Abstract
This study examined the mechanisms by which H2S modulates coronary microvascular resistance and myocardial perfusion at rest and in response to cardiac ischemia. Experiments were conducted in isolated coronary arteries and in open-chest anesthetized dogs. We found that the H2S substrate L-cysteine (1-10 mM) did not alter coronary tone of isolated arteries in vitro or coronary blood flow in vivo. In contrast, intracoronary (ic) H2S (0.1-3 mM) increased coronary flow from 0.49 ± 0.08 to 2.65 ± 0.13 ml/min/g (P□0.001). This increase in flow was unaffected by inhibition of Kv channels with 4-aminopyridine (P=0.127) but was attenuated (0.23 ± 0.02 to 1.13 ± 0.13 ml/min/g) by the KATP channel antagonist glibenclamide (P□0.001). Inhibition of NO synthesis (L-NAME) did not attenuate coronary responses to H2S. Immunohistochemistry revealed expression of cystathionine gamma-lyase (CSE), an endogenous H2S enzyme, in myocardium. Inhibition of CSE with β-cyano-L-alanine (10 µM) had no effect on baseline coronary flow or responses to a 15 sec coronary occlusion (P=0.82). These findings demonstrate that exogenous H2S induces potent, endothelial-independent dilation of the coronary microcirculation predominantly through the activation of KATP channels, however, our data do not support a functional role for endogenous H2S in the regulation of coronary microvascular resistance.
Keywords: coronary circulation, reactive hyperemia, K channels
Introduction
The endogenous gasotransmitter hydrogen sulfide (H2S) is known to exert a variety of effects on the cardiovascular system (14, 37, 41). In particular, H2S has been shown to be a vasodilator in multiple vascular beds (10, 17, 22, 39, 44, 45) and to influence the physiologic regulation of vascular tone (15, 40) and blood pressure (44, 46). Other studies have also demonstrated that H2S acts as a negative inotrope (14, 37, 41). Thus, H2S could protect the heart from ischemic injury by mediating the balance between myocardial oxygen delivery and metabolism. The potential cardioprotective actions of H2S are supported by data indicating that exogenous administration of H2S donors protects against a loss of contractile function and diminishes myocardial infarct size/necrosis in animal models of ischemia reperfusion injury (6, 19, 32, 47). Although these protective effects are associated with improvements in coronary endothelial dependent and independent microvascular reactivity (32), the direct effects of H2S on the coronary circulation have not been specifically examined.
This investigation was designed to elucidate the mechanisms by which H2S influences myocardial perfusion and to define the role of H2S in the regulation of coronary microvascular resistance at rest and in response to a brief episode of cardiac ischemia. Experiments tested the hypothesis that H2S induces dose-dependent coronary vasodilation via endothelial-dependent production of nitric oxide (NO) (1, 11), and that endogenous production of H2S contributes to the control of coronary blood flow in normal and/or ischemic hearts. Additional studies were also conducted to examine whether H2S elicits increases in coronary blood flow through activation of ATP-sensitive K+ (KATP) channels or voltage-dependent K+ (Kv) channels, both of which have been shown to modulate vascular responses to H2S in non-coronary vascular beds (28, 36, 45, 49) and in response to myocardial ischemia (5, 7, 12). Findings from this investigation provide novel insight in to the mechanisms and functional significance of H2S in the regulation of coronary blood flow in vivo.
Methods
This investigation was approved by the IUPUI Institutional Animal Care and Use Committee in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85–23, Revised 1996). All animals studied were lean mongrel dogs weighing between 20 and 30 kg. Following completion of experimental protocols, hearts were fibrillated and excised as recommended by the American Veterinary Medical Association Guide on Euthanasia (June 2007).
Immunohistochemistry
Immunohistochemical (IHC) analyses were performed in conjunction with Indiana University Health Pathology Laboratory (Indianapolis, IN). Briefly, liver and cardiac tissues were harvested immediately post mortem, rinsed in saline and transferred to 10% formalin. Formalin fixed tissues were then exposed to primary IgG antibodies against cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) at manufacturer recommended concentrations (Sigma Aldrich, St. Louis, Missouri). Slides were imaged at 10× magnification on a Nikon Eclipse 80i microscope and images captured with a Nikon DS-Fi1 and associated Nikon Elements software.
Isometric tension studies
Canine hearts were excised upon sacrifice and the aorta cannulated to perfuse the coronary tree with 4°C, Ca2+-free Krebs solution (131.5 mM NaCl, 5 mM KCl, 1.2 mM NaH2PO4, 1.2 mM MgCl2, 25 mM NaHCO3, 10 mM glucose) in order to rinse the excised heart of blood and blood proteins. After perfusion, coronary arteries were grossly dissected from the heart, and further isolated from surrounding myocardium and adventitia using a dissecting microscope. Following adventitial removal, arteries were cut into 3 mm rings and mounted in water-jacketed organ baths filled with a Ca2+-containing Krebs solution (131.5 mM NaCl, 5 mM KCl, 1.2 mM NaH2PO4, 1.2 mM MgCl2, 25 mM NaHCO3, 10 mM glucose, 4 mM CaCl2) at 37°C. Optimal length (passive tension) was assessed by contractions of isolated arteries to 60 mM KCl. Passive tension was increased in gram increments until there was <10% change in active tension development to 60 mM KCl (typical optimal passive tension equaled ~4 g). Once optimal passive tension was obtained, arteries were then pre-constricted with 1 µM U46619 and stimulated with either NaHS (1-10 mM) or the H2S substrate L-cysteine (1-10 mM). Changes in vascular tone were measured as a percent change from maximal tension developed in response to 1 µM U46619.
Surgical preparation
Dogs were initially sedated with morphine (3 mg/kg, subcutaneously) and anesthetized with α-chloralose (100 mg/kg, intravenously). The animals were then intubated and mechanically ventilated (Harvard respirator) with oxygen-supplemented room air. A catheter was placed into the thoracic aorta via the right femoral artery to measure aortic blood pressure and heart rate. The left femoral artery was catheterized to supply blood to an extracorporeal perfusion system used to perfuse the left anterior descending (LAD) artery at a controlled pressure (100 mmHg). A catheter was also inserted into the right femoral vein for injection of supplemental anesthetic, heparin and sodium bicarbonate. Arterial blood gases were analyzed periodically throughout the experimental protocol and adjustments were made as needed to maintain blood gas parameters within normal physiological limits. A left lateral thoracotomy was performed to expose the heart, and the LAD was isolated distal to its first major diagonal branch. Following heparin administration (500 U/kg, intravenously), the LAD was cannulated with a stainless steel cannula connected to an extracorporeal perfusion system. Coronary perfusion pressure (CPP) was regulated by a servo-controlled roller pump, held constant at 100 mmHg. Coronary blood flow was continuously measured by an inline Transonic Systems flow transducer (Ithaca, NY, USA). Data were continuously recorded on IOX data acquisition software from Emka Technologies (Falls Church, VA, USA).
Experimental Protocol
Following coronary cannulation, hemodynamic parameters were allowed to stabilize for 30 min. Basal coronary blood flow and hematocrit were then determined, and based on these parameters, an aqueous solution of NaHS was infused into the LAD perfusion line at controlled rates in order to achieve coronary plasma NaHS concentrations of 100µM, 300 µM, 1 mM, and 3mM. Animals were also subjected to an L-cysteine dose response curve (100 µM, 300 µM, 1 mM and 3 mM). Coronary flow responses as well as heart rate and blood pressure were monitored throughout the course of the dose response curves. Following a 15 min washout period, animals were then subjected to an identical NaHS dose response curve in the presence of the NO synthase inhibitor L-NG-Nitroarginine methyl ester (L-NAME, ~35 µg/ml, ic), the general Kv channel blocker 4-aminopyridine (4AP, 0.3mM, ic) or the KATP channel inhibitor glibenclamide (3mg/kg, iv). In a subset of animals (n = 3), coronary reactive hyperemic responses were measured following a 15 second occlusion of the LAD in the absence and presence of the CSE enzyme inhibitor β-cyano L-alanine (BCA, 10µM, ic). Hyperemic responses were measured until coronary flow reached baseline values. All drugs (Sigma Aldrich, St Louis, MO, USA) with the exception of glibenclamide (dissolved in equal parts of ethanol, propylene glycol, 1N NaOH) were dissolved in saline and infused in to the coronary perfusion line. Data on the systemic hemodynamic effects of each of these drugs are provided in Table 1.
Table 1.
Effects of inhibition of selected signaling pathways on baseline hemodynamic variables in anesthetized, open-chest dogs.
| Heart Rate (beats/min) |
Systolic Pressure Coronary Blood Flow (mmHg) (ml/min) |
Diastolic Pressure (mmHg) |
Mean Pressure (mmHg) |
|
|---|---|---|---|---|
| Baseline | 114 ± 6 | 74 ± 15 | 93 ± 8 | 99 |
| ± 26 | 0.47 ± 0.09 | |||
| L-NAME | 114 ± 6 | 79 ± 10 | 96 ± 7 | 95 |
| ± 16 | 0.50 ± 0.11 | |||
| Baseline | 112 ± 7 | 81 ± 15 | 96 ± 8 | 98 |
| ± 12 | 0.55 ± 0.15 | |||
| 4AP | 107 ± 14 | 76 ± 13 | 90 ± 14 | 92 ± 10 |
| 0.34 ± 0.07 | ||||
| Baseline | 107 ± 7 | 74 ± 8 | 89 ± 8 | 86 |
| ± 12 | 0.40 ± 0.04 | |||
| Glibenclamide | 122 ± 4* | 90 ± 6* | 103 ± 5* | 78 ± 10 |
| 0.25 ± 0.03 | ||||
| Baseline | 132 ± 15 | 90 ± 14 | 106 ± 14 | 79 |
| ± 24 | 0.27 ± 0.04 | |||
| BCA | 125 ± 17 | 83 ± 15 | 100 ± 16 | 90 ± 31 |
| 0.31 ± 0.04 | ||||
Values are mean ± SE.
indicates P < 0.05 vs. respective baseline. (L-NAME n = 3, 4AP n = 3, Glibenclamide n =5 , BCA n = 3).
Statistical analyses
Data are presented as mean ± SE. Statistical comparisons were made by a one-way or two-way repeated measures analysis of variance (ANOVA) as appropriate (Sigma Plot 11.0 Software). If statistical differences (P < 0.05) in these analyses were noted, a Student-Newman-Keuls multiple comparison test was performed. Reactive hyperemic volumes were calculated as area under the curve using Prism software (GraphPad Software).
Results
Tissue Immunohistochemistry
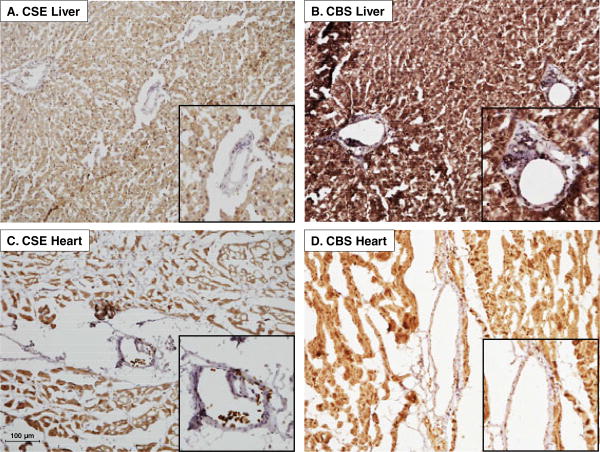
Consistent with previous findings (2, 16), liver tissue sections stained positive for both of the H2S producing enzymes cystathionine γ-lyase (CSE) (Figure 1A) and cystathionine β-synthase (CBS) (Figure 1B). However, expression of these enzymes within liver vasculature was relatively modest (see inset). Left ventricular myocardial tissue also stained positive for CSE (Figure 1C) and CBS (Figure 1D). However, CSE and CBS were not prominently expressed in the coronary vasculature (see inset).
Figure 1.
Representative immunohistochemistry showing expression of the H2S producing enzyme cystathionine γ-lyase (CSE) CSE in liver (A) and cardiac (C) tissue samples. Positive staining for the H2S producing enzyme cystathionine β-synthase (CBS) in liver (B) and heart (D). Insets: Magnification of microvessels show modest staining for both CSE and CBS in liver and cardiac tissue samples.
Isometric Tension Studies
Isometric tension recordings were performed on isolated coronary artery rings pre-constricted with the thromboxane A2 mimetic U46619 (1 µM). In these pre-contracted rings, administration of the H2S substrate L-cysteine (1-10 mM; n = 3) tended to increase isometric tension ~15% (Figure 2A) while NaHS (1-10 mM; n = 3) tended to diminish active tension development ~5% (Figure 2B). However, neither L-cysteine (P = 0.27) nor NaHS (P = 0.44) significantly altered coronary artery tension relative to U46619 treatment alone.
Figure 2.
Representative isometric tension recordings show modest effects of L-cysteine (n=3) (A) and NaHS (n=3) (B) on tension of isolated coronary artery rings pre-constricted with U46619. In-vivo intracoronary infusion of L-cysteine had no effect on basal coronary blood flow (C) while intracoronary infusion of NaHS significantly augmented coronary flow at plasma concentrations >300 µM (D).
Effects of H2S on Coronary Blood Flow In Vivo
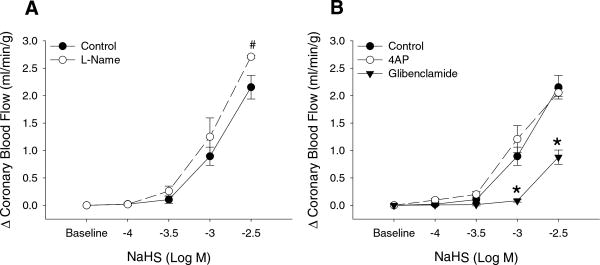
Intracoronary administration of L-cysteine (0.1-3.0 mM) had no effect on baseline coronary blood flow (Figure 2C). In contrast, infusion of NaHS (n = 5) dose-dependently increased coronary blood flow from 0.49 ± 0.09 ml/min/g at baseline to 2.65 ± 0.15 ml/min/g at the highest (3 mM) concentration of NaHS (Figure 2D; P < 0.001). This vasodilator response was not significantly diminished by inhibition of NO synthase with L-NAME (Figure 3A; n = 3) or by blockade of Kv channels with 4AP (Figure 3B; n = 3). However, administration of the KATP channel antagonist glibenclamide significantly impaired coronary vasodilation to 3mM H2S by ~ 70% (P < 0.001) (Figure 3B; n = 5).
Figure 3.
Inhibition of NO synthase with L-Name produced a modest increase the coronary blood flow response to intracoronary NaHS (n=3) (A). Coronary vasodilation to NaHS was unaffected by the inhibition of voltage-dependent K+ channels with 4-AP (n=3) while blockade of KATP channels with glibenclamide (n=5) markedly reduced NaHS-induced dilation in the coronary circulation. # P < 0.05; * P < 0.001.
Inhibition of H2S Producing Enzyme CSE
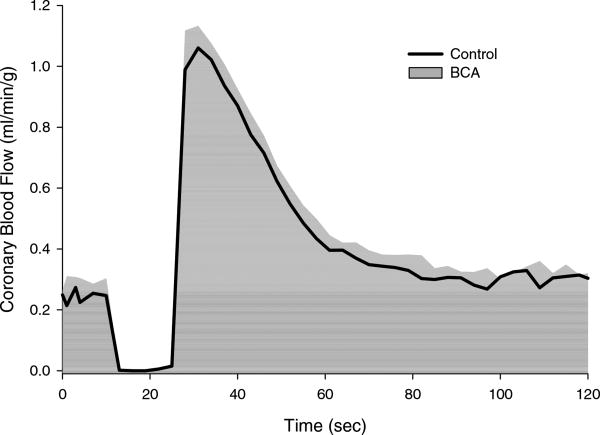
Administration of the CSE enzyme inhibitor BCA (10 µM; n = 3) had no effect on baseline hemodynamic parameters (Table 1). The effect of CSE inhibition on the coronary blood flow response to a 15 sec coronary artery occlusion (i.e. coronary reactive hyperemia) is shown in Figure 4. BCA did not significantly affect the reactive hyperemic response as evidenced by no alterations in the peak vasodilatory response, flow volume of repayment or in the repayment of the coronary flow debt following the inhibition of CSE (P = 0.82) (Table 2).
Figure 4.
Inhibition of the H2S producing enzyme cystathionine γ-lyase (CSE) with β-cyano-L-alanine (BCA) (n=3) did not significantly alter the coronary blood flow response to a 15 sec coronary artery occlusion.
Table 2.
Effect of CSE inhibition on the coronary blood flow response to a 15 sec coronary artery occlusion.
| Control | BCA | |
|---|---|---|
| Peak Flow (ml/min/g) | 1.33 ± 0.13 | 1.36 ± 0.11 |
| Debt Area (ml/g) | 0.09 ± 0.01 | 0.10 ± 0.02 |
| Repayment Area (ml/g) | 18.1 ± 1.8 | 18.2 ± 1.6 |
| Repayment/debt Ratio (%) | 359 ± 35 | 323 ± 48 |
Values are mean ± SE. (Control n = 3, BCA n = 3).
Discussion
This study was designed to delineate the mechanisms by which H2S modulates coronary microvascular resistance and myocardial perfusion at rest and in response to transient cardiac ischemia. The major novel findings of the investigation include: 1) prominent expression of the H2S producing enzymes CBS and CSE in canine myocardium; 2) infusion of the key H2S substrate L-cysteine failed to significantly alter coronary vascular tone of isolated conduit arteries in vitro or microvessels in vivo; 3) intracoronary administration of H2S dose-dependently increases coronary blood flow (~ 5 fold) via activation of KATP channels; 4) H2S mediated coronary vasodilation occurs independent of endothelial NO production or Kv channel activation and is largely absent in conduit coronary arteries; 5) inhibition of endogenous CSE has no effect on the regulation of coronary blood flow at rest or in response to a brief coronary artery occlusion. Taken together, these findings indicate that exogenous H2S induces potent dilation of the canine coronary microcirculation predominantly through a KATP channel dependent (NO independent) mechanism. However, our data support no functional role for endogenous H2S in the regulation of baseline coronary resistance or ischemic coronary vasodilation.
Functional expression of H2S producing enzymes in canine hearts
Our IHC studies demonstrate the prominent expression the H2S producing enzymes (CBS and CSE) in canine myocardium (Figure 1). This finding is consistent with other studies which have documented the presence of CSE in rat and mouse liver and cardiac tissue (14, 16, 34, 46), and expression of CBS in rodent hearts (9, 35). IHC also revealed relatively low levels of CSE and CBS expression in the liver and cardiac microcirculation (Figure 1C & 1D); which is consistent with little/no effect of the H2S substrate L-cysteine on tone of isolated coronary arteries (Figure 2A) or on coronary blood flow in vivo (Figure 2C). This lack of a coronary response to L-cysteine is in contrast with the recent findings of Leffler et al. who documented dose-dependent dilation to L-cysteine in cerebral pial arterioles in newborn swine (22); i.e. differences in the functional relevance of endogenous H2S production likely exist between the cerebral and coronary circulation.
Although H2S is known to be a vasodilator in a variety of vascular beds (10, 17, 31, 36, 49), primarily in the cerebral circulation (22, 23, 25, 26, 39), no study has directly examined coronary vasodilation in response to NaHS administration in vivo. We found that NaHS significantly increased coronary blood flow in a concentration dependent manner (Figure 2D). Interestingly, our findings indicate marked regional differences in coronary conduit vs. microvascular responsiveness to H2S as isolated coronary arteries responded only ~5% to 3 mM NaHS while coronary flow increased ~500% in response to the same concentration of NaHS.
Mechanism of H2S-mediated coronary vasodilation
Earlier studies in peripheral arteries suggest that H2S mediated dilation occurs via an endothelial-dependent mechanism (10, 48) that converges on the activation of smooth muscle KATP (22, 25, 28, 29, 49, 50) and/or KCa channels (17, 26). However, the pathways responsible for the effects of H2S in the coronary circulation have not been delineated. In the current study, we found that intracoronary administration H2S (plasma concentration 3 mM) induced an ~5-fold increase in coronary blood flow (Figure 2D). This increase in coronary flow is not related to endothelial-production of NO as administration of the NOS inhibitor L-NAME, at a dose we previously demonstrated to attenuate NO-mediated coronary vasodilation in dogs (5, 20), did not diminish the coronary response to H2S (Figure 3A). Infusion of the voltage-dependent K+ channel antagonist 4-aminopyridine (4AP) also had no effect on H2S mediated coronary vasodilation (Figure 3B). However, inhibition of KATP channels with glibenclamide significantly reduced the increase in coronary blood flow to H2S by nearly 70% (Figure 3B). Therefore, our findings demonstrate that exogenous H2S acts as a potent endothelial-independent vasodilator in the coronary circulation predominantly via activation of smooth muscle KATP channels. Given the prominent role of Kv and KATP channels in the regulation of coronary blood flow (3, 4, 13, 43), and the relatively modest effect of KCa channel inhibition on coronary responses in vivo (7, 8, 21) we elected to focus the present studies on H2S mediated activation of coronary Kv and KATP channels. Since earlier studies in other vascular beds have documented a role for KCa channels in H2S-induced dilation (18, 24, 27, 42), further systematic experiments are needed to specifically examine the contribution of specific KCa channels (BKCa, IKCa, SKCa) to H2S mediated increases in coronary blood flow.
Role of endogenous H2S in control of coronary blood flow
As outlined above, the lack of a coronary response to the H2S substrate L-cysteine (Figure 2) does not support an active role for endogenous H2S in the regulation of coronary microvascular resistance. However, alterations in the physiologic state of the myocardium, such as ischemia, have been shown to increase endogenous production of H2S and limit myocardial ischemia-reperfusion injury (38). In order to examine the role of H2S in ischemic coronary vasodilation, coronary reactive hyperemia studies were conducted in the absence and presence of the CSE inhibitor BCA (30, 33). Findings from these experiments indicate that 10 µM BCA, an effective dose capable of inhibiting liver H2S synthesizing activity (30), had no effect on baseline coronary blood flow (Table 1) or on any aspect of the coronary reactive hyperemic response (Figure 4); i.e. peak vasodilator response or the overall debt to repayment ratio (Table 2). Therefore, although there is evidence to support a role for endogenous H2S in mitigating myocardial ischemia-reperfusion injury (19, 32, 38, 51), there are little/no data to support that endogenous H2S is an active regulator of coronary vasomotor tone at rest or following a brief episode of myocardial ischemia.
Conclusions
Findings from this investigation are the first to show that exogenous H2S induces potent, endothelial NO-independent dilation of the canine coronary microcirculation, predominantly through the activation of KATP channels. Despite the pronounced effects of exogenous H2S, our data do not support a functional role for endogenous H2S in the regulation of baseline coronary resistance or ischemic coronary vasodilation. Such findings do not negate prior studies regarding the cardioprotective effects of endogenous H2S in the ischemic heart (38), but rather indicate that H2S-related improvements in cardiac function and ischemic injury are not mediated by alterations in myocardial perfusion per se. Therefore, although exogenous H2S demonstrates the capacity for robust coronary dilator responses, there was no demonstrable physiologic role for H2S as a coronary signaling molecule in this study.
Acknowledgments
This work was supported by a National Institutes of Health grant, HL092245. Dr. Goodwill was supported by National Institutes of Health T32HL079995. Dr. Owen was supported by National Institutes of Health T32DK064466. Dr. Moberly was supported by the IU Medical Scientist Training Program and Dr. Berwick was supported by the American Heart Association 10PRE4230035.
References
- 1.Altaany Z, Yang G, Wang R. Crosstalk between hydrogen sulfide and nitric oxide in endothelial cells. Journal of cellular and molecular medicine. 2013 doi: 10.1111/jcmm.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao L, Vlcek C, Paces V, Kraus JP. Identification and tissue distribution of human cystathionine beta-synthase mRNA isoforms. Archives of biochemistry and biophysics. 1998;350:95–103. doi: 10.1006/abbi.1997.0486. [DOI] [PubMed] [Google Scholar]
- 3.Berwick ZC, Dick GM, Moberly SP, Kohr MC, Sturek M, Tune JD. Contribution of voltage-dependent K(+) channels to metabolic control of coronary blood flow. Journal of molecular and cellular cardiology. 2012;52:912–919. doi: 10.1016/j.yjmcc.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berwick ZC, Moberly SP, Kohr MC, Morrical EB, Kurian MM, Dick GM, Tune JD. Contribution of voltage-dependent K+ and Ca2+ channels to coronary pressure-flow autoregulation. Basic research in cardiology. 2012;107:264. doi: 10.1007/s00395-012-0264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berwick ZC, Payne GA, Lynch B, Dick GM, Sturek M, Tune JD. Contribution of adenosine A(2A) and A(2B) receptors to ischemic coronary dilation: role of K(V) and K(ATP) channels. Microcirculation. 2010;17:600–607. doi: 10.1111/j.1549-8719.2010.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bian JS, Yong QC, Pan TT, Feng ZN, Ali MY, Zhou S, Moore PK. Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. The Journal of pharmacology and experimental therapeutics. 2006;316:670–678. doi: 10.1124/jpet.105.092023. [DOI] [PubMed] [Google Scholar]
- 7.Borbouse L, Dick GM, Payne GA, Berwick ZC, Neeb ZP, Alloosh M, Bratz IN, Sturek M, Tune JD. Metabolic syndrome reduces the contribution of K+ channels to ischemic coronary vasodilation. American journal of physiology Heart and circulatory physiology. 2010;298:H1182–1189. doi: 10.1152/ajpheart.00888.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borbouse L, Dick GM, Payne GA, Payne BD, Svendsen MC, Neeb ZP, Alloosh M, Bratz IN, Sturek M, Tune JD. Contribution of BK(Ca) channels to local metabolic coronary vasodilation: Effects of metabolic syndrome. American journal of physiology Heart and circulatory physiology. 2010;298:H966–973. doi: 10.1152/ajpheart.00876.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen P, Poddar R, Tipa EV, Dibello PM, Moravec CD, Robinson K, Green R, Kruger WD, Garrow TA, Jacobsen DW. Homocysteine metabolism in cardiovascular cells and tissues: implications for hyperhomocysteinemia and cardiovascular disease. Advances in enzyme regulation. 1999;39:93–109. doi: 10.1016/s0065-2571(98)00029-6. [DOI] [PubMed] [Google Scholar]
- 10.Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. American journal of physiology Heart and circulatory physiology. 2004;287:H2316–2323. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- 11.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Modis K, Panopoulos P, Asimakopoulou A, Gero D, Sharina I, Martin E, Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9161–9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dick GM, Bratz IN, Borbouse L, Payne GA, Dincer UD, Knudson JD, Rogers PA, Tune JD. Voltage-dependent K+ channels regulate the duration of reactive hyperemia in the canine coronary circulation. American journal of physiology Heart and circulatory physiology. 2008;294:H2371–2381. doi: 10.1152/ajpheart.01279.2007. [DOI] [PubMed] [Google Scholar]
- 13.Dick GM, Tune JD. Role of potassium channels in coronary vasodilation. Experimental biology and medicine. 2010;235:10–22. doi: 10.1258/ebm.2009.009201. [DOI] [PubMed] [Google Scholar]
- 14.Geng B, Yang J, Qi Y, Zhao J, Pang Y, Du J, Tang C. H2S generated by heart in rat and its effects on cardiac function. Biochemical and biophysical research communications. 2004;313:362–368. doi: 10.1016/j.bbrc.2003.11.130. [DOI] [PubMed] [Google Scholar]
- 15.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochemical and biophysical research communications. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 16.Ishii I, Akahoshi N, Yu XN, Kobayashi Y, Namekata K, Komaki G, Kimura H. Murine cystathionine gamma-lyase: complete cDNA and genomic sequences, promoter activity, tissue distribution and developmental expression. The Biochemical journal. 2004;381:113–123. doi: 10.1042/BJ20040243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson-Weaver O, Osmond JM, Riddle MA, Naik JS, Bosc LV, Walker BR, Kanagy NL. Hydrogen sulfide dilates rat mesenteric arteries by activating endothelial large-conductance Ca2+-activated K+ channels and smooth muscle Ca2+ sparks. American journal of physiology Heart and circulatory physiology. 2013;304:H1446–1454. doi: 10.1152/ajpheart.00506.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson-Weaver O, Paredes DA, Gonzalez Bosc LV, Walker BR, Kanagy NL. Intermittent hypoxia in rats increases myogenic tone through loss of hydrogen sulfide activation of large-conductance Ca(2+)-activated potassium channels. Circulation research. 2011;108:1439–1447. doi: 10.1161/CIRCRESAHA.110.228999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansen D, Ytrehus K, Baxter GF. Exogenous hydrogen sulfide (H2S) protects against regional myocardial ischemia-reperfusion injury--Evidence for a role of K ATP channels. Basic research in cardiology. 2006;101:53–60. doi: 10.1007/s00395-005-0569-9. [DOI] [PubMed] [Google Scholar]
- 20.Knudson JD, Dincer UD, Dick GM, Shibata H, Akahane R, Saito M, Tune JD. Leptin resistance extends to the coronary vasculature in prediabetic dogs and provides a protective adaptation against endothelial dysfunction. American journal of physiology Heart and circulatory physiology. 2005;289:H1038–1046. doi: 10.1152/ajpheart.00244.2005. [DOI] [PubMed] [Google Scholar]
- 21.Kurian MM, Berwick ZC, Tune JD. Contribution of IKCa channels to the control of coronary blood flow. Experimental biology and medicine. 2011;236:621–627. doi: 10.1258/ebm.2011.010351. [DOI] [PubMed] [Google Scholar]
- 22.Leffler CW, Parfenova H, Basuroy S, Jaggar JH, Umstot ES, Fedinec AL. Hydrogen sulfide and cerebral microvascular tone in newborn pigs. American journal of physiology Heart and circulatory physiology. 2011;300:H440–447. doi: 10.1152/ajpheart.00722.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leffler CW, Parfenova H, Jaggar JH, Wang R. Carbon monoxide and hydrogen sulfide: gaseous messengers in cerebrovascular circulation. Journal of applied physiology. 2006;100:1065–1076. doi: 10.1152/japplphysiol.00793.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Zang Y, Fu S, Zhang H, Gao L, Li J. H2S relaxes vas deferens smooth muscle by modulating the large conductance Ca2+ -activated K+ (BKCa) channels via a redox mechanism. The journal of sexual medicine. 2012;9:2806–2813. doi: 10.1111/j.1743-6109.2012.02879.x. [DOI] [PubMed] [Google Scholar]
- 25.Liang GH, Adebiyi A, Leo MD, McNally EM, Leffler CW, Jaggar JH. Hydrogen sulfide dilates cerebral arterioles by activating smooth muscle cell plasma membrane KATP channels. American journal of physiology Heart and circulatory physiology. 2011;300:H2088–2095. doi: 10.1152/ajpheart.01290.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang GH, Xi Q, Leffler CW, Jaggar JH. Hydrogen sulfide activates Ca2+ sparks to induce cerebral arteriole dilatation. The Journal of physiology. 2012;590:2709–2720. doi: 10.1113/jphysiol.2011.225128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang GH, Xi Q, Leffler CW, Jaggar JH. Hydrogen sulfide activates Ca(2)(+) sparks to induce cerebral arteriole dilatation. The Journal of physiology. 2012;590:2709–2720. doi: 10.1113/jphysiol.2011.225128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu WQ, Chai C, Li XY, Yuan WJ, Wang WZ, Lu Y. The cardiovascular effects of central hydrogen sulfide are related to K(ATP) channels activation. Physiological research / Academia Scientiarum Bohemoslovaca. 2011;60:729–738. doi: 10.33549/physiolres.932092. [DOI] [PubMed] [Google Scholar]
- 29.Lowicka E, Beltowski J. Hydrogen sulfide (H2S) - the third gas of interest for pharmacologists. Pharmacological reports : PR. 2007;59:4–24. [PubMed] [Google Scholar]
- 30.Mok YY, Atan MS, Yoke Ping C, Zhong Jing W, Bhatia M, Moochhala S, Moore PK. Role of hydrogen sulphide in haemorrhagic shock in the rat: protective effect of inhibitors of hydrogen sulphide biosynthesis. British journal of pharmacology. 2004;143:881–889. doi: 10.1038/sj.bjp.0706014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circulation research. 2011;109:1259–1268. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osipov RM, Robich MP, Feng J, Liu Y, Clements RT, Glazer HP, Sodha NR, Szabo C, Bianchi C, Sellke FW. Effect of hydrogen sulfide in a porcine model of myocardial ischemia-reperfusion: comparison of different administration regimens and characterization of the cellular mechanisms of protection. Journal of cardiovascular pharmacology. 2009;54:287–297. doi: 10.1097/FJC.0b013e3181b2b72b. [DOI] [PubMed] [Google Scholar]
- 33.Pfeffer M, Ressler C. Beta-cyanoalanine, an inhibitor of rat liver cystathionase. Biochemical pharmacology. 1967;16:2299–2308. doi: 10.1016/0006-2952(67)90217-1. [DOI] [PubMed] [Google Scholar]
- 34.Qipshidze N, Metreveli N, Mishra PK, Lominadze D, Tyagi SC. Hydrogen sulfide mitigates cardiac remodeling during myocardial infarction via improvement of angiogenesis. International journal of biological sciences. 2012;8:430–441. doi: 10.7150/ijbs.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robert K, Vialard F, Thiery E, Toyama K, Sinet PM, Janel N, London J. Expression of the cystathionine beta synthase (CBS) gene during mouse development and immunolocalization in adult brain. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2003;51:363–371. doi: 10.1177/002215540305100311. [DOI] [PubMed] [Google Scholar]
- 36.Schleifenbaum J, Kohn C, Voblova N, Dubrovska G, Zavarirskaya O, Gloe T, Crean CS, Luft FC, Huang Y, Schubert R, Gollasch M. Systemic peripheral artery relaxation by KCNQ channel openers and hydrogen sulfide. Journal of hypertension. 2010;28:1875–1882. doi: 10.1097/HJH.0b013e32833c20d5. [DOI] [PubMed] [Google Scholar]
- 37.Sitdikova GF, Khaertdinov NN, Zefirov AL. Role of calcium and potassium channels in effects of hydrogen sulfide on frog myocardial contractility. Bulletin of experimental biology and medicine. 2011;151:163–166. doi: 10.1007/s10517-011-1280-5. [DOI] [PubMed] [Google Scholar]
- 38.Sivarajah A, McDonald MC, Thiemermann C. The production of hydrogen sulfide limits myocardial ischemia and reperfusion injury and contributes to the cardioprotective effects of preconditioning with endotoxin, but not ischemia in the rat. Shock. 2006;26:154–161. doi: 10.1097/01.shk.0000225722.56681.64. [DOI] [PubMed] [Google Scholar]
- 39.Streeter E, Hart J, Badoer E. An investigation of the mechanisms of hydrogen sulfide-induced vasorelaxation in rat middle cerebral arteries. Naunyn-Schmiedeberg's archives of pharmacology. 2012 doi: 10.1007/s00210-012-0779-2. [DOI] [PubMed] [Google Scholar]
- 40.Sun Y, Tang CS, Du JB, Jin HF. Hydrogen sulfide and vascular relaxation. Chinese medical journal. 2011;124:3816–3819. [PubMed] [Google Scholar]
- 41.Sun YG, Cao YX, Wang WW, Ma SF, Yao T, Zhu YC. Hydrogen sulphide is an inhibitor of L-type calcium channels and mechanical contraction in rat cardiomyocytes. Cardiovascular research. 2008;79:632–641. doi: 10.1093/cvr/cvn140. [DOI] [PubMed] [Google Scholar]
- 42.Tang G, Yang G, Jiang B, Ju Y, Wu L, Wang R. HS Is an Endothelium-Derived Hyperpolarizing Factor. Antioxidants & redox signaling. 2013 doi: 10.1089/ars.2012.4805. [DOI] [PubMed] [Google Scholar]
- 43.Tune JD, Richmond KN, Gorman MW, Feigl EO. K(ATP)(+) channels, nitric oxide, and adenosine are not required for local metabolic coronary vasodilation. American journal of physiology Heart and circulatory physiology. 2001;280:H868–875. doi: 10.1152/ajpheart.2001.280.2.H868. [DOI] [PubMed] [Google Scholar]
- 44.Wagner CA. Hydrogen sulfide: a new gaseous signal molecule and blood pressure regulator. Journal of nephrology. 2009;22:173–176. [PubMed] [Google Scholar]
- 45.Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 46.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Z, Huang H, Liu P, Tang C, Wang J. Hydrogen sulfide contributes to cardioprotection during ischemia-reperfusion injury by opening K ATP channels. Canadian journal of physiology and pharmacology. 2007;85:1248–1253. doi: 10.1139/Y07-120. [DOI] [PubMed] [Google Scholar]
- 48.Zhao W, Wang R. H(2)S-induced vasorelaxation and underlying cellular and molecular mechanisms. American journal of physiology Heart and circulatory physiology. 2002;283:H474–480. doi: 10.1152/ajpheart.00013.2002. [DOI] [PubMed] [Google Scholar]
- 49.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. The EMBO journal. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhong GZ, Li YB, Liu XL, Guo LS, Chen ML, Yang XC. Hydrogen sulfide opens the KATP channel on rat atrial and ventricular myocytes. Cardiology. 2010;115:120–126. doi: 10.1159/000260073. [DOI] [PubMed] [Google Scholar]
- 51.Zhu XY, Yan XH, Chen SJ. H(2)S protects myocardium against ischemia/reperfusion injury and its effect on c-Fos protein expression in rats. Sheng li xue bao : [Acta physiologica Sinica] 2008;60:221–227. [PubMed] [Google Scholar]