Abstract
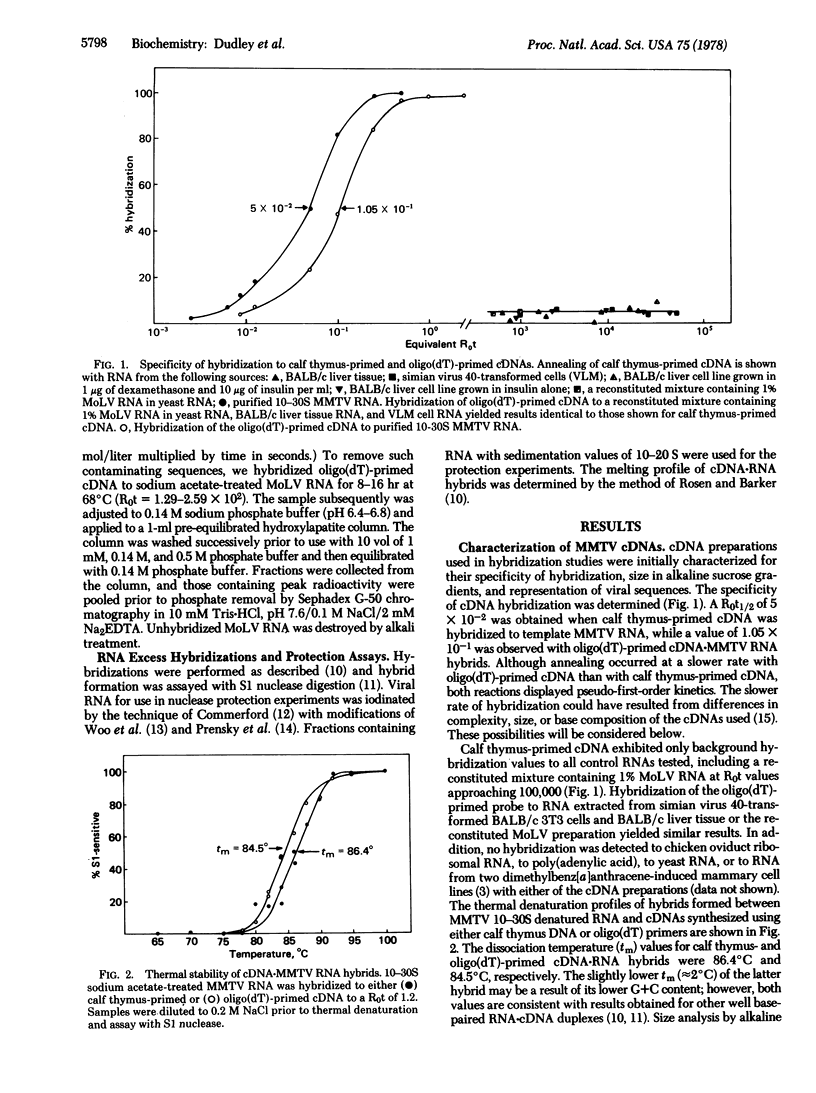
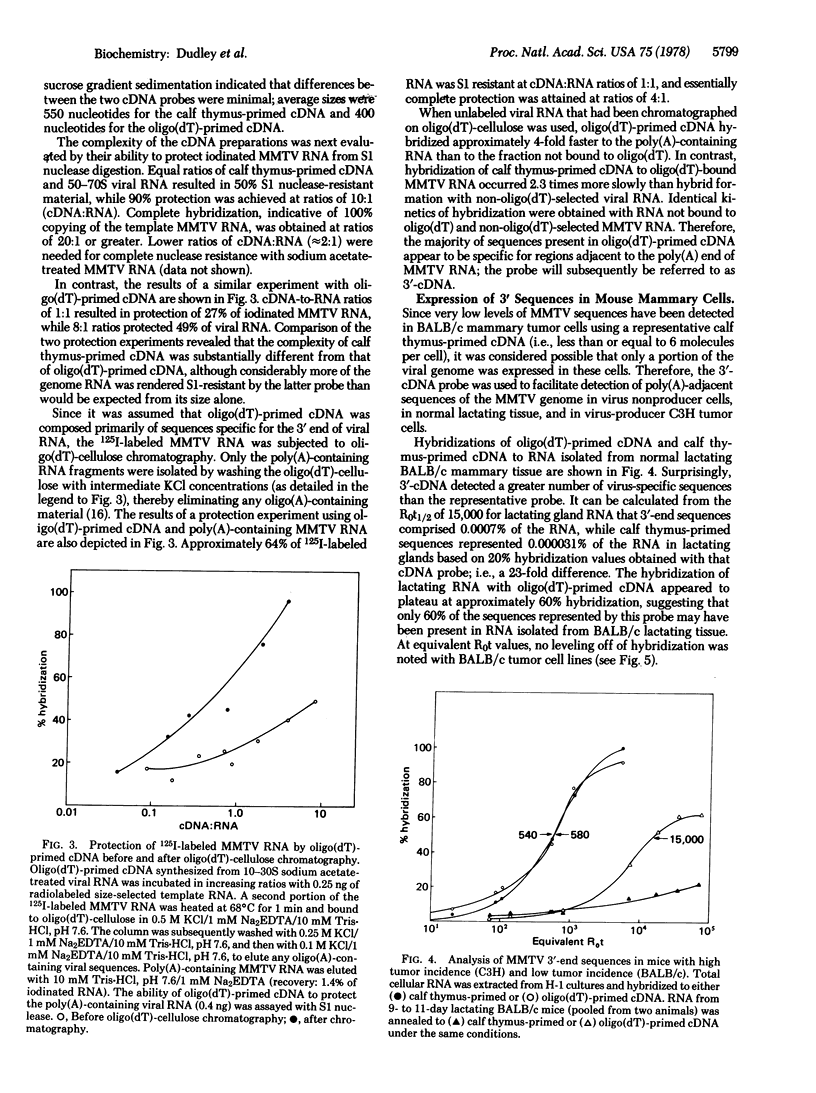
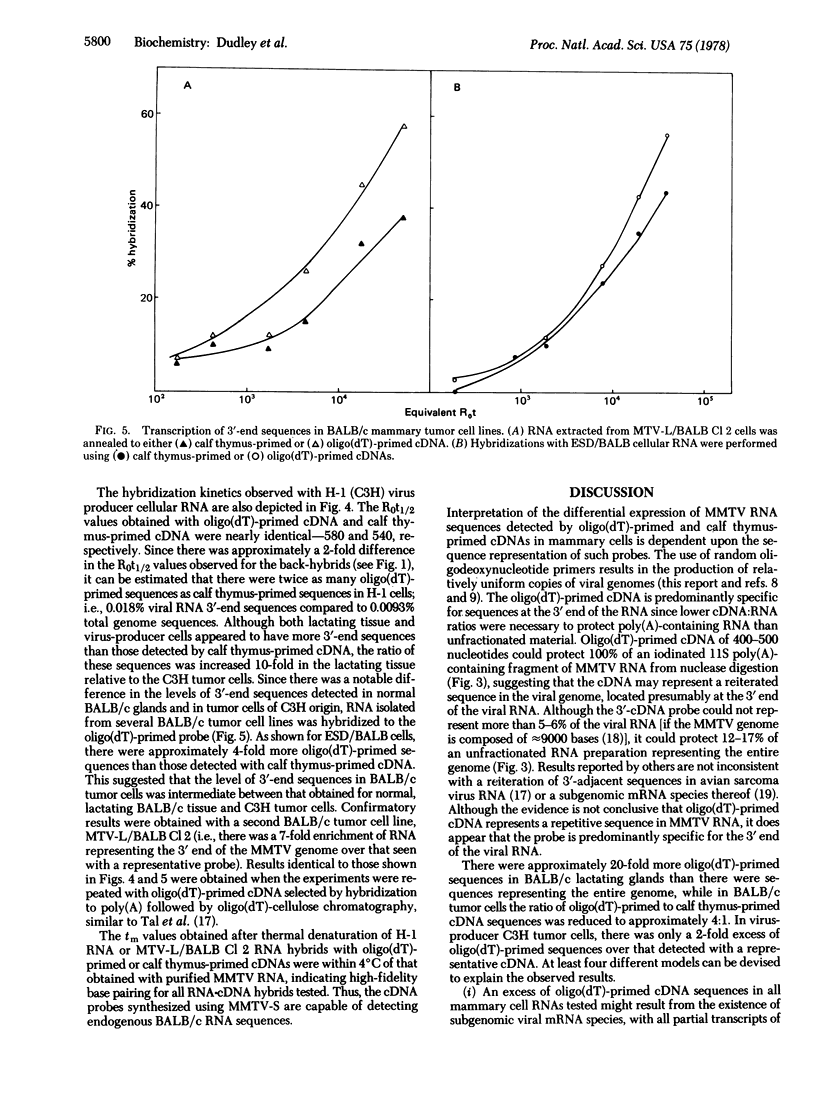
Two DNA probes representative of either the entire mouse mammary tumor virus (MMTV) genome or the poly(A)-adjacent sequences at the 3′ end of MMTV RNA were synthesized with calf thymus DNA or oligo(dT) primers, respectively. These probes were used to study the expression of endogenous MMTV sequences in several BALB/c mammary tumor cell lines, in normal lactating BALB/c tissue, and in a cloned C3H tumor cell line. Both probes were characterized with respect to their rates of hybridization with template RNA, their size as determined by alkaline sucrose gradient centrifugation, and the thermal stability of the cDNA·MMTV RNA hybrids. In addition, the ability of the calf thymus oligodeoxy-nucleotide- or oligo(dT)-primed probes to protect 125I-labeled MMTV RNA or 125I-labeled poly(A)-adjacent MMTV RNA sequences from S1 nuclease digestion was determined. Hybridization analysis with these two probes indicated that (i) there were approximately 20-fold more oligo(dT)-primed sequences in BALB/c lactating tissue than there were sequences representing the entire genome; (ii) in BALB/c tumor cells, the oligo(dT):random oligonucleotide-primed cDNA sequence ratio was reduced to 4:1; and (iii) in virus-producer C3H tumor cells, there was only a 2-fold excess of oligo(dT)-primed sequences over that observed with a representative cDNA. These results are consistent with the presence of subgenomic viral mRNA species, integration of partial proviral copies, or altered mRNA processing.
Keywords: representative cDNA, 3′-cDNA, BALB, c lactating tissue, BALB, c and C3H mammary tumor cell lines
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Dhar R., Laub O., Horowitz M., Khoury G. Novel mechanism for RNA maturation: the leader sequences of simian virus 40 mRNA are not transcribed adjacent to the coding sequences. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3686–3690. doi: 10.1073/pnas.74.9.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berget S. M., Moore C., Sharp P. A. Spliced segments at the 5' terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butel J. S., Dudley J. P., Medina D. Comparison of the growth properties in vitro and transplantability of continuous mouse mammary tumor cell lines and clonal derivatives. Cancer Res. 1977 Jun;37(6):1892–1900. [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Drohan W., Colcher D., Schochetman G., Schlom J. Distribution of Mason-Pfizer virus-specific sequences in the DNA of primates. J Virol. 1977 Jul;23(1):36–43. doi: 10.1128/jvi.23.1.36-43.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds M., Caramela M. G. The isolation and characterization of adenosine monophosphate-rich polynucleotides synthesized by Ehrlich ascites cells. J Biol Chem. 1969 Mar 10;244(5):1314–1324. [PubMed] [Google Scholar]
- Fareed G. C., Davoli D. Molecular biology of papovaviruses. Annu Rev Biochem. 1977;46:471–522. doi: 10.1146/annurev.bi.46.070177.002351. [DOI] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan J. J., Harris S. E., Woo S. L., Robberson D. L., O'Malley B. W. The synthesis and properties of the complete complementary DNA transcript of ovalbumin mRNA. Biochemistry. 1976 Jan 13;15(1):223–233. doi: 10.1021/bi00646a034. [DOI] [PubMed] [Google Scholar]
- Owens R. B., Smith H. S., Hackett A. J. Epithelial cell cultures from normal glandular tissue of mice. J Natl Cancer Inst. 1974 Jul;53(1):261–269. doi: 10.1093/jnci/53.1.261. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Prensky W., Steffensen D. M., Hughes W. L. The use of iodinated RNA for gene localization. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1860–1864. doi: 10.1073/pnas.70.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen J. M., Barker S. W. Quantitation of casein messenger ribonucleic acid sequences using a specific complementary DNA hybridization probe. Biochemistry. 1976 Nov 30;15(24):5272–5280. doi: 10.1021/bi00669a012. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Woo S. L., Comstock J. P. Regulation of casein messenger RNA during the development of the rat mammary gland. Biochemistry. 1975 Jul;14(13):2895–2903. doi: 10.1021/bi00684a016. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Woo S. L., Holder J. W., Means A. R., O'Malley B. W. Preparation and preliminary characterization of purified ovalbumin messenger RNA from the hen oviduct. Biochemistry. 1975 Jan 14;14(1):69–78. doi: 10.1021/bi00672a012. [DOI] [PubMed] [Google Scholar]
- Schlom J., Colcher D., Drohan W., Kettmann R., Michalides R., Vlahakis G., Young J. Differences in mouse mammary tumor viruses. Relationship to early and late occurring mammary tumors. Cancer. 1977 Jun;39(6 Suppl):2727–2733. doi: 10.1002/1097-0142(197706)39:6<2727::aid-cncr2820390660>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Schlom J., Michalides R., Kufe D., Hehlmann R., Spiegelman S., Bentvelzen P., Hageman P. A comparative study of the biologic and molecular basis of murine mammary carcinoma: a model for human breast cancer. J Natl Cancer Inst. 1973 Aug;51(2):541–551. [PubMed] [Google Scholar]
- Schochetman G., Schlom J. Independent polypeptide chain initiation sites for the synthesis of different classes of proteins for an RNA tumor virus: mouse mammary tumor virus. Virology. 1976 Sep;73(2):431–441. doi: 10.1016/0042-6822(76)90404-9. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Cohen J. C., Varmus H. E., Yamamoto K. R., Ringold G. M. Mapping of linear and circular forms of mouse mammary tumor virus DNA with restriction endonucleases: evidence for a large specific deletion occurring at high frequency during circularization. Proc Natl Acad Sci U S A. 1978 May;75(5):2112–2116. doi: 10.1073/pnas.75.5.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal J., Kung H. J., Varmus H. E., Bishop J. M. Characterization of DNA complementary to nucleotide sequences adjacent to poly(A) at the 3'-terminus of the avian sarcoma virus genome. Virology. 1977 Jun 1;79(1):183–197. doi: 10.1016/0042-6822(77)90344-0. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Quintrell N., Medeiros E., Bishop J. M., Nowinski R. C., Sarkar N. H. Transcription of mouse mammary tumor virus genes in tissues from high and low tumor incidence mouse strains. J Mol Biol. 1973 Oct 5;79(4):663–679. doi: 10.1016/0022-2836(73)90070-3. [DOI] [PubMed] [Google Scholar]
- Weiss S. R., Varmus H. E., Bishop J. M. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977 Dec;12(4):983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]
- Woo S. L., Rosen J. M., Liarakos C. D., Choi Y. C., Busch H., Means A. R., O'Malley Physical and chemical characterization of purified ovalbumin messenger RNA. J Biol Chem. 1975 Sep 10;250(17):7027–7039. [PubMed] [Google Scholar]
- Young H. A., Shih T. Y., Scolnick E. M., Parks W. P. Steroid induction of mouse mammary tumor virus: effect upon synthesis and degradation of viral RNA. J Virol. 1977 Jan;21(1):139–146. doi: 10.1128/jvi.21.1.139-146.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


