–20. doi: 10.1097/PAI.0b013e31802fab75. [DOI] [PubMed] [Google Scholar]
- 25.Kerr KM. Pulmonary preinvasive neoplasia. J Clin Pathol. 2001;54(4):257–71. doi: 10.1136/jcp.54.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Travis WD, Brambilla E, Noguchi M, et al. Diagnosis of lung adenocarcinoma in resected specimens: implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch Pathol Lab Med. 2013;137(5):685–705. doi: 10.5858/arpa.2012-0264-RA. [DOI] [PubMed] [Google Scholar]
- 27.Sheikh HA, Fuhrer K, Cieply K, Yousem S. p63 expression in assessment of bronchioloalveoler proliferations of the lung. Mod Pathol. 2004;17(9):1134–40. doi: 10.1038/modpathol.3800163. [DOI] [PubMed] [Google Scholar]
- 28.Narahashi T, Niki T, Wang T, et al. Cytoplasmic localization of p63 is associated with poor patient survival in lung adenocarcinoma. Histopathology. 2006;49(4):349–57. doi: 10.1111/j.1365-2559.2006.02507.x. [DOI] [PubMed] [Google Scholar]


