Abstract
Objectives
To identify salient characteristics of frailty that increase risk of death in depressed elders.
Design
Data from the Nordic Research on Ageing Study.
Setting
Research sites in Denmark, Sweden, and Finland.
Participants
Sample included 1027 75-year-old adults, 436 men and 591 women.
Main Outcome Measure
Time of death was obtained, providing a maximum survival time of 11.08 years (initial evaluation took place between 1988-1991).
Results
Depressed elders showed greater baseline impairments in each frailty characteristic (gait speed, grip strength, physical activity levels, and fatigue). Simultaneous models including all four frailty characteristics showed slow gait speed (HR, 1.84; 95% CI, 1.05-3.21) and fatigue (HR, 1.94; 95% CI, 1.11-3.40) associated with faster progression to death in depressed women; none of the frailty characteristics in the simultaneous model were associated with death in depressed men. In women, the effect of impaired gait speed on mortality rates nearly doubled when depression was present (mortality rates, nondepressed women: no gait impairment =26%; slow gait =40%; depressed women: no gait impairment=32%; slow gait =58%). A similar pattern was observed for fatigue.
Conclusions
The confluence of specific characteristics of frailty (fatigue and slow gait speed) and depressive illness is associated with an increased risk of death in older adults; this association is particularly strong in older depressed women. Future research should investigate whether multimodal interventions targeting depressive illness, mobility deficits, and fatigue can decrease mortality and improve quality of life in older depressed individuals with characteristics of the syndrome of frailty.
Frailty is a syndrome defined by weakness, fatigue, low physical activity, slowed gait, and unintentional weight loss. These declines across multiple physiologic systems1 develop incrementally with weakness emerging early in the process and weight loss and fatigue representing a final pathway towards frailty.1-4 The syndrome, a clinical marker for disease and/or physiological decline, is associated with greater depressive symptoms and disability.1, 5, 6 Frailty characteristics are prevalent in the community, with estimates of prefrailty (1-2 characteristics) or frailty (3+ characteristics) as high as 7% of community dwelling elders over the age of 65, and 18% of those over the age of 80. Three year follow-up data2 showed that prefrail elders had more than a 3-fold higher risk of death compared with non-frail elders; frail elders had a 6-fold higher risk of death. Thus a significant proportion of community dwelling elderly are at a “tipping point” towards dire outcomes.
Depression is another disease prevalent in older adults associated with disability and increased mortality; It is estimated that 2.6% of community-dwelling elders suffer from a mood disorder, a rate believed to be an underestimation due to underdiagnosis, the presence of subthreshold symptoms, and a lack of high-risk older adults assessed.7 The diagnosis and treatment of late life depression is complicated by increased risk of comorbid disability, medical disorders, and cognitive impairment.8-15
There is phenomenological overlap between late life depression and frailty, with symptoms common to both depression (weight loss, decreased physical activities, low energy) and frailty (fatigue, decreased leisure activities, weight loss).16 The Cardiovascular Health Study1 reported that the rate of depressive symptoms increased proportional to the number of frailty characteristics present.1 Yet despite these associations, there has been little research focused on this high-risk clinical population.16-21 Therefore, although characteristics of frailty have been predictive of mortality in nondepressed older adults, it is not known if these same characteristics are predictive in depressed elders or whether the presence of depression increases the risk of mortality in these individuals.
Using data from the Nordic Research on Ageing study (NORA22-24), we investigated the effect of the presence of frailty characteristics on mortality in older adults with differing degrees of depressive symptomatology. We hypothesized that in the depressed sample low grip strength and gait speed rather than fatigue and low physical activities (characteristics of frailty that overlap with symptoms of depression) would predict mortality.
Methods
Participants
The purpose of the NORA study was to determine the functional capacity of 75-year-olds from Glostrup in Denmark, Göteborg in Sweden, and Jyväskylä in Finland.5, 22-24 Data were obtained in January 2012 for 1204 older adults who were 75 years of age at the time of evaluation (conducted between 1988 and 1991). The sample used in this study consisted of 1027 older adults. Participants were excluded because of missing baseline depression data or missing data on all of the four frailty characteristics.
Measures
FRAILTY CHARACTERISTICS
Individuals were coded as having a frailty characteristic if they scored in the bottom 25th percentile of the total sample of 1027 older adults on that particular assessment.1, 2 Participants were coded as missing if they did not have a value on a frailty assessment. Missingness was coded for the survival analyses to investigate the potential meaning of missing data.
Grip strength
Handgrip strength was assessed in kilograms (kg) of force. Men with grip strength ≤ 36.71 kg and women ≤ 20.90 kg were coded as having the frailty characteristic of low grip strength. 173 of the 1027 were coded as missing.
Gait speed
Ten-meter walking time was coded as meters per second. Men with gait speed ≤ 1.43 m/s and women ≤ 1.25 m/s were coded as having the frailty characteristic of slow gait speed. 145 of the 1027 were coded as missing.
Fatigue
Fatigue was assessed by the Avlund Mob-T Scale that ranges from 0 to 6 with high scores denoting greater fatigue.25 Participants who scored 4 or greater on the scale were coded as having the frailty characteristic of fatigue. 188 of the 1027 were coded as missing.
Physical activity levels
Self-reported physical activity levels ranged from 0-6, with high scores denoting greater physical activity.26 If participants scored 1 or 2, they were coded as having the frailty characteristic of low physical activity levels. 75 of the 1027 were coded as missing.
DEPRESSION
Depressive symptoms were assessed using the Center for Epidemiologic Studies Depression Scale (CES-D27). The scale ranges from 0 to 60 with high scores indicative of greater depressive symptomatology. Scores were presented categorically as nondepressed (CES-D ≤ 9), mildly depressed (CES-D between 10-15), and depressed (CES-D ≥ 16). A cut-off score of ≥ 16 correlates with the presence of a depressive disorder.28, 29
MORTALITY
Time of death for all participants who died following initial assessment was obtained from the official register or from hospital records. Death records were last updated in the fall of 2000, providing a maximum follow-up from initial evaluation of 11.08 years (survival: mean [SD], 10.48 years [.59]; mortality: mean [SD], 5.44 years [2.83]).
Secondary variables (instrumental activities of daily living [IADLs], number of chronic illnesses, and neuropsychological measures of processing speed30, 31 and executive function10, 12 [Digit Symbol Substitution Test of the Wechsler Adult Intelligence Scale-Revised32 and Raven’s Progressive Matrices33]) are included in preliminary analyses.
Statistical Analysis
Analysis of variance or χ2 tests were used to detect differences across the three depressed groups and between men and women for baseline continuous and categorical variables. Post hoc analyses compared only nondepressed and depressed groups. Nonparametric Kruskal-Wallis or Mann-Whitney U procedures were used for variables with skewed distributions across groups. Survival time from baseline was the main outcome, with time observed either to death or to last follow up. We estimated survival curves using the Kaplan-Meier method to examine the pattern in survival time and used log rank tests to detect group differences in the survival curves. An initial omnibus site-stratified Cox proportional hazard model was conducted examining the relationship between gender, total frailty characteristics, and depressive symptoms in the total sample, χ25= 80.52, P < .001. This model identified a main effect of total frailty characteristics, Wald χ21= 7.76, P = .005 (HR, 1.62; 95% CI, 1.15-2.26) and a gender × depressive symptoms interaction, Wald χ21= 5.95, P = .015 (HR, 1.04; 95% CI, 1.01-1.07). Given the significant gender × depression interaction, gender-stratified Cox proportional hazard models were used to examine the effects of frailty characteristics, depression, and depression by frailty characteristic interactions on survival time. Dummy coded variables for frailty, missing data, and depression status were used for single predictor models. For these single predictor models, Bonferroni correction on the false-positive error rate was used to account for multiple comparisons (α of .05 adjusted for four frailty characteristics: α = .0125). Multiple predictor models were used to explore the simultaneous effects of the baseline frailty characteristics in nondepressed and depressed men and women. Hazard ratios with 95% confidence interval were derived to aid interpretation. Age was not entered into the models because participants were all 75 years of age at baseline. Education was not entered because it was coded differently across the three research sites and missing in most participants. Models were conducted with and without number of chronic baseline illnesses entered as a covariate.
Results
Frailty and Depression at Baseline
Baseline demographics, frailty characteristics (total number and values on each individual characteristic), IADLs, number of chronic illnesses, and neuropsychological variables differed by depressed group (Table 1). Total number of frailty characteristics increased across groups (H2 = 28.74, P < .001) with the depressed group having more frailty characteristics than the nondepressed group. Individual frailty characteristics gait speed (F2, 879 = 14.13, P < .001), physical activity level (F2, 949 = 14.73, P < .001), grip strength (F2, 851 = 21.90, P < .001), and levels of fatigue (H2,= 54.05, P < .001) differed by depression status, with each more impaired in the depressed than the nondepressed group. Additionally, IADLs (H2 = 71.28, P < .001), number of chronic illnesses (H2 = 27.85, P < .001), Digit Symbol Substitution Test (F2, 907 = 26.91, P < .001) and Raven’s Progressive Matrices (F2, 909 = 20.99, P < .001) differed by depression status, with the depressed group showing greater impairment than the nondepressed group. Mortality rates were related to depression status in the sample of 1027 elderly, χ22 = 6.93, P = .03, with the rate in the depressed group (54%) greater than that of the nondepressed group (45%).
Table 1.
Baseline characteristics for nondepressed, mildly depressed, and depressed groupsa
| Nondepressed | Mildly Depressed | Depressed | |
|---|---|---|---|
| Variables | (n = 543) | (n = 223) | (n = 261) |
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Sex, No. M/F (% F) | 278/265 (49%) | 74/149 (67%) | 84/177 (68%) b |
| Mortality, No. Alive/Dead (% Dead) | 299/244 (45%) | 125/98 (44%) | 120/141 (54%) b |
| CES-D | 4.14 (3.07) | 12.47 (1.68) | 23.26 (7.26) b |
| Number of Chronic Illnessesτ | n = 280 | n = 141 | n = 156 |
| 1.95 (1.44) | 2.31 (1.42) | 2.69 (1.49) b | |
| Neuropsychological scores | |||
| Digit Symbol | n = 493 | n = 193 | n = 224 |
| 30.30 (12.26) | 25.81 (10.11) | 24.09 (10.21) b | |
| Raven’s Matrices (number correct) | n = 489 | n = 195 | n = 228 |
| 17.23 (4.45) | 15.99 (4.23) | 14.96 (4.73) b | |
| Functioning | |||
| IADLsτ | 1.44 (2.16) | 2.01 (2.48) | 3.31 (3.44) b |
| Frailty Characteristics | |||
| Total Frailty Characteristicsτ | n = 389 | n = 149 | n = 155 |
| Mean | .91 (0.99) | .91 (1.01) | 1.43 (1.11) b |
| No. ≥ 3 frailty characteristics (% Frail) |
27 (7%) | 15 (10%) | 33 (21%) |
| Gait Speed (m/s) | n = 479 | n = 186 | n = 217 |
| Mean | 1.63 (0.41) | 1.56 (0.38) | 1.45 (0.41) b |
| Frailty, No. Yes/No (% Frail) | 163/316 (34%) | 64/122 (34%) | 107/110 (49%) b |
| Grip Strength (kg/f) | n = 460 | n = 187 | n = 207 |
| Mean | 35.28 (12.57) | 29.69 (10.20) | 30.07 (11.75) b |
| Frailty, No. Yes/No (% Frail) | 95/365 (21%) | 57/130 (31%) | 70/137 (34%) b |
| Fatigue Levelsτ | n = 470 | n = 179 | n = 190 |
| Mean | 1.57 (2.09) | 1.75 (1.99) | 2.84 (2.22) b |
| Frailty, No. Yes/No (% Frail) | 104/366 (22%) | 35/144 (20%) | 82/108 (43%) b |
| Physical Activity Levels | n = 509 | n = 207 | n = 236 |
| Mean | 3.10 (0.91) | 2.92 (0.93) | 2.70 (1.01) b |
| Frailty, No. Yes/No (% Frail) | 118/391 (23%) | 64/143 (31%) | 101/135 (43%) b |
Abbreviations: CES-D = Center for Epidemiologic Studies Depression Scale; Nondepressed = CES-D less than or equal to 10; Mildly depressed = CES-D greater than or equal to 10 but less than 16; and Depressed = CES-D greater than or equal to 16; Digit Symbol = Digit Symbol Substitution Test of the Wechsler Adult Intelligence Scale-Revised; Raven’s Matrices = Raven’s Progressive Matrices; IADLs = Instrumental Activities of Daily Living.
Data are presented as means (SD) unless otherwise indicated. Neuropsychological scores are raw scores. Analysis of variance F-tests or χ2 tests were used to detect overall group differences across the three depressed groups for continuous and categorical variables, but only two post hoc group comparisons (nondepressed vs. depressed) were made. Sample sizes are indicated for variables with missing data.
Nonparametric Kruskal-Wallis tests were used to detect overall and pairwise group differences for variables with skewed distributions.
Significant difference in post hoc parametric or nonparametric comparisons between nondepressed and depressed individuals (P ≤ .05).
Baseline differences were also observed between men and women (Table 2). Women presented with greater depressive symptoms, more impairment in IADLs, and worse performance on Raven’s Progressive Matrices compared with men. There were no gender differences on number of chronic illnesses or Digit Symbol Substitution Test. More women had gait impairment, greater fatigue, and lower physical activity levels compared with men; no gender differences were observed, however, for impaired grip strength (Table 2). Mortality rates were related to gender, χ22 = 20.68, P < .001; more men died (55%) than did women (41%).
Table 2.
Baseline characteristics for men and women in the Nordic Research on Ageing Projecta
| Women | Men | |
|---|---|---|
| Variable | (n = 591) | (n = 436) |
| Mean (SD) | Mean (SD) | |
| CES-D | 12.29 (9.51) | 8.81 (8.06) b |
| Number of Chronic Illnesses | n = 350 | n = 227 |
| 2.24 (1.50) | 2.22 (1.44) | |
| Neuropsychological scores | ||
| Digit Symbol | n = 515 | n = 395 |
| 28.65 (11.77) | 28.05 (11.54) | |
| Raven’s Matrices (number correct) | n = 519 | n = 393 |
| 15.79 (4.30) | 17.21 (4.79) b | |
| Functioning | ||
| IADLsτ | 2.33 (2.73) | 1.66 (2.67) b |
| Frailty Characteristics | ||
| Total Frailty Characteristicsτ | n = 382 | n = 311 |
| Mean | 1.12 (1.05) | .91 (1.01) b |
| No. ≥ 3 frailty characteristics (% Frail) | 52 (14%) | 23 (7%) |
| Gait Speed (m/s) | n = 479 | n = 377 |
| Mean | 1.47 (0.35) | 1.71 (0.44) b |
| Frailty, No. Yes/No (% Frail) | 235/270 (49%) | 99/278 (26%) |
| Grip Strength (kg/f) | n = 482 | n = 372 |
| Mean | 25.03 (6.82) | 42.86 (10.04) b |
| Frailty, No. Yes/No (% Frail) | 124/358 (26%) | 98/274 (26%) |
| Fatigue Levelsτ | n = 463 | n = 376 |
| Mean | 2.00 (2.14) | 1.77 (2.18) b |
| Frailty, No. Yes/No (% Frail) | 128/335 (28%) | 93/283 (25%) |
| Physical Activity Levels | n = 542 | n = 410 |
| Mean | 2.89 (0.88) | 3.06 (1.04) b |
| Frailty, No. Yes/No (% Frail) | 176/366 (33%) | 107/303 (26%) |
Abbreviations: CES-D = Center for Epidemiologic Studies Depression Scale; Digit Symbol = Digit Symbol Substitution Test of the Wechsler Adult Intelligence Scale-Revised; Raven’s Matrices = Raven’s Progressive Matrices; IADLs = Instrumental Activities of Daily Living.
Data are presented as means (SD) unless otherwise indicated. Neuropsychological scores are raw scores. Sample sizes are indicated for variables with missing data. Independent sample t-tests or χ2 tests were used to detect gender differences for continuous or categorical variables.
Nonparametric Mann-Whitney U tests were used to detect gender differences for variables with skewed distributions.
Significant difference between men and women (P ≤ .05).
To investigate the relationship between depression and individual frailty characteristics on mortality, a series of site-stratified gender specific Cox regression models were conducted.
Gait Speed
Cox regression models with depression, gait speed, and missing gait speed data predicted survival in men (n = 362; χ25 = 46.98, P < .001) and women (n = 442; χ23 = 42.19, P < .001). In men, there was a main effect of gait impairment (Wald χ21= 11.32, P = .001; HR, 1.85; 95% CI, 1.29-2.64) and a depression by missing gait data interaction (Wald χ21= 7.29, P = .007; HR, 3.39; 95% CI, 1.40-8.21). In women, there was a main effect of depression (Wald χ21= 6.33, P = .012; HR, 1.47; 95% CI, 1.09-1.98), gait impairment (Wald χ21= 15.51, P < .001; HR, 1.99; 95% CI, 1.41-2.81), and missing gait data (Wald χ21= 28.84, P < .001; HR, 3.07; 95% CI, 2.04-4.63); no interactions were observed. The inclusion of number of chronic health illnesses as a covariate did not impact the model in men; in women, its inclusion diminished the effect of depression status on survival.
Grip Strength
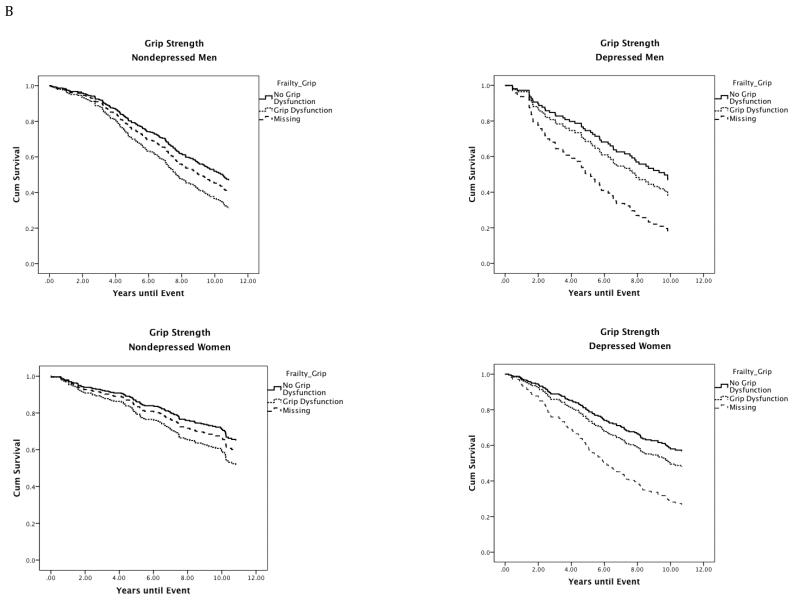
Cox regression models with depression, grip strength, and missing grip strength data predicted survival in men (n = 362; χ25 = 22.59, P < .001) and women (n = 442; χ23 = 30.43, P < .001). In men, there was a depression by missing grip strength data interaction (Wald χ21= 8.57, P = .003; HR, 3.66; 95% CI, 1.54-8.71). In women, there was a main effect of grip impairment (Wald χ21= 6.30, P = .012; HR, 1.59; 95% CI, 1.11-2.82), and missing grip strength data (Wald χ21= 18.70, P < .001; HR, 2.24; 95% CI, 1.55-3.22); no interactions were observed. The inclusion of the covariate did not impact the model in men or women.
Fatigue
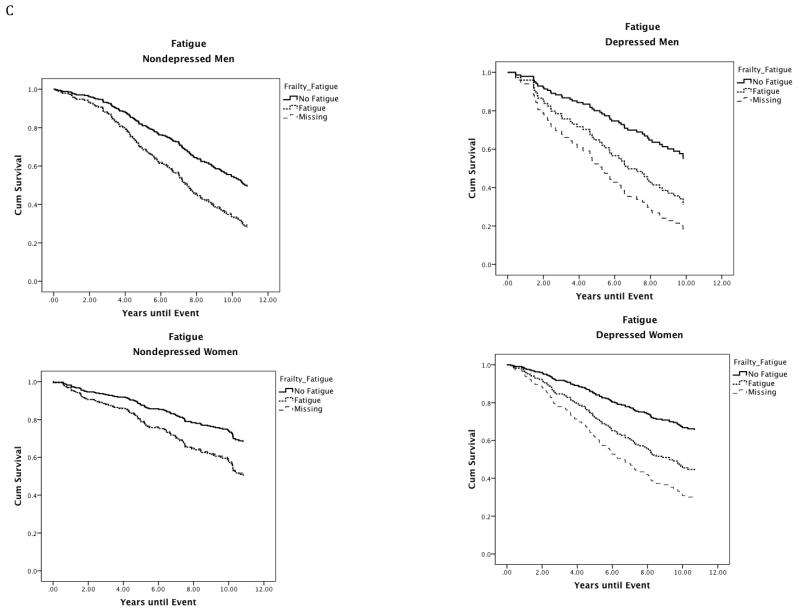
Cox regression models with depression, fatigue, and missing fatigue data predicted survival in men (n = 362; χ2 = 39.88, P < .001) and women (n = 442; χ25 3 = 31.69, P < .001). In men, there was a main effect of fatigue (Wald χ21= 8.03, P = .005; HR, 1.75; 95% CI, 1.19-2.57) and missing fatigue data (Wald χ21= 7.86, P = .005; HR, 2.95; 95% CI, 1.22-3.10). In women, there was a main effect of fatigue (Wald χ21= 11.61, P = .001; HR, 1.87; 95% CI, 1.30-2.67) and missing fatigue data (Wald χ21= 17.86, P < .001; HR, 2.21; 95% CI, 1.53-3.19). No interactions were observed. The inclusion of the covariate did not impact the model in men or women.
Physical Activity Levels
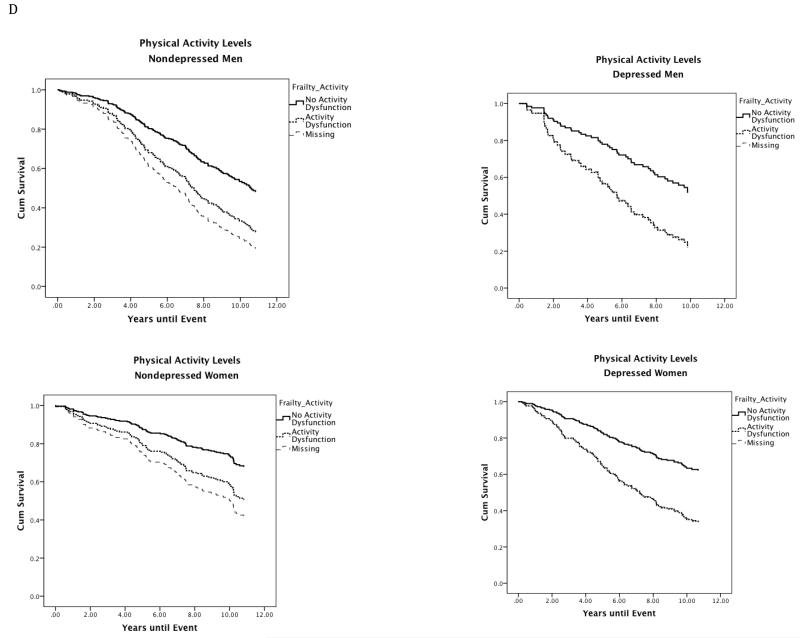
Cox regression models with depression, physical activity, and missing physical activity data predicted survival in men (n = 362; χ23 = 37.38, P < .001) and women (n = 442; χ23 = 33.50, P < .001). In men, there was a main effect of low physical activity (Wald χ21= 27.69, P < .001; HR, 2.37; 95% CI, 1.72-3.26) and missing physical activity data (Wald χ21= 12.54, P < .001; HR, 2.64; 95% CI, 1.54-5.53). In women, there was a main effect of low physical activity (Wald χ21= 19.82, P < .001; HR, 2.11; 95% CI, 1.52-2.93), and missing physical activity data (Wald χ21= 8.05, P = .005; HR, 1.97; 95% CI, 1.23-3.15). The inclusion of the covariate did not impact either model.
Gender, Frailty, and Depression
The survival curves displayed in Figure 1 illustrate that frailty characteristics, missing data on these frailty characteristics, and depression affect survival in both men and women. To explore the most salient frailty characteristics in predicting mortality in both nondepressed and depressed men and women, four multiple-predictor, site-stratified models were conducted. Each model [nondepressed men (n = 278; χ28 = 33.95, P < .001), nondepressed women (n = 265; χ28 = 25.20, P = .001), depressed men (n = 84; χ28 = 31.51, P < .001), and depressed women (n = 177; χ28 = 32.00, P < .001)] predicted risk of death.
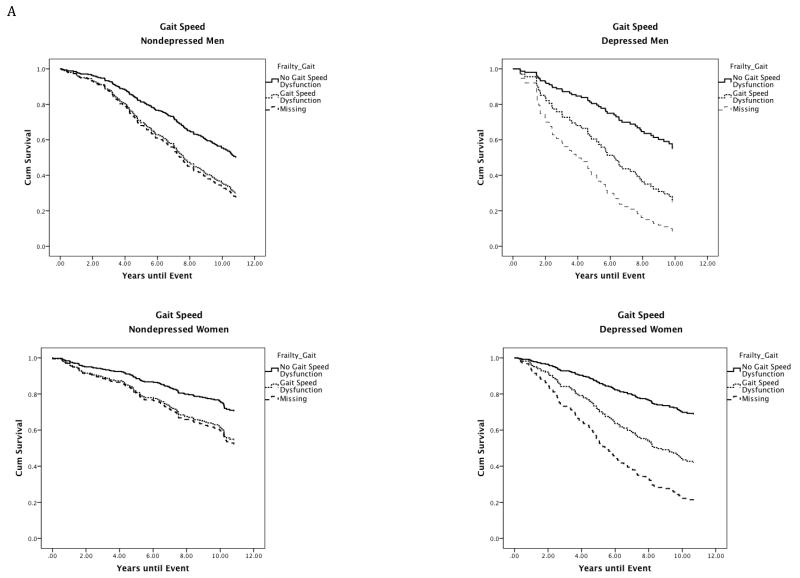
Figure 1.
Site stratified single predictor Kaplan-Meier survival curves for individual frailty characteristics in depressed and nondepressed men and women.
Note. Nondepressed = Center for Epidemiologic Studies Depression Scale less than or equal to 9; Depressed = Center for Epidemiologic Studies Depression Scale greater than or equal to 16; Gait Speed: No Gait Speed = Walking speed in meters per second in the gender-specific top 75th percentile; Gait Speed Dysfunction = Walking speed in meters per second in the gender-specific bottom 25th percentile (men: less than or equal to 1.43 m/s; women: less than or equal to 1.25 m/s); Grip Strength: No Grip Dysfunction = Grip strength in kilograms of force in the gender-specific top 75th percentile; Grip Dysfunction = Grip strength in kilograms of force in the gender-specific bottom 25th percentile (men: less than or equal to 36.71 kg; Women: less than or equal to 20.90 kg); Fatigue: No Fatigue = Individuals who scored less than 4 on the fatigue scale (denoting less exhaustion on select activities) scored in the top 75th percentile. Fatigue: Individuals who scored 4 or greater on the Fatigue scale (denoting greater Fatigue) scored in the bottom 25th percentile; Physical Activity Levels: No Physical Activity Dysfunction = Individuals who responded to the statement: Free time activity over the past year is best described as: moderate physical activity about 3 hours per week (3), moderate physical activity over 4 hours per week or intense physical activity up to 4 hours per week (4), active sports at least 3 hours per week (4), competitive sports (6) were in the top 75th percentile. Physical Activity Dysfunction = Individuals who responded to the statement Free time activity over the past year is best described as: mainly sitting in one place (1) or light physical activity (2) were in the bottom 25th percentile.
The pattern differed by gender and depression status (Table 3). For nondepressed men low physical activity, missing physical activity data, and impaired gait speed increased risk of death after controlling for the effect of all other frailty characteristics. In nondepressed women only impaired grip strength was predictive of death. In depressed men only missing fatigue data increased risk of death. In depressed women, however, impaired gait speed and fatigue were associated with higher risk of death, although the overall effect of fatigue was not significant due to the lack of effect of missing data on the fatigue assessment. Inclusion of number of chronic health illnesses as a covariate did not contribute to either depressed model. In nondepressed men, its inclusion decreased the effect of fatigue and gait impairment on survival. In the nondepressed women, its inclusion decreased the effect of low physical activity and fatigue, and increased the effect of gait and grip impairment on survival.
Table 3.
Site stratified proportional hazards models with multiple predictors of frailty characteristics for depressed and nondepressed men and women.
| Nondepressed (n=543) |
Depressed (n=261) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men (n=278) |
Women (n=265) |
Men (n=84) |
Women (n=177) |
|||||||||
| Frailty Predictors | Wald χ2 |
Hazard ratio (95% CI) |
p Value |
Wald χ2 |
Hazard ratio (95% CI) |
p Value |
Wald χ2 |
Hazard ratio (95% CI) |
p Value |
Wald χ2 |
Hazard ratio (95% CI) |
p Value |
| Low Physical Activities | 10.48 | 0.005 | 1.25 | 0.535 | 1.81 | 0.404 | 3.66 | 0.160 | ||||
| Yes vs. No | 7.27 | 1.76 (1.17, 2.65) | 0.007 | 1.13 | 1.34 (0.78, 2.28) | 0.287 | 1.61 | 1.68 (0.76, 3.74) | 0.204 | 2.86 | 1.56 (0.93, 2.61) | 0.091 |
| Missing vs. No | 4.85 | 2.80 (1.12, 7.01) | 0.028 | 0.01 | 0.95 (0.36, 2.50) | 0.917 | 0.09 | 1.25 (0.29, 5.46) | 0.765 | 0.19 | 0.81 (0.32, 2.05) | 0.662 |
| Fatigue | 4.90 | 0.086 | 3.67 | 0.160 | 6.46 | 0.040 | 5.62 | 0.060 | ||||
| Yes vs. No | 3.82 | 1.51 (1.00, 2.28) | 0.051 | 2.73 | 1.54 (0.92, 2.56) | 0.098 | 1.02 | 1.48 (0.69, 3.15) | 0.313 | 5.40 | 1.94 (1.11, 3.40) | 0.020 |
| Missing vs. No | 2.24 | 1.46 (0.89, 2.41) | 0.134 | 1.96 | 1.52 (0.85, 2.74) | 0.161 | 6.29 | 2.90 (1.26, 6.67) | 0.012 | 2.81 | 1.66 (0.92, 3.00) | 0.094 |
| Slow Gait Speed | 5.99 | 0.050 | 1.89 | 0.388 | 2.17 | 0.338 | 6.69 | 0.035 | ||||
| Yes vs. No | 4.96 | 1.54 (1.05, 2.26) | 0.026 | 1.04 | 1.30 (0.79, 2.14) | 0.307 | 1.01 | 1.52 (0.67, 3.45) | 0.315 | 4.59 | 1.84 (1.05, 3.21) | 0.032 |
| Missing vs. No | 1.92 | 1.71 (0.80, 3.64) | 0.166 | 1.38 | 1.85 (0.66, 5.18) | 0.240 | 1.95 | 2.67 (0.67, 10.59) | 0.163 | 5.47 | 2.71 (1.18, 6.26) | 0.019 |
| Low Grip Strength | 2.34 | 0.310 | 4.92 | 0.085 | 0.64 | 0.725 | 1.60 | 0.452 | ||||
| Yes vs. No | 0.01 | 1.02 (0.65, 1.59) | 0.934 | 4.64 | 1.83 (1.06, 3.18) | 0.031 | 0.63 | 1.35 (0.64, 2.81) | 0.429 | 0.43 | 1.19 (0.70, 2.02) | 0.513 |
| Missing vs. No | 2.19 | 0.55 (0.25, 1.22) | 0.139 | 0.85 | 1.52 (0.63, 3.69) | 0.355 | 0.05 | 1.15 (0.33, 4.02) | 0.827 | 1.46 | 1.59 (0.75, 3.36) | 0.228 |
Cox proportional hazard models were used to explore the simultaneous effects of the baseline frailty characteristics in separate models for nondepressed and depressed men and women. Wald χ2 values are listed; (df = 2 for the overall effect of each frailty characteristic, df= 1 for each subgroup comparison); Hazard ratios (and 95% CI) are for multiple predictor models with the inclusion of other frailty characteristics as covariates.
As depicted in Figure 1A, depression did little to alter the effect of impaired gait speed on mortality in men (mortality rates, nondepressed men: no gait impairment = 48%; gait impairment = 75%; depressed men: no gait impairment=45%; gait impairment = 71%). In women, however, the effect of impaired gait speed on mortality nearly doubled when depression was present (mortality rates, nondepressed women: no gait impairment = 26%; gait impairment = 40%; depressed women: no gait impairment=32%; gait impairment = 58%). A similar pattern was observed for fatigue (Figure 1C).
Conclusions
The syndrome of frailty and depressive illness are each associated with increased disability and death, but the confluence of each has rarely been studied.1, 2, 4, 16, 19, 20, 31, 34-41 This study investigated the relationship between depressive symptoms, characteristics of frailty, and mortality in 75-year old men and women. The results show that 1) the effect of each frailty characteristic, missing data on each frailty characteristic, and depression status on progression to death differed between men and women; 2) in depressed men, missing data on the assessment of gait speed, grip strength, or fatigue resulted in an increased risk of death; and 3) the combined effect of depression and characteristics of frailty, specifically fatigue and impaired gait speed, on mortality is particularly deleterious for older women.
The study hypothesis that impaired gait speed and grip strength would be the most salient characteristics for predicting mortality in the older depressed group was only partially observed. Low grip strength was only predictive of mortality in women, independent of depression status, and only when none of the other frailty characteristics were considered. Subgroup analysis showed that mobility disturbance and fatigue were strongly associated with increased risk of death in older depressed women. These results are consistent with past research demonstrating the impact fatigue and worsening mobility have on prognosis,24, 42-46 although these studies did not account for depression. Frailty characteristics have been shown to induce a self-perpetuating cycle that develop incrementally with weakness emerging early in the process and weight loss and fatigue representing a final pathway towards frailty.1-3 Fatigue, however, is a common symptom in late life depression and can effect prognosis in elderly people.47 These findings, particularly those observed in depressed women, emphasize the importance of accounting for the presence of depression in frailty research; depressed elders may have a different level of impairment and a different clinical course.
The presence of depressive illness in the context of frailty has until recently21 been largely ignored.16, 19, 20 The adverse effect of depression on frailty characteristics in this study however was pervasive, with depressive symptoms associated with increased weakness, mobility deficits, and fatigue, as well as illness burden, and neuropsychological impairment.11, 12, 21, 48-51 Although these findings highlight the strength and severity of these associations, currently the nature of the relationship between the syndrome of frailty and depressive illness remains unclear. There are a number of possible models to explain the depressed-frail phenotype. First, the two conditions may be unrelated to each other but frequently coexist in the elderly (as with cataracts and arthritis). Second, one condition may be a risk factor for developing the other. In this model it is critical to know whether their onset is concurrent or whether one generally precedes the other. Third, the relationship maybe an illusion; two disorders that appear to be distinct are really different manifestations of the same disorder. For example, there is now evidence to suggest that depression is an early symptom of Huntington’s disease rather than a separable diagnosis.52 Longitudinal data need to be examined to test these models.
Research examining the nature of the relationship between depressive illness and the syndrome of frailty in late life will have treatment implications. Currently, there are multiple nonpharmacological treatments such as exercise or nutritional interventions53-57 that have been designed to improve strength in lower extremities to improve mobility and decrease fall potential.4 Questions remain however about the utility of these interventions in depressed elders, as these studies typically exclude patients with depressive illness and the overall effectiveness of exercise interventions as a treatment for depression is at best mixed.58-60 Likewise, although antidepressant medications such as selective serotonin reuptake inhibitors (SSRIs) have been shown to be effective for the treatment of late life depression,61 there is currently no evidence that these medications can effectively treat characteristics of frailty, particularly characteristics such as slowed gait speed that are strongly associated with mortality. Given these findings, an important direction for future research would be an investigation of the effectiveness of antidepressant medication on frailty characteristics in older depressed frail patients, as well as the feasibility and effectiveness of multimodal interventions (exercise and antidepressant medication) to improve prognosis in older depressed prefrail and frail individuals. Although side effect profiles in older depressed patients appear similar to those of younger depressed patients,62 caution must be taken with SSRI use in a prefrail or frail geriatric population as treatment with these medications is associated with increased risk of falls,63 hyponatremia, and bleeding.
There are important limitations to this study. The NORA study included 75-year old men and women from Glostrup in Denmark, Göteborg in Sweden and Jyväskylä in Finland; the generalizability of these results to other localities may be limited. Also, the use of the CES-D as a diagnostic tool for depressive illness is suboptimal. The cut-off of ≥ 16 used to denote depression, however, has been shown to have 100% sensitivity and 88% specificity for major depressive disorder in elders.27-29 Of note, individuals with missing data on frailty characteristics do not appear to represent a random sample; we can only hypothesize the meaning behind the missing data and its relationship to outcome, which is included in supplemental material online. Additionally, this investigation has limited power to assess interactions between depression and each of the frailty characteristics in the multiple predictor models due to sample size constraints. As such, subgroup analyses were used and interpretation of these results should be made with caution. Future research is needed to better understand the interrelationships between depression and individual frailty characteristics and their impact on prognosis.
In conclusion, this study showed that the confluence of characteristics of frailty (fatigue, slow gait speed) and depressive illness resulted in an increased risk of death in older adults; specifically, the presence of depression nearly doubled the effect of fatigue and slow gait speed on mortality rates in older women. Future research should investigate whether multimodal interventions targeting depressive illness, mobility deficits, and fatigue can decrease mortality and improve quality of life in older depressed individuals with characteristics of the syndrome of frailty.
Supplementary Material
Acknowledgements
This research was supported by grants T32 MH20004. The Nordic Research on Ageing Project was supported by the Nordea-Foundation, The Danish Medical Research Council, Denmark, Academy of Finland, Ministry of Education, Ministry of Social Affairs and Health, Social Insurance Institution, and the city of Jyväskylä, Finland.
Dr. Roose has served as a consultant for Medtronics. Dr. Devanand has received research support from Novartis and Eli Lilly, and has served as a consultant to Bristol Myers Squibb and Sanofi-Aventis. These sponsors had no role in this current manuscript.
Dr. Brown had full access to all of the data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
from the National Institute of Mental Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Drs. Brown, Liu, Fieo, Avlund, Rutherford, Rantanen, and Sneed have nothing to disclose. Dr. Roose has served as a consultant for Medtronics. Dr. Devanand has received research support from Novartis and Eli Lilly, and has served as a consultant to Bristol Myers Squibb and Sanofi-Aventis. These sponsors had no role in this current manuscript.
References
- 1.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006 Mar;61(3):262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 3.Fried LP, Ettinger WH, Lind B, Newman AB, Gardin J, Cardiovascular Health Study Research Group Physical disability in older adults: a physiological approach. J Clin Epidemiol. 1994 Jul;47(7):747–760. doi: 10.1016/0895-4356(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 4.Xue QL, Bandeen-Roche K, Varadhan R, Zhou J, Fried LP. Initial manifestations of frailty criteria and the development of frailty phenotype in the Women’s Health and Aging Study II. J Gerontol A Biol Sci Med Sci. 2008 Sep;63(9):984–990. doi: 10.1093/gerona/63.9.984. [DOI] [PubMed] [Google Scholar]
- 5.Rantanen T, Avlund K, Suominen H, Schroll M, Frandin K, Pertti E. Muscle strength as a predictor of onset of ADL dependence in people aged 75 years. Aging Clin Exp Res. 2002 Jun;14(3 Suppl):10–15. [PubMed] [Google Scholar]
- 6.Rantanen T, Sakari-Rantala R, Heikkinen E. Muscle strength before and mortality after a bone fracture in older people. Scand J Med Sci Sports. 2002 Oct;12(5):296–300. doi: 10.1034/j.1600-0838.2002.102100.x. [DOI] [PubMed] [Google Scholar]
- 7.Gum AM, King-Kallimanis B, Kohn R. Prevalence of mood, anxiety, and substance-abuse disorders for older Americans in the national comorbidity survey-replication. Am J Geriatr Psychiatry. 2009 Sep;17(9):769–781. doi: 10.1097/JGP.0b013e3181ad4f5a. [DOI] [PubMed] [Google Scholar]
- 8.Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997 Oct;54(10):915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 9.Alexopoulos GS, Meyers BS, Young RC, Kakuma T, Silbersweig D, Charlson M. Clinically defined vascular depression. Am J Psychiatry. 1997 Apr;154(4):562–565. doi: 10.1176/ajp.154.4.562. [DOI] [PubMed] [Google Scholar]
- 10.Sneed JR, Culang ME, Keilp JG, Rutherford BR, Devanand DP, Roose SP. Antidepressant medication and executive dysfunction: a deleterious interaction in late-life depression. Am J Geriatr Psychiatry. 2010 Feb;18(2):128–135. doi: 10.1097/JGP.0b013e3181c796d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sneed JR, Rindskopf D, Steffens DC, Krishnan KR, Roose SP. The vascular depression subtype: evidence of internal validity. Biol Psychiatry. 2008 Sep 15;64(6):491–497. doi: 10.1016/j.biopsych.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sneed JR, Roose SP, Keilp JG, Krishnan KR, Alexopoulos GS, Sackeim HA. Response inhibition predicts poor antidepressant treatment response in very old depressed patients. Am J Geriatr Psychiatry. 2007 Jul;15(7):553–563. doi: 10.1097/JGP.0b013e3180302513. [DOI] [PubMed] [Google Scholar]
- 13.de Groot JC, de Leeuw FE, Oudkerk M, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and depressive symptoms in elderly adults. Arch Gen Psychiatry. 2000 Nov;57(11):1071–1076. doi: 10.1001/archpsyc.57.11.1071. [DOI] [PubMed] [Google Scholar]
- 14.Taylor WD, Steffens DC, MacFall JR, et al. White matter hyperintensity progression and late-life depression outcomes. Arch Gen Psychiatry. 2003 Nov;60(11):1090–1096. doi: 10.1001/archpsyc.60.11.1090. [DOI] [PubMed] [Google Scholar]
- 15.Sheline YI, Pieper CF, Barch DM, et al. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010 Mar;67(3):277–285. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mezuk B, Edwards L, Lohman M, Choi M, Lapane K. Depression and frailty in later life: a synthetic review. Int J Geriatr Psychiatry. 2011 Oct 7; doi: 10.1002/gps.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang SS, Weiss CO, Xue QL, Fried LP. Patterns of comorbid inflammatory diseases in frail older women: the Women’s Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2010 Apr;65(4):407–413. doi: 10.1093/gerona/glp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang SS, Weiss CO, Xue QL, Fried LP. Association between inflammatory-related disease burden and frailty: Results from the Women’s Health and Aging Studies (WHAS) I and II. Arch Gerontol Geriatr. 2011 Jul 13; doi: 10.1016/j.archger.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mezuk B, Lohman M, Dumenci L, Lapane KL. Are Depression and Frailty Overlapping Syndromes in Mid- and Late-life? A Latent Variable Analysis. Am J Geriatr Psychiatry. 2012 May 16; doi: 10.1016/j.jagp.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz IR. Depression and frailty: the need for multidisciplinary research. Am J Geriatr Psychiatry. 2004 Jan-Feb;12(1):1–6. [PubMed] [Google Scholar]
- 21.Lakey SL, LaCroix AZ, Gray SL, et al. Antidepressant use, depressive symptoms, and incident frailty in women aged 65 and older from the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2012 May;60(5):854–861. doi: 10.1111/j.1532-5415.2012.03940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kauppinen M, Davidsen M, Valter S. Design, material and methods in the NORA study. Nordic Research on Ageing. Aging Clin Exp Res. 2002 Jun;14(3 Suppl):5–9. [PubMed] [Google Scholar]
- 23.Schroll M, Steen B, Berg S, Heikkinen E, Viidik A. NORA--Nordic research on ageing. Functional capacity of 75-year-old men and women in three Nordic localities. Dan Med Bull. 1993 Nov;40(5):618–624. [PubMed] [Google Scholar]
- 24.Avlund K, Rantanen T, Schroll M. Tiredness and subsequent disability in older adults: The role of walking limitations. J Gerontol A Biol Sci Med Sci. 2006 Nov;61(11):1201–1205. doi: 10.1093/gerona/61.11.1201. [DOI] [PubMed] [Google Scholar]
- 25.Avlund K, Kreiner S, Schultz-Larsen K. Functional ability scales for the elderly. European Journal of Public Health. 1996;6:35–42. [Google Scholar]
- 26.Grimby G. Physical activity and muscle training in the elderly. Acta Med Scand Suppl. 1986;711:233–237. doi: 10.1111/j.0954-6820.1986.tb08956.x. [DOI] [PubMed] [Google Scholar]
- 27.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 28.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977 Sep;106(3):203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- 29.Beekman AT, Deeg DJ, Van Limbeek J, Braam AW, De Vries MZ, Van Tilburg W. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in The Netherlands. Psychol Med. 1997 Jan;27(1):231–235. doi: 10.1017/s0033291796003510. [DOI] [PubMed] [Google Scholar]
- 30.Brown PJ, Devanand DP, Liu X, Caccappolo E. Functional impairment in elderly patients with mild cognitive impairment and mild Alzheimer disease. Arch Gen Psychiatry. 2011 Jun;68(6):617–626. doi: 10.1001/archgenpsychiatry.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown PJ, Liu X, Sneed JR, Pimontel MA, Devanand DP, Roose SP. Speed of Processing and Depression Affect Function in Older Adults With Mild Cognitive Impairment. Am J Geriatr Psychiatry. 2012 Apr 27; doi: 10.1016/j.jagp.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.W DA. Manual for the Wechsler Adult Intelligence Scale-Revised. Psychological Corporation; New York: 1981. [Google Scholar]
- 33.Raven J, Raven JC, Court JH. Manual for Raven’s Progressive Matrices and Vocabulary Scales. Harcourt Assessment; San Antonio, TX: 2003. updated 2004. [Google Scholar]
- 34.Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. N Engl J Med. 2010 Apr 1;362(13):1173–1180. doi: 10.1056/NEJMoa0909087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin CC, Li CI, Chang CK, et al. Reduced health-related quality of life in elders with frailty: a cross-sectional study of community-dwelling elders in Taiwan. PLoS One. 2011;6(7):e21841. doi: 10.1371/journal.pone.0021841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruce ML. Depression and disability in late life: directions for future research. Am J Geriatr Psychiatry. 2001;9(2):102–112. Spring. [PubMed] [Google Scholar]
- 37.Chapman DP, Perry GS. Depression as a major component of public health for older adults. Prev Chronic Dis. 2008 Jan;5(1):A22. [PMC free article] [PubMed] [Google Scholar]
- 38.Gallo JJ, Rebok GW, Tennsted S, Wadley VG, Horgas A. Linking depressive symptoms and functional disability in late life. Aging Ment Health. 2003;7(14578009):469–480. doi: 10.1080/13607860310001594736. [DOI] [PubMed] [Google Scholar]
- 39.Gurland B, Wilder BE, Berkman C. Depression and disability in the elderly: reciprocal relations and changes with age. Int J Geriatr Psychiatry. 1988;3:163–179. [Google Scholar]
- 40.Kennedy G. The dynamics of depression and disability. American Journal of Geriatric Psychiatry. 2001;9(2):99–101. [PubMed] [Google Scholar]
- 41.Kennedy GJ, Kelman HR, Thomas C. The emergence of depressive symptoms in late life: the importance of declining health and increasing disability. J Community Health. 1990 Apr;15(2):93–104. doi: 10.1007/BF01321314. [DOI] [PubMed] [Google Scholar]
- 42.Shamliyan T, Talley KM, Ramakrishnan R, Kane RL. Association of frailty with survival: A systematic literature review. Ageing Res Rev. 2012 Mar 12; doi: 10.1016/j.arr.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Verghese J, Holtzer R, Lipton RB, Wang C. Mobility stress test approach to predicting frailty, disability, and mortality in high-functioning older adults. J Am Geriatr Soc. 2012 Oct;60(10):1901–1905. doi: 10.1111/j.1532-5415.2012.04145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng H, Gurland BJ, Maurer MS. Self-reported lack of energy (anergia) among elders in a multiethnic community. J Gerontol A Biol Sci Med Sci. 2008 Jul;63(7):707–714. doi: 10.1093/gerona/63.7.707. [DOI] [PubMed] [Google Scholar]
- 45.White DK, Neogi T, Nevitt MC, et al. Trajectories of Gait Speed Predict Mortality in Well-Functioning Older Adults: The Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2012 Oct 9; doi: 10.1093/gerona/gls197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manty M, de Leon CF, Rantanen T, et al. Mobility-related fatigue, walking speed, and muscle strength in older people. J Gerontol A Biol Sci Med Sci. 2012 May;67(5):523–529. doi: 10.1093/gerona/glr183. [DOI] [PubMed] [Google Scholar]
- 47.Meeks TW, Vahia IV, Lavretsky H, Kulkarni G, Jeste DV. A tune in “a minor” can “b major”: a review of epidemiology, illness course, and public health implications of subthreshold depression in older adults. J Affect Disord. 2011 Mar;129(1-3):126–142. doi: 10.1016/j.jad.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brandler TC, Wang C, Oh-Park M, Holtzer R, Verghese J. Depressive Symptoms and Gait Dysfunction in the Elderly. Am J Geriatr Psychiatry. 2011 Mar 17; doi: 10.1097/JGP.0b013e31821181c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bruce ML, Seeman TE, Merrill SS, Blazer DG. The impact of depressive symptomatology on physical disability: MacArthur Studies of Successful Aging. Am J Public Health. 1994 Nov;84(11):1796–1799. doi: 10.2105/ajph.84.11.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glassman AH. Depression and cardiovascular comorbidity. Dialogues Clin Neurosci. 2007;9(1):9–17. doi: 10.31887/DCNS.2007.9.1/ahglassman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Butters MA, Young JB, Lopez O, et al. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci. 2008;10(3):345–357. doi: 10.31887/DCNS.2008.10.3/mabutters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krogias C, Strassburger K, Eyding J, et al. Depression in patients with Huntington disease correlates with alterations of the brain stem raphe depicted by transcranial sonography. J Psychiatry Neurosci. 2011 May;36(3):187–194. doi: 10.1503/jpn.100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rolland Y, Dupuy C, Abellan van Kan G, Gillette S, Vellas B. Treatment strategies for sarcopenia and frailty. Med Clin North Am. 2011 May;95(3):427–438. doi: 10.1016/j.mcna.2011.02.008. ix. [DOI] [PubMed] [Google Scholar]
- 54.Waters DL, Baumgartner RN, Garry PJ, Vellas B. Advantages of dietary, exercise-related, and therapeutic interventions to prevent and treat sarcopenia in adult patients: an update. Clin Interv Aging. 2010;5:259–270. doi: 10.2147/cia.s6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Binder EF, Schechtman KB, Ehsani AA, et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc. 2002 Dec;50(12):1921–1928. doi: 10.1046/j.1532-5415.2002.50601.x. [DOI] [PubMed] [Google Scholar]
- 56.Pahor M, Blair SN, Espeland M, et al. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006 Nov;61(11):1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 57.Rejeski WJ, Miller ME, King AC, et al. Predictors of adherence to physical activity in the Lifestyle Interventions and Independence for Elders pilot study (LIFE-P) Clin Interv Aging. 2007;2(3):485–494. [PMC free article] [PubMed] [Google Scholar]
- 58.Teri L, McCurry SM, Logsdon RG, Gibbons LE, Buchner DM, Larson EB. A randomized controlled clinical trial of the Seattle Protocol for Activity in older adults. J Am Geriatr Soc. 2011 Jul;59(7):1188–1196. doi: 10.1111/j.1532-5415.2011.03454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brenes GA, Williamson JD, Messier SP, et al. Treatment of minor depression in older adults: a pilot study comparing sertraline and exercise. Aging Ment Health. 2007 Jan;11(1):61–68. doi: 10.1080/13607860600736372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Forsman AK, Schierenbeck I, Wahlbeck K. Psychosocial interventions for the prevention of depression in older adults: systematic review and meta-analysis. J Aging Health. 2011 Apr;23(3):387–416. doi: 10.1177/0898264310378041. [DOI] [PubMed] [Google Scholar]
- 61.Sneed JR, Rutherford BR, Rindskopf D, Lane DT, Sackeim HA, Roose SP. Design makes a difference: a meta-analysis of antidepressant response rates in placebo-controlled versus comparator trials in late-life depression. Am J Geriatr Psychiatry. 2008 Jan;16(1):65–73. doi: 10.1097/JGP.0b013e3181256b1d. [DOI] [PubMed] [Google Scholar]
- 62.Roose SP, Sackeim HA. Late Life Depression. Oxford University Press; New York: 2004. [Google Scholar]
- 63.Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009 Nov 23;169(21):1952–1960. doi: 10.1001/archinternmed.2009.357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.