Abstract
Objective
While several studies have found an association between excessive gestational weight gain and obesity later in life, to the best of our knowledge, no studies have explored the role of gestational weight gain events across the life course.
Design and Methods
We describe how the prevalence of mid-life obesity (BMI≥30 at age 40 or 41) among women varies by life course patterns of gestational weight gain (using 2009 IOM guidelines) in the USA’s National Longitudinal Survey of Youth 1979 cohort.
Results
Among women who reported 1–3 births before age 40, the prevalence of mid-life obesity increased with a rising number of excessive gestational weight gain events: from none (23.4%, n=875), to one (37.6%, n=707), to two (46.8%, n=427), and to three (54.6%, n=108), p<0.00005 for trend. Obesity prevalence was similar for the same number of excessive gestational weight gain events, regardless of parity. No clear pattern emerged for the sequencing of excessive gestational weight gain event(s) and later obesity.
Conclusions
In our descriptive exploratory study, excessive gestational weight gain events appear to be associated with increased prevalence of obesity for parous women, suggesting the importance of preventive interventions regardless of timing of pregnancy-related weight changes over the life course.
Keywords: gestational weight gain, life course, obesity, pregnancy, women
Introduction
The Institute of Medicine (IOM) 2009 guidelines 1 distinguish between inadequate, adequate, and excessive gestational weight gain as having different implications for the health of the mother and of the child. Excessive weight gain is associated with several short-term weight-related outcomes, including higher post-partum weight retention 2,3, higher birth weight 4, and childhood obesity 5.
Several birth cohort analyses have found that excessive gestational weight gain is associated with obesity later in life 6, but these studies 7–10 measure gestational weight gain in only one pregnancy per woman. Two studies have considered gestational weight gain events from two consecutive pregnancies 11,12, but to the best of our knowledge, no studies have explored gestational weight gain events over a woman’s life course. Our primary goal is to explore the combined role of both gestational weight gain and parity: whether women with multiple pregnancies with excessive gestational weight gain have an elevated prevalence of midlife obesity. A second goal of the paper is to explore whether the number of excessive gestational weight gain pregnancies or the pattern of excessive gestational weight gain over the life course (for example, the first, or the most recent) is associated with midlife obesity prevalence, inspired by social epidemiology research characterizing patterns of multiple exposures 13–15.
Materials and Methods
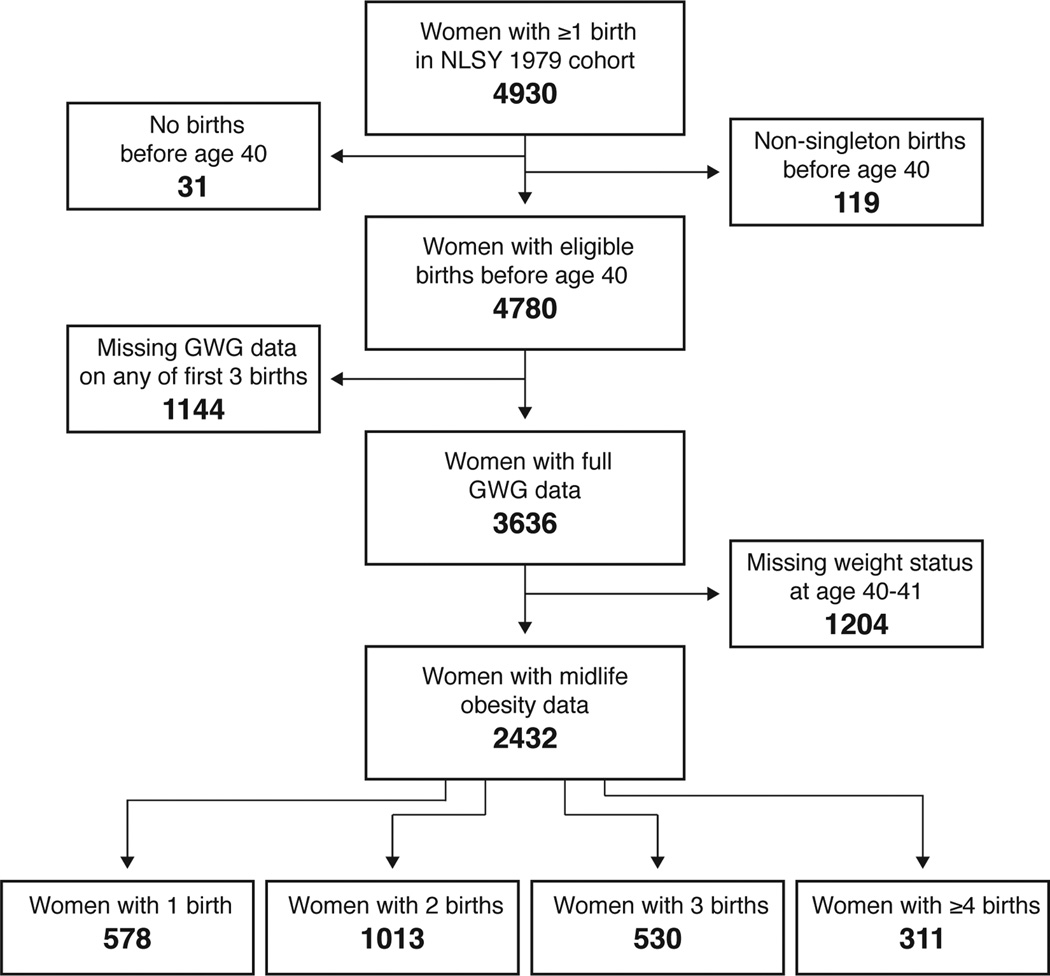
We examined excessive gestational weight gain across multiple pregnancies among participants of the National Longitudinal Survey of Youth 1979 (NLSY79) cohort (unweighted). The NLSY79 is a national sample of individuals who were between the ages of 14 and 21 in 1979 (11). Through 2010, 4930 women enrolled in the NLSY cohort had reported at least one birth (and therefore had a gestational weight gain event) and were followed at least to age 40. Of these, 4808 women reported only singleton births (11,042 births). Our outcome of interest was obesity at age 40; therefore, we excluded women whose births all took place at age ≥40 (n=31). However, we included three women whose births were all singleton prior to age 40 even though they also had additional births after age 40, leaving 4780 women eligible for analysis (Figure 1). Of the mothers in our sample, 24.8% (n=1185) reported one birth, 40.7% (n=1942) reported two births, 22.5% (n=1075) reported three births, 8.2% (n=392) reported four births, and 3.9% (n=186) reported five or more births (maximum number of births reported was 11).
Figure 1.
Participant eligibility for analytic sample.
For each birth, women self-reported their pre-pregnancy weight and weight at delivery, and gestational weight gain was calculated as the difference between these two weights. We used pre-pregnancy weight and self-reported height to calculate each woman’s pre-pregnancy body mass index (BMI) in order to categorize her gestational weight gain as inadequate (low weight gain), adequate, or excessive (high weight gain) according to 2009 IOM guidelines (6). Our classifications take into account gestational age at delivery 16,17, and hence, gestational weight gain (GWG) could not be calculated for births with implausible gestational age values (≥ 44 weeks or ≤ 22 weeks). We excluded women with missing GWG data on any of their first three births (n=1144 births) (Figure 1).
Each woman’s self-reported weight at age 40 or 41 and her most recently reported height were used to calculate BMI in mid-life. Women with BMI ≥30 kg/m2 were classified as obese (12). Midlife obesity data were available for 66.9% of eligible women with full GWG histories (2432/3636) (Figure 1).
For this descriptive analysis, we presented categories of GWG (inadequate, adequate, or excessive) for up to three births occurring before age 40. We then estimated the prevalence of obesity at age 40 among women with each possible combination of number of excessive GWG births. We calculated continuity-corrected confidence intervals for our prevalence estimates using an online tool (http://www.vassarstats.net/prop1.html) to ensure that the confidence intervals would be between 0 and 100%, because prevalence estimates must be between 0 and 100%18. We used Fisher’s Exact Test and test of trend to test for statistically significant differences in the prevalence of obesity between groups.
Results
The mothers with full data on gestational weight gain and recorded obesity status in midlife were, on average, similar to those in the initially eligible population (as outlined in figure 1). Among those with full data, mean age at first birth (22.9 years), mean total number of births (2.3), and proportion attaining 16 or more less of education by age 25 (13.2%) were nearly identical to those characteristics in the eligible population (age at first birth: 22.7 years; total births: 2.3; and ≥16 years of education: 13.2%). In the population with complete data, there was a slightly higher proportion of Black non-Hispanic mothers (29.7% versus 25.2%) and a slightly lower proportion of mothers with fewer than 12 years of education at age 25 (16.4% versus 18.4%).
Of the included women (n=3636), 43.9% of their pregnancies (n=7811 births) resulted in excessive GWG. More than half (59.2%) of these women experienced at least one excessive GWG event. Excessive GWG events occurred in similar proportions across self-reported racial/ethnic groups: 44.5% for non-Black non-Hispanic (i.e., White, Asian, other) women (n=4623 births); 41.1% for non-Hispanic Black women (n=2049); and 46.4% for non-Black Hispanic women (n=1139).
When we consider excessive GWG prevalence by birth order, the prevalence of excessive GWG decreased as birth order increased (p for trend=0.107), with 45.7% of first births (n=3636), 42.3% of second births (n=2575), 42.9% of third births (n=1127), 42.8% of fourth births (n=327), and 37.1% of fifth births (n=97) having excessive GWG. When we look at racial/ethnic subgroups, the trends were not statistically significant (p for trend for Hispanics = 0.129, p for trend for non-Black non-Hispanics = 0.163, p for trend for Blacks = 0.845).
When we consider the prevalence of excessive GWG by mother, the likelihood of ever experiencing excessive GWG increased with an increasing number of recorded births (p for trend <0.00005): the proportion of women who ever experienced a birth associated with excessive gestational weight gain was 47.9% among women with one birth (n=1061), 61.9% among women with two births (n=1448), 64.7% among women with three births (n=731), 67.9% of women with four births (n=280), and 72.4% among women with five or more births (n=116). These findings were similar across racial/ethnic groups, and the p for trend was significant for non-Black non-Hispanics (p=0.002) and non-Hispanic Blacks (p= 0.04) but not non-Black Hispanics (p=0.14).
Gestational weight gain experience tracked moderately with GWG in subsequent births. Among women with exactly two births (n=1448), excessive GWG in the first birth (n=685) was most often followed by excessive GWG in the second: 66.4% excessive GWG, versus 21.3% adequate GWG and 12.3% inadequate GWG. Adequate GWG in the first birth (n=421) was most often followed by adequate GWG in the second: 43.0% adequate GWG, versus 33.7% excessive GWG and 23.2% inadequate. Likewise, inadequate GWG in the first birth (n=342) was most often followed by inadequate GWG in the second: 53.8% inadequate GWG, versus 26.0% adequate GWG and 20.2% inadequate GWG.
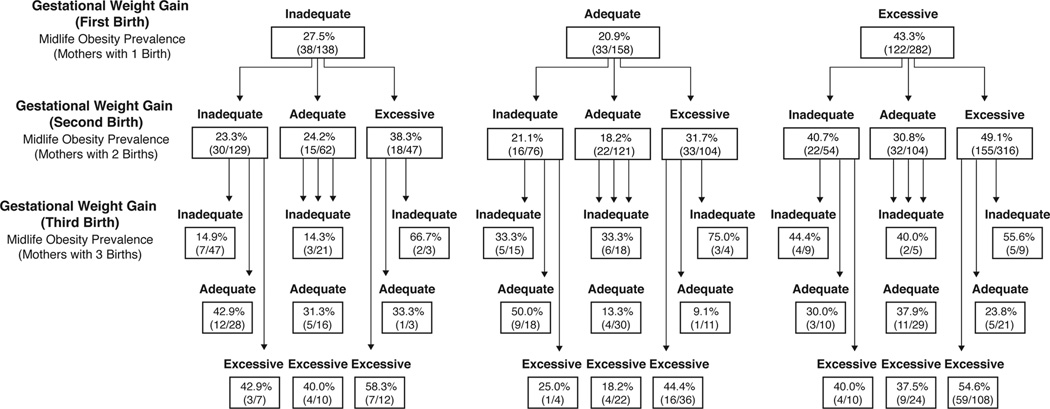
Figure 2 presents the prevalence of mid-life obesity according to histories of gestational weight gain over a woman’s first three births. The overall prevalence of obesity in this sample was 35.4% (n=2432 women with complete GWG data). The prevalence of obesity at age 40 was greatest among those experiencing excessive gestational weight gain, regardless of GWG experiences in previous births. This pattern did not hold only in groups of small sample size (n ≤ 10). The figure can be used to examine the prevalence of obesity at age 40 for any combination of categories of GWG and number of births. For example, following from the upper left block of “Inadequate” to the far left block below “Inadequate” to the next far left block “Inadequate”, the figure shows that among women with 3 births, 14.9% of women (7/47) are obese at age 40 if they had inadequate GWG for all three of their pregnancies. Similarly, the pathways in the figure can be followed to show that among women who reported three births, the prevalence of obesity at age 40 was smaller when all three GWG experiences were categorized as adequate (13.3%; 4/30) and larger when all three were excessive 54.6% (59/108). For women reporting two births, the prevalence of mid-life obesity was also greatest when both births resulted in excessive gain (49.1%, 155/316), in comparison to two adequate GWG events (18.2%, 22/121) or two inadequate GWG events (23.3%, 30/129).
Figure 2.
Midlife obesity by gestational weight gain patterns over the first three births among NLSY79 mothers.2
2 In order to identify the prevalence of obesity for a combination of GWG categories and number of births, follow from the top of the figure down rows depending on the number of births of interest. For example, following from the upper left block of “Inadequate” to the far left block below “Inadequate” to the next far left block “Inadequate”, the figure shows that among women with 3 births, all 3 with inadequate GWG, 14.9% of women (7/47) are obese at age 40.
Overall, there was a significant relationship between the ordered combination of excessive GWG births and prevalence of obesity (Fisher's Exact Test p<0.001). Collapsing across GWG patterns with the same number of excessive GWG events, there was a significant relationship between number of excessive GWG events and prevalence of obesity (Fisher's Exact Test p<0.001). However, when looking within GWG patterns with the same number of excessive GWG events, the individual GWG pattern was not significantly related to prevalence of obesity in either the patterns with one excessive GWG event (Fisher's Exact Test p=0.42) or the patterns with two excessive GWG events (Fisher's Exact Test p=0.64).
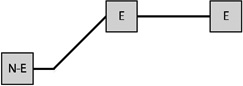
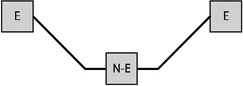
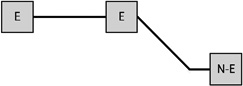
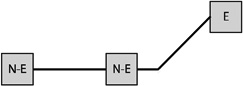
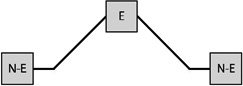
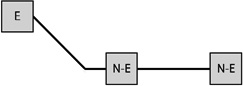
Table 1 presents the prevalence of obesity for each of eight possible GWG patterns for women with three births. Among women reporting three births and a single excessive GWG, the obesity prevalence was 37.7% when the excessive GWG occurred in the first birth (n=53), 33.3% when the excessive GWG occurred in the second (n=21), and 27.9% when the third gestational weight gain was excessive (n=43). Among women reporting three births and two excessive GWGs, obesity prevalence varied according to whether the excessive GWGs occurred in the first two births (33.3%, n=30), the first and third (38.2%, n=34), or the final two (47.9%, n=48).
Table 1.
The prevalence of obesity at age 40 for each trajectory of gestational weight gains for women who had three births:
| Trajectory | Number of women with this trajectory (n) |
Prevalence of obesity |
Continuity- corrected 95% CI |
|---|---|---|---|
| 108 | 54.6% | 44.8%–64.1% | |
 |
48 | 47.9% | 33.5%–62.3% |
 |
34 | 38.2% | 22.7%–56.4% |
 |
30 | 33.3% | 17.9%–52.9% |
 |
43 | 27.9% | 15.8%–43.9% |
 |
21 | 33.3% | 15.5%–56.9% |
 |
53 | 37.7% | 25.1%–52.1% |
| 193 | 26.4% | 20.5%–33.3% |
Notes: E indicates excessive gestational weight gain; N-E indicates not excessive gestational weight gain.
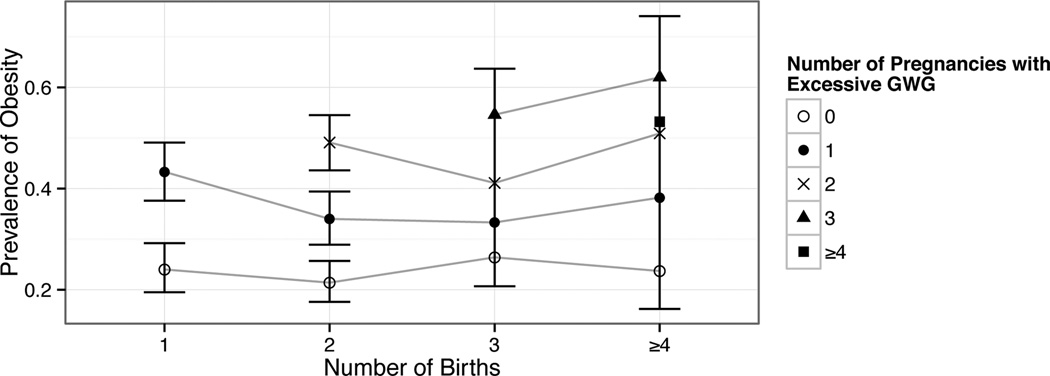
Figure 3 presents the prevalence of mid-life obesity organized by the total number of births and the total number of excessive GWG events prior to age 40, among women with complete GWG data through their first three births (see table 2 for the numerical prevalence estimates and confidence intervals). The prevalence of obesity was higher with increasing excessive GWG events at all numbers of total births (p for trend <0.005 for each number of total births (one, two, three, and four or more)). However, for any given number of excessive GWG events, the prevalence of obesity was not higher with an increasing number of births. For example, among women with zero excessive GWG events, obesity prevalence ranged from 21%-26% depending on parity, whereas the prevalence of obesity among women with one excessive GWG event ranged from 33%–43%, the prevalence of obesity among women with two excessive GWG events ranged from 41%–51%, and the prevalence of obesity among women with three excessive GWG events ranged from 55-62% (figure 3).
Figure 3.
The prevalence of obesity for each number of births, by number of excessive GWG events.
Table 2.
Prevalence of Obesity (BMI ≥30) at Age 40 by Birth History and Number of Excessive GWG Events1
| Number of Excessive GWG Events Before Age 40 | |||||
|---|---|---|---|---|---|
| Number of Births Before Age 40 |
0 | 1 | 2 | 3 | ≥4 |
| 1 | 24.0% 19.5%–29.2% (71/296) |
43.3% 37.6%–49.1% (122/282) |
n/a | n/a | n/a |
| 2 | 21.4% 17.6%–25.7% (83/388) |
34.0% 28.9%–39.4% (105/309) |
49.1% 43.6%–54.5% (155/316) |
n/a | n/a |
| 3 | 26.4% 20.7%–33.1% (51/193) |
33.3% 25.4%–42.3% (39/117) |
41.1% 32.4%–50.3% (46/112) |
54.6% 45.2%–63.7% (59/108) |
n/a |
| ≥4 | 23.7% 16.2%–33.2% (22/93) |
38.2% 27.6%–50.1% (26/68) |
50.9% 37.9%–63.9% (27/53) |
62.0% 48.2%–74.1% (31/50) |
53.2% 39.2%–66.7% (25/47) |
This table reports the proportion and continuity-corrected 95% CI for all NLSY79 women for whom no data were missing for their first three births and for whom BMI information at age 40 or 41 was available. These are the numbers that are illustrated in figure 2.
Discussion
Our findings illustrate how experiencing excessive gestational weight gain is correlated with mid-life obesity, with a higher prevalence of obesity as the number of excessive GWG events accumulates. In particular, stratified by the number of excessive GWG events, the prevalence of mid-life obesity was not higher with greater parity; whereas, holding parity constant, the number of excessive GWG events was strongly linked to mid-life obesity prevalence. It did not appear that the order in which excessive GWG events occurs was an important predictor of mid-life obesity.
Other studies have focused on the association between parity and obesity in mid-life19–21 but have not included data on GWG, while, to the best of our knowledge, most studies of GWG and midlife obesity are birth cohorts that have information on only one pregnancy. Our findings, which include data on GWG over a woman’s entire life course, suggest that the number of births per se do not necessarily put women at higher risk for obesity; instead, the accumulated number of excessive gestational weight gain experiences appears to be a more important factor.
Our study had several limitations, including that we relied on self-reported measures of weight and that there was loss to follow up over the course of the study. Additionally, given the small cell sizes for many of the gestational weight gain patterns illustrated in figure 2, the specific prevalence estimates may be imprecise. However, our primary strength was that we had a long follow-up (to age 40) over the life course with a national sample that included multiple pregnancies. The NLSY cohort covers the emergence of the obesity epidemic, and we are not able to determine whether data from earlier or contemporary populations would yield similar findings, so we encourage future researchers with access to other populations to conduct similar analyses.
We note that this study was exploratory and hypothesis-generating in nature; we encourage future research to test the hypotheses generated with multivariable models, including that ever experiencing excessive gestational weight gain, as well as the number of excessive gestational weight gain events, may be associated with obesity later in life. We also encourage future researchers to examine what factors may modify (e.g., pre-pregnancy BMI) or mediate (e.g., lactation behaviors) the relationship between excessive gestational weight gain and midlife obesity.
Some studies have focused on the first birth as a key intervention point for the prevention of excessive gestational weight gain 22, while other randomized controlled trials did not differentiate based on birth order 23,24. Our results confirm that women who gain excessively in the first birth are more likely to gain excessively in later pregnancies. From the standpoint of reducing obesity in mid-life, our findings are consistent with the idea that preventing excessive weight gain in any pregnancy in the birth order may be associated with reducing midlife obesity. With this in mind, we encourage health care providers and public health practitioners to think of every pregnancy as an opportunity to promote healthy gestational weight gain and prevent obesity.
Acknowledgements
This work was supported by the National Institute of Minority Health and Health Disparities at the National Institutes of Health (grant 5R01MD6104-2).
Funding: This work was supported by the National Institute of Minority Health and Health Disparities at the National Institutes of Health (grant 5R01MD6104-2).
Footnotes
Conflict of interest: the authors have no conflicts of interest to declare.
References
- 1.Rasmussen KM, Yaktine AL. Weight gain during pregnancy: reexamining the guidelines. National Academies Press; 2009. Institute Of Medicine. [PubMed] [Google Scholar]
- 2.Olson CM, Strawderman MS, Hilton PS, Pearson TA. Gestational weight gain and postpartum behaviors associated with weight change from early pregnancy to 1 y postpartum. International Journal of Obesity. 2003;27:117–127. doi: 10.1038/sj.ijo.0802156. [DOI] [PubMed] [Google Scholar]
- 3.Margerison Zilko CE, Rehkopf D, Abrams B. Association of maternal gestational weight gain with short- and long-term maternal and child health outcomes. American Journal of Obstetrics and Gynecology. 2010;202:574.e1–574.e8. doi: 10.1016/j.ajog.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Siega-Riz AM, et al. A systematic review of outcomes of maternal weight gain according to the Institute of Medicine recommendations: birthweight, fetal growth, and postpartum weight retention. American Journal of Obstetrics and Gynecology. 2009;201:339.e1–339.e14. doi: 10.1016/j.ajog.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Nehring I, Lehmann S, Kries R. Gestational weight gain in accordance to the IOM/NRC criteria and the risk for childhood overweight: a meta-analysis. Pediatric Obesity. 2012 doi: 10.1111/j.2047-6310.2012.00110.x. [DOI] [PubMed] [Google Scholar]
- 6.Nehring I, Schmoll S, Beyerlein A, Hauner H, Kries von R. Gestational weight gain and long-term postpartum weight retention: a meta-analysis. The American Journal of Clinical Nutrition. 2011;94:1225–1231. doi: 10.3945/ajcn.111.015289. [DOI] [PubMed] [Google Scholar]
- 7.Mamun AA, et al. Associations of excess weight gain during pregnancy with long-term maternal overweight and obesity: evidence from 21 y postpartum follow-up. American Journal of Clinical Nutrition. 2010;91:1336–1341. doi: 10.3945/ajcn.2009.28950. [DOI] [PubMed] [Google Scholar]
- 8.Fraser A, et al. Associations of gestational weight gain with maternal body mass index, waist circumference, and blood pressure measured 16 y after pregnancy: the Avon Longitudinal Study of Parents and Children (ALSPAC) American Journal of Clinical Nutrition. 2011;93:1285–1292. doi: 10.3945/ajcn.110.008326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rooney BL, Schauberger CW, Mathiason MA. Impact of perinatal weight change on long-term obesity and obesity-related illnesses. Obstetrics & Gynecology. 2005;106:1349–1356. doi: 10.1097/01.AOG.0000185480.09068.4a. [DOI] [PubMed] [Google Scholar]
- 10.Amorim AR, Rössner S, Neovius M, Lourenço PM, Linné Y. Does excess pregnancy weight gain constitute a major risk for increasing long-term BMI? Obesity (Silver Spring Md.) 2007;15:1278–1286. doi: 10.1038/oby.2007.149. [DOI] [PubMed] [Google Scholar]
- 11.Chin JR, et al. Gestational weight gain in consecutive pregnancies. American Journal of Obstetrics and Gynecology. 2010;203:279.e1–279.e6. doi: 10.1016/j.ajog.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 12.Waring ME, Moore Simas TA, Liao X. Gestational weight gain within recommended ranges in consecutive pregnancies: a retrospective cohort study. Midwifery. 2013;29:550–556. doi: 10.1016/j.midw.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mishra G, et al. A structured approach to modelling the effects of binary exposure variables over the life course. International Journal of Epidemiology. 2008;38:528–537. doi: 10.1093/ije/dyn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hallqvist J, Lynch J, Bartley M, Lang T, Blane D. Can we disentangle life course processes of accumulation, critical period and social mobility? An analysis of disadvantaged socio-economic positions and myocardial infarction in the Stockholm Heart Epidemiology Program. Social Science & Medicine. 2004;58:1555–1562. doi: 10.1016/S0277-9536(03)00344-7. [DOI] [PubMed] [Google Scholar]
- 15.Lê F, Roux AD, Morgenstern H. Effects of child and adolescent health on educational progress. Soc Sci Med. 2013;76:57–66. doi: 10.1016/j.socscimed.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Bodnar LM, et al. Should gestational weight gain recommendations be tailored by maternal characteristics? American Journal of Epidemiology. 2011;174:136–146. doi: 10.1093/aje/kwr064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siega-Riz AM, Hobel CJ. Predictors of Poor Maternal Weight Gain from Baseline Anthropometric, Psychpsocial, and Demographic Information in a Hispanic Population. Journal of the American Dietetic Association. 1997;97:1264–1268. doi: 10.1016/s0002-8223(97)00303-9. [DOI] [PubMed] [Google Scholar]
- 18.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Statistics in Medicine. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 19.Luoto R, Männistö S, Raitanen J. Ten-Year Change in the Association Between Obesity and Parity: Results From the National FINRISK Population Study. Gender Medicine. 2011;8:399–406. doi: 10.1016/j.genm.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Melzer K, Schutz Y. Pre-pregnancy and pregnancy predictors of obesity. International Journal of Obesity. 2010;34:S44–S52. doi: 10.1038/ijo.2010.239. [DOI] [PubMed] [Google Scholar]
- 21.Weng HH, Bastian LA, Taylor DH, Moser BK, Ostbye T. Number of children associated with obesity in middle-aged women and men: results from the health and retirement study. J Womens Health (Larchmt) 2004;13:85–91. doi: 10.1089/154099904322836492. [DOI] [PubMed] [Google Scholar]
- 22.Kinnunen TI, et al. Preventing excessive weight gain during pregnancy – a controlled trial in primary health care. European Journal of Clinical Nutrition. 2007;61:884–891. doi: 10.1038/sj.ejcn.1602602. [DOI] [PubMed] [Google Scholar]
- 23.Skouteris H, et al. Preventing excessive gestational weight gain: a systematic review of interventions. Obesity Reviews. 2010;11:757–768. doi: 10.1111/j.1467-789X.2010.00806.x. [DOI] [PubMed] [Google Scholar]
- 24.Phelan S, et al. Randomized trial of a behavioral intervention to prevent excessive gestational weight gain: the Fit for Delivery Study. American Journal of Clinical Nutrition. 2011;93:772–779. doi: 10.3945/ajcn.110.005306. [DOI] [PMC free article] [PubMed] [Google Scholar]