Abstract
An increasing number of T1a renal cell carcinomas are being diagnosed in recent years, in part due to incidental detection from the increased use of cross-sectional imaging. Although partial nephrectomy is still considered the primary treatment for these small renal masses, percutaneous ablation is now being performed as a standard therapeutic, nephron-sparing approach in patients who are poor surgical candidates. Clinical studies to date have demonstrated that percutaneous ablation is an effective therapy with acceptable outcomes and low risk in the appropriate clinical settings. This article will review various clinical aspects regarding the percutaneous ablation of small renal masses, including patient selection, preprocedural preparations, and the procedural considerations of commonly employed ablative technologies. Specific techniques such as radiofrequency ablation, cryoablation, microwave ablation, irreversible electroporation, and high-intensity focused ultrasound will be addressed in detail. In addition, the technical and oncologic outcomes of percutaneous ablation will be discussed and referenced to that of partial nephrectomy.
Keywords: renal mass, percutaneous ablation, radiofrequency ablation, cryoablation, interventional radiology
Objectives: Upon completion of this article, the reader will be able to describe patient selection and preparation for percutaneous ablation of a small renal mass. In addition, the reader should be able to identify the technical aspects surrounding the most commonly employed ablative strategies.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Background, Indications, and Alternatives to Percutaneous Ablation
There will be an estimated 65,150 new cases of renal cell carcinoma (RCC) in 2013 with approximately 13,680 deaths attributable to this disease.1 The increased incidence of RCC in the United States is, in large part, secondary to the incidental detection of small, localized RCCs in patients who undergo cross-sectional imaging for an unrelated complaint.2 3 4 5 Classically, the gold standard for the treatment of even small, localized tumors in the kidney has been radical nephrectomy. However, given that these incidentally detected RCCs have been shown to be smaller, of lower grade, and associated with longer disease-free survival,6 nephron-sparing treatments such as partial nephrectomy, laparoscopic ablation, and percutaneous ablation are becoming more commonly used in clinical practice. Indeed, all three alternatives to radical nephrectomy have been shown to effectively treat small renal masses with decreased rates of complication and less detriment to patient renal function.3 6 7 8 9 10 11 12 However, only percutaneous ablation offers the patient with a small renal mass the opportunity for cure without being subject to operative risks, which is especially important for patients who are poor surgical candidates or who are at increased risk for multiple RCCs (such as in Von Hippel–Lindau syndrome). Moreover, ablation is a viable alternative to surgery in patients with chronic kidney disease (CKD), patients with comorbid conditions that render them poor candidates for surgery, and patients with a solitary kidney. According to current understanding, the ideal candidate for percutaneous ablation would have a renal mass smaller than 4 cm in diameter in a noncentral location in the kidney and be without acute illness, uncorrected coagulopathy, tumor extension beyond Gerota fascia or into the renal vein, or metastatic disease.13 In addition, in a small number of cases, radiofrequency (RF) ablation has been successfully applied to treat tumor-related hematuria in an attempt to ablate the interface of the tumor and collecting system to coagulate the bleeding surface of a tumor.
Definition of a Small Renal Mass
Early evaluations of ablative therapies for small renal masses commonly categorized masses as “small” if they measured less than 3 cm in diameter. This assessment was based on a then commonly applied categorization scheme in the evaluation of small liver tumors, specifically hepatocellular carcinoma. However, as experience and techniques matured, evaluation of larger series showed that masses greater than 3 cm (in some cases up to 4 cm) were amenable to ablation.3 12 Moreover, the American Joint Committee on Cancer (AJCC) staging system for RCC classifies a T1a tumor as one that measures up to 4 cm.14 Thus, both the technical capabilities of tumor ablation and the staging system converge upon 4 cm as an appropriate threshold value for the definition of a small renal mass. Thus, the term “small renal mass” and “T1a tumor” will be used interchangeably for the remainder of this discussion. When reporting results from other authors who used a different size cutoff, the values used will be specified with the results.
Preablative Considerations: Clinical Work-Up and Biopsy
Patients considered candidates for percutaneous image-guided renal tumor ablation are typically evaluated by both an interventional radiologist and an urologist before the procedure. In some situations, a consultation with a medical oncologist may also be warranted. The interventional radiologist ideally meets the patient at a clinic visit during which a history and physical examination is obtained. Imaging findings, laboratory work (including tests of hemostasis and renal function), and pathology results, if available, can also be reviewed with the patient at that time. At the authors' institution, an international normalized ratio (INR), partial thromboplastin time (PTT), and complete blood count are obtained before the procedure. It is preferred that the INR be < 1.5 and the PTT and platelet count be within normal limits, although, platelet counts as low as 50,000/µL may be acceptable in certain situations. All anticoagulation medications are discontinued for a period of time sufficient to normalize coagulation. If normalization is not possible, fresh frozen plasma and platelets can be administered.
The interventional radiologist formulates a plan for ablation with the patient that includes the approach to be used, the form of ablative therapy, the type of anesthesia to be used, and any appropriate adjunctive measures (such as hydrodissection or pyeloperfusion). In general, there are three types of anesthesia employed during percutaneous ablation: general anesthesia with endotracheal intubation, deep sedation with an anesthetist present, and moderate intravenous (IV) sedation. The choice of sedation method is dependent on several factors including the patients' cardiorespiratory status, metabolic status, ability to protect their airway, and personal preferences. At the authors' institution, the use of moderate IV sedation is preferred, in which small doses of anxiolytics and anesthetics are administered by radiology nursing under the supervision of the interventional radiologist. However, some patients, with significant comorbidities may benefit from more advanced monitoring by anesthesiology personnel. Anesthesiologists may choose to intubate patients with a significant risk of aspiration. Finally, all risks, alternatives, and benefits are discussed with the patient (or health care proxy) and informed consent is obtained.
There is still some degree of controversy that exists surrounding the role of percutaneous biopsy of small renal masses, especially when definite characterization by contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) is available. Although some argue that the biopsy results are unlikely to change the clinical management of the patient,15 others suggest that complications from percutaneous ablation can be avoided if a benign diagnosis can be obtained through percutaneous biopsy before ablation.16 There are several advantages to preablative biopsy of small renal masses. For example, reports have shown that up to 37% of biopsied renal masses are benign oncocytomas or lipid-poor angiomyolipomas,17 18 which may mean that a substantial number of patients would undergo unnecessary preablative evaluation, anesthesia, and ablation if not for preablative biopsy. In addition, the inclusion of these benign renal masses in any research evaluating the outcomes of percutaneous ablation for small RCCs would necessarily over-estimate its efficacy.18 In addition, preablative biopsies are able to provide both the patient and referring physician with a definitive diagnosis rather than a presumptive diagnosis based on imaging features. Furthermore, an actual tissue diagnosis allows future treatments and surveillance to be tailored to tumor subtypes and grades if such information is available from the biopsy.19 The case for preablative biopsy is further strengthened by the fact that improvements in biopsy techniques and histologic analysis and immunohistochemistry stains have raised the positive predictive values to 95 to 100% for the diagnosis of RCC.19 20 Performing a percutaneous biopsy of a small renal mass in advance of the ablative session, rather than on the day of the ablation, typically provides the pathologist more time to analyze the specimen and apply special immunocytochemical stains sometimes needed to diagnose renal neoplasms.21 Useful clinic information can be obtained from the biopsy procedure itself. For example, the actual procedure could provide the interventional radiologist with a better understanding of how the patient will tolerate the ablation, the optimal position for ablation, the best percutaneous approach to the lesion, and how much IV sedation and analgesia might be required. However, if the prebiopsy probability of RCC is extremely high, then performing a biopsy on the day of the ablation may also be a reasonable approach.
One must also consider the clinical scenario in which the preablation biopsy comes back as “negative” in the setting of a solid enhancing renal mass without features of angiomyolipoma. In this situation, a repeat biopsy could be considered, depending on the degree of clinical suspicion, or close interval follow-up imaging performed to assess for change in size. Another clinical dilemma occasionally encountered is that of a mass with “oncocytic elements”; in such cases, we manage solid renal masses with oncocytic elements as malignant neoplasms unless the pathologist can make a definitive diagnosis of oncocytoma.
Ablation Procedure and Techniques
The majority of percutaneous ablations can be performed on an outpatient basis; however, in certain situations such as complicated cases or if there is slow patient recovery from sedation, the interventional radiologist may consider hospitalizing the patient for overnight observation. The patients arrive for the procedure having ingested nothing by mouth for at least 8 hours. An IV line is started by radiology nursing for the administration of fluids, antibiotics (if used), other medications, and blood products (if necessary). The authors do not routinely give antibiotics unless the patient has an ileal loop diversion or the case is being performed with pyeloperfusion.
At the authors' institution, the patient is preferentially positioned with the ipsilateral side down on the table, if possible, to try to limit the amount of respiratory motion in the kidney. However, other patient positions, including prone, supine, and ipsilateral side up, may also be used. Once the safest, most direct percutaneous route to the mass has been identified by preliminary imaging, the patient's skin is prepped and draped in standard sterile fashion. Renal ablation can be safely performed using ultrasound (US), CT, or MRI guidance. Most commonly, percutaneous ablations are performed under CT guidance, with US available, if needed. MRI guidance requires that all ablation equipment, as well as all patients monitoring equipment, be MR compatible.
In some cases, adjacent organs (such as the bowel) will need to be displaced from the kidney to prevent injury during the ablation. This can be achieved with the introduction of fluid (hydrodissection), carbon dioxide gas, or balloons into the abdomen. When the percutaneous path to the tumor is clear, some operators will choose to place the needle applicators within the tumor before organ displacement since placement of the needle applicators may move the tumor, provide a lever to move the tumor, or cause a small amount of hemorrhage, which may all provide the separation from adjacent structures needed to safely proceed with ablation. Other operators have advocated opacifying hydrodissecting fluid to make it more readily visible on CT22; this approach also has the advantage of making the fluid more easily distinguishable from fluid in the bowel and from the iceball created during cryoablation.
The choice of the ablative therapy and specific equipment to be used should be made in consultation with the patient at the time of the initial clinic visit. The most commonly used ablative agents are RF and cryoablation16; however, other technologies, such as microwave (MW) ablation, irreversible electroporation (IRE), and high-intensity focused US (HIFU) are being evaluated. Each agent has advantages and will be discussed in more detail below.
Radiofrequency Ablation
RF ablation is the most commonly used and studied mode of ablation (Figs. 1 and 2). It delivers a current to tissue causing ionic agitation of intracellular molecules, resulting in frictional heating.23 Currently, there are a variety of commercially available RF generators and probe configurations which all aim to achieve coagulation necrosis in the defined treatment area. This typically occurs at approximately 60 to 100°C over a period of approximately 8 to 16 minutes, depending on the RF ablation system used. RF ablation has been shown to be most efficacious when employed for noncentral tumors that are less than 3 to 4 cm in diameter.24 The perinephric fat is thought to act as an insulating material, making thermal ablation particularly effective in peripheral tumors.16 Centrally located tumors are somewhat less effectively treated with RF ablation, which could be due to the “heat-sink” phenomenon from hilar vessels and the urinary collecting system.3 It may also be due to less aggressive ablative treatments in this location by the interventional radiologist who may be worried about causing damage to the renal collecting system.25 26 27
Figure 1.
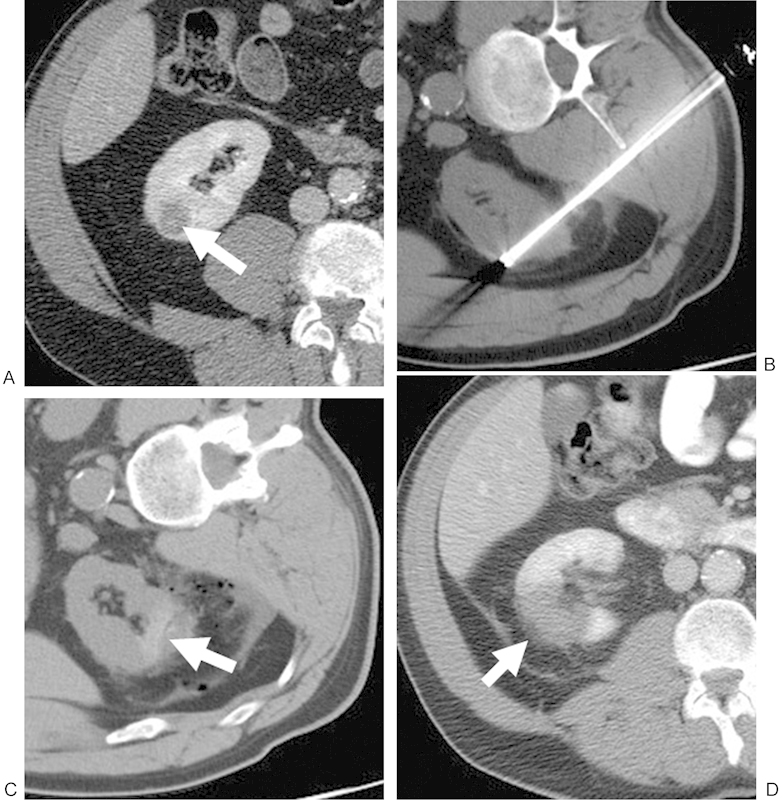
(A) Contrast-enhanced CT of a 56-year-old man shows a 1.9-cm RCC in the right lower pole (white arrow). The patient was referred for RF because of a complex cardiac history. (B) Noncontrast CT with the patient right-side/ipsilateral-side down demonstrating a posterior approach with the RF probe within the lesion. (C) Noncontrast postablation CT demonstrates areas of increased attenuation (white arrow) in the ablation zone, consistent with blood products, in addition to expected postprocedural changes adjacent to the kidney. This is a common postablation appearance. (D) Contrast-enhanced CT performed approximately 1 month after ablation shows no evidence of enhancement in the ablation zone (white arrow), consistent with a completely treated lesion. CT, computed tomography; RCC, renal cell carcinoma; RF, radiofrequency.
Figure 2.
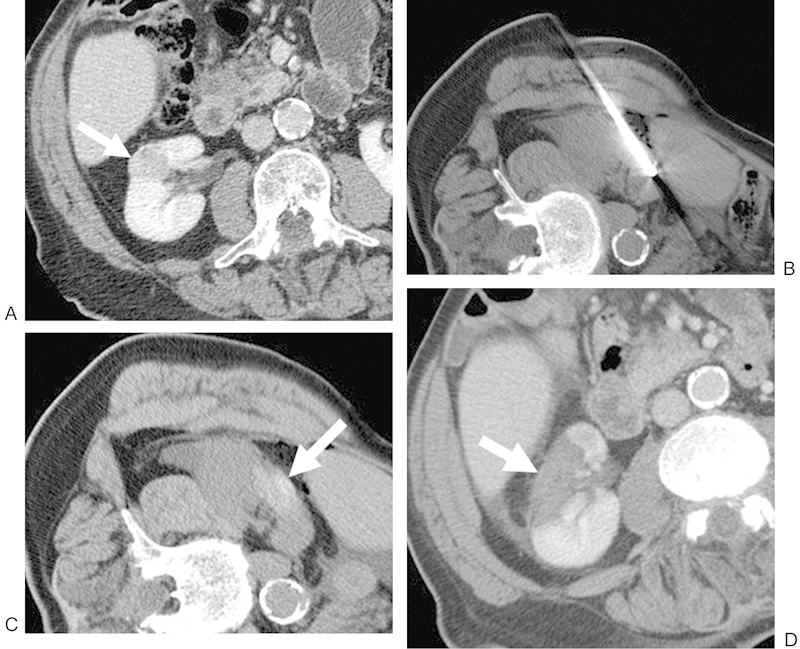
(A) Contrast-enhanced CT of an 83-year-old man shows a 2.3-cm RCC in the interpolar region of the right kidney that extends toward the renal sinus fat (white arrow). The patient was referred for RF because of an overall poor functional status. (B) Noncontrast CT with the patient right-side/ipsilateral-side up demonstrating a lateral approach with the RF probe within the lesion. (C) Noncontrast postablation CT demonstrates areas of increased attenuation (white arrow) in the ablation zone, consistent with blood products, in addition to expected postprocedural changes adjacent to the kidney. This is a common postablation appearance. (D) Contrast-enhanced CT performed approximately 1 month after ablation shows no evidence of enhancement in the ablation zone (white arrow), consistent with a completely treated lesion. CT, computed tomography; RCC, renal cell carcinoma; RF, radiofrequency.
The effects of RF ablation on the renal collecting system have been shown to be mitigated by “pyeloperfusion” techniques.28 29 In this clinical scenario, the patient has a ureteral stent placed on the ipsilateral side on the morning of the ablation. During the ablation, a cold dextrose solution is dripped through the stent, helping to prevent ureteral injury. Dextrose is used to provide a nonionic solution that insulates the collecting system from the electrical current applied during RF ablation; similarly, saline solution is avoided because of its ionic nature and potential for electrical current conduction. The dextrose perfusate solution drains via a bladder catheter while new cold dextrose is perfused into the stent. In general, the authors perform RF ablation with pyeloperfusion if the tumor is within 1.5 cm of the ureter or ureteropelvic junction.
RF ablation of a volume of tumor can be achieved by performing multiple overlapping ablations, but this requires a methodical approach. Generally, one can begin the ablation process along a defined margin of the tumor and sequentially move the RF probe toward the opposite margin until an acceptable volume of tissues has been treated. In addition, recent studies have shown that a larger volume of tissue can be treated with a single ablation by employing bipolar probes and having multiple electrodes activated sequentially30; while initial results with these newer techniques have been promising, further research into their clinical use is needed.
Cryoablation
Cryoablation relies on depressurization of argon gas at the tip of the cryoprobe to achieve rapid cooling to temperatures below − 30 to − 40°C.31 Alternating freeze-thaw cycles result in cell death secondary to cellular dehydration, cell membrane rupture, and vascular thrombosis.31 This can typically be achieved by applying two freeze-thaw cycles consisting of 10 to 15 minutes of freezing followed by 8 to 10 minutes of thawing (Fig. 3). The ablation zone for cryoablation is planned such that the entire area of the tumor is covered plus a 5 to 10 mm margin, remembering that the visible iceball has a nonlethal leading edge that extends approximately 5 mm into the iceball. If multiple cryoprobes are used, they are typically placed approximately 1.5 cm apart to achieve optimal results. Moreover, cryoprobes should not be placed more than 1 cm from the edge of the tumor and no more than 2 cm from the nearest adjacent cryoprobe. Even though cryoablation results in less tissue destruction per probe than RF, there are important advantages to using cryoablation. One significant advantage of cryoablation is the ability to tailor the size and shape of the ablated area during the procedure. For example, if multiple cryoprobes are employed, then the interventional radiologist has the ability to activate some probes while leaving others off, a function available on only some RF generators. In addition, the interventional radiologist is able to actively monitor the treated area by CT, US, or MR through the formation of the iceball. Thus, the ablation zone can be expanded to untreated areas or moved away from nontarget structures based on the imaging appearance of the iceball. Furthermore, cryoablation does not use grounding pads on the patient's skin, as is done in RF, which reduces the possibility of patient skin burns. Of note, warm pyeloperfusion can be performed for cryoablation in a manner similar to chilled pyeloperfusion in RF ablation for tumors near the renal collecting system. In this scenario, normal saline solution can be employed since there is no electric current directly involved in the treatment application.
Figure 3.
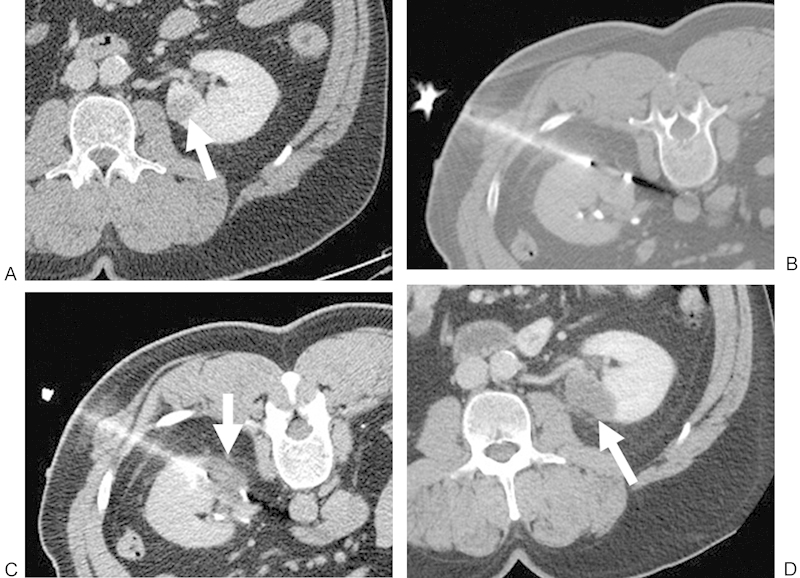
(A) Contrast-enhanced CT of a 55-year-old man shows a 1.8-cm RCC in the lower pole of the left kidney (white arrow). The patient was referred for cryoablation of the left renal lesion because of prior right nephrectomy for RCC. (B) Noncontrast CT with the patient in prone position demonstrating a posterolateral approach with the cryoprobe within the lesion. (C) Noncontrast CT demonstrates the low-attenuation “iceball” in the ablation zone after a 10-minute freeze (white arrow). (D) Contrast-enhanced CT performed approximately 1 month after ablation shows no evidence of enhancement in the ablation zone (white arrow), consistent with a completely treated lesion. CT, computed tomography; RCC, renal cell carcinoma; RF, radiofrequency.
Microwave Ablation
MW ablation probes offer several advantages over traditional RF probes including the ability to more rapidly generate high temperatures. Moreover, when multiple probes are used, then each probe can be controlled individually and no grounding pads are placed on the patient's skin, reducing the possibility of skin burns. MW ablation was first reported for renal tumors in 2007.32 This phase I clinical study using a single 10 minutes ablation demonstrated complete tumor cell destruction without skip areas in the ablation zones while achieving ablation volumes of 27 and 105 cm3 for single and three-pronged probes, respectively. Recent clinical studies using MW ablation in RCC have been mixed. For example, Carrafiello et al performed MW ablation of small renal masses in 12 patients with contrast-enhanced US guidance.33 The mean tumor size in this group was 2 cm with the largest tumor being 2.9 cm. The investigators followed these patients for 6 months after MW ablation with contrast-enhanced CT, reporting no severe complications with the procedure and no evidence of recurrence in any patient. However, Castle et al treated 10 patients with small renal masses with either laparoscopic or percutaneous MW ablation.34 This latter study reported intraprocedural complications in 20% of patients, postprocedural complications in 40% of patients, and recurrence in 38% of patients followed out to 17.9 months on average. More research is needed to identify the safety profile and role of MW ablation for the treatment of small renal masses.
High-Intensity Focused Ultrasound
HIFU is a relatively new thermal ablation technology that has yet to gain Food and Drug Administration approval and is not used in the United States. HIFU causes coagulative necrosis in renal tumors through the use of high-intensity US beams (100–10,000 W/cm2) that are directed toward millimeter-sized focal zones within the renal tumor.35 Tissue damage to adjacent structures is limited because US beam intensities outside of the focal zone are much lower. A recent review of HIFU for RCC identified several limitations, including: technical and anatomic difficulties in delivering HIFU beams to a small renal mass, tracking the lesion during treatment, nonuniform ablation, and clinical efficacies of only 57 to 67%.36 Certainly, additional technical advancements are necessary before HIFU becomes commonplace in the treatment of small renal masses.
Irreversible Electroporation
Electroporation is a process by which small pores are created in the cellular membrane through the application of a brief but high voltage across the membrane.37 These small pores destabilize the cellular membrane, thereby increasing its permeability. “Reversible” electroporation has been used in the laboratory setting and, to a lesser extent, in the clinical setting for several decades as researchers have taken advantage of the transient increase in membrane permeability to deliver DNA, chemotherapeutic drugs, and other molecules.37 Before 2006, IRE was an undesirable consequence of “reversible” electroporation; however, Edd et al were the first to describe the process of IRE as a therapeutic alternative to thermal ablation.38 In this initial report, normal hepatic parenchyma underwent IRE in a murine model demonstrating microvascular occlusion, endothelial cell necrosis, and diapedesis on pathologic analysis with sharp margins between treated and untreated tissues. Early demonstrations of IRE for RCC in animal models have also been promising. For example, Tracy et al performed 24 laparoscopic IREs in a porcine model of RCC and found no evidence of cellular viability by nicotinamide adenine dinucleotide staining on histologic analysis.39 Similar results were reported by Deodhar et al who performed 29 percutaneous ablations in a porcine model under CT guidance.40 Histologic analysis in this report showed evidence of necrotic tissue in the ablated areas with early evidence of fibrosis 3 weeks after IRE. Importantly, no evidence of thermal, renal pelvic, urinary collecting system, or vascular injury was identified. Pech et al were the first to perform a phase I clinical trial of IRE in RCC.41 In this report, six patients with renal tumors measuring less than 3.5 cm in maximal diameter underwent intraoperative IRE just before definitive surgical resection of the renal masses. General anesthesia was required because of the prerequisite need for muscle relaxation before IRE. In this report, IRE was performed with electrocardiographic synchronization; the authors reported only one case of intraoperative supraventricular tachycardia with no postoperative sequela and no significant changes to the hemodynamic status of any patient, thus demonstrating an excellent safety profile. Unfortunately, since tumors were immediately resected and analyzed after IRE, cellular viability studies in the treated regions were unreliable. Further study is warranted to confirm the safety profile of IRE in RCC as well as to determine its treatment efficacy, especially in comparison to more established methods such as thermal ablation, cryoablation, and partial nephrectomy.
Technical and Oncologic Outcomes after Percutaneous Ablation of the Small Renal Mass
Technical Outcomes
Since the initial description of a percutaneous method to ablate small renal masses in 1999, multiple studies in patients have demonstrated both thermal ablation and cryoablation to be technically feasible, efficacious, and safe.3 10 12 42 43 44 45 46 47 48 For example, Gervais et al reported a 100% technical success rate for all 52 thermally ablated tumors smaller than 3 cm and a 93% technical success rate for tumors between 3 and 5 cm.3 This series had only a 25% technical success rate for tumors larger than 5 cm and reported that 50% of tumors larger than 3 cm required more than one treatment. A noncentral location of tumor was also found to be an independent predictor of treatment success. Zagoria et al also reported a 100% technical success rate for RF ablation in renal tumors less than 3.6 cm in size, although 7% of the tumors in this group required a second ablative session to achieve complete necrosis.42 This series also demonstrated a statistically significant inverse relationship between tumor size and technical success. Similarly, other larger series evaluating the technical feasibility of RF ablation have reported success rates ranging from 79 to 100%.43 44 45 46 It should be noted that even though Breen et al only reported a 79% success rate after initial RF ablation, they were able to achieve a 91% technical success rate after an additional RF ablation session.44 The complication rates in these series ranged from 4.1 to 11%, with the majority of complications being minor. Comparable outcomes with regard to feasibility, technical success, and safety for cryoablation of small renal masses have also been achieved.10 47 48 For example, Atwell et al did a retrospective analysis of technical outcomes after performing 115 cryoablations in 100 patients.47 This series reported a technical success rate of 97% (with the mean tumor size being 3.3 cm) and only seven complications. Moreover, Hinshaw et al10 and Georgiades et al48 both reported a 100% technical success rate for cryoablation of 30 and 51 small renal masses, respectively.
Oncologic Outcomes
Despite the technical efficacy and excellent safety profiles associated with ablative technologies, demonstrating the long-term oncologic effectiveness of ablation has been complicated by limited patient follow-up, small sample sizes, the inclusion of patients with hereditary RCC syndromes, and lack of a definitive pathologic diagnosis in many studies.12 Recently, Psutka et al12 retrospectively reviewed their experience with percutaneous RF ablation of small renal masses in 185 patients with biopsy-proven RCC over a mean follow-up period of 6.4 years after treatment to assess long-term oncologic outcomes. In this series, the mean tumor size was 3 cm, although 22.7% of tumors in the series were greater than 4 cm (i.e., T1b tumors). Local recurrence occurred in 6.5% of patients overall at a mean of 2.5 years after RF ablation, with a 5-year recurrence-free survival of 96% for T1a tumors and 92% for T1b tumors (p = 0.02). A small minority (2.2%) of patients developed metastatic disease at an average of 1.5 years after therapy corresponding to a metastatic-free survival rate of 99.4% at 5 years, which was also greater in patients with T1b tumors (p = 0.011). This results in a 5-year disease-free survival rate of 91.5 and 74.5% for patients with T1a and T1b tumors, respectively (p = 0.003). Cancer-specific survival was 100 and 97% for patients with T1a and T1b tumors, respectively (p = 0.01), at 5 years. These long-term data demonstrating excellent oncologic outcomes after RF ablation, especially for T1a tumors, concur with other reports in the literature from smaller studies with only moderate-term follow-up that have reported local recurrence-free survivals of 80 to 100% and metastatic-free survivals of 93 to 100%.6 49 50 51
Perhaps a more salient question is raised when comparing the oncologic outcomes of percutaneous RF ablation and partial nephrectomy. Olweny et al52 retrospectively compared patients with pathologically proven solitary T1a tumors treated with either partial nephrectomy or RF ablation. Even though the RF cohort was significantly older and had more medical comorbidities than the partial nephrectomy cohort, they found no statistically significant difference in overall survival, cancer-specific survival, disease-free survival, metastatic-free survival, or local recurrence at 5 years. Furthermore, a larger analysis of the Surveillance, Epidemiology and End Results database of patients with T1a RCC, which included 578 patients who underwent RF ablation and 4,402 patients who underwent partial nephrectomy, failed to demonstrate any statistically significant difference in cancer-free or overall survival between the two groups.53 Although additional data, including larger, randomized studies with long-term outcomes, are needed, these early reports comparing the oncologic effectiveness of RF ablation to partial nephrectomy are encouraging, especially when the decreased periprocedural risks of percutaneous ablation are considered.
Comparing Ablative Methods
Even though cryoablation was the first method of ablation employed for RCC, RF ablation is now the most widely studied ablative technology for RCC. However, while both technologies enjoy high technical success rates, there has been relative difficulty in directly comparing their effectiveness.54 Accumulated experience and data have shown that any differences in efficacy are likely to be small; it has been estimated that approximately 900 patients would need to be enrolled in such a trial to gain adequate power. However, the combination of a relatively rare tumor with the excellent outcomes associated with partial nephrectomy leaves a smaller patient population available for comparative studies. These factors have made the design and execution of a trial comparing these two technologies elusive.54 Despite these difficulties, some attempts have been made to retrospectively compare the oncologic effectiveness of RF ablation and cryoablation. Kunkle and Uzzo reported that 12.9 and 5.2% of patients experienced local recurrence of their T1a tumor after RF ablation and cryoablation, respectively, suggesting RF ablation to be the superior modality.55 Conversely, El Dib et al found no statistically significant difference between the effectiveness of RF ablation and cryoablation.56 Clearly, further research into this matter is warranted. Unfortunately, the obstacles in comparing RF ablation to cryoablation also apply when trying to assess newer ablative technologies such as MW ablation, HIFU, or IRE. With current understanding, it is unlikely that these small efficacy differences between ablative technologies would have any significant clinical impact.54 It is more likely that operators would base their decision on which technology to use on comfort level, availability, and tumor location rather than small efficacy differences.
Utilization of Percutaneous Ablation for Small Renal Masses
The current gold standard for the treatment of small renal masses remains surgical excision, with a growing reliance on nephron-sparing techniques that preserve renal function, improve all-cause mortality, and have similar oncologic outcomes when compared with total nephrectomy.7 8 However, percutaneous ablation is becoming an increasingly used treatment option for the small renal mass, particularly in patients with comorbid conditions that make them poor candidates for surgery, CKD, or hereditary syndromes that place them at risk for multiple RCCs. For example, the utilization rate of ablation for small renal masses increased each year between 2005 and 2007, the last date for which data was available.57 In addition, Yang et al reported utilization growth from 1 to 11% from 1998 to 2008 for tumors less than 2 cm in size, and growth from 2 to 9% over the same time period for tumors between 2.1 and 4 cm in size.58 The full potential for future growth in the use of ablation for the treatment of small renal masses is yet to be determined. On the contrary, it could be argued that thermal and cryoablation are relatively mature technologies that face the difficult task of trying to supplant partial nephrectomy. On the contrary, as the patient population ages and the use of cross-sectional imaging increases, it could also be argued that the role of ablation will grow as more small renal masses will be detected in patients with increasingly complex medical histories. Moreover, improvements in existing ablative technologies, the development of new technologies, and increased operator experience with ablation could potentially culminate in an oncologically superior treatment modality without the operative morbidity and mortality of partial nephrectomy.
Summary
Percutaneous ablation of small (T1a) renal masses is an effective therapeutic option for selected patients. At current, the most widely used ablative technologies are RF and cryoablation with a growing experience in MW ablation. Complete ablation of these masses by imaging can be achieved in a large majority of cases. Local recurrence after RF ablation has been shown to be slightly higher at 5 years than with partial nephrectomy, although these differences have not reached statistical significance.
Acknowledgment
Dr. Gervais has received research support from Covidien.
References
- 1.National Cancer Institute. Kidney cancer home page Available at: http://www.cancer.gov/cancertopics/types/kidney. Accessed September 25, 2013
- 2.Pantuck A J, Zisman A, Belldegrun A S. The changing natural history of renal cell carcinoma. J Urol. 2001;166(5):1611–1623. [PubMed] [Google Scholar]
- 3.Gervais D A, McGovern F J, Arellano R S, McDougal W S, Mueller P R. Radiofrequency ablation of renal cell carcinoma: part 1, indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol. 2005;185(1):64–71. doi: 10.2214/ajr.185.1.01850064. [DOI] [PubMed] [Google Scholar]
- 4.Zagoria R J, Hawkins A D, Clark P E. et al. Percutaneous CT-guided radiofrequency ablation of renal neoplasms: factors influencing success. AJR Am J Roentgenol. 2004;183(1):201–207. doi: 10.2214/ajr.183.1.1830201. [DOI] [PubMed] [Google Scholar]
- 5.Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology. 1998;51(2):203–205. doi: 10.1016/s0090-4295(97)00506-2. [DOI] [PubMed] [Google Scholar]
- 6.Levinson A W Su L-M Agarwal D et al. Long-term oncological and overall outcomes of percutaneous radio frequency ablation in high risk surgical patients with a solitary small renal mass J Urol 20081802499–504., discussion 504 [DOI] [PubMed] [Google Scholar]
- 7.Huang W C Elkin E B Levey A S Jang T L Russo P Partial nephrectomy versus radical nephrectomy in patients with small renal tumors—is there a difference in mortality and cardiovascular outcomes? J Urol 2009181155–61., discussion 61–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller D C Schonlau M Litwin M S Lai J Saigal C S; Urologic Diseases in America Project. Renal and cardiovascular morbidity after partial or radical nephrectomy Cancer 20081123511–520. [DOI] [PubMed] [Google Scholar]
- 9.Bandi G, Hedican S P, Nakada S Y. Current practice patterns in the use of ablation technology for the management of small renal masses at academic centers in the United States. Urology. 2008;71(1):113–117. doi: 10.1016/j.urology.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 10.Hinshaw J L, Shadid A M, Nakada S Y, Hedican S P, Winter T C III, Lee F T Jr. Comparison of percutaneous and laparoscopic cryoablation for the treatment of solid renal masses. AJR Am J Roentgenol. 2008;191(4):1159–1168. doi: 10.2214/AJR.07.3706. [DOI] [PubMed] [Google Scholar]
- 11.Zlotta A R, Wildschutz T, Raviv G. et al. Radiofrequency interstitial tumor ablation (RITA) is a possible new modality for treatment of renal cancer: ex vivo and in vivo experience. J Endourol. 1997;11(4):251–258. doi: 10.1089/end.1997.11.251. [DOI] [PubMed] [Google Scholar]
- 12.Psutka S P, Feldman A S, McDougal W S, McGovern F J, Mueller P, Gervais D A. Long-term oncologic outcomes after radiofrequency ablation for T1 renal cell carcinoma. Eur Urol. 2013;63(3):486–492. doi: 10.1016/j.eururo.2012.08.062. [DOI] [PubMed] [Google Scholar]
- 13.Zagoria R J. Imaging-guided radiofrequency ablation of renal masses. Radiographics. 2004;24 01:S59–S71. doi: 10.1148/rg.24si045512. [DOI] [PubMed] [Google Scholar]
- 14.American Cancer Society. Kidney cancer home page Available at: http://www.cancer.org/cancer/kidneycancer/detailedguide/kidney-cancer-adult-staging. Accessed September 25, 2013
- 15.Zagoria R J. Imaging of small renal masses: a medical success story. AJR Am J Roentgenol. 2000;175(4):945–955. doi: 10.2214/ajr.175.4.1750945. [DOI] [PubMed] [Google Scholar]
- 16.Uppot R N, Silverman S G, Zagoria R J, Tuncali K, Childs D D, Gervais D A. Imaging-guided percutaneous ablation of renal cell carcinoma: a primer of how we do it. AJR Am J Roentgenol. 2009;192(6):1558–1570. doi: 10.2214/AJR.09.2582. [DOI] [PubMed] [Google Scholar]
- 17.Frank I, Blute M L, Cheville J C, Lohse C M, Weaver A L, Zincke H. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170(6 Pt 1):2217–2220. doi: 10.1097/01.ju.0000095475.12515.5e. [DOI] [PubMed] [Google Scholar]
- 18.Tuncali K, vanSonnenberg E, Shankar S, Mortele K J, Cibas E S, Silverman S G. Evaluation of patients referred for percutaneous ablation of renal tumors: importance of a preprocedural diagnosis. AJR Am J Roentgenol. 2004;183(3):575–582. doi: 10.2214/ajr.183.3.1830575. [DOI] [PubMed] [Google Scholar]
- 19.Wood B J, Khan M A, McGovern F, Harisinghani M, Hahn P F, Mueller P R. Imaging guided biopsy of renal masses: indications, accuracy and impact on clinical management. J Urol. 1999;161(5):1470–1474. doi: 10.1016/s0022-5347(05)68929-x. [DOI] [PubMed] [Google Scholar]
- 20.Beland M D, Mayo-Smith W W, Dupuy D E, Cronan J J, DeLellis R A. Diagnostic yield of 58 consecutive imaging-guided biopsies of solid renal masses: should we biopsy all that are indeterminate? AJR Am J Roentgenol. 2007;188(3):792–797. doi: 10.2214/AJR.06.0356. [DOI] [PubMed] [Google Scholar]
- 21.Silverman S G, Gan Y U, Mortele K J, Tuncali K, Cibas E S. Renal masses in the adult patient: the role of percutaneous biopsy. Radiology. 2006;240(1):6–22. doi: 10.1148/radiol.2401050061. [DOI] [PubMed] [Google Scholar]
- 22.DeBenedectis C M, Beland M D, Dupuy D E, Mayo-Smith W W. Utility of iodinated contrast medium in hydrodissection fluid when performing renal tumor ablation. J Vasc Interv Radiol. 2010;21(5):745–747. doi: 10.1016/j.jvir.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg S N, Gazelle G S. Radiofrequency tissue ablation: physical principles and techniques for increasing coagulation necrosis. Hepatogastroenterology. 2001;48(38):359–367. [PubMed] [Google Scholar]
- 24.Best S L, Park S K, Youssef R F. et al. Long-term outcomes of renal tumor radio frequency ablation stratified by tumor diameter: size matters. J Urol. 2012;187(4):1183–1189. doi: 10.1016/j.juro.2011.11.096. [DOI] [PubMed] [Google Scholar]
- 25.Zorn K C, Orvieto M A, Mikhail A A. et al. Case report: radiofrequency ablation-induced renal-pelvic obstruction resulting in nephrectomy. J Endourol. 2007;21(9):1059–1063. doi: 10.1089/end.2006.0296. [DOI] [PubMed] [Google Scholar]
- 26.Chen S H, Mouraviev V, Raj G V, Marguet C G, Polascik T J. Ureteropelvic junction obliteration resulting in nephrectomy after radiofrequency ablation of small renal cell carcinoma. Urology. 2007;69(5):e3–e5. doi: 10.1016/j.urology.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 27.Johnson D B, Saboorian M H, Duchene D A, Ogan K, Cadeddu J A. Nephrectomy after radiofrequency ablation-induced ureteropelvic junction obstruction: potential complication and long-term assessment of ablation adequacy. Urology. 2003;62(2):351–352. doi: 10.1016/s0090-4295(03)00361-3. [DOI] [PubMed] [Google Scholar]
- 28.Cantwell C P, Wah T M, Gervais D A. et al. Protecting the ureter during radiofrequency ablation of renal cell cancer: a pilot study of retrograde pyeloperfusion with cooled dextrose 5% in water. J Vasc Interv Radiol. 2008;19(7):1034–1040. doi: 10.1016/j.jvir.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Wah T M, Koenig P, Irving H C, Gervais D A, Mueller P R. Radiofrequency ablation of a central renal tumor: protection of the collecting system with a retrograde cold dextrose pyeloperfusion technique. J Vasc Interv Radiol. 2005;16(11):1551–1555. doi: 10.1097/01.RVI.0000175322.05225.0a. [DOI] [PubMed] [Google Scholar]
- 30.Neuhaus J, Blachut L, Rabenalt R. et al. Efficiency analysis of bipolar and multipolar radiofrequency ablation in an in vivo porcine kidney model using three-dimensional reconstruction of histologic section series. J Endourol. 2011;25(5):859–867. doi: 10.1089/end.2010.0578. [DOI] [PubMed] [Google Scholar]
- 31.Levy D, Avallone A, Jones J S. Current state of urological cryosurgery: prostate and kidney. BJU Int. 2010;105(5):590–600. doi: 10.1111/j.1464-410X.2010.09235.x. [DOI] [PubMed] [Google Scholar]
- 32.Clark P E, Woodruff R D, Zagoria R J, Hall M C. Microwave ablation of renal parenchymal tumors before nephrectomy: phase I study. AJR Am J Roentgenol. 2007;188(5):1212–1214. doi: 10.2214/AJR.05.2190. [DOI] [PubMed] [Google Scholar]
- 33.Carrafiello G, Mangini M, Fontana F. et al. Single-antenna microwave ablation under contrast-enhanced ultrasound guidance for treatment of small renal cell carcinoma: preliminary experience. Cardiovasc Intervent Radiol. 2010;33(2):367–374. doi: 10.1007/s00270-009-9745-x. [DOI] [PubMed] [Google Scholar]
- 34.Castle S M, Salas N, Leveillee R J. Initial experience using microwave ablation therapy for renal tumor treatment: 18-month follow-up. Urology. 2011;77(4):792–797. doi: 10.1016/j.urology.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 35.Olweny E O, Cadeddu J A. Novel methods for renal tissue ablation. Curr Opin Urol. 2012;22(5):379–384. doi: 10.1097/MOU.0b013e328355ecf5. [DOI] [PubMed] [Google Scholar]
- 36.Nabi G, Goodman C, Melzer A. High intensity focused ultrasound treatment of small renal masses: Clinical effectiveness and technological advances. Indian J Urol. 2010;26(3):331–337. doi: 10.4103/0970-1591.70561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gehl J. Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand. 2003;177(4):437–447. doi: 10.1046/j.1365-201X.2003.01093.x. [DOI] [PubMed] [Google Scholar]
- 38.Edd J F, Horowitz L, Davalos R V, Mir L M, Rubinsky B. In vivo results of a new focal tissue ablation technique: irreversible electroporation. IEEE Trans Biomed Eng. 2006;53(7):1409–1415. doi: 10.1109/TBME.2006.873745. [DOI] [PubMed] [Google Scholar]
- 39.Tracy C R, Kabbani W, Cadeddu J A. Irreversible electroporation (IRE): a novel method for renal tissue ablation. BJU Int. 2011;107(12):1982–1987. doi: 10.1111/j.1464-410X.2010.09797.x. [DOI] [PubMed] [Google Scholar]
- 40.Deodhar A, Monette S, Single G W Jr. et al. Renal tissue ablation with irreversible electroporation: preliminary results in a porcine model. Urology. 2011;77(3):754–760. doi: 10.1016/j.urology.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 41.Pech M, Janitzky A, Wendler J J. et al. Irreversible electroporation of renal cell carcinoma: a first-in-man phase I clinical study. Cardiovasc Intervent Radiol. 2011;34(1):132–138. doi: 10.1007/s00270-010-9964-1. [DOI] [PubMed] [Google Scholar]
- 42.Zagoria R J, Traver M A, Werle D M, Perini M, Hayasaka S, Clark P E. Oncologic efficacy of CT-guided percutaneous radiofrequency ablation of renal cell carcinomas. AJR Am J Roentgenol. 2007;189(2):429–436. doi: 10.2214/AJR.07.2258. [DOI] [PubMed] [Google Scholar]
- 43.Mayo-Smith W W, Dupuy D E, Parikh P M, Pezzullo J A, Cronan J J. Imaging-guided percutaneous radiofrequency ablation of solid renal masses: techniques and outcomes of 38 treatment sessions in 32 consecutive patients. AJR Am J Roentgenol. 2003;180(6):1503–1508. doi: 10.2214/ajr.180.6.1801503. [DOI] [PubMed] [Google Scholar]
- 44.Breen D J, Rutherford E E, Stedman B. et al. Management of renal tumors by image-guided radiofrequency ablation: experience in 105 tumors. Cardiovasc Intervent Radiol. 2007;30(5):936–942. doi: 10.1007/s00270-007-9090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farrell M A, Charboneau W J, DiMarco D S. et al. Imaging-guided radiofrequency ablation of solid renal tumors. AJR Am J Roentgenol. 2003;180(6):1509–1513. doi: 10.2214/ajr.180.6.1801509. [DOI] [PubMed] [Google Scholar]
- 46.Su Li, Jarrett T W, Chan D Y, Kavoussi L R, Solomon S B. Percutaneous computed tomography-guided radiofrequency ablation of renal masses in high surgical risk patients: preliminary results. Urology. 2003;61(4) 01:26–33. doi: 10.1016/s0090-4295(03)00118-3. [DOI] [PubMed] [Google Scholar]
- 47.Atwell T D Farrell M A Leibovich B C et al. Percutaneous renal cryoablation: experience treating 115 tumors J Urol 200817962136–2140., discussion 2140–2141 [DOI] [PubMed] [Google Scholar]
- 48.Georgiades C S, Hong K, Bizzell C, Geschwind J F, Rodriguez R. Safety and efficacy of CT-guided percutaneous cryoablation for renal cell carcinoma. J Vasc Interv Radiol. 2008;19(9):1302–1310. doi: 10.1016/j.jvir.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 49.Zagoria R J, Pettus J A, Rogers M, Werle D M, Childs D, Leyendecker J R. Long-term outcomes after percutaneous radiofrequency ablation for renal cell carcinoma. Urology. 2011;77(6):1393–1397. doi: 10.1016/j.urology.2010.12.077. [DOI] [PubMed] [Google Scholar]
- 50.Tracy C R, Raman J D, Donnally C, Trimmer C K, Cadeddu J A. Durable oncologic outcomes after radiofrequency ablation: experience from treating 243 small renal masses over 7.5 years. Cancer. 2010;116(13):3135–3142. doi: 10.1002/cncr.25002. [DOI] [PubMed] [Google Scholar]
- 51.McDougal W S, Gervais D A, McGovern F J, Mueller P R. Long-term followup of patients with renal cell carcinoma treated with radiofrequency ablation with curative intent. J Urol. 2005;174(1):61–63. doi: 10.1097/01.ju.0000162046.45024.2b. [DOI] [PubMed] [Google Scholar]
- 52.Olweny E O, Park S K, Tan Y K, Best S L, Trimmer C, Cadeddu J A. Radiofrequency ablation versus partial nephrectomy in patients with solitary clinical T1a renal cell carcinoma: comparable oncologic outcomes at a minimum of 5 years of follow-up. Eur Urol. 2012;61(6):1156–1161. doi: 10.1016/j.eururo.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Choueiri T K, Schutz F A, Hevelone N D. et al. Thermal ablation vs surgery for localized kidney cancer: a Surveillance, Epidemiology, and End Results (SEER) database analysis. Urology. 2011;78(1):93–98. doi: 10.1016/j.urology.2011.01.068. [DOI] [PubMed] [Google Scholar]
- 54.Gervais D A. Cryoablation versus radiofrequency ablation for renal tumor ablation: time to reassess? J Vasc Interv Radiol. 2013;24(8):1135–1138. doi: 10.1016/j.jvir.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 55.Kunkle D A, Uzzo R G. Cryoablation or radiofrequency ablation of the small renal mass : a meta-analysis. Cancer. 2008;113(10):2671–2680. doi: 10.1002/cncr.23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El Dib R, Touma N J, Kapoor A. Cryoablation vs radiofrequency ablation for the treatment of renal cell carcinoma: a meta-analysis of case series studies. BJU Int. 2012;110(4):510–516. doi: 10.1111/j.1464-410X.2011.10885.x. [DOI] [PubMed] [Google Scholar]
- 57.Kowalczyk K J, Choueiri T K, Hevelone N D. et al. Comparative effectiveness, costs and trends in treatment of small renal masses from 2005 to 2007. BJU Int. 2013;112(4):E273–E280. doi: 10.1111/j.1464-410X.2012.11776.x. [DOI] [PubMed] [Google Scholar]
- 58.Yang G, Villalta J D, Meng M V, Whitson J M. Evolving practice patterns for the management of small renal masses in the USA. BJU Int. 2012;110(8):1156–1161. doi: 10.1111/j.1464-410X.2012.10969.x. [DOI] [PubMed] [Google Scholar]


