Abstract
Image-guided percutaneous thermal ablation is a safe and effective nephron-sparing alternative to surgical resection for the treatment of small renal tumors. Assessment of treatment efficacy relies heavily on interval follow-up imaging after treatment. Contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI) both play a pivotal role in evaluating the treatment zone, identifying residual tumor, and detecting early and delayed procedure-related complications. This article discusses a surveillance imaging protocol for patients who undergo percutaneous thermal ablation of renal tumors, and also illustrates the typical appearances of both successfully treated tumors and residual disease on contrast-enhanced CT or MRI. In addition, it discusses the imaging appearance of potential early and delayed treatment-related complications to facilitate their prompt detection and management.
Keywords: thermal ablation renal mass, ablation imaging follow-up
Objectives: Upon completion of this article, the reader will be able to identify the typical CT and MRI features of renal tumors successfully treated with RFA or cryoablation, as well as the typical appearance of residual tumor on follow-up imaging. In addition, the reader should be able to identify signs of treatment-related complications that may appear on early or delayed follow-up imaging.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
The incidence of renal cell carcinoma is rising, likely due to an increase in the number of incidental small renal masses detected on cross-sectional imaging. Current estimates for 2013 project a rate of more than 65,150 new cases and 13,680 deaths from renal cell carcinoma in the United States.1 For early-stage tumors ≤ 4 cm in size, nephron-sparing surgical resection is now considered by most urologists to be the gold standard treatment. Percutaneous thermal ablation has generally been reserved for patients at high operative risk due to age or multiple medical comorbidities, or for patients with underlying renal insufficiency, a solitary kidney, or a hereditary predisposition to tumor multiplicity that necessitates a maximum nephron-sparing treatment approach.2 This paradigm, however, is shifting. New compelling data show that 5-year cancer-specific survival rates for T1a renal tumors treated with ablation are comparable to rates achieved with surgical excision.3 4 5 This has spurred increased utilization of thermal ablation as a first-line therapy in younger patients as an alternative to more invasive surgical resection.4
Cryotherapy and radiofrequency ablation (RFA) are the most frequently used methods of thermal ablation of small renal masses. Each can be performed using a minimally invasive percutaneous approach or by open or laparoscopic surgical techniques.6 Cryotherapy relies on the Joule–Thompson effect of pressurized gasses to induce ultracold temperatures (approximately −160°C) to freeze tissue. Alternating cycles of freezing and thawing result in intracellular dehydration, protein denaturation, destruction of cell membranes, and subsequent cell death.7 RFA uses an alternating current created between a conducting electrode and a grounding source to produce thermal energy at the electrode tip. When radiofrequency pulses are applied, an oscillating current is created between the conducting electrode and the grounding source (usually a grounding pad placed on the patient's skin). Heating occurs as energy is transferred from the oscillating current of electrons to the ionic molecules of the tissue due to inherent electrical resistance within the tissue itself. When the treated tissue achieves cytotoxic temperatures in the range of 50 to 100°C, tissue desiccation and protein denaturation (coagulation necrosis) ensue, resulting in cell death.8
Unlike surgical resection, the success of percutaneous ablation treatment cannot be measured histologically as there is no excised specimen to assess treatment margins for residual tumor. Instead, the success of thermal ablation therapy is established by findings at the ablation site on posttreatment imaging. Imaging surveillance with contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) is essential for determining initial treatment success, detecting residual tumor at the treatment site, or new metachronous tumors that may develop after treatment. As the use of minimally invasive image-guided thermal ablation treatments for renal tumors increases, radiologists should become familiar with the appearance of successfully treated tumors and residual disease on follow-up imaging. Recognizing the signs of potential early and delayed complications on imaging follow-up is also essential to ensure timely management and prevent long-term adverse sequelae.
Postablation Imaging Assessment of Treatment Efficacy
Technical Success
The fundamental goal of thermal ablation is to achieve complete necrosis of the target tumor indicated by the absence of enhancement at the treatment site. However, microscopic foci of residual disease may not be detectable until they grow large enough to be detected on CT or MRI, which can take months or years to occur.9 10 11
Standard practice in thermal ablation for renal tumors is to extend the treatment margins 5 to 10 mm beyond the target tumor in all dimensions to decrease the likelihood of residual microscopic tumor at the margins of the ablation zone.10 Although the literature has commonly referred to the delayed appearance of viable tumor in the ablation zone as “recurrent” disease, it more likely represents growth of occult foci of residual disease that were spared during the original treatment. To increase the likelihood of complete tumor destruction at the time of treatment, the ablation zone should always be larger than the target tumor, at least on initial follow-up imaging studies.9 10 11
There are limited data on the long-term efficacy of percutaneous thermal ablation treatment for renal tumors. Studies on short-term results for RFA report several common findings: (1) tumors < 4 cm in diameter can reliably be treated in a single treatment session without residual viable tumor noted on immediate follow-up imaging,12 13 14 15 16 17 18 19 20 (2) tumors located in an exophytic location have lower rates of residual tumor on follow-up imaging when compared with tumors located centrally within the kidney,14 19 and (3) tumors > 3 cm in diameter have higher rates of local recurrence (delayed appearance of residual tumor) than smaller tumors on imaging follow-up after initial successful treatment.14 15 17 19 20
Surveillance Imaging
The technical success of treatment is established based on the appearance of the ablation zone on the first follow-up imaging study. The timing of the initial imaging assessment for treatment efficacy varies based on operator preference, but typically ranges from immediately (day 0) to up to 1 month after treatment.21 22 Some authors recommend imaging assessment as soon as possible after ablation, both to establish a baseline appearance of the treatment zone and to rapidly identify residual tumor that may prompt immediate retreatment.21
Long-term interval surveillance imaging after treatment is essential for identifying slow growing foci of residual tumor in the treatment zone (local recurrence), as well as to identify delayed complications and detect any new tumors that may develop outside of the treatment site. Guidelines regarding the duration and specific timing of interval follow-up imaging have not been well established.23 Available data within the literature suggest that most residual tumor is detected within the first 3 months after treatment, emphasizing the need for early short interval follow-up.24 Microscopic residual tumor may remain occult for months to years on follow-up imaging due to indolent growth, however, and occult residual tumor can eventually be identified as “local recurrence” in up to 10.5% of cases on follow-up imaging.12 13 14 15 16 17 18 19 20 24 25 26 27 28 29 30 31 32 The majority of cases are identified in the first 12 months after treatment, but residual tumor at the treatment site has been reported up to 2.5 years after ablation.18 19 24 28 These data highlight the need for long-term imaging surveillance in patients who undergo thermal ablation treatment of renal cell carcinoma, but the endpoint remains undefined.
On the basis of the experience and the above reported data, the authors suggest surveillance CT or MRI at 1, 3, 6, and 12 months after treatment for the first year, every 6 months for the second year, and then annually thereafter. The authors often perform an immediate helical CT acquisition through the kidneys after ablation to assess the appearance of the ablation zone and exclude any immediate complication, but seldom use intravenous (IV) contrast in this setting so as to avoid the potential additive nephrotoxic effect of the contrast to the thermal injury associated with the treatment itself. The first formal multiphase contrast-enhanced imaging study is performed at 1 month postablation to establish a baseline appearance of the treatment zone and exclude residual tumor. After 2 years of follow-up, surveillance imaging is then performed annually to monitor for slow-growing residual tumor at the treatment site or the development of new tumors at other locations within either kidney. Metachronous tumors are known to occur in up to 20% of patients referred for RFA of renal cell carcinoma.33
Dedicated Computed tomography and Magnetic Resonance Imaging Protocols
At the authors' institution, posttreatment imaging is routinely performed with CT. MRI is reserved for patients who are allergic to iodinated contrast, younger patients who undergo multiple treatments for multiple, metachronous hereditary tumors, or those who underwent pretreatment MRI, in which case subsequent MRI follow-up allows for more accurate comparison. The authors' CT protocol consists of helical multidetector acquisition performed before and after IV administration of 100 mL of low-osmolar contrast injected at a rate of 3 to 4 mL/s. In patients with chronic renal insufficiency (estimated glomerular filtration rate (eGFR) < 60 mL/min), only 50 mL of low-osmolar contrast is used, and the patient is prehydrated with 500 mL of IV normal saline (0.9% sodium chloride) before and following the exam (total 1 L) to minimize nephrotoxicity. For patients with a history of cardiac disease or congestive heart failure, patients are hydrated with half normal saline (0.45% sodium chloride). The initial unenhanced CT acquisition is performed from the diaphragm to the iliac crest. This is followed by dynamic contrast-enhanced imaging in the (1) corticomedullary phase 90 seconds after contrast injection and (2) excretory phase 4 minutes after contrast injection. The addition of delayed (excretory phase) imaging is helpful to evaluate the collecting system for injury related to the treatment. It should be noted that the authors do not routinely perform an arterial phase acquisition (at 30 seconds after contrast injection) because the sensitivity of the corticomedullary phase of enhancement is comparable to arterial phase imaging in detecting residual viable tumor, and the elimination of the arterial phase acquisition spares unnecessary radiation dose to the patient. A soft tissue reconstruction algorithm is used to generate 2.5-mm-thick contiguous slices through each helical acquisition dataset in the axial and coronal planes.
Posttreatment MRI is routinely performed on either a 1.5-T or 3-T unit, using a surface coil whenever possible. Imaging is performed before and after IV administration of a gadolinium-based contrast agent at a dose of 0.1 mmol per kg of body weight. The authors' protocol includes T2-weighted single-shot fast-spin echo images; T1-weighted dual, echo gradient echo (chemical shift) images; and T1-weighted, fat-saturated, spoiled gradient echo images performed before and after IV administration of contrast at 1, 2, and 3 minutes. Subtracted contrast-enhanced datasets are routinely generated by the technologists. Diffusion-weighted images are also obtained with b values of 50 and 500.
Imaging Findings during Treatment
Radiofrequency Ablation
Axial CT-fluoroscopic images or truncated helical acquisitions are performed during RFA, mainly to confirm positioning of the electrodes during treatment rather than to monitor the extent of the ablation. Noncontrast CT is limited at detecting changes within the treated tissue; however, it may become slightly hypodense during heating. This is often followed by the appearance of stranding within the perinephric fat and mild thickening of the pararenal fascia. At higher temperatures, charring and vaporization may occur, resulting in small locules of gas in and around the treated tumor and adjacent perinephric fat34 35 (Fig. 1).
Figure 1.

CT-fluoroscopic imaging during RFA. (A) Cluster RF electrode in position within a partially exophytic renal mass (arrow). (B) Immediately after treatment the tumor is slightly hypodense with central hyperdensity (coagulative necrosis). Foci of gas are seen around the treated tumor from tissue vaporization that often occurs at high temperatures. CT, computed tomography; RFA, radiofrequency ablation.
Cryoablation
The changes in tissue density that occur during cryoablation are more evident than RFA on CT. The decrease in density of soft tissue can be visualized as it freezes, allowing for more accurate monitoring of the treatment margins34 (Fig. 2). It should be noted, however, that the visualized margin of the cryozone does not correlate exactly with the zone of lethal cellular injury. There is a sublethal zone of injury at the margin of the visualized treatment zone where temperatures are not low enough to cause cell death. Therefore, the treatment margin is extended 1 cm beyond the visible margin of the targeted tumor to ensure efficacy.36
Figure 2.

CT-fluoroscopic imaging during cryoablation. (A) Pretreatment noncontrast CT shows a large, partially exophytic RCC in the right kidney (arrows). (B) Images obtained during treatment demonstrate multiple cryoprobes positioned within the mass. The cryozone can be visualized as a well-demarcated region of hypodensity in the surrounding tissue (arrows). CT, computed tomography; RCC, renal cell carcinoma.
Imaging Findings Immediately after Treatment
Radiofrequency Ablation
Routine use of IV contrast for immediate posttreatment imaging is not essential for preliminary assessment of the treatment zone. CT performed within hours of RFA typically shows extensive stranding within the perinephric fat and mild thickening of the pararenal fascia. There is often focal hyperdensity within the region of the treated tumor due to heat-induced protein denaturation and coagulative necrosis. Foci of gas may be extensive within the treated tumor and throughout the adjacent perinephric fat.34 37 If contrast is administered, the ablation zone appears as a well-demarcated region without enhancement and should completely include the target tumor (Fig. 3). Perinephric or subcapsular hemorrhage is commonly seen and usually appears as a crescent-shaped hyperdense fluid collection (> 60 HU) immediately abutting the kidney or treatment zone.
Figure 3.

Contrast-enhanced CT performed immediately after RFA of the tumor depicted in Fig. 1 shows the ablation zone as a well-demarcated region without enhancement (arrows), surrounding the target tumor with adequate margins. CT, computed tomography; RFA, radiofrequency ablation.
Following administration of IV contrast, active hemorrhage may occasionally be seen as contrast extravasation into the perinephric space. Despite the concerning appearance of this finding, it is usually self-limited. However, it is recommended that the patient's vital signs be strictly monitored, and an additional delayed noncontrast CT scan should be performed after 15 to 20 minutes to ascertain stability of the perinephric hematoma. Any sign of hemodynamic instability or a significant increase in the size of the hematoma on delayed imaging should prompt aggressive fluid or blood product resuscitation. Emergent angiography and embolization should be considered in cases where patients fail to respond appropriately to conservative treatment measures.
The hallmark of successful thermal ablation is the absence of enhancement within the treated tumor.13 If IV contrast is administered immediately after treatment, great care must be taken when interpreting the significance of any residual enhancement in the treated tumor. It has been reported that mild, homogenous enhancement (> 10 HU) may be seen throughout the treated tissue within the ablation zone on immediate posttreatment imaging and should not be mistaken for residual viable tumor.38 To avoid confusion, the authors usually wait to administer IV contrast until 1 month after treatment, and subsequently obtain an additional early follow-up imaging study at 3 months.
On immediate posttreatment MRI, treated tumor typically has increased signal on T1-weighted images and decreased signal on T2-weighted images as a result of coagulative necrosis and tissue desiccation (Fig. 4). On in-phase and opposed-phase imaging, a well-demarcated thin rim of dark signal may be seen demarcating the margin of the thermal ablation zone.34 37 39 40 The hallmark of tumor viability is residual nodular enhancement; however, in patients who are unable to receive IV contrast T1 and T2 signal characteristics on unenhanced MRI may serve as a surrogate measure by which to assess the size and extent of the treatment zone.
Figure 4.

Immediate post-RFA MRI appearance of a successfully treated tumor. (A) Axial T1-weighted image shows increased signal within the treated tumor due to coagulative necrosis (arrows). (B) Axial T2-weighted image shows predominantly decreased signal within the treated tumor due to tissue desiccation, with small foci of T2-bright signal dispersed throughout the treatment zone (arrows). (C) Axial precontrast and (D) postcontrast T1-weighted, fat-saturated spoiled gradient echo images (VIBE) show no significant enhancement within the treated tumor. MRI, magnetic resonance imaging; RFA, radiofrequency ablation.
Cryoablation
The appearance of tumors treated with cryoablation differs considerably from that of tumors treated with RFA on immediate follow-up CT imaging. After multiple freeze-thaw cycles and removal of the cryoprobe(s), the iceball usually persists as a well-demarcated region of hypodensity that should include and extend beyond the margins of the targeted tumor by at least 5 mm.18 Hemorrhage within the treatment zone is common, the degree of which is usually greater than that seen with RFA.34 41
If IV contrast is administered, a thin (1–2 mm) homogeneous rim of enhancing tissue is commonly seen surrounding the treatment zone as a result of reactive hyperemia within the sublethal zone42 43 (Fig. 5). Although this finding has also been described immediately after RFA, it is more commonly visualized after cryoablation. Benign periablational enhancement may subsequently appear as a concentric, symmetric, and uniform process with smooth inner margins, unlike residual viable tumor that has a more focal, irregular, and nodular appearance23 (Fig. 6). It has been reported that 15 to 20% of ablated tumors may show some residual mild enhancement within the ablation zone in the first few months after treatment; this should not be interpreted as residual tumor but rather monitored on subsequent follow-up imaging to ascertain resolution.44 45
Figure 5.

Immediate postcryoablation CT appearance of a successfully treated tumor. (A) Axial noncontrast image shows hypodensity throughout the treated tumor (arrows). (B) A thin homogeneous rim of enhancing tissue (arrow) is commonly seen surrounding the treatment zone as a result of reactive hyperemia within the sublethal zone. The treated tumor in this case does not significantly enhance. CT, computed tomography.
Figure 6.

Benign periablational enhancement after cryoablation. (A) Axial contrast-enhanced CT image performed at 1 month posttreatment shows a thin, concentric rim of tissue surrounding the ablation zone with uniform enhancement and smooth margins (arrow). This should not be confused with residual tumor, which typically has a more irregular, nodular pattern of enhancement. (B) Subsequent imaging performed 5 months later shows complete resolution of the enhancing rim. CT, computed tomography.
On MRI, any residual iceball within the treatment zone appears as a well-demarcated signal void on T1-weighted and T2-weighted images. A rim of T2 signal hyperintensity may be seen within thawed tissues at the margins of the treatment zone. Acute hemorrhage may appear in the treatment zone as iso- to hyperintense on T1-weighted images and variable in signal intensity on T2-weighted images. The treatment is deemed a technical success if there is no enhancement within the treated tumor after IV contrast administration.34 46 As with CT imaging performed immediately after cryoablation, benign periablational enhancement is a common finding at the margins of the ablation zone on MRI, which slowly resolves and rarely persists beyond 3 months.43
Imaging Findings during Follow-Up
An important concept when following renal tumors after ablation is to compare the posttreatment contrast-enhanced images with contrast-enhanced images of the tumor performed before treatment. Determining the location and extent of tumor enhancement on pretreatment imaging is critical to accurately assess posttreatment success.
Radiofrequency Ablation
CT imaging performed at 1 month or more after RFA treatment typically demonstrates a characteristic change in the appearance of the treated tumor.47 The treated lesion will appear as a nonenhancing soft tissue mass immediately surrounded by a thick rind of fat and then a thin peripheral rim of fibrous tissue. This appearance has been referred to as the “bull's eye” or “halo” sign and is often associated with atrophy of the adjacent renal parenchyma11 47 48 (Fig. 7). This can be seen on both CT and MRI and has been described in up to 75% of treated lesions, most commonly when the treated lesion is exophytic.48 49 50 51 The “halo sign” is typically associated with tumors that are treated using a percutaneous, image-guided approach rather than those who are treated by an open or laparoscopic surgical approach. This is likely because the perinephric fat is often separated from the surface of the tumor and spared from treatment when using a surgical approach.47
Figure 7.

The “halo” sign after RFA. (A) Axial noncontrast CT image, and (B) axial and (C) coronal contrast-enhanced images performed at 3 months after RFA show the treated tumor as a central nonenhancing soft tissue mass immediately surrounded by a thick rind of fat and then a thin peripheral rim of fibrous tissue (arrow). CT, computed tomography.
The “halo sign” typically appears an average of 6 months after RFA and persists on all subsequent follow-up imaging. Although the central treated tumor mass tends to gradually decrease in size over time, the surrounding halo usually maintains a thin, uniform, curvilinear morphology and defines the boundary of the ablation zone, separating it from the adjacent perinephric fat. The rind of fat within the halo surrounding the treated tumor mass may be of variable thickness48 (Fig. 8). The appearance of the “halo sign” may resemble that of an angiomyolipoma, and its recognition by radiologists as a typical finding after RFA is important to avoid a false diagnosis.
Figure 8.
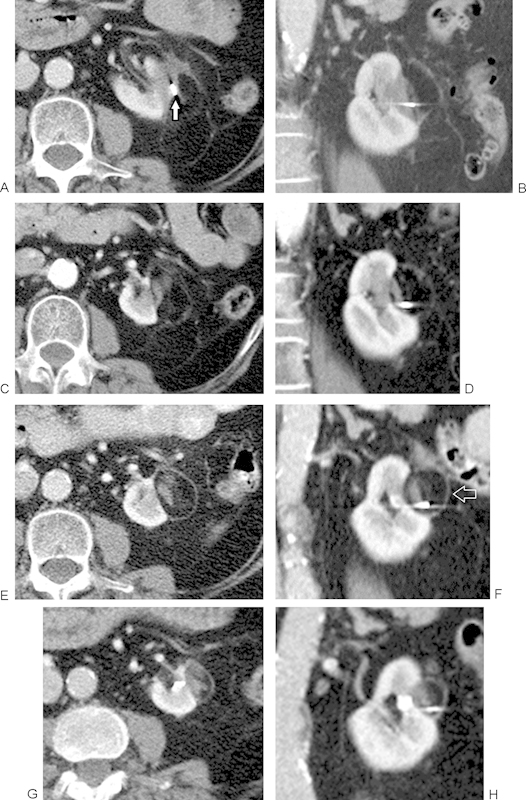
Persistent appearance of the “halo” sign over time. Axial and coronal contrast-enhanced CT images of a treated tumor acquired at (A, B) 1 month, (C, D) 6 months, (E, F) 18 months, and (G, H) 30 months after RFA. Although the central treated tumor mass tends to gradually decrease in size over time, the surrounding halo persists and maintains a thin, uniform shape, defining the boundary between the ablation zone and the adjacent perinephric fat (open arrow, F). A metallic fiducial marker coil is seen within the treated tumor mass (arrow), originally placed during biopsy to assist in localization of the lesion using CT-fluoroscopy during treatment. CT, computed tomography.
On MRI, successfully treated tumors typically maintain the T1 signal hyperintensity commonly associated with coagulative necrosis. On T2-weighted sequences, the treated tumor tends to remain hypointense relative to normal renal parenchyma, although T2 signal may be somewhat heterogeneous and contain small internal foci of increased signal due to evolving necrosis.34 37 39 40 47 49 50 51 As on CT follow-up imaging, the “halo sign” is commonly seen on MRI after percutaneous RFA treatment. The T1 and T2 signal intensity of the surrounding thin soft tissue halo can be variable, but it is typically hypointense to normal renal parenchyma on both sequences. The central treated tumor mass should appear predominantly T2 dark and nonenhancing50 (Fig. 9). The signal characteristics of the fat adjacent to the ablation zone and within the halo are not altered by tissue heating during treatment and will therefore suppress normally when using fat-suppression techniques. As a result, caution should be taken when using fat-suppressed T2-weighted images in a follow-up imaging protocol as it may be difficult to distinguish the treated tumor from suppressed surrounding fat, which may lead to the false perception of tumor enlargement during interval follow-up.34
Figure 9.

Appearance of the “halo” sign on MRI after RFA. (A) Coronal T2-weighted image showing dark signal within the thin peripheral soft tissue halo and within the central treated tumor mass (arrows). Axial T1-weighted images acquired (B) without and (C) with intravenous contrast demonstrate the treated tumor to be T1-bright (due to coagulative necrosis) and nonenhancing (arrows). MRI, magnetic resonance imaging; RFA, radiofrequency ablation.
After RFA, the treated tumor mass gradually decreases in size by up to 30% over the first 6 months,40 49 but further interval decrease in size is minimal on subsequent follow-up imaging studies.21 Any increase in the size of the treated tumor mass should raise concern for growth of residual tumor within the treatment zone.21
Benign periablational enhancement may be seen up to 3 months posttreatment on both CT and MRI. Early on, this is attributed to reactive hyperemia in the tissues surrounding the ablation zone where thermal injury was insufficient to achieve complete cell death. Later, it is due to a localized giant cell-mediated granulomatous reaction to tissue heating.52 Any nodular enhancement that develops within or at the margin of the ablation zone and persists beyond 3 months may still represent granulomatous tissue, but should be considered suspicious for residual tumor (Fig. 10).
Figure 10.

Benign granulomatous response resembling delayed appearance of residual tumor at the treatment margin. Axial contrast-enhanced CT images of a treated tumor acquired at (A) 1 month and (B) 7 months after RFA treatment show no residual tumor in the treatment zone. (C) Subsequent follow-up imaging performed at 19 months shows development of a suspicious, nodular focus of enhancing soft tissue at the margin of the ablation zone considered suspicious for residual tumor (arrow). Subsequent percutaneous needle biopsy confirmed benign granulomatous tissue and no evidence of residual tumor. CT, computed tomography; RFA, radiofrequency ablation.
Cryoablation
CT findings noted within the treated tumor on immediate postablation imaging may persist for several months after treatment without significant interval change. Hemorrhage and stranding around the ablation zone typically resolve within 1 to 3 months. The treated tumor mass usually maintains its hypodense appearance with respect to the normal renal parenchyma. The “halo sign” may also be seen after percutaneous cryoablation but is usually less pronounced than that seen with RFA.37 45
Tumor involution is much more common and occurs to a greater degree after cryoablation than it does after RFA.34 36 37 43 45 49 During freezing, proteins within the treated tissue are not denatured and are therefore readily resorbed by host immune cells, resulting in less inflammation and fibrosis in the treatment zone.53 One study reported an average decrease in size of the ablation zone of 26% at 3 months, 56% at 1 year, and 75% at 3 years after laparoscopic cryoablation, with 38% of ablation zones no longer detectable by MRI by 3 years after treatment.43 Tumor involution tends to be the rule rather than the exception after cryoablation, and therefore any interval growth of the treated tumor mass on follow-up imaging should be considered suspicious for residual tumor45 (Fig. 11).
Figure 11.

Tumor involution after cryoablation. Coronal contrast-enhanced CT images acquired at (A) 3 months, (B) 2 years, and (C) 5 years after successful cryoablation treatment show gradual involution of the nonenhancing treated tumor mass over time. CT, computed tomography.
The appearance of the treated tumor on MRI may change over time. Within hours after treatment, ablated tumors typically demonstrate variable T1 and T2 signal intensity, but then at subsequent follow-up tend to appear isointense to normal renal parenchyma on T1-weighted images and predominantly dark or heterogeneously isointense on T2-weighted images34 36 (Fig. 12). A thin rim of dark signal may be seen between the treated lesion and the adjacent normal renal parenchyma on T2-weighted images. This finding tends to resolve over time as the treated tumor involutes.36
Figure 12.
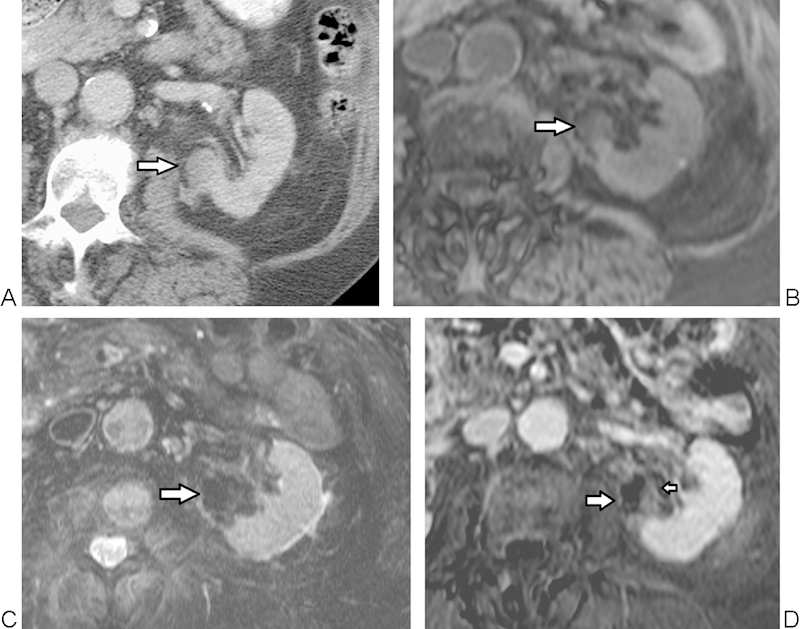
MRI appearance of a tumor successfully treated with cryoablation. (A) Axial contrast-enhanced CT image before treatment shows a central tumor along the medial aspect of the left kidney (arrow). At 3 months after cryoablation, (B) T1-weighted image shows heterogeneous, predominantly isointense signal (arrow), and (C) T2-weighted image shows predominantly dark signal within the treated tumor (arrow). (D) Postcontrast subtracted image shows mild homogeneous benign central enhancement (small arrow), enhancing less than normal renal cortex, and benign periablational enhancement (large arrow). These findings may be seen transiently after cryoablation, and subsequently resolved in this patient on 6-month follow-up imaging. MRI, magnetic resonance imaging.
Benign periablational enhancement is a common finding after cryoablation and may persist for several months after treatment but is rarely seen beyond 6 months.42 43 Up to 20% of successfully ablated tumors may show some residual mild enhancement within the ablation zone in the first few months after treatment.44 45
Residual Disease
Residual tumor is essentially undetectable on noncontrast CT and MRI. The hallmark of residual tumor is the presence or development of an enhancing nodule or crescent of tissue within the treatment zone on follow-up imaging.34 Enhancement of greater than 10 HU on CT or qualitative enhancement on postcontrast MRI is suspicious for residual tumor9 (Fig. 13) As mentioned previously, ablated tissue is often bright on T1-weighted images; for this reason, routine use of subtraction imaging is necessary to detect subtle enhancement, as a successfully treated tumor will appear completely dark on well-registered subtraction images34 (Fig. 14). Foci of residual tumor in the treatment zone are most commonly nodular or irregular in appearance and typically increase in size over time.23 34 Interpreting both the corticomedullary and delayed (excretory) phases of enhancement on follow-up imaging, as well as meticulous comparison to the appearance of the tumor on pretreatment contrast-enhanced imaging, is helpful to differentiate residual tumor from benign postablation changes. Diffusion-weighted imaging (DWI) may also be helpful to identify foci of residual tumor when contrast enhancement is difficult to ascertain, or when contrast cannot be administered due to renal insufficiency. Tumor tissue may demonstrate restricted diffusion, appearing as bright signal relative to normal renal cortex on DWI images obtained at high b values (500–1,000 s/mm2) and as dark signal on corresponding ADC images.54
Figure 13.

Appearance of residual tumor at the treatment site. Axial (A) noncontrast and (B) contrast-enhanced CT images performed through the treatment site 3 months after RFA demonstrate a subtle nodular focus of enhancing soft tissue (arrow) representing residual disease. CT, computed tomography.
Figure 14.

Utility of enhanced subtraction imaging for detecting residual disease on MRI. Axial (A) and coronal (B) fat-saturated, T1-weighted, subtracted contrast-enhanced images obtained through the treatment site at 3 months post-RFA show definitive focal, nodular tissue enhancement (arrows) within the medial aspect of an otherwise devascularized treatment zone (dark signal), indicative of residual tumor. T1-bright signal due to coagulative necrosis will not appear in the treatment zone on subtraction images, which facilitates detection of true enhancement in the residual tumor. MRI, magnetic resonance imaging; RFA, radiofrequency ablation.
The presence of enhancement within the ablated tumor zone after treatment raises suspicion for residual disease; however, the absence of enhancement does not confirm treatment efficacy, especially if the tumor was treated using RFA. One study showed 6 of 13 patients (46%) with biopsy-proven residual tumor at 6 months after RFA treatment had no evidence of suspicious enhancement on CT or MRI before biopsy.55 This study included only a small series of patients, however, and was conducted early in the ablation experience when less aggressive treatment strategies may have contributed to higher rates of residual tumor. Nevertheless, any increase in the size of the treated lesion is suspicious for residual tumor, even in the absence of any corresponding enhancement.
When assessing for residual disease after treatment, it is critical to review the pretreatment and intraprocedural imaging to assess the tumor size, location, and probe placement during treatment. These features help identify regions at risk for residual disease.34 Understanding the general capabilities and limitations of different thermal ablation modalities and of the specific ablation devices used during a particular treatment can also improve detection and prevent false-positive diagnoses.
Delayed Imaging Appearance of Treatment-Related Complications
Most complications associated with percutaneous thermal ablation of renal masses are minor and do not require an escalation in the level of patient care. Minor complications include postprocedural pain, transient hematuria, mild perinephric hemorrhage, and transient neuropathy related to thermal injury to the genitofemoral nerve or intercostal nerves adjacent to the psoas or paraspinal muscle groups.56 Major complications are rare and are typically due to thermal injury of nontarget organs (bowel or ureteral injury), pneumothorax, and bleeding.41 57 58 A meta-analysis of 1,180 treated tumors demonstrated a lower rate of major complications with percutaneous treatment compared with surgically assisted ablation (3.1 vs. 7.4%).59
The incidence of bowel injury is low, but injuries to the colon and duodenum have been reported during treatment of anterior renal masses.41 57 For RFA, in particular, the risk for nontarget injury to bowel is greatest when there is less than 10 mm of interposed fat between the target lesion and the adjacent bowel loop.60 Injury to the bowel may appear as focal thickening of the bowel wall with associated inflammatory changes in the mesenteric fat, or even free intraperitoneal air in the setting of perforation. Hydrodissection techniques to displace bowel and adjacent organs away from exophytic tumors have been described to minimize the risk of thermal injury during treatment. The fluid, consisting of D5W with or without the addition of nonionic contrast, is reabsorbed within hours of administration and is not seen on follow-up imaging studies.61 62
Massive bleeding as an immediate complication of percutaneous RFA treatment is defined as hemorrhage associated with hemodynamic instability requiring blood transfusion. This is very rare, estimated to occur in fewer than 1% of renal ablations.58 A small to moderate amount of perinephric hemorrhage may be seen in up to 5% of patients during follow-up and may persist for several months63 (Fig. 15).
Figure 15.
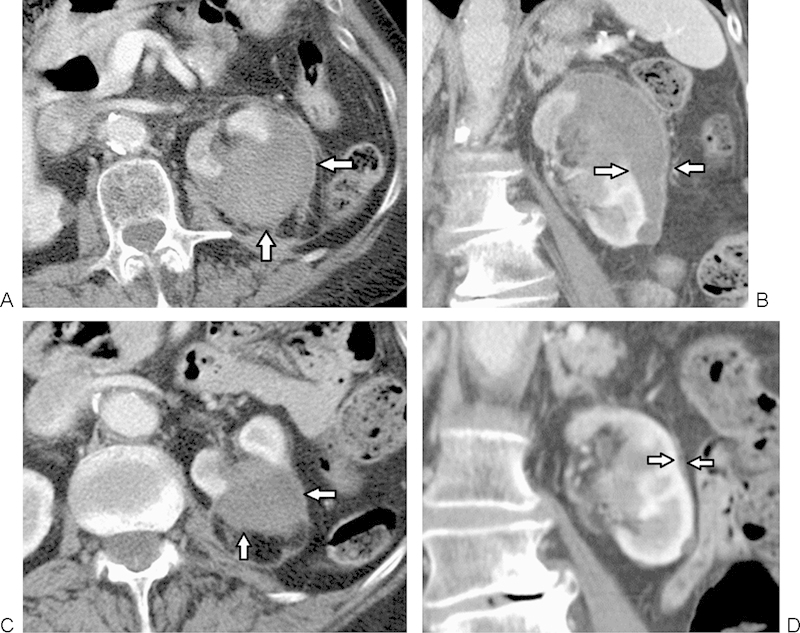
Subcapsular hematoma. (A, B) Axial and coronal contrast-enhanced CT images obtained through the treatment site 3 weeks after percutaneous RFA of a renal tumor show a moderate-sized subcapsular hematoma (arrows). (C, D) Subsequent follow-up imaging performed at 6 months after treatment shows slow partial resolution of the hematoma without significant residual mass effect on the kidney (arrows). CT, computed tomography.
Injury to the collecting system is more common with RFA than with cryoablation and is typically associated with the treatment of central tumors.52 57 59 Such injuries may not be apparent on immediate posttreatment imaging, and usually manifest weeks to months later as ureteral thickening, periureteral stranding, or development of hydronephrosis. The appearance of a new perinephric fluid collection should raise suspicion for urine leak and is confirmed by accumulation of IV contrast within the collection on delayed imaging34 (Fig. 16). Although unusual, chyluria may present as an incidental finding of a fat-fluid level within the bladder on CT after renal ablation due to injury to the periforniceal lymphatics and subsequent formation of a lymphatic-forniceal fistula. Patients are usually asymptomatic, but may complain of “foamy” appearance to the urine. The majority of patients are treated conservatively, however, if significant hypoproteinemia develops, surgical ligation of the lymphatic ducts may be necessary to prevent immune deficiency.64
Figure 16.

Delayed appearance of injury to the collecting system after RFA. (A) Axial contrast-enhanced CT image through the treatment site performed in the corticomedullary phase shows a small crescent-shaped hypodense fluid collection adjacent to the treatment zone (arrow). (B) Delayed, excretory phase imaging shows accumulation of excreted contrast within the collection (arrow), confirming injury to the collecting system and urine leakage into the perinephric space. CT, computed tomography; RFA, radiofrequency ablation.
Conclusions
CT and MRI play a pivotal role in the assessment of treatment efficacy after percutaneous thermal ablation of renal masses. The hallmark of successful treatment is the absence of any enhancement within the mass on follow-up imaging. Areas of irregular, nodular, or crescentic soft tissue enhancement within or at the periphery of the ablation zone should raise suspicion for residual disease. Long-term follow-up is necessary to detect slow growing residual disease, as well as metachronous tumors and delayed complications from treatment, including ureteral stricture and urine leak.
References
- 1.Altanta, GA: American Cancer Society; 2013. American Cancer Society. Cancer Facts and Figures 2013. [Google Scholar]
- 2.Licht M R, Novick A C, Goormastic M. Nephron sparing surgery in incidental versus suspected renal cell carcinoma. J Urol. 1994;152(1):39–42. doi: 10.1016/s0022-5347(17)32810-0. [DOI] [PubMed] [Google Scholar]
- 3.Hui G C, Tuncali K, Tatli S, Morrison P R, Silverman S G. Comparison of percutaneous and surgical approaches to renal tumor ablation: metaanalysis of effectiveness and complication rates. J Vasc Interv Radiol. 2008;19(9):1311–1320. doi: 10.1016/j.jvir.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Salas N, Ramanathan R, Dummett S, Leveillee R J. Results of radiofrequency kidney tumor ablation: renal function preservation and oncologic efficacy. World J Urol. 2010;28(5):583–591. doi: 10.1007/s00345-010-0562-2. [DOI] [PubMed] [Google Scholar]
- 5.Takaki H, Yamakado K, Soga N. et al. Midterm results of radiofrequency ablation versus nephrectomy for T1a renal cell carcinoma. Jpn J Radiol. 2010;28(6):460–468. doi: 10.1007/s11604-010-0451-z. [DOI] [PubMed] [Google Scholar]
- 6.Thumar A B Trabulsi E J Lallas C D Brown D B Thermal ablation of renal cell carcinoma: triage, treatment, and follow-up J Vasc Interv Radiol 201021(8, Suppl):S233–S241. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann N E, Bischof J C. The cryobiology of cryosurgical injury. Urology. 2002;60(2) 01:40–49. doi: 10.1016/s0090-4295(02)01683-7. [DOI] [PubMed] [Google Scholar]
- 8.Gazelle G S, Goldberg S N, Solbiati L, Livraghi T. Tumor ablation with radio-frequency energy. Radiology. 2000;217(3):633–646. doi: 10.1148/radiology.217.3.r00dc26633. [DOI] [PubMed] [Google Scholar]
- 9.Gervais D A, McGovern F J, Wood B J, Goldberg S N, McDougal W S, Mueller P R. Radio-frequency ablation of renal cell carcinoma: early clinical experience. Radiology. 2000;217(3):665–672. doi: 10.1148/radiology.217.3.r00dc39665. [DOI] [PubMed] [Google Scholar]
- 10.Zagoria R J, Hawkins A D, Clark P E. et al. Percutaneous CT-guided radiofrequency ablation of renal neoplasms: factors influencing success. AJR Am J Roentgenol. 2004;183(1):201–207. doi: 10.2214/ajr.183.1.1830201. [DOI] [PubMed] [Google Scholar]
- 11.Gervais D A, McGovern F J, Arellano R S, McDougal W S, Mueller P R. Renal cell carcinoma: clinical experience and technical success with radio-frequency ablation of 42 tumors. Radiology. 2003;226(2):417–424. doi: 10.1148/radiol.2262012062. [DOI] [PubMed] [Google Scholar]
- 12.Pavlovich C P, Walther M M, Choyke P L. et al. Percutaneous radio frequency ablation of small renal tumors: initial results. J Urol. 2002;167(1):10–15. [PMC free article] [PubMed] [Google Scholar]
- 13.Farrell M A, Charboneau W J, DiMarco D S. et al. Imaging-guided radiofrequency ablation of solid renal tumors. AJR Am J Roentgenol. 2003;180(6):1509–1513. doi: 10.2214/ajr.180.6.1801509. [DOI] [PubMed] [Google Scholar]
- 14.Ahrar K, Matin S, Wood C G. et al. Percutaneous radiofrequency ablation of renal tumors: technique, complications, and outcomes. J Vasc Interv Radiol. 2005;16(5):679–688. doi: 10.1097/01.RVI.0000153589.10908.5F. [DOI] [PubMed] [Google Scholar]
- 15.Clark T W, Malkowicz B, Stavropoulos S W. et al. Radiofrequency ablation of small renal cell carcinomas using multitined expandable electrodes: preliminary experience. J Vasc Interv Radiol. 2006;17(3):513–519. doi: 10.1097/01.RVI.0000204853.75376.2C. [DOI] [PubMed] [Google Scholar]
- 16.Breen D J, Rutherford E E, Stedman B. et al. Management of renal tumors by image-guided radiofrequency ablation: experience in 105 tumors. Cardiovasc Intervent Radiol. 2007;30(5):936–942. doi: 10.1007/s00270-007-9090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zagoria R J, Traver M A, Werle D M, Perini M, Hayasaka S, Clark P E. Oncologic efficacy of CT-guided percutaneous radiofrequency ablation of renal cell carcinomas. AJR Am J Roentgenol. 2007;189(2):429–436. doi: 10.2214/AJR.07.2258. [DOI] [PubMed] [Google Scholar]
- 18.Levinson A W Su L M Agarwal D et al. Long-term oncological and overall outcomes of percutaneous radio frequency ablation in high risk surgical patients with a solitary small renal mass J Urol 20081802499–504., discussion 504 [DOI] [PubMed] [Google Scholar]
- 19.Ferakis N, Bouropoulos C, Granitsas T, Mylona S, Poulias I. Long-term results after computed-tomography-guided percutaneous radiofrequency ablation for small renal tumors. J Endourol. 2010;24(12):1909–1913. doi: 10.1089/end.2009.0639. [DOI] [PubMed] [Google Scholar]
- 20.del Cura J L, Zabala R, Iriarte J I, Unda M. Treatment of renal tumors by percutaneous ultrasound-guided radiofrequency ablation using a multitined electrode: effectiveness and complications. Eur Urol. 2010;57(3):459–465. doi: 10.1016/j.eururo.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 21.Ganguli S, Brennan D D, Faintuch S, Rayan M E, Goldberg S N. Immediate renal tumor involution after radiofrequency thermal ablation. J Vasc Interv Radiol. 2008;19(3):412–418. doi: 10.1016/j.jvir.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 22.Rutherford E E, Cast J E, Breen D J. Immediate and long-term CT appearances following radiofrequency ablation of renal tumours. Clin Radiol. 2008;63(2):220–230. doi: 10.1016/j.crad.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg S N, Grassi C J, Cardella J F. et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology. 2005;235(3):728–739. doi: 10.1148/radiol.2353042205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matin S F, Ahrar K, Cadeddu J A. et al. Residual and recurrent disease following renal energy ablative therapy: a multi-institutional study. J Urol. 2006;176(5):1973–1977. doi: 10.1016/j.juro.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Bosniak M A, Birnbaum B A, Krinsky G A, Waisman J. Small renal parenchymal neoplasms: further observations on growth. Radiology. 1995;197(3):589–597. doi: 10.1148/radiology.197.3.7480724. [DOI] [PubMed] [Google Scholar]
- 26.Mayo-Smith W W, Dupuy D E, Parikh P M, Pezzullo J A, Cronan J J. Imaging-guided percutaneous radiofrequency ablation of solid renal masses: techniques and outcomes of 38 treatment sessions in 32 consecutive patients. AJR Am J Roentgenol. 2003;180(6):1503–1508. doi: 10.2214/ajr.180.6.1801503. [DOI] [PubMed] [Google Scholar]
- 27.Su Li, Jarrett T W, Chan D Y, Kavoussi L R, Solomon S B. Percutaneous computed tomography-guided radiofrequency ablation of renal masses in high surgical risk patients: preliminary results. Urology. 2003;61(4) 01:26–33. doi: 10.1016/s0090-4295(03)00118-3. [DOI] [PubMed] [Google Scholar]
- 28.Varkarakis I M Allaf M E Inagaki T et al. Percutaneous radio frequency ablation of renal masses: results at a 2-year mean followup J Urol 20051742456–460., discussion 460 [DOI] [PubMed] [Google Scholar]
- 29.Gervais D A, McGovern F J, Arellano R S, McDougal W S, Mueller P R. Radiofrequency ablation of renal cell carcinoma: part 1, indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol. 2005;185(1):64–71. doi: 10.2214/ajr.185.1.01850064. [DOI] [PubMed] [Google Scholar]
- 30.Gervais D A, Arellano R S, McGovern F J, McDougal W S, Mueller P R. Radiofrequency ablation of renal cell carcinoma: part 2, lessons learned with ablation of 100 tumors. AJR Am J Roentgenol. 2005;185(1):72–80. doi: 10.2214/ajr.185.1.01850072. [DOI] [PubMed] [Google Scholar]
- 31.Park S H, Yoon S K, Cho J H. et al. Radiofrequency ablation treatment for renal cell carcinoma: early clinical experience. Korean J Radiol. 2008;9(4):340–347. doi: 10.3348/kjr.2008.9.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiraoka K, Kawauchi A, Nakamura T, Soh J, Mikami K, Miki T. Radiofrequency ablation for renal tumors: our experience. Int J Urol. 2009;16(11):869–873. doi: 10.1111/j.1442-2042.2009.02378.x. [DOI] [PubMed] [Google Scholar]
- 33.Beland M D, Wolf F J, Grand D J, Dupuy D E, Mayo-Smith W W. Incidence of multiple sporadic renal cell carcinomas in patients referred for renal radiofrequency ablation: implications for imaging follow-up. AJR Am J Roentgenol. 2011;197(3):671–675. doi: 10.2214/AJR.10.6044. [DOI] [PubMed] [Google Scholar]
- 34.Wile G E Leyendecker J R Krehbiel K A Dyer R B Zagoria R J CT and MR imaging after imaging-guided thermal ablation of renal neoplasms Radiographics 2007272325–339., discussion 339–340 [DOI] [PubMed] [Google Scholar]
- 35.Saliken J, Cohen J, Miller R, Rothert M. Laboratory evaluation of ice around a 3mm AccuProbe. Cryobiology. 1995;32:285–295. [Google Scholar]
- 36.Remer E M, Weinberg E J, Oto A, O'Malley C M, Gill I S. MR imaging of the kidneys after laparoscopic cryoablation. AJR Am J Roentgenol. 2000;174(3):635–640. doi: 10.2214/ajr.174.3.1740635. [DOI] [PubMed] [Google Scholar]
- 37.Kawamoto S, Permpongkosol S, Bluemke D A, Fishman E K, Solomon S B. Sequential changes after radiofrequency ablation and cryoablation of renal neoplasms: role of CT and MR imaging. Radiographics. 2007;27(2):343–355. doi: 10.1148/rg.272065119. [DOI] [PubMed] [Google Scholar]
- 38.Javadi S, Ahrar J U, Ninan E, Gupta S, Matin S F, Ahrar K. Characterization of contrast enhancement in the ablation zone immediately after radiofrequency ablation of renal tumors. J Vasc Interv Radiol. 2010;21(5):690–695. doi: 10.1016/j.jvir.2009.12.400. [DOI] [PubMed] [Google Scholar]
- 39.Boss A, Clasen S, Kuczyk M. et al. Magnetic resonance-guided percutaneous radiofrequency ablation of renal cell carcinomas: a pilot clinical study. Invest Radiol. 2005;40(9):583–590. doi: 10.1097/01.rli.0000174473.32130.28. [DOI] [PubMed] [Google Scholar]
- 40.Merkle E M, Nour S G, Lewin J S. MR imaging follow-up after percutaneous radiofrequency ablation of renal cell carcinoma: findings in 18 patients during first 6 months. Radiology. 2005;235(3):1065–1071. doi: 10.1148/radiol.2353040871. [DOI] [PubMed] [Google Scholar]
- 41.Johnson D B, Solomon S B, Su L M. et al. Defining the complications of cryoablation and radio frequency ablation of small renal tumors: a multi-institutional review. J Urol. 2004;172(3):874–877. doi: 10.1097/01.ju.0000135833.67906.ec. [DOI] [PubMed] [Google Scholar]
- 42.Beemster P, Phoa S, Wijkstra H, de la Rosette J, Laguna P. Follow-up of renal masses after cryosurgery using computed tomography; enhancement patterns and cryolesion size. BJU Int. 2008;101(10):1237–1242. doi: 10.1111/j.1464-410X.2007.07437.x. [DOI] [PubMed] [Google Scholar]
- 43.Gill I S, Remer E M, Hasan W A. et al. Renal cryoablation: outcome at 3 years. J Urol. 2005;173(6):1903–1907. doi: 10.1097/01.ju.0000158154.28845.c9. [DOI] [PubMed] [Google Scholar]
- 44.Stein A J, Mayes J M, Mouraviev V, Chen V H, Nelson R C, Polascik T J. Persistent contrast enhancement several months after laparoscopic cryoablation of the small renal mass may not indicate recurrent tumor. J Endourol. 2008;22(11):2433–2439. doi: 10.1089/end.2008.0261. [DOI] [PubMed] [Google Scholar]
- 45.Allen B C, Remer E M. Percutaneous cryoablation of renal tumors: patient selection, technique, and postprocedural imaging. Radiographics. 2010;30(4):887–900. doi: 10.1148/rg.304095134. [DOI] [PubMed] [Google Scholar]
- 46.Harada J, Dohi M, Mogami T. et al. Initial experience of percutaneous renal cryosurgery under the guidance of a horizontal open MRI system. Radiat Med. 2001;19(6):291–296. [PubMed] [Google Scholar]
- 47.Matsumoto E D, Watumull L, Johnson D B. et al. The radiographic evolution of radio frequency ablated renal tumors. J Urol. 2004;172(1):45–48. doi: 10.1097/01.ju.0000132124.01060.0c. [DOI] [PubMed] [Google Scholar]
- 48.Schirmang T, Mayo-Smith W, Dupuy D, Beland M, Grand D. Kidney neoplasms: renal halo sign after percutaneous radiofrequency ablation—incidence and clinical importance in 101 consecutive patients. Radiology. 2009;251(1):263–269. doi: 10.1148/radiol.2531082257. [DOI] [PubMed] [Google Scholar]
- 49.Kawamoto S, Solomon S B, Bluemke D A, Fishman E K. Computed tomography and magnetic resonance imaging appearance of renal neoplasms after radiofrequency ablation and cryoablation. Semin Ultrasound CT MR. 2009;30(2):67–77. doi: 10.1053/j.sult.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Svatek R S, Sims R, Anderson J K, Abdel-Aziz K, Cadeddu J A. Magnetic resonance imaging characteristics of renal tumors after radiofrequency ablation. Urology. 2006;67(3):508–512. doi: 10.1016/j.urology.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 51.Davenport M S, Caoili E M, Cohan R H. et al. MRI and CT characteristics of successfully ablated renal masses: imaging surveillance after radiofrequency ablation. AJR Am J Roentgenol. 2009;192(6):1571–1578. doi: 10.2214/AJR.08.1303. [DOI] [PubMed] [Google Scholar]
- 52.Johnson D B, Saboorian M H, Duchene D A, Ogan K, Cadeddu J A. Nephrectomy after radiofrequency ablation-induced ureteropelvic junction obstruction: potential complication and long-term assessment of ablation adequacy. Urology. 2003;62(2):351–352. doi: 10.1016/s0090-4295(03)00361-3. [DOI] [PubMed] [Google Scholar]
- 53.Maiwand M O. The role of cryosurgery in palliation of tracheo-bronchial carcinoma. Eur J Cardiothorac Surg. 1999;15(6):764–768. doi: 10.1016/s1010-7940(99)00121-9. [DOI] [PubMed] [Google Scholar]
- 54.Saremi F, Knoll A N, Bendavid O J, Schultze-Haakh H, Narula N, Sarlati F. Characterization of genitourinary lesions with diffusion-weighted imaging. Radiographics. 2009;29(5):1295–1317. doi: 10.1148/rg.295095003. [DOI] [PubMed] [Google Scholar]
- 55.Weight C J Kaouk J H Hegarty N J et al. Correlation of radiographic imaging and histopathology following cryoablation and radio frequency ablation for renal tumors J Urol 200817941277–1281., discussion 1281–1283 [DOI] [PubMed] [Google Scholar]
- 56.Boss A, Clasen S, Kuczyk M. et al. Thermal damage of the genitofemoral nerve due to radiofrequency ablation of renal cell carcinoma: a potentially avoidable complication. AJR Am J Roentgenol. 2005;185(6):1627–1631. doi: 10.2214/AJR.04.1946. [DOI] [PubMed] [Google Scholar]
- 57.Park B K, Kim C K. Complications of image-guided radiofrequency ablation of renal cell carcinoma: causes, imaging features and prevention methods. Eur Radiol. 2009;19(9):2180–2190. doi: 10.1007/s00330-009-1399-1. [DOI] [PubMed] [Google Scholar]
- 58.Heye S, Maleux G, Van Poppel H, Oyen R, Wilms G. Hemorrhagic complications after nephron-sparing surgery: angiographic diagnosis and management by transcatheter embolization. AJR Am J Roentgenol. 2005;184(5):1661–1664. doi: 10.2214/ajr.184.5.01841661. [DOI] [PubMed] [Google Scholar]
- 59.Hui G C, Tuncali K, Tatli S, Morrison P R, Silverman S G. Comparison of percutaneous and surgical approaches to renal tumor ablation: metaanalysis of effectiveness and complication rates. J Vasc Interv Radiol. 2008;19(9):1311–1320. doi: 10.1016/j.jvir.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 60.Ginat D T, Saad W E. Bowel displacement and protection techniques during percutaneous renal tumor thermal ablation. Tech Vasc Interv Radiol. 2010;13(2):66–74. doi: 10.1053/j.tvir.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 61.Arellano R S, Garcia R G, Gervais D A, Mueller P R. Percutaneous CT-guided radiofrequency ablation of renal cell carcinoma: efficacy of organ displacement by injection of 5% dextrose in water into the retroperitoneum. AJR Am J Roentgenol. 2009;193(6):1686–1690. doi: 10.2214/AJR.09.2904. [DOI] [PubMed] [Google Scholar]
- 62.DeBenedectis C M, Beland M D, Dupuy D E, Mayo-Smith W W. Utility of iodinated contrast medium in hydrodissection fluid when performing renal tumor ablation. J Vasc Interv Radiol. 2010;21(5):745–747. doi: 10.1016/j.jvir.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 63.Rhim H, Dodd G D III, Chintapalli K N. et al. Radiofrequency thermal ablation of abdominal tumors: lessons learned from complications. Radiographics. 2004;24(1):41–52. doi: 10.1148/rg.241025144. [DOI] [PubMed] [Google Scholar]
- 64.Kaur H, Matin S F, Javadi S. et al. Chyluria after radiofrequency ablation of renal tumors. J Vasc Interv Radiol. 2011;22(7):924–927. doi: 10.1016/j.jvir.2011.02.014. [DOI] [PubMed] [Google Scholar]


