Abstract
Renal arterial embolization (RAE) performed for the treatment of renal masses has been proven to be a safe and effective technique, with several decades of experience. RAE is well tolerated with few complications, particularly if the time interval from embolization to surgery is reduced to less than 48 hours. Review of the literature suggests that RAE is also extremely effective for palliation of symptoms in the setting of nonoperative advanced stage renal cell carcinoma. In addition, this technique plays a large role in the management of angiomyolipomas that are symptomatic or at risk of spontaneous rupture. To date, RAE has not been evaluated in a randomized controlled setting, which has contributed to its underutilization. All of these potential benefits warrant the need for prospective studies for further validation.
Keywords: embolization, renal cell carcinoma, angiomyolipoma, preoperative, interventional radiology
Objectives: Upon completion of this article, the reader will be able to describe the role of embolization of renal cell carcinoma and angiomyolipoma, both as adjunctive as well as definitive therapy.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Renal cell carcinoma (RCC) is the most common malignant renal tumor, comprising an estimated 2 to 3% of all malignancies with an estimated 64,770 new cases diagnosed in the United States1 in 2012. Common symptoms associated with RCC include hematuria, flank pain, and a palpable mass. Patients presenting with symptoms usually have advanced stage disease. The natural history of RCC has changed, with the majority (∼70%) now diagnosed incidentally on routine abdominal imaging.2 Despite the associated stage migration due to increase in incidental detection, there remain a significant proportion of patients presenting with advanced stage disease. Approximately 30 to 40% of patients with renal tumors will either present with or later develop metastatic disease, and death rate from RCC remains high, with 13,570 disease-related deaths1 2 in 2012.
Prognosis significantly changes with stage, ranging from a 5-year overall survival (OS) of 90% for localized disease to 10% for advanced stage disease.1 For localized RCC, surgical resection is the treatment of choice. Partial nephrectomy (nephron-sparing surgery) to preserve renal function is preferred over radical nephrectomy if technically feasible and has been shown to not compromise survival outcomes.1 2 For patients unable to undergo surgery, local ablative therapies remain an option.
Renal angiomyolipoma (AML) is the most common mesenchymal renal tumor, with a reported incidence of 0.3 to 3% in the general population.3 4 Renal AMLs can occur either in a sporadic form or in association with tuberous sclerosis (TS). AMLs are generally considered to be benign; though in rare cases, it can be locally aggressive with either extension into the renal vein and inferior vena cava or dissemination into adjacent lymph nodes.4 AMLs are typically asymptomatic and found incidentally on imaging. However, a minority present with hematuria, flank pain, or spontaneous hemorrhage. Thus, treatment of symptomatic AMLs or those at risk of hemorrhage is recommended.5 6 7
Almgård first popularized renal artery embolization (RAE) in the 1970s and initial interest in the technique resulted in multiple published series in the 1980s and 1990s.8 9 10 Indications for RAE for the treatment of renal masses include as an adjunctive preoperative treatment before nephrectomy for primary kidney masses; palliation of symptoms related to advanced stage RCC; and primary treatment of AML.
Anatomic and Technical Considerations
Multiple previous review articles have detailed the anatomic and technical considerations for RAE.9 10 11 Classically, single renal arteries arise from the abdominal aorta at the level of the L1–L2 interspace. The main renal arteries branch into anterior and posterior divisions, followed by segmental, lobar, interlobar, and arcuate arteries. Variant anatomy can occur in greater than 30% of the general population, with either early division of the main renal arteries or extrarenal arteries further subdivided into accessory (hilar) or aberrant (polar) entry into the kidney.
Arterial vascular access is typically gained via the right common femoral artery and a vascular sheath (5 French) placed. Through the vascular sheath, aortography can be performed to locate the origin of the renal arteries with a flush catheter placed slightly superior to the expected origin of the renal arteries. Selection of the renal arteries is performed with shaped catheters such as a SOS-shaped, Cobra, or Simmons-shaped catheter. Superselective catheterization of the renal arteries can be performed with a microcatheter and microwire. Embolization materials can be introduced into the desired target vessels. If ethanol is chosen as the embolic material, occlusion balloon catheter delivery systems are typically used.
There is wide variation in embolization agents used for RAE as highlighted in Table 1. No specific agent has been demonstrated to be more efficacious than others in the setting of RAE. Several principles specific to RAE should be noted in choosing an embolic agent. First, vessels in the kidney are considered “end arteries” without significant intrarenal collaterals. Second, RCCs are hypervascular tumors that recruit extrarenal collateral vascular supply. As such, utilization of an agent that results in permanent small vessel/capillary bed occlusion/sclerosis such as NBCA glue, ethanol, PVA, or Embospheres (Merit Medical, South Jordan, UT) in addition to large vessel occlusion with coils is desired.11 12 13 14 15
Table 1. Case series published in the English literature, in order of year of publication, agents used for therapeutic embolization, number of patients, and indication for embolization.
| First author | Year of publication | Method | Number of patients | Preoperative | Palliative | Not specified |
|---|---|---|---|---|---|---|
| Almgård8 | 1977 | Muscle particles | 38 | 29 | 9 | |
| Frasson53 | 1978 | Gelfoam | 45 | 35 | 10 | |
| Schulman54 | 1980 | Coils Gelfoam Gelfoam + coils ICBA |
3 10 9 4 |
26 | 2 | |
| Frasson55 | 1981 | Coils Gelfoam Gelfoam + coils ICBA Muscle particles |
6 226 3 7 2 |
241 | 41 | |
| Giuliani56 | 1981 | Coils Gelatin foam ICBA |
1 14 25 |
40 | ||
| Kato57 | 1981 | Encaps MMC + gel sponge | 33 | 23 | 10 | |
| Mobilio58 | 1981 | Gelfoam ICBA |
41 1 |
41 1 |
||
| Wallace38 | 1981 | Gelfoam + coils | 100 | 74 | 26 | |
| LeGuillou59 | 1982 | Not specified | 247 | 203 | 44 | |
| Teasdale60 | 1982 | Coils Gelfoam Gelfoam + coils |
3 22 3 |
26 | 2 | |
| Bono61 | 1983 | Avitene Gelfoam ICBA |
47 48 4 |
47 | 48 | 4 |
| Nakano62 | 1983 | Gelatin sponge ICBA |
21 | 12 | 9 | |
| Ekelund63 | 1984 | Ethanol | 20 | 6 | 14 | |
| Kaisary64 | 1984 | Coils Gelatin sponge Lyodura Thrombin Combinations of above |
25 | 25 | ||
| Kurth65 | 1984 | Coils Ethanol Gelfoam ICBA Thrombin |
25 | 25 | ||
| McIvor66 | 1984 | Coils Dura particles Gelfoam Thrombin |
29 | 29 | ||
| Mebust67 | 1984 | Coils + gelatin sponge Ethanol |
41 5 |
40 | 6 | |
| Christensen68 | 1985 | Coils | 36 | 36 | ||
| Gottesman69 | 1985 | Coil + Gelfoam | 30 | 30 | ||
| Klimberg70 | 1985 | Ethanol | 25 | 24 | 1 | |
| Lammer71 | 1985 | Coils Ethanol Gelfoam Ivalon |
4 7 85 25 |
81 | 40 | |
| Leinonen72 | 1985 | Ethanol Gelfoam |
12 18 |
10 11 |
2 7 |
|
| Weigel73 | 1985 | Coils Ethanol Gelfoam |
22 | 22 | ||
| Chudácek74 | 1986 | Ethibloc Gelaspon Vilan |
30 | 30 | ||
| Karwowski75 | 1987 | Gelfoam Gelfoam + coils |
81 39 |
30 | 39 | |
| Kurth76 | 1987 | Coils Ethanol Gelfoam ICBA |
33 | 33 | ||
| Nurmi77 | 1987 | Ethanol Ethanol + coils Gelfoam Gelfoam + coils ICBA ICBA + coils ICBA + coils + Gelfoam |
4 3 3 2 10 2 1 |
25 | ||
| Stoesslein78 | 1988 | Detachable balloons Histoacryl Ivalon Combinations |
147 | 100 | 47 | |
| Swanson79 | 1988 | Coils Gelfoam Ivalon Gelfoam + BCG |
134 11 |
145 | ||
| Kato80 | 1989 | Encaps MMC Encaps MMC + Gelfoam |
44 129 |
99 | 74 | |
| Lanigan81 | 1992 | Ethanol | 35 | 35 | ||
| Bakal82 | 1993 | Ethanol Ethanol + coils Ethanol + Gelfoam |
22 1 1 |
22 1 1 |
||
| Park83 | 1994 | Ethanol | 27 | 27 |
Results
Current indications for RAE include palliation for advanced stage RCC; preoperative embolization before nephrectomy; treatment for angiomyolipoma; and as an adjunctive therapy to ablation for RCC.
To date, no prospective randomized controlled clinical trials have been performed to evaluate the role of RAE in these settings. In 1999, Kalman and Varenhorst published a survey of the pertinent literature on RAE, which was later adapted by Madoff et al in 2006.9 10
Table 1 highlights some of the limitations of the previous evidence accumulated in the 20th century. These studies were limited by design, many being observational with significant variation in both patient selection criteria and embolic agents used, making it difficult to draw firm conclusions.
Given the wide variation in results and lack of strong clinical evidence, preoperative RAE has largely fallen out of favor. Scattered case series have been published since 2000 as summarized in Table 2, and have not altered the role of RAE.
Table 2. Case series published in the English literature since 2000 for renal arterial embolization performed in the setting of renal cell carcinoma, in order of year of publication, number of patients, indication for embolization, conclusions, and study limitations.
| First author | Year of publication | Number of patients | Indication | Study conclusions | Study limitations |
|---|---|---|---|---|---|
| Zielinski17 | 2000 | 118 | Preoperative | RAE with improved overall 5- and 10-y survival as compared with 116 case-matched controls (62 vs. 47, 35 vs. 23%) particularly for T2, T3, and node-positive disease | Single center Variability in time delay from embolization to surgery (Only 56% performed 1–3 d before surgery) Variability in embolization agents |
| Onishi22 | 2001 | 24 | Palliation | RAE effective in palliation with 75% improvement in symptoms and increased overall survival (229 vs. 116 d) compared with similar controls | 7-mo overall survival of RAE poorer than 17.8 mo reported for cytoreductive nephrectomy, though cohort of this study had poorer performance status |
| Munro21 | 2003 | 25 | Palliation | RAE effective in palliation with 70% improvement in symptoms | |
| Maxwell19 | 2007 | 19 | Palliation | RAE effective for palliation with 18/19 symptom control | |
| Schwartz15 | 2007 | 121 | 66 preoperative 15 AML 13 vascular lesions 8 palliation 19 other |
RAE well tolerated with minimal complications, and suggestion of improved intraoperative times and decreased blood loss in the perioperative setting | Outcomes study with no matched controls for comparison |
| May16 | 2009 | 227 | Preoperative | RAE with no difference in overall survival and trend toward worsened cancer-specific survival as compared with oncologic matched controls | Poor control matching with controls taken from 1992 to 2006 as compared with a RAE historical group (1992–1997) Coils used as the sole embolic agent in 95% of cases |
| Subramanian18 | 2009 | 135 | Preoperative advanced stage RCC with IVC involvement | RAE with no measurable benefit in reducing blood loss or perioperative complications, with increased perioperative mortality and trend toward increased transfusion requirement | Poor control matching with embolization group with increased ASA scores in 3–4 (97 vs. 90%), increased vascular tumor thrombus extent (66 vs. 48%) level III–IV, and use of vascular bypass (52 vs. 28%) |
| Mukund20 | 2010 | 8 | Palliation | 88% effective at palliation of symptoms | Small series of case reports |
Abbreviations: AML, angiomyolipoma; IVC, inferior vena cava; RAE, renal artery embolization; RCC, renal cell carcinoma.
Preoperative Embolization
Benefits of RAE in the preoperative setting include a decrease in perioperative blood loss, creation of a tissue plane of edema facilitating dissection, and reduction in tumor bulk including extent of vascular thrombus, when present. Wide variation in reporting markers such as reduction in intraoperative blood loss, transfusion requirements, surgical procedure time, surgical complications, and survival outcomes has limited its use to local practice patterns10 (Fig. 1).
Figure 1.
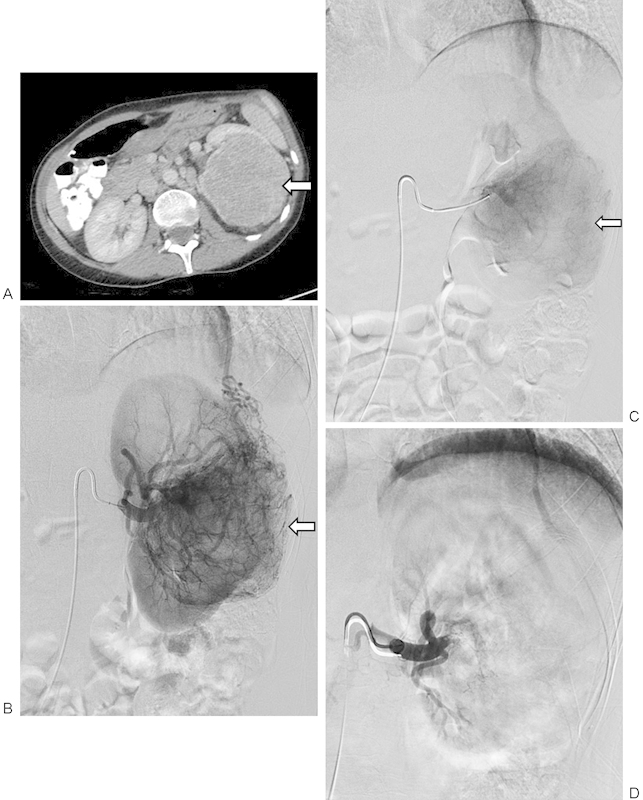
A 53-year-old woman with 11.7 cm left renal cell carcinoma that underwent preoperative left renal arterial embolization followed by uneventful left nephrectomy with a reported total estimated blood loss of 300 mL. (A) Axial contrast-enhanced computed tomography image of the enhancing left renal mass (arrow). (B) Selective left renal arteriogram demonstrating a large hypervascular renal cell carcinoma (arrow) before embolization. (C) Microcatheter-mediated embolization of the hypervascular mass using tris-acryl gelatin microspheres to stasis. Note the decreased regions of tumor vascularity (arrow). (D) Postembolization selective left renal arteriogram demonstrating near-complete embolization of the left renal artery to stasis.
In an observational study by Schwartz et al performed at the authors' institution, there was a perceived benefit of both decreased intraoperative transfusions and operative time.15 The study population included those with advanced stage disease with mean tumor size of 11.2 cm. Almost half of all patients had vascular invasion, with reported blood loss of only 1,048 mL (median 725 mL) and average patient transfusion requirements over their entire hospital course of 3.9 units. In the subset of patients who did not have vascular invasion, mean blood loss was lower (mean 647 mL, median 425 mL). Described complications were minor (none requiring hospitalization) and surgical times and survival outcomes were not analyzed.
Recent studies by May et al and Zielinski et al include case-controlled matched historical cohorts with survival outcomes that are contradictory.16 17 May et al concluded no survival benefit from RAE, while Zielinski et al concluded significant survival benefit. This difference may be a result of different selection processes of the control groups. In the study by Zielinski et al, controls were chosen from the same historical cohort and matched in age, sex, tumor size, grade, and stage. In the report by May et al, controls were chosen from the timeframe of 1992 to 2006, with baseline oncology characteristics matched based on propensity scores to the RAE cohort performed within the timeframe of 1992 to 1997. In both series, surgical complications were similar in both control and RAE groups; intraoperative blood loss and intraoperative times were not directly evaluated. May et al did demonstrate increased postoperative transfusion requirements in the RAE cohort.
Subramanian et al provided comparisons of those undergoing preoperative RAE to controls in regard to intraoperative times, blood loss, and immediate perioperative complication and mortality rates.18 In this study, preoperative RAE was associated with increased median operative times (390 vs. 313 minutes), median intraoperative transfusion requirements of 8 versus 4 units of packed red blood cells, and perioperative mortality of 13% versus 3%. However, the control population was not equal with the preoperative RAE group, with the latter being composed of patients of higher tumor stage, American Society of Anesthesiology (ASA) scores, and need for the use of intraoperative cardiopulmonary bypass.
Palliation
Several recent small series (n = 8–25 patients) have focused on RAE in the setting of palliation,19 20 21 22 demonstrating favorable results for symptom control. Treatment indications commonly included palliation of hematuria and/or flank pain, and less commonly control of paraneoplastic syndromes and palpable masses. Mukund and Gamanagatti demonstrated symptom control in 7/8 (88%) patients, with gross hematuria controlled in 6/7 (86%) patients and flank pain controlled in 1 patient at 6 months.20 Maxwell et al demonstrated symptom control in 18/19 (95%) patients with hematuria controlled in 13/13 (100%) patients and flank pain in 8/9 (89%) patients.19 Munro et al also demonstrated a 70% effectiveness in symptom control, with only a 12% rate of recurrent hematuria requiring hospital admission.21 Onishi et al22 compared 30 historical controls with 24 patients who underwent RAE who were matched for age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, and stage. Similar to the other studies, RAE was 75% effective in symptomatic control (14 patients with gross hematuria and 5 patients with flank pain). Interestingly, the RAE group showed a statistically significant median survival benefit of 229 versus 116 days for the control group, suggestive of a possible improvement in outcome either through a cyto-reductive effect or via immunomodulation. This result, while encouraging, is still inferior to surgical palliation, with 7-month median survival in the RAE group versus 17.8 months median survival gained in the setting of palliative nephrectomy.23
RAE in the setting of palliation is well tolerated, although postembolization syndrome is common after RAE (reported in 14/24 (57%) patients in one study). Postembolization syndrome is well controlled with antipyretics, analgesics, and antiemetics, usually resolving in 3 days. Median hospital stay after RAE is 4 to 5 days.
Treatment for Angiomyolipoma
RAE is well established as the first-line therapy for the treatment of AML. Indications for RAE include symptomatic AMLs such as those with prior or active hemorrhage, flank pain, hematuria, or mass effect. AMLs larger than 4 cm have been shown to be associated with symptoms (80–90%) and spontaneous hemorrhage (50–60%). Therefore, prophylactic treatment of AMLs greater than 4 cm serves as another indication for RAE24 25 26 (Fig. 2). Multiple retrospective series have been published since 2000 that provide long-term follow-up confirming safety and long-term efficacy of RAE for AML (Table 3).
Figure 2.
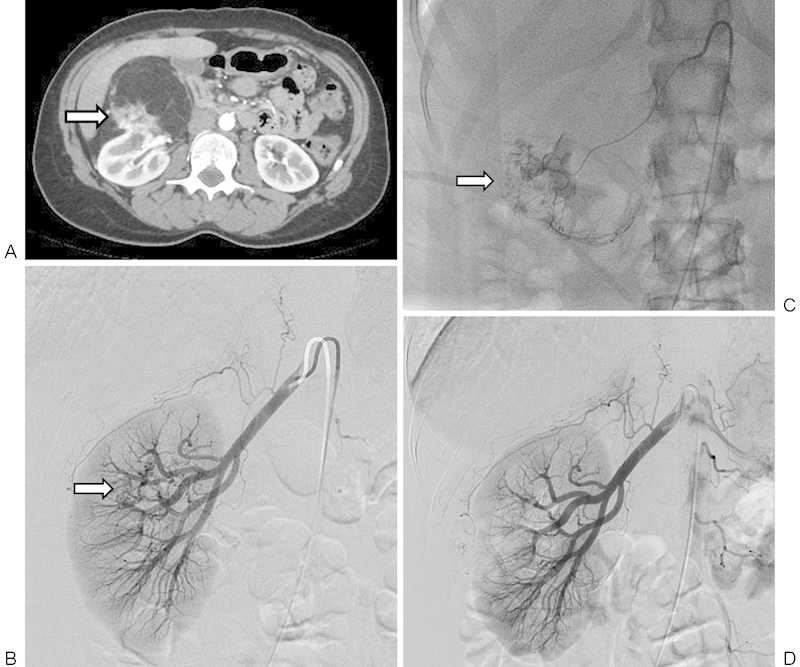
A 45-year-old woman with incidental 9.4 cm right renal angiomyolipoma that underwent renal arterial embolization. (A) Axial contrast-enhanced computed tomography image of angiomyolipoma demonstrating predominantly fatty components, vascular components (arrow), and origin of the mass from the kidney. (B) Selective right renal arteriogram demonstrating a small area of heterogeneity and hypervascularity overlying the midpole region of the right kidney (arrow). (C) Microcatheter-mediated embolization of the hypervascular aspects of the mass using tris-acryl gelatin microspheres to stasis (arrow). (D) Postembolization selective right renal arteriogram demonstrating complete embolization of the right AML.
Table 3. Case series published in the English literature since 2000 for renal arterial embolization performed in the setting of angiomyolipoma, in order of year of publication, number of patients, mean size, indication for embolization, embolization agents and outcomes.
| First author | Year of publication | Number of patients | Mean size (cm) | Indication | Agents | Outcomes |
|---|---|---|---|---|---|---|
| Ewalt36 | 2005 | 16 with 27 AMLs (16 TS) |
Not specified:4–21 range | 13 symptomatic 3 asymptomatic |
PVA or Embospheres (50–750 μM) + fibered coils | No documented recurrence with 7 patients followed up 3–9 y |
| Kothary5 | 2005 | 19 with 30 AMLs (9 sporadic/10 TS) | Not specified | 16 symptomatic 3 asymptomatic |
Ethanol + lipiodol (1 also with coils) |
31.6% recurrence rate all in TS group with mean f/u 51.5 mo Median time to recurrence 78.7 mo (mean 81.3) |
| Williams37 | 2006 | 16 with 20 AMLs (16 TS) |
Not specified | PVA (200–1,200 μM) + liquid coils (3 also with stainless steel coils) | No documented recurrence or rehemorrhage with mean f/u of 40 mo | |
| Lenton33 | 2008 | 17 (5 sporadic/12 TS) |
Not specified | 13 symptomatic 4 asymptomatic |
PVA (350–500 μM) +/− coils | 29% recurrent treatment rate with repeat embolization |
| Chick84 | 2010 | 34 (16 with multifocal AML) (25 sporadic/9 TS) |
11.9 (2.9–24.4) |
26 symptomatic 8 asymptomatic |
Ethanol + lipiodol (2 also with PVA and 3 also with coils) |
15% recurrence rate with mean f/u of 44.2 mo. 2 patients with repeat embolization and 3 with surgery offered |
| Lee85 | 2009 | 11 (7 sporadic/4 TS) | 8.6 (4.5–12.8) | 9 symptomatic 2 asymptomatic |
Gelfoam + coils | 18% recurrence rate with mean f/u of 28.2 mo. 1 recurrence with nephrectomy at 40 mo another with repeat embolization at 2 mo and 7 y |
| Ramon6 | 2009 | 41 with 48 AMLs (33 sporadic/8 TS) | 10.3 (2.5–20) | 21 symptomatic 21 asymptomatic |
Ethanol + PVA (50–150 μM) and coils in 7 cases | 29% predicted recurrence rate at 5 y from Kaplan–Meyer estimates (71% reembolization free survival) and 3 with surgery offered (94% surgery free) |
| Takebayashi86 | 2009 | 10 with 10 AMLs (10 Sporadic) | 7.0 (4.5–11.0) | 10 asymptomatic | Ethanol | No documented recurrences at mean f/u of 2.2 y |
| Bishay87 | 2010 | 16 with 23 AMLs (4 sporadic/12 TS) | 15 (10–25) | Ethanol + lipiodol | 19% recurrence rate requiring repeat embolization with mean f/u of 29 mo (1 failure with hemorrhage at 59 mo requiring repeat embolization) | |
| Chan35 | 2011 | 27 with 28 AMLs (26 sporadic/1 TS) | 10.9 (4–30) | 15 symptomatic 13 asymptomatic |
Microcoils Ethanol + lipiodol Ethanol + coils PVA (50–150 μM) + coils |
37% recurrence rate at 5 y from Kaplan–Meyer estimates (63% treatment free survival) and 4 with surgery performed (85% surgery free) Size > 10 cm predictive of failure requiring surgery |
| Chatziioannou88 | 2012 | 10 with 12 AMLs (8 sporadic/2 TS) | 8 (5–12) | 7 symptomatic 3 asymptomatic |
PVA + coils or Embospheres +/− coils | 20% recurrence/failure rate at mean f/u of 9 mo with 1 failure needing urgent surgery at 4 d and 1 recurrence needing repeat embolization at 6 mo |
| Patatas89 | 2013 | 13 with 13 AMLs (11 sporadic/2 TS) | 6.2 (4.2–10) | Not specified (12 performed electively) | Microcoils, Embospheres (500–700 μM), or a combination of both | 23% recurrence/retreatment rate with a median f/u of 46 mo |
Abbreviations: AML, angiomyolipoma; PVA, polyvinyl alcohol; TS, tuberous sclerosis.
These studies show overall durable tumor treatments with low surgical salvage rates, with recurrence rates ranging from 0 to 37%. The majority of recurrences are successfully managed with repeat RAE. Complications are rare with no episodes of renal insufficiency attributable to embolization documented in any series.27 Postembolization syndrome is the most common complication, occurring in up to 64% of patients in one series. Most cases are self-limited and successfully treated with antipyretics and nonsteroidal anti-inflammatories. Major reported complications are limited to abscess formation, coagulative necrosis, and self-limited nontarget embolization to normal renal parenchyma, all successfully treated without long-term adverse sequelae.
The proven efficacy of nephron sparing surgery (NSS) in the setting of RCC has renewed interest in surgical options for AML, and multiple recent series evaluating the use of NSS as an alternative to embolization for AMLs have been published.28 29 30 31 Proponents of NSS as compared with embolization for AML argue that NSS provides more durable outcomes and also offers pathology in case of malignancy. However, NSS has increased long-term adverse sequelae associated with the procedure, with Boorjian et al reporting a 5% urinary leak rate and Heidenreich et al reporting a 7% urinary fistula rate.28 30 Therefore, NSS is currently limited to the setting of failed RAE.
The choice of embolization agent for AML is controversial. Common agents include ethanol, PVA, and Embospheres (Merit Medical). The use of Onyx (Covidien, Plymouth, MN) in AML treatment has also been reported.32 The utility of coils remains unclear with some authors advocating their use, and others suggesting that their use promotes collateral formation around the level of occlusion. Lenton et al reported a high intraprocedural aneurysm rupture rate (30%) as compared with most groups that reported no episodes of rupture. These authors conveyed that this may be attributed to the use of PVA alone rather than PVA and coils.33 Villalta et al found that smaller microspheres (< 150 μm) were associated with both an increased recurrence rate requiring repeat embolization (odds ratio (OR) 5.88) and risk of pulmonary complications; these complications resulted in the authors advocating for larger sized particles.34
Large AMLs (those sized 10 cm or greater) are considered more resistant to RAE. In a series reported by Chan et al, subgroup analysis demonstrated that recurrences necessitating surgery only occurred when treating AMLs > 10 cm in size.35 Larger tumors are thought to be more difficult to embolize due to their size, multiple feeding vessels, and greater difficultly in isolating them from normal renal parenchyma.
While several series suggest efficacy of treatment of AMLs associated with TS,36 37 active surveillance of this high-risk group is prudent. In fact, Kothary et al suggest that AMLs treated with RAE in this group may be associated with higher rate of regrowth.5
Future Directions
Significant variation in the literature exists for the optimal time from embolization to nephrectomy. In their review of the literature, Kalman and Varenhorst concluded that the optimal delay from embolization to nephrectomy should be less than 48 hours.9 Postembolization syndrome (characterized by lumbar pain, nausea, and fever) occurs in a majority of patients 1 to 3 days postprocedure, and can be minimized with nephrectomy performed within this timeframe. Additionally, surgery is technically more difficult 72 hours after embolization, which is thought to be secondary to collateral vessel formation.38 Controversy surrounds the optimal timing of resection; some authors suggest a delay of 24 to 48 hours after embolization, which allows edema to develop facilitating surgical dissection, while other authors have suggested that there should be as minimal delay as possible to prevent collateral vessel formation.13 14 15
Reducing the time between RAE and surgery can minimize postembolization syndrome. In a small series by Lin et al, 8 patients underwent concomitant RAE and surgical resection as compared with 14 control patients who underwent conventional staged preoperative embolization.39 The concomitant group had no instances of postinfarction syndrome as compared with 36% in the staged group, thereby increasing patient comfort, decreasing hospital stay, and as a result reducing health care costs. Another series by Carvajal et al studied seven patients who underwent concomitant RAE and surgical resection.40 This study was limited to tumors > 13 cm in size (Stage II, IIIA–B), but did demonstrate safety and increased patient comfort.
Combined Therapies
In treatment of primary hepatic malignancies, there is a growing body of evidence indicating that transarterial embolization therapy in conjunction with localized ablative therapies result in improved patient outcomes as compared with either monotherapy alone.41 42 43 The two therapies are thought to be synergistic, with the decreased blood flow from embolization resulting in decreased heat loss during radiofrequency ablation.44 45 RAE may also serve as a useful adjunctive technique to thermal ablative therapies performed in the setting of nonoperable RCC. The synergistic effect of the two modalities may also have a positive impact on immunomodulatory mechanisms. Li et al combined cryoablation and RAE, and showed a decrease in T-regulatory cells thought to be related to a large volume of cell necrosis and release of tumor antigens.46 The promotion of T-regulatory cells and inflammation is thought to be one method by which tumor cells can subvert normal immune regulation methods.47 In clinical practice, the role of RAE in combination with local ablation setting is still largely undefined, with only a few case reports and small series performed demonstrating feasibility and safety.48 49 50 51 52
Conclusion
RAE is a safe and effective technique that is well tolerated with few complications, particularly if the time interval from embolization to surgery is reduced to less than 48 hours.
To date, RAE has not been evaluated in a randomized controlled setting, which has contributed to its underutilization. RAE serves as the initial treatment in the management of symptomatic AMLs or those at risk for spontaneous rupture (> 4 cm). In addition, RAE is extremely effective for palliation of symptoms in the setting of nonoperative advanced stage RCC. Potential benefits of this procedure for preoperative RCC treatment include reduction in intraoperative blood loss and operative time by facilitating dissection. Finally, there is suggestion of improved survival benefit after RAE, possibly through immunomodulatory mechanisms. All of these potential benefits warrant the need for prospective studies for further validation.
References
- 1.Motzer R J, Agarwal N, Beard C. et al. Kidney cancer. J Natl Compr Canc Netw. 2011;9(9):960–977. doi: 10.6004/jnccn.2011.0082. [DOI] [PubMed] [Google Scholar]
- 2.Russo P. The role of surgery in the management of early-stage renal cancer. Hematol Oncol Clin North Am. 2011;25(4):737–752. doi: 10.1016/j.hoc.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Arenson A M, Graham R T, Shaw P, Srigley J, Herschorn S. Angiomyolipoma of the kidney extending into the inferior vena cava: sonographic and CT findings. AJR Am J Roentgenol. 1988;151(6):1159–1161. doi: 10.2214/ajr.151.6.1159. [DOI] [PubMed] [Google Scholar]
- 4.Katabathina V S, Vikram R, Nagar A M, Tamboli P, Menias C O, Prasad S R. Mesenchymal neoplasms of the kidney in adults: imaging spectrum with radiologic-pathologic correlation. Radiographics. 2010;30(6):1525–1540. doi: 10.1148/rg.306105517. [DOI] [PubMed] [Google Scholar]
- 5.Kothary N, Soulen M C, Clark T W. et al. Renal angiomyolipoma: long-term results after arterial embolization. J Vasc Interv Radiol. 2005;16(1):45–50. doi: 10.1097/01.RVI.0000143769.79774.70. [DOI] [PubMed] [Google Scholar]
- 6.Ramon J, Rimon U, Garniek A. et al. Renal angiomyolipoma: long-term results following selective arterial embolization. Eur Urol. 2009;55(5):1155–1161. doi: 10.1016/j.eururo.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 7.Sauk S, Zuckerman D A. Renal artery embolization. Semin Intervent Radiol. 2011;28(4):396–406. doi: 10.1055/s-0031-1296082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almgård L E, Slezak P. Treatment of renal adenocarcinoma by embolization: a follow-up of 38 cases. Eur Urol. 1977;3(5):279–281. doi: 10.1159/000472115. [DOI] [PubMed] [Google Scholar]
- 9.Kalman D, Varenhorst E. The role of arterial embolization in renal cell carcinoma. Scand J Urol Nephrol. 1999;33(3):162–170. doi: 10.1080/003655999750015934. [DOI] [PubMed] [Google Scholar]
- 10.Madoff D, Verma R, Ahrar K. Germany: Springer-Verlag; 2006. Embolotherapy for organ ablation; pp. 201–20. [Google Scholar]
- 11.Sauk S, Zuckerman D A. Renal artery embolization. Semin Intervent Radiol. 2011;28(4):396–406. doi: 10.1055/s-0031-1296082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ginat D T, Saad W E, Turba U C. Transcatheter renal artery embolization: clinical applications and techniques. Tech Vasc Interv Radiol. 2009;12(4):224–239. doi: 10.1053/j.tvir.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Loffroy R, Rao P, Kwak B K. et al. Transcatheter arterial embolization in patients with kidney diseases: an overview of the technical aspects and clinical indications. Korean J Radiol. 2010;11(3):257–268. doi: 10.3348/kjr.2010.11.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loffroy R Rao P Ota S Geschwind J F Renal artery embolisation prior to radical nephrectomy for renal cell carcinoma: when, how and why? Br J Radiol 201083991630, author reply 631–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz M J, Smith E B, Trost D W, Vaughan E D Jr. Renal artery embolization: clinical indications and experience from over 100 cases. BJU Int. 2007;99(4):881–886. doi: 10.1111/j.1464-410X.2006.06653.x. [DOI] [PubMed] [Google Scholar]
- 16.May M, Brookman-Amissah S, Pflanz S, Roigas J, Hoschke B, Kendel F. Pre-operative renal arterial embolisation does not provide survival benefit in patients with radical nephrectomy for renal cell carcinoma. Br J Radiol. 2009;82(981):724–731. doi: 10.1259/bjr/17514226. [DOI] [PubMed] [Google Scholar]
- 17.Zielinski H, Szmigielski S, Petrovich Z. Comparison of preoperative embolization followed by radical nephrectomy with radical nephrectomy alone for renal cell carcinoma. Am J Clin Oncol. 2000;23(1):6–12. doi: 10.1097/00000421-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Subramanian V S, Stephenson A J, Goldfarb D A, Fergany A F, Novick A C, Krishnamurthi V. Utility of preoperative renal artery embolization for management of renal tumors with inferior vena caval thrombi. Urology. 2009;74(1):154–159. doi: 10.1016/j.urology.2008.12.084. [DOI] [PubMed] [Google Scholar]
- 19.Maxwell N J, Saleem Amer N, Rogers E, Kiely D, Sweeney P, Brady A P. Renal artery embolisation in the palliative treatment of renal carcinoma. Br J Radiol. 2007;80(950):96–102. doi: 10.1259/bjr/31311739. [DOI] [PubMed] [Google Scholar]
- 20.Mukund A, Gamanagatti S. Ethanol ablation of renal cell carcinoma for palliation of symptoms in advanced disease. J Palliat Med. 2010;13(2):117–120. doi: 10.1089/jpm.2009.0243. [DOI] [PubMed] [Google Scholar]
- 21.Munro N P, Woodhams S, Nawrocki J D, Fletcher M S, Thomas P J. The role of transarterial embolization in the treatment of renal cell carcinoma. BJU Int. 2003;92(3):240–244. doi: 10.1046/j.1464-410x.2003.04314.x. [DOI] [PubMed] [Google Scholar]
- 22.Onishi T, Oishi Y, Suzuki Y, Asano K. Prognostic evaluation of transcatheter arterial embolization for unresectable renal cell carcinoma with distant metastasis. BJU Int. 2001;87(4):312–315. doi: 10.1046/j.1464-410x.2001.00070.x. [DOI] [PubMed] [Google Scholar]
- 23.Tigrani V S, Reese D M, Small E J, Presti J C Jr, Carroll P R. Potential role of nephrectomy in the treatment of metastatic renal cell carcinoma: a retrospective analysis. Urology. 2000;55(1):36–40. doi: 10.1016/s0090-4295(99)00395-7. [DOI] [PubMed] [Google Scholar]
- 24.Halpenny D, Snow A, McNeill G, Torreggiani W C. The radiological diagnosis and treatment of renal angiomyolipoma-current status. Clin Radiol. 2010;65(2):99–108. doi: 10.1016/j.crad.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Oesterling J E, Fishman E K, Goldman S M, Marshall F F. The management of renal angiomyolipoma. J Urol. 1986;135(6):1121–1124. doi: 10.1016/s0022-5347(17)46013-7. [DOI] [PubMed] [Google Scholar]
- 26.Soulen M C, Faykus M H Jr, Shlansky-Goldberg R D, Wein A J, Cope C. Elective embolization for prevention of hemorrhage from renal angiomyolipomas. J Vasc Interv Radiol. 1994;5(4):587–591. doi: 10.1016/s1051-0443(94)71558-x. [DOI] [PubMed] [Google Scholar]
- 27.Lee S Y, Hsu H H, Chen Y C. et al. Evaluation of renal function of angiomyolipoma patients after selective transcatheter arterial embolization. Am J Med Sci. 2009;337(2):103–108. doi: 10.1097/MAJ.0b013e31817f6dd9. [DOI] [PubMed] [Google Scholar]
- 28.Boorjian S A, Frank I, Inman B. et al. The role of partial nephrectomy for the management of sporadic renal angiomyolipoma. Urology. 2007;70(6):1064–1068. doi: 10.1016/j.urology.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 29.Fazeli-Matin S, Novick A C. Nephron-sparing surgery for renal angiomyolipoma. Urology. 1998;52(4):577–583. doi: 10.1016/s0090-4295(98)00236-2. [DOI] [PubMed] [Google Scholar]
- 30.Heidenreich A, Hegele A, Varga Z, von Knobloch R, Hofmann R. Nephron-sparing surgery for renal angiomyolipoma. Eur Urol. 2002;41(3):267–273. doi: 10.1016/s0302-2838(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 31.Yip S K, Tan P H, Cheng W S, Li M K, Foo K T. Surgical management of angiomyolipoma: nephron-sparing surgery for symptomatic tumour. Scand J Urol Nephrol. 2000;34(1):32–35. doi: 10.1080/003655900750016850. [DOI] [PubMed] [Google Scholar]
- 32.Katsanos K, Sabharwal T, Ahmad F, Dourado R, Adam A. Onyx embolization of sporadic angiomyolipoma. Cardiovasc Intervent Radiol. 2009;32(6):1291–1295. doi: 10.1007/s00270-008-9481-7. [DOI] [PubMed] [Google Scholar]
- 33.Lenton J, Kessel D, Watkinson A F. Embolization of renal angiomyolipoma: immediate complications and long-term outcomes. Clin Radiol. 2008;63(8):864–870. doi: 10.1016/j.crad.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Villalta J D, Sorensen M D, Durack J C, Kerlan R K, Stoller M L. Selective arterial embolization of angiomyolipomas: a comparison of smaller and larger embolic agents. J Urol. 2011;186(3):921–927. doi: 10.1016/j.juro.2011.04.082. [DOI] [PubMed] [Google Scholar]
- 35.Chan C K, Yu S, Yip S, Lee P. The efficacy, safety and durability of selective renal arterial embolization in treating symptomatic and asymptomatic renal angiomyolipoma. Urology. 2011;77(3):642–648. doi: 10.1016/j.urology.2010.08.040. [DOI] [PubMed] [Google Scholar]
- 36.Ewalt D H, Diamond N, Rees C. et al. Long-term outcome of transcatheter embolization of renal angiomyolipomas due to tuberous sclerosis complex. J Urol. 2005;174(5):1764–1766. doi: 10.1097/01.ju.0000177497.31986.64. [DOI] [PubMed] [Google Scholar]
- 37.Williams J M, Racadio J M, Johnson N D, Donnelly L F, Bissler J J. Embolization of renal angiomyolipomata in patients with tuberous sclerosis complex. Am J Kidney Dis. 2006;47(1):95–102. doi: 10.1053/j.ajkd.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 38.Wallace S, Chuang V P, Swanson D. et al. Embolization of renal carcinoma. Radiology. 1981;138(3):563–570. doi: 10.1148/radiology.138.3.7465831. [DOI] [PubMed] [Google Scholar]
- 39.Lin P H, Terramani T T, Bush R L, Keane T E, Moore R G, Lumsden A B. Concomitant intraoperative renal artery embolization and resection of complex renal carcinoma. J Vasc Surg. 2003;38(3):446–450. doi: 10.1016/s0741-5214(03)00429-4. [DOI] [PubMed] [Google Scholar]
- 40.Carvajal R, Orgaz A, Leal J I. et al. Renal embolization and nephrectomy in a single surgical act in high-risk renal tumor pathology. Ann Vasc Surg. 2011;25(2):222–228. doi: 10.1016/j.avsg.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 41.Peng Z W, Zhang Y J, Chen M S. et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31(4):426–432. doi: 10.1200/JCO.2012.42.9936. [DOI] [PubMed] [Google Scholar]
- 42.Peng Z W, Zhang Y J, Liang H H, Lin X J, Guo R P, Chen M S. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology. 2012;262(2):689–700. doi: 10.1148/radiol.11110637. [DOI] [PubMed] [Google Scholar]
- 43.Ni J Y, Liu S S, Xu L F, Sun H L, Chen Y T. Transarterial chemoembolization combined with percutaneous radiofrequency ablation versus TACE and PRFA monotherapy in the treatment for hepatocellular carcinoma: a meta-analysis. J Cancer Res Clin Oncol. 2013;139(4):653–659. doi: 10.1007/s00432-012-1369-x. [DOI] [PubMed] [Google Scholar]
- 44.Peng Z W, Chen M S. Transcatheter arterial chemoembolization combined with radiofrequency ablation for the treatment of hepatocellular carcinoma. Oncology. 2013;84 01:40–43. doi: 10.1159/000345888. [DOI] [PubMed] [Google Scholar]
- 45.Rossi S, Garbagnati F, Lencioni R. et al. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000;217(1):119–126. doi: 10.1148/radiology.217.1.r00se02119. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Guo Z, Liu C F. et al. Effect of transcatheter renal arterial embolization combined with cryoablation on regulatory CD4+CD25+ T lymphocytes in the peripheral blood of patients with advanced renal carcinoma. Cryobiology. 2012;65(1):56–59. doi: 10.1016/j.cryobiol.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Finn O J. Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol. 2012;(23) 08:6–9. doi: 10.1093/annonc/mds256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamakado K, Nakatsuka A, Kobayashi S. et al. Radiofrequency ablation combined with renal arterial embolization for the treatment of unresectable renal cell carcinoma larger than 3.5 cm: initial experience. Cardiovasc Intervent Radiol. 2006;29(3):389–394. doi: 10.1007/s00270-004-0090-9. [DOI] [PubMed] [Google Scholar]
- 49.Arima K Yamakado K Kinbara H Nakatsuka A Takeda K Sugimura Y Percutaneous radiofrequency ablation with transarterial embolization is useful for treatment of stage 1 renal cell carcinoma with surgical risk: results at 2-year mean follow up Int J Urol 2007147585–590., discussion 590 [DOI] [PubMed] [Google Scholar]
- 50.Mondshine R T, Owens S, Mondschein J I, Cizman B, Stavropoulos S W, Clark T W. Combination embolization and radiofrequency ablation therapy for renal cell carcinoma in the setting of coexisting arterial disease. J Vasc Interv Radiol. 2008;19(4):616–620. doi: 10.1016/j.jvir.2007.12.444. [DOI] [PubMed] [Google Scholar]
- 51.Woodrum D A, Atwell T D, Farrell M A, Andrews J C, Charboneau J W, Callstrom M R. Role of intraarterial embolization before cryoablation of large renal tumors: a pilot study. J Vasc Interv Radiol. 2010;21(6):930–936. doi: 10.1016/j.jvir.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 52.Nakasone Y, Kawanaka K, Ikeda O, Tamura Y, Yamashita Y. Sequential combination treatment (arterial embolization and percutaneous radiofrequency ablation) of inoperable renal cell carcinoma: single-center pilot study. Acta Radiol. 2012;53(4):410–414. doi: 10.1258/ar.2012.110413. [DOI] [PubMed] [Google Scholar]
- 53.Frasson F, Fugazzola C, Bianchi G. et al. Selective arterial embolization in renal tumors. Radiol Clin (Basel) 1978;47(4):239–251. [PubMed] [Google Scholar]
- 54.Schulman C C, Struyven J, Giannakopoulos X, Mathieu J. Preoperative embolization of renal tumors—comparison of different methods. Eur Urol. 1980;6(3):154–157. doi: 10.1159/000473315. [DOI] [PubMed] [Google Scholar]
- 55.Frasson F, Roversi R A, Simonetti G, Ziviello M. Embolization of renal tumors. A survey of the Italian experience : 282 patients. Ann Radiol (Paris) 1981;24(5):396–399. [PubMed] [Google Scholar]
- 56.Giuliani L, Carmignani G, Belgrano E, Puppo P, Quattrini S. Usefulness of preoperative transcatheter embolization in kidney tumors. Urology. 1981;17(5):431–434. doi: 10.1016/0090-4295(81)90182-5. [DOI] [PubMed] [Google Scholar]
- 57.Kato T, Nemoto R, Mori H, Takahashi M, Harada M. Arterial chemoembolization with mitomycin C microcapsules in the treatment of primary or secondary carcinoma of the kidney, liver, bone and intrapelvic organs. Cancer. 1981;48(3):674–680. doi: 10.1002/1097-0142(19810801)48:3<674::aid-cncr2820480303>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 58.Mobilio G, Cavalli A, Bianchi G. Preoperative arterial occlusion in renal tumors: 3 years experience. Int Urol Nephrol. 1981;13(1):25–33. doi: 10.1007/BF02082068. [DOI] [PubMed] [Google Scholar]
- 59.LeGuillou M, Merland J J. The indications of embolisation in renal tumor: what remains to be said? Prog Clin Biol Res. 1982;100:603–607. [PubMed] [Google Scholar]
- 60.Teasdale C, Kirk D, Jeans W D, Penry J B, Tribe C T, Slade N. Arterial embolisation in renal carcinoma: a useful procedure? Br J Urol. 1982;54(6):616–619. doi: 10.1111/j.1464-410x.1982.tb13608.x. [DOI] [PubMed] [Google Scholar]
- 61.Bono A V, Caresano A. The role of embolization in the treatment of kidney carcinoma. Eur Urol. 1983;9(6):334–337. doi: 10.1159/000474117. [DOI] [PubMed] [Google Scholar]
- 62.Nakano H, Nihira H, Toge T. Treatment of renal cancer patients by transcatheter embolization and its effects on lymphocyte proliferative responses. J Urol. 1983;130(1):24–27. doi: 10.1016/s0022-5347(17)50935-0. [DOI] [PubMed] [Google Scholar]
- 63.Ekelund L, Ek A, Forsberg L. et al. Occlusion of renal arterial tumor supply with absolute ethanol. Experience with 20 cases. Acta Radiol Diagn (Stockh) 1984;25(3):195–201. doi: 10.1177/028418518402500307. [DOI] [PubMed] [Google Scholar]
- 64.Kaisary A V, Williams G, Riddle P R. The role of preoperative embolization in renal cell carcinoma. J Urol. 1984;131(4):641–646. doi: 10.1016/s0022-5347(17)50556-x. [DOI] [PubMed] [Google Scholar]
- 65.Kurth K H, Cinqualbre J, Oliver R T, Schulman C C. Embolization and subsequent nephrectomy in metastatic renal cell carcinoma. Prog Clin Biol Res. 1984;153:423–436. [PubMed] [Google Scholar]
- 66.McIvor J, Kaisary A V, Williams G, Grant R W. Tumour infarction after pre-operative embolisation of renal carcinoma. Clin Radiol. 1984;35(1):59–64. doi: 10.1016/s0009-9260(84)80239-1. [DOI] [PubMed] [Google Scholar]
- 67.Mebust W K, Weigel J W, Lee K R, Cox G G, Jewell W R, Krishnan E C. Renal cell carcinoma—angioinfarction. J Urol. 1984;131(2):231–235. doi: 10.1016/s0022-5347(17)50320-1. [DOI] [PubMed] [Google Scholar]
- 68.Christensen K, Dyreborg U, Andersen J F, Nissen H M. The value of transvascular embolization in the treatment of renal carcinoma. J Urol. 1985;133(2):191–193. doi: 10.1016/s0022-5347(17)48877-x. [DOI] [PubMed] [Google Scholar]
- 69.Gottesman J E, Crawford E D, Grossman H B, Scardino P, McCracken J D. Infarction-nephrectomy for metastatic renal carcinoma. Southwest oncology group study. Urology. 1985;25(3):248–250. doi: 10.1016/0090-4295(85)90321-8. [DOI] [PubMed] [Google Scholar]
- 70.Klimberg I, Hunter P, Hawkins I F, Drylie D M, Wajsman Z. Preoperative angioinfarction of localized renal cell carcinoma using absolute ethanol. J Urol. 1985;133(1):21–24. doi: 10.1016/s0022-5347(17)48768-4. [DOI] [PubMed] [Google Scholar]
- 71.Lammer J, Justich E, Schreyer H, Pettek R. Complications of renal tumor embolization. Cardiovasc Intervent Radiol. 1985;8(1):31–35. doi: 10.1007/BF02552637. [DOI] [PubMed] [Google Scholar]
- 72.Leinonen A. Embolization of renal carcinoma. Comparison between the early results of Gelfoam and absolute ethanol embolization. Ann Clin Res. 1985;17(6):299–305. [PubMed] [Google Scholar]
- 73.Weigel J W, Mebust W K, Foret J D. et al. Treatment of renal cell carcinoma with renal infarction, delayed nephrectomy, medroxyprogesterone, and xenogeneic immune RNA. Urology. 1985;25(2):103–105. doi: 10.1016/0090-4295(85)90522-9. [DOI] [PubMed] [Google Scholar]
- 74.Chudácek Z, Zavázal V. Palliative embolization of renal tumors. I. Immunologic reaction to the embolization. Radiol Diagn (Berl) 1986;27(2):211–213. [PubMed] [Google Scholar]
- 75.Karwowski A, Wojtowicz J. Long-term results of embolization in renal tumors. Radiol Diagn (Berl) 1987;28(4):533–535. [PubMed] [Google Scholar]
- 76.Kurth K H, Debruyne F M, Hall R R. et al. Embolization and postinfarction nephrectomy in patients with primary metastatic renal adenocarcinoma. Eur Urol. 1987;13(4):251–255. doi: 10.1159/000472789. [DOI] [PubMed] [Google Scholar]
- 77.Nurmi M, Satokari K, Puntala P. Renal artery embolization in the palliative treatment of renal adenocarcinoma. Scand J Urol Nephrol. 1987;21(2):93–96. doi: 10.3109/00365598709180300. [DOI] [PubMed] [Google Scholar]
- 78.Stoesslein F, Schwenke A, Muenster W. Percutaneous transluminal embolization for improved prognosis of renal cell carcinoma—dependence on tumor stages. Cardiovasc Intervent Radiol. 1988;11(2):91–96. doi: 10.1007/BF02577067. [DOI] [PubMed] [Google Scholar]
- 79.Swanson D A, Wallace S. Surgery of metastatic renal cell carcinoma and use of renal infarction. Semin Surg Oncol. 1988;4(2):124–128. [PubMed] [Google Scholar]
- 80.Kato T, Sato K, Abe R, Moriyama M. The role of embolization/chemoembolization in the treatment of renal cell carcinoma. Prog Clin Biol Res. 1989;303:697–705. [PubMed] [Google Scholar]
- 81.Lanigan D, Jurriaans E, Hammonds J C, Wells I P, Choa R G. The current status of embolization in renal cell carcinoma—a survey of local and national practice. Clin Radiol. 1992;46(3):176–178. doi: 10.1016/s0009-9260(05)80440-4. [DOI] [PubMed] [Google Scholar]
- 82.Bakal C W, Cynamon J, Lakritz P S, Sprayregen S. Value of preoperative renal artery embolization in reducing blood transfusion requirements during nephrectomy for renal cell carcinoma. J Vasc Interv Radiol. 1993;4(6):727–731. doi: 10.1016/s1051-0443(93)71958-2. [DOI] [PubMed] [Google Scholar]
- 83.Park J H, Kim S H, Han J K, Chung J W, Han M C. Transcatheter arterial embolization of unresectable renal cell carcinoma with a mixture of ethanol and iodized oil. Cardiovasc Intervent Radiol. 1994;17(6):323–327. doi: 10.1007/BF00203951. [DOI] [PubMed] [Google Scholar]
- 84.Chick C M, Tan B S, Cheng C. et al. Long-term follow-up of the treatment of renal angiomyolipomas after selective arterial embolization with alcohol. BJU Int. 2010;105(3):390–394. doi: 10.1111/j.1464-410X.2009.08813.x. [DOI] [PubMed] [Google Scholar]
- 85.Lee S Y, Hsu H H, Chen Y C. et al. Embolization of renal angiomyolipomas: short-term and long-term outcomes, complications, and tumor shrinkage. Cardiovasc Intervent Radiol. 2009;32(6):1171–1178. doi: 10.1007/s00270-009-9637-0. [DOI] [PubMed] [Google Scholar]
- 86.Takebayashi S, Horikawa A, Arai M, Iso S, Noguchi K. Transarterial ethanol ablation for sporadic and non-hemorrhaging angiomyolipoma in the kidney. Eur J Radiol. 2009;72(1):139–145. doi: 10.1016/j.ejrad.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 87.Bishay V L, Crino P B, Wein A J. et al. Embolization of giant renal angiomyolipomas: technique and results. J Vasc Interv Radiol. 2010;21(1):67–72. doi: 10.1016/j.jvir.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 88.Chatziioannou A, Gargas D, Malagari K. et al. Transcatheter arterial embolization as therapy of renal angiomyolipomas: the evolution in 15 years of experience. Eur J Radiol. 2012;81(9):2308–2312. doi: 10.1016/j.ejrad.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 89.Patatas K, Robinson G J, Ettles D F, Lakshminarayan R. Patterns of renal angiomyolipoma regression post embolisation on medium- to long-term follow-up. Br J Radiol. 2013;86(1024):2.0120633E7. doi: 10.1259/bjr.20120633. [DOI] [PMC free article] [PubMed] [Google Scholar]


