Abstract
In light of evidence linking radical nephrectomy and consequent suboptimal renal function to adverse cardiovascular events and increased mortality, research into nephron-sparing techniques for renal masses widely expanded in the past two decades. The American Urological Association (AUA) guidelines now explicitly list partial nephrectomy as the standard of care for the management of T1a renal tumors. Because of the increasing utilization of cross-sectional imaging, up to 70% of newly detected renal masses are stage T1a, making them more amenable to minimally invasive nephron-sparing therapies including laparoscopic and robotic partial nephrectomy and ablative therapies. Cryosurgery has emerged as a leading option for renal ablation, and compared with surgical techniques it offers benefits in preserving renal function with fewer complications, shorter hospitalization times, and allows for quicker convalescence. A mature dataset exists at this time, with intermediate and long-term follow-up data available. Cryosurgical recommendations as a first-line therapy are made at this time in limited populations, including elderly patients, patients with multiple comorbidities, and those with a solitary kidney. As more data emerge on oncologic efficacy, and technical experience and the technology continue to improve, the application of this modality will likely be extended in future treatment guidelines.
Keywords: renal masses, cryoablation, cryosurgery, laparoscopic cryoablation
Objectives: Upon completion of this article, the reader will be able to identify trends in evolution of surgical management for stage T1a renal masses, and discuss the evolving current status and future directions of renal cryoablation.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Although renal cell carcinoma (RCC) represents only approximately 2% of adult cancers, it is the most lethal of the common urologic malignancies.1 In 2008, there were nearly 55,000 new cases of kidney cancer diagnosed, resulting in 13,010 deaths.2 Common risk factors include tobacco smoking and obesity, both of which are prevalent in today's society. The biology of these tumors is relatively variable and ranges from slow-growing indolent tumors to faster growing malignant tumors with aggressive features. The classic physical exam findings of flank pain, hematuria, and palpable mass are actually quite rare today and tend to denote advanced disease. Much more commonly, renal tumors are detected incidentally at earlier stages due to the increased utilization of cross-sectional imaging. Some reports have estimated that as many as 70% of these newly diagnosed renal tumors are stage T1a, making them more amenable to minimally invasive nephron-sparing therapies including laparoscopic partial nephrectomy (LPN) and ablative therapies.3 Typically, of the small renal masses found, up to 80% represent RCC,4 with approximately 65% having clear cell histology; however, up to 15 to 20% of these enhancing small renal masses are benign. This combination of increasing incidence through early detection and significant percentage of benignity leads to management dilemmas.
Cryosurgery, also known as cryoablation, offers benefits in preserving renal function with fewer complications, shorter hospitalization times, and allows for quicker convalescence.3 Sophisticated imaging modalities such as intraoperative laparoscopic ultrasound have helped further increase the efficacy of renal cryotherapy. Advances in technology and increased experience with cryoprobes have served to make this a viable alternative to excisional procedures. This article will explain cryosurgery and review the current literature regarding oncologic efficacy and morbidity benefits of performing laparoscopic cryoablation (LCA) for small renal masses.
How Cryoablation Works
Cryoablation causes direct cell damage at the tissue level by producing intra- and extracellular ice, which results in direct disruption of the cell membrane. Over time this leads to coagulation necrosis, fibrosis, and scarring. Temperatures between −40 and −20°C are ideal and these are generally achieved 5 to 6 mm inside the edge of the forming ice ball. These extreme temperatures also cause an indirect effect of tumor killing that is due to microvasculature thrombosis, occlusion, ischemia, and eventually cell death.5 Performing a second thawing and freezing cycle has been shown to maximize the amount of cell death. Slow thawing, with a rate limited to no more than 20°C/minute, has been demonstrated as the most effective method of tumor killing. This allows more time for the osmolar effects, recrystallization effects, prolonged oxidative stress, and growth of crystals to take effect. Current cryotherapy equipment is based on the Joule–Thomson effect, which describes temperature changes in gases that occur during expansion. Argon is one example of a gas that cools during expansion, while helium warms during expansion.6 It is this combination of gases that is used for freezing and thawing in most current systems.
Preoperative Planning/Probe Placement
The number and size of the cryoprobes placed depends on the size and configuration of the mass. Smaller probes minimize the risk of bleeding; however, larger probes result in larger ice balls. Recently, the use of multiple smaller probes has increased variety and size of tumors that can be treated. When mobilization of the kidney is feasible, laparoscopic ultrasound probes are placed on the contralateral side of the kidney for visualization (Fig. 1).7 This can be used for identification of the lesion, and for guiding biopsy needle and cryoprobe placement (Figs. 2 and 3). The tip of the probe must extend beyond the deep margin of the mass, which can be confirmed with intraoperative ultrasound.
Figure 1.
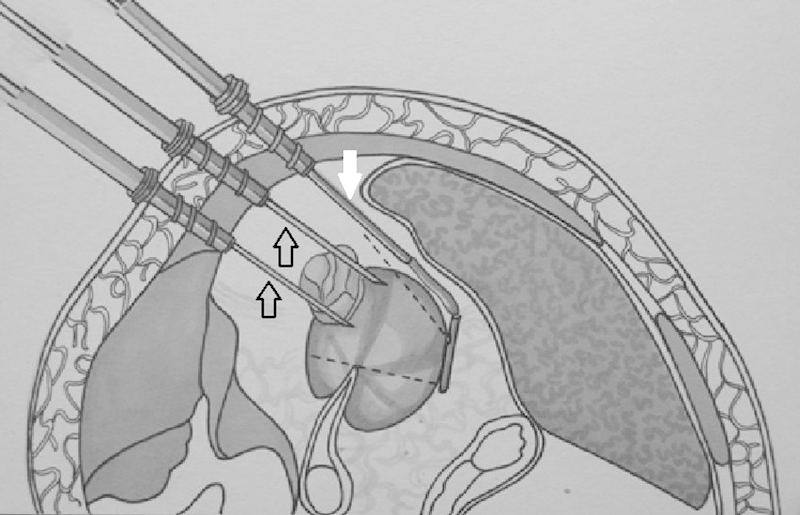
Schematic of laparoscopic cryoablation demonstrating two cryoablation needles (open arrows) within a renal mass with contralateral positioning of the laparoscopic ultrasound probe (solid white arrow).
Figure 2.
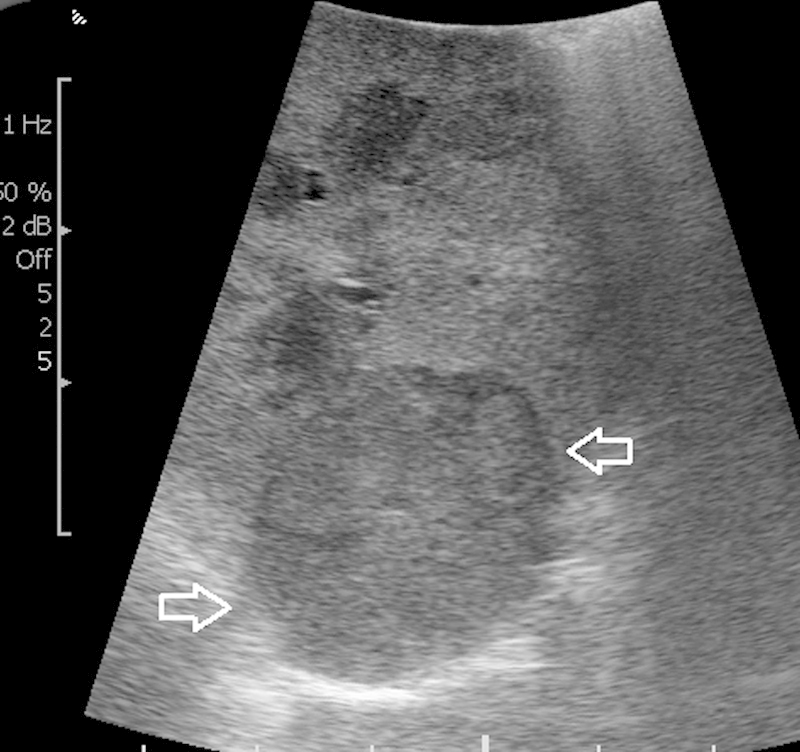
Intraoperative image obtained with contralateral positioning of laparoscopic ultrasound probe clearly demonstrates the partially exophytic renal mass (arrows).
Figure 3.
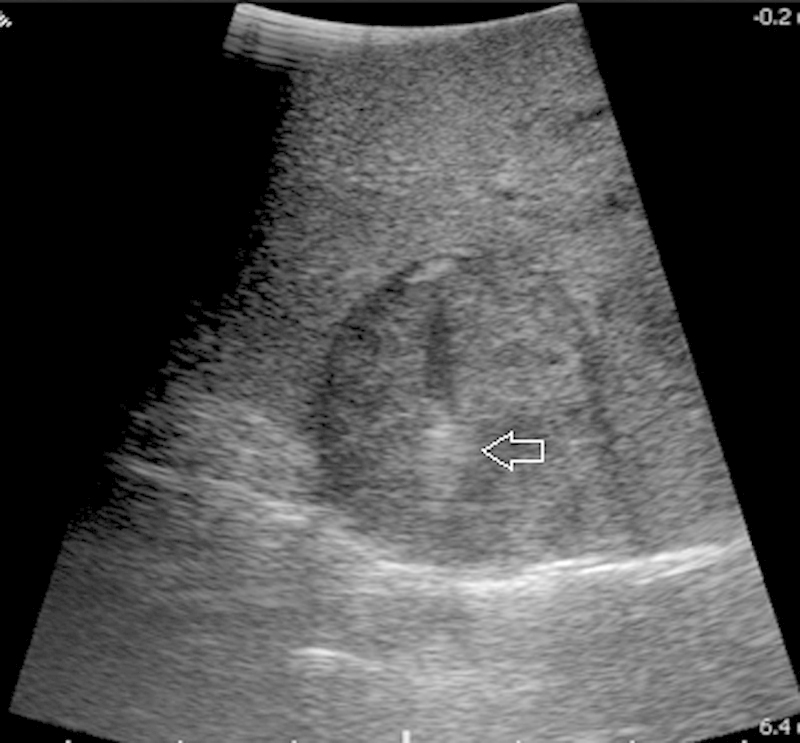
Image obtained during real-time probe placement, with the probe seen (arrow) advancing into the mass.
Tumors immediately adjacent to large blood vessels may be subject to warmer temperatures due to heat sink effect, which should be taken into consideration during treatment planning. A transperitoneal approach is generally used for anterior and anteromedial tumors, whereas a retroperitoneal approach permits access to posterior and posterolateral tumors.8 Direct laparoscopic visualization allows for monitoring of the growing ice (Fig. 4), and helps ensure that vital structures are not frozen or damaged. Surgicel (Ethicon, Somerville, NJ) and/or fibrin glue are frequently placed over the field after completion of thawing and probe removal.
Figure 4.
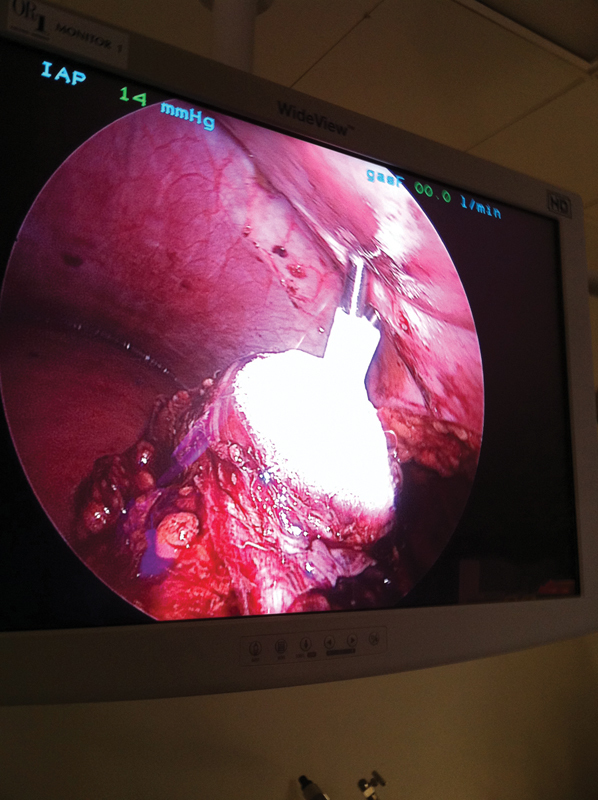
Intraoperative monitoring of the forming ice ball, with two probes placed directly through the skin after palpation.
Patient Selection
Treatment options and alternatives are discussed in detail with every patient. Partial nephrectomy is currently considered the treatment of choice in those patients who can tolerate it.2 However, the American Urological Association considers ablation as a viable therapy for T1a renal tumors, particularly in elderly patients with multiple comorbidities who are unfit for partial nephrectomy.2 Cryoablation confers additional benefits over excision such as preservation of renal function9 and lower perioperative complication rates when compared with partial nephrectomy2; this should be taken into consideration when making individualized recommendations for a patient. In general, one probe is needed for each centimeter of tumor diameter to be treated. The location of the mass is a major factor in determining if the mass should be ablated laparoscopically or percutaneously. In general, masses that are located posteriorly or posterolaterally may be very amenable to percutaneous treatment, whereas masses that are anteriorly positioned or adjacent to vital structures may be best approached laparoscopically. Experienced percutaneous operators may be able to access anterior or upper pole lesions, or utilize techniques to dissect away structures to create a safe margin for ablation, so cases should be reviewed on an individual basis with the operator performing the cryosurgery. In patients in whom treatment is required and general anesthesia is considered risky, percutaneous cryoablation may be a preferred treatment, as it can be performed with sedation and analgesia. Please refer to the Gunn et al article in this issue of the Seminars in Interventional Radiology for further discussion.10
Literature Review
A recent meta-analysis determined that small renal masses demonstrated an average growth rate of 0.3 cm a year,11 suggesting that active surveillance is a reasonable approach in elderly patients with multiple comorbidities who do not wish to pursue treatment; in those patients who do wish to pursue treatment, LCA is a viable minimally invasive alternative. Kunkle et al analyzed 99 studies representing 6,471 lesions and demonstrated that LCA had a relative risk of 7.45 for local tumor recurrence as compared with LPN.11 Many of these studies use criteria for recurrence established by Weight et al,12 who demonstrated a high correlation between radiologic and histopathological results, specifically with respect to enhancement on posttreatment magnetic resonance imaging (MRI). In this analysis, 192 lesions in 176 patients were treated with LCA with a 6-month success rate of 93.8%. Percutaneous biopsy was obtained in 97 of these patients. All of the patients who had scans demonstrating enhancement had evidence of residual viable tumor cells on the corresponding biopsy, and none of the biopsies that were performed on treatment sites without enhancement revealed viable tumor, thus demonstrating postcryoablation MRI as a reliable method to screen for recurrence. Although Kunkle's meta-analysis concluded that there was a slightly higher occurrence of residual and recurrent disease with LCA, the progression to metastatic disease did not significantly differ, suggesting LCA is still a viable oncologic approach for treating selected small renal masses.11
The landmark paper by Aron et al demonstrated a 92% 5-year disease-specific survival as well as 84% overall survival in 80 patients treated by LCA by a single surgeon.13 These authors also demonstrated an average of nearly 90% decrease in diameter of the cryoablation zone by 5 years. Complete radiographic disappearance was noted in 73% of the patients in this study. None of the lesions grew, including lesions that had imaging suspicious for local recurrence. A recent single institution study from Washington University by Tanagho et al14 mirrored Aron's findings, with 80, 100, and 76.2% disease-free survival, cancer-specific survival, and overall survival, respectively, with a mean follow-up of 76 months.
Additional benefits of LCA over partial LPN include decreased blood loss, no need for renal hilar clamping, decreased urine leaks/stenting, and improved access to endophytic tumors. The morbidity benefits of LCA have been documented by several authors. Desai et al concluded that partial nephrectomy was associated with greater blood loss and delayed complications when compared with LCA.15 A smaller study performed by Hruby et al comparing 12 LPNs with 11 LCAs demonstrated decreased operating time, decreased blood loss, decreased perioperative complications, and decreased hospital stay for the cryoablation group, with equivalent disease recurrence in both groups at 1 year.16 In a meta-analysis comparing complications of LCA versus PN (both open and laparoscopic), investigators found a nearly 10-fold risk higher relative risk of major complications for PN versus LCA.17
The oncologic efficacy of LCA compared with LPN remains the major focus in determining the application of this technique. As noted above, Kunkle et al analyzed 99 studies representing 6,471 lesions and demonstrated that LCA had a relative risk of 7.45 for local tumor recurrence as compared with LPN.11 A recent meta-analysis by Klatte et al again demonstrated a 4.82 relative risk of local tumor recurrence following LCA versus LPN in an analysis comparing 5,379 small renal masses treated by LPN and 1,406 small renal masses treated with LCA17; in this analysis, larger tumor size represented the main risk factor for recurrence. Tsivian et al demonstrated a 4-fold increase in local recurrence with LCA for each 1 cm increase in size.18
Specific cryoablation technique may have a meaningful impact on oncologic efficacy and complication rates. A recent publication from Guazzoni et al demonstrated benefits from changing to smaller cryoprobes with subsequent utilization of multiple cryoprobes halfway through their study. In addition to increasing the diameter of tumor that could potentially be treated, the use of multiple cryoprobes decreased the incidence of major bleeding, with no major bleeds seen after converting to the smaller probes.19 This was a retrospective analysis of 131 small renal masses in 123 patients, of which 44 underwent LCA. Of these latter 44 patients, the cancer-specific survival rate was 100% and overall survival was 93.2% with a mean follow-up of 61 months.19 Several recent studies have provided evidence for the usage of multiple smaller probes as a way of increasing effective tumor kill. Wyler et al used up to 6 thin cryoprobes, allowing for extension of the coldest isothermal line.20 His preliminary results of retroperitoneoscopic-assisted cryotherapy for small renal masses at a mean follow-up of 21 months demonstrated no recurrence in 12 patients, with 2 patients dying of unrelated disease.20 Littrup et al demonstrated the synergistic effects of multiple cryoprobes allowing for a lethal ice diameter of 35 to 70% higher than a single cryoprobe.21 Young et al confirmed this finding, concluding that the ice ball volumes increased in size more than expected when pulses were used in tandem, demonstrating that cryolesions that are created by multiple simultaneous 1.47-mm probes have a synergistic effect.22
Effect on Renal Function
One of the main benefits of LCA is the preservation of normal renal parenchyma. LCA is an excellent option for treatment of small renal masses in those patients with chronic renal insufficiency. Tsivian et al concluded that there was no significant change in chronic renal insufficiency category at any time in the 2 years follow-up of 67 patients status post-LCA.9 Tanagho et al also demonstrated preservation of renal function with relatively stable glomerular filtration rates (GFR) nearly 4 years post-LCA (GFR 68 mL/min pre-op vs. 65 mL/min post-op).14 These benefits are particularly crucial in patients with solitary kidneys and elderly patients with multiple comorbidities.
Another potential application for cryotherapy is in the situation of a tumor in a solitary kidney. Haber et al analyzed patients with solitary kidneys who were treated with LPN (n = 48) compared with LCA (n = 30).23 These authors found that estimated glomerular filtration rates (eGFR) decreased more with LPN (14.5 vs. 7.3%). Other findings in this study were that LPN was associated with increased blood loss and greater postoperative complications (22.9 vs. 6.7%, p = 0.07) compared with LCA. Despite the decreased morbidity with LCA, there were greater recurrence rates in the LCA group (13.3 vs. 0%). The 5-year overall survival was comparable at 93 versus 88%, but there was a significant difference in disease-specific survival (100% in the LPN group vs. 88% in the LCA group). The authors concluded that although LCA is technically easier with fewer complications and better eGFR in the long term, LPN demonstrated superior oncologic efficacy. Not all studies have reproduced these findings, however. A recent retrospective analysis by Panumatrassame et al demonstrated that LCA provided better perioperative outcomes with less blood loss, shorter hospital stay, and fewer complications, but the eGFR rates were not significantly different between groups treated with LPN versus LCA.24
LPN and LCA affect renal function by potentially separate mechanisms. With LPN, renal hilar clamping causing renal ischemia and actual parenchymal resection represent two separate mechanisms for potential decreases in eGFR. With LCA, normal parenchymal tissue surrounding the cryolesion may be inadvertently damaged while freezing the tumor.24 Again there have been varying results reported in the literature. Turna et al compared three minimally invasive nephron-sparing techniques concluding that LPN had better intermediate-term oncologic efficacy while having significantly decreased eGFR at 6 months compared with LCA and RFA.25
Discussion
Advances in and increased utilization of cross-sectional imaging in the past few decades have led to widespread paradigm shifts in the surgical management of small renal masses. Data have emerged demonstrating the long-term value to patients of nephron preservation. Radical nephrectomy, which traditionally has represented to gold standard for the treatment of renal cancers, has been replaced by partial nephrectomy, with sufficient research demonstrating surgical feasibility as well as equivalent oncologic efficacy. Advances in nephron-sparing surgery including laparoscopic and robotic techniques have emerged, and LPN in experienced hands has shown equivalent oncologic outcomes to open partial nephrectomy. Of interest, as experience has increased and techniques evolved, superior overall outcomes have been demonstrated; as reported in the paper by Gill et al, in which his cohort of 800 patients were divided into three chronological eras, the most recent era of patients had superior functional outcomes with shorter warm ischemia times and fewer complications, despite the masses in this group being more complex, both with larger and more centrally located masses.26
Toward the endpoint of improved renal preservation and less invasive procedures, ablative techniques including cryosurgery have been developed and widely studied. Across many series, such techniques have demonstrated shorter hospitalization times, faster recovery, and lower complication rates, and improved preservation of renal function. A mature dataset exists, with multiple investigators reporting intermediate- and long-term oncologic results with excellent cancer-specific survival. Multiple analyses have shown a slightly higher relative risk of local recurrence with cryoablation, which may require retreatment; however, increased metastatic progression relative to partial nephrectomy has not been demonstrated. Of note, cryosurgery equipment and the understanding of techniques have progressed in recent years, with improved equipment and synergy of lethal ice by multiple cryoprobes shown to occur. The reports with the longest follow-up to date for LCA patients used a single probe in 50 out of 62 patients,14 and in 64 out of 80 patients.13 This makes their results extremely encouraging, as patients were treated with potentially suboptimal technique and primitive equipment compared with that which is available today, but still with very favorable results.
While LPN should still be considered the gold standard given its superior oncologic efficacy, multiple studies have concluded that LCA is a viable treatment alternative for T1a renal tumors, particularly in elderly patients or those with multiple comorbidities who are considered poor candidates for surgery. LCA has fewer perioperative complications and better preserved renal function than LPN. As such, LCA may also be an excellent alternative in patients with renal insufficiency, patients with solitary or transplant kidneys, and patients with recurrent tumors due to hereditary conditions such as Von Hippel–Lindau syndrome. With increased operator experience and better understanding of cryoprobe technology, cancer-specific survival and disease-free survival rates after LCA will likely continue to improve. The long-term data available at this time, while encouraging, remain scarce. Additional long-term data must emerge and prospective trials with more modern techniques and equipment will need to be conducted for the role of LCA in the treatment of SRMs to be fully elucidated.
Footnotes
Funding There are no sources of financial support associated with this review.
References
- 1.Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology. 1998;51(2):203–205. doi: 10.1016/s0090-4295(97)00506-2. [DOI] [PubMed] [Google Scholar]
- 2.AUA Guideline for Management of Clinical Stage 1 Renal Mass Available at: http://www.auanet.org/education/guidelines/renal-mass.cfm
- 3.Berger A, Kamoi K, Gill I S, Aron M. Cryoablation for renal tumors: current status. Curr Opin Urol. 2009;19(2):138–142. doi: 10.1097/MOU.0b013e328323f618. [DOI] [PubMed] [Google Scholar]
- 4.El Dib R, Touma N J, Kapoor A. Cryoablation vs radiofrequency ablation for the treatment of renal cell carcinoma: a meta-analysis of case series studies. BJU Int. 2012;110(4):510–516. doi: 10.1111/j.1464-410X.2011.10885.x. [DOI] [PubMed] [Google Scholar]
- 5.Williams S K, de la Rosette J, Landman J, Keeley F X. Cryoablation of small renal tumors. European Association of Urology. 2007;5:206–218. [Google Scholar]
- 6.Ahmed M, Brace C L, Lee F T Jr, Goldberg S N. Principles of and advances in percutaneous ablation. Radiology. 2011;258(2):351–369. doi: 10.1148/radiol.10081634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill I S, Novick A C, Soble J J. et al. Laparoscopic renal cryoablation: initial clinical series. Urology. 1998;52(4):543–551. doi: 10.1016/s0090-4295(98)00309-4. [DOI] [PubMed] [Google Scholar]
- 8.Weld K J, Figenshau R S, Venkatesh R. et al. Laparoscopic cryoablation for small renal masses: three-year follow-up. Urology. 2007;69(3):448–451. doi: 10.1016/j.urology.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 9.Tsivian M M, Caso J, Kimura M, Polascik T J. Renal function outcomes after laparoscopic renal cryoablation. J Endourol. 2011;25(8):1287–1291. doi: 10.1089/end.2011.0017. [DOI] [PubMed] [Google Scholar]
- 10.Gunn A, Gervais D. Percutaneous ablation of the small renal mass—techniques and outcomes. Semin Intervent Radiol. 2014;31(1):33–41. doi: 10.1055/s-0033-1363841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunkle D A Egleston B L Uzzo R G Excise, ablate or observe: the small renal mass dilemma—a meta-analysis and review J Urol 200817941227–1233., discussion 1233–1234 [DOI] [PubMed] [Google Scholar]
- 12.Weight C J Kaouk J H Hegarty N J et al. Correlation of radiographic imaging and histopathology following cryoablation and radio frequency ablation for renal tumors J Urol 200817941277–1281., discussion 1281–1283 [DOI] [PubMed] [Google Scholar]
- 13.Aron M, Kamoi K, Remer E. et al. Laparoscopic renal cryoablation: 8-year, single surgeon outcomes. Urology. 2010;183:889–895. doi: 10.1016/j.juro.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 14.Tanagho Y S, Roytman T M, Bhayani S B. et al. Laparoscopic cryoablation of renal masses: single-center long-term experience. Urology. 2012;80(2):307–314. doi: 10.1016/j.urology.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 15.Desai M M Aron M Gill I S Laparoscopic partial nephrectomy versus laparoscopic cryoablation for the small renal tumor Urology 200566(5, Suppl):23–28. [DOI] [PubMed] [Google Scholar]
- 16.Hruby G, Reisiger K, Venkatesh R, Yan Y, Landman J. Comparison of laparoscopic partial nephrectomy and laparoscopic cryoablation for renal hilar tumors. Urology. 2006;67(1):50–54. doi: 10.1016/j.urology.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 17.Klatte T, Grubmüller B, Waldert M, Weibl P, Remzi M. Laparoscopic cryoablation versus partial nephrectomy for the treatment of small renal masses: systematic review and cumulative analysis of observational studies. Eur Urol. 2011;60(3):435–443. doi: 10.1016/j.eururo.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Tsivian M, Lyne J C, Mayes J M, Mouraviev V, Kimura M, Polascik T J. Tumor size and endophytic growth pattern affect recurrence rates after laparoscopic renal cryoablation. Urology. 2010;75(2):307–310. doi: 10.1016/j.urology.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 19.Guazzoni G, Cestari A, Buffi N. et al. Oncologic results of laparoscopic renal cryoablation for clinical T1a tumors: 8 years of experience in a single institution. Urology. 2010;76(3):624–629. doi: 10.1016/j.urology.2010.03.078. [DOI] [PubMed] [Google Scholar]
- 20.Wyler S F, Sulser T, Ruszat R. et al. Intermediate-term results of retroperitoneoscopy-assisted cryotherapy for small renal tumours using multiple ultrathin cryoprobes. Eur Urol. 2007;51(4):971–979. doi: 10.1016/j.eururo.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 21.Littrup P J, Jallad B, Vorugu V. et al. Lethal isotherms of cryoablation in a phantom study: effects of heat load, probe size, and number. J Vasc Interv Radiol. 2009;20(10):1343–1351. doi: 10.1016/j.jvir.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young J L, McCormick D W, Kolla S B. et al. Are multiple cryoprobes additive or synergistic in renal cryotherapy? Urology. 2012;79(2):e1–e6. doi: 10.1016/j.urology.2011.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haber G P, Lee M C, Crouzet S, Kamoi K, Gill I S. Tumour in solitary kidney: laparoscopic partial nephrectomy vs laparoscopic cryoablation. BJU Int. 2012;109(1):118–124. doi: 10.1111/j.1464-410X.2011.10287.x. [DOI] [PubMed] [Google Scholar]
- 24.Panumatrassamee K, Kaouk J H, Autorino R. et al. Cryoablation versus minimally invasive partial nephrectomy for small renal masses in the solitary kidney: impact of approach on functional outcomes. J Urol. 2013;189(3):818–822. doi: 10.1016/j.juro.2012.09.075. [DOI] [PubMed] [Google Scholar]
- 25.Turna B, Kaouk J H, Frota R. et al. Minimally invasive nephron sparing management for renal tumors in solitary kidneys. J Urol. 2009;182(5):2150–2157. doi: 10.1016/j.juro.2009.07.066. [DOI] [PubMed] [Google Scholar]
- 26.Gill I S, Kamoi K, Aron M, Desai M M. 800 Laparoscopic partial nephrectomies: a single surgeon series. J Urol. 2010;183(1):34–41. doi: 10.1016/j.juro.2009.08.114. [DOI] [PubMed] [Google Scholar]


