Abstract
Oral delivery of Gram positive bacteria, often derived from the genera Lactobacillus or Bifidobacterium, can modulate immune function. Although the exact mechanisms remain unclear, immunomodulatory effects may be elicited through the direct interaction of these bacteria with the intestinal epithelium or resident dendritic cell (DC) populations. We analyzed the immune activation properties of Lactobacilli and Bifidobacterium species and made the surprising observation that cellular responses in vitro were differentially influenced by the presence of serum, specifically the extracellular vesicle (EV) fraction. In contrast to the tested Lactobacilli species, tested Bifidobacterium species induce TLR2/6 activity which is inhibited by the presence of EVs. Using specific TLR ligands, EVs were found to enhance cellular TLR2/1 and TLR4 responses while TLR2/6 responses were suppressed. No effect could be observed on cellular TLR5 responses. We determined that EVs play a role in bacterial aggregation, suggesting that EVs interact with bacterial surfaces. EVs were found to slightly enhance DC phagocytosis of Bifidobacterium breve whereas phagocytosis of Lactobacillus rhamnosus was virtually absent upon serum EV depletion. DC uptake of a non-microbial substance (dextran) was not affected by the different serum fractions suggesting that EVs do not interfere with DC phagocytic capacity but rather modify the DC-microbe interaction. Depending on the microbe, combined effects of EVs on TLR activity and phagocytosis result in a differential proinflammatory DC cytokine release. Overall, these data suggest that EVs play a yet unrecognized role in host-microbe responses, not by interfering in recipient cellular responses but via attachment to, or scavenging of, microbe-associated molecular patterns. EVs can be found in any tissue or bodily fluid, therefore insights into EV-microbe interactions are important in understanding the mechanism of action of potential probiotics and gut immune homeostasis.
Introduction
Mammals live in symbiosis with commensal bacteria and it has long been recognized that host-bacterial associations yield mutual benefits [1]. Over the last years it has become clear that the microbiota play a critical role in the maintenance of gut homeostasis and immunological tolerance [2]. Accumulating evidence suggests that changes in the gut microbiota composition occur and may be causally involved in an array of immunopathologies such as inflammatory bowel diseases (IBD) and systemic immune diseases such as rheumatoid arthritis, type 1 diabetes and allergic diseases [3]. Strategies aimed at influencing dysbiosis via the oral administration of lactic acid bacteria (LAB), such as strains from the Bifidobacterium and Lactobacillus genera, have been proven beneficial in IBD [4] and atopic diseases [5]. Although their exact mechanism of action still remains obscure [6], LAB administration has been shown to enhance the barrier function of intestinal epithelial cells (IEC)[7] and to induce regulatory T-cells both systemically [8] and within the large intestine [9], most likely via ligation of specific Toll-like receptors (TLR) [10]. IEC form a barrier separating commensal bacteria and host connective tissue. Next to their barrier function, recognition of bacteria by IECs contributes to gut immune homeostasis via the release of soluble factors that regulate immune-cell function [11]. Dendritic cells (DC) residing in the lamina propria sample the microbiota by extending dendrites between IECs into the lumen [12] and interact directly with bacteria passing through M-cells, a special subset of IEC found within Peyer’s patches [13]. DCs express a full complement of pattern recognition receptors (PRR) that allows for the direct recognition and activation by microbes. As DCs are potent stimulators of naïve T-cells, microbial induced DC activation in concert with IEC soluble factors shape T-cell polarization towards effector and regulatory populations [11], [14]. TLRs are part of the larger family of PRRs and are best known for their involvement in the recognition of microbe-associated molecular patterns (MAMP) [15]. Depending on the type, species or strain of bacteria, it carries ligands for various TLRs on its surface [16]. LAB cell wall constituents typically include lipoteichoic acids (LTA), peptidoglycan (PG) and lipoproteins (LP), which are all able to interact with TLR2 [17].
Extracellular vesicles (EV) recently have gained scientists’ interest due to their role in cell to cell communication locally or at a distance. EVs typically range in diameter from 30 nm to 1 µm and can be regarded as cargo containers used to exchange biomolecules and genetic information able to both activate or inhibit recipient cells [18], [19]. The term EVs encompasses all types of secreted vesicles including exosomes, microvesicles and ectosomes [20] which originate from a broad range of cell types, including IEC [21] and dendritic cells [22]. Currently, there is no information available on the half-life of EVs, but EVs originating from epithelial, tumor and hematopoietic cells have been isolated from bodily fluids such as human plasma [23], serum [24], bronchoalveolar lavage fluid [25] and milk [26]. This suggests that EV form stable structures able to convey their information over long distances, playing a role in normal physiology and disease pathogenesis [27]. Immunosuppressive effects of EVs collected from different body fluids have been reported. For instance, EVs isolated from breast milk have been found to inhibit IFNγ production by activated PBMCs and by increasing the number of regulatory T-cells [26]. Additionally, placenta-derived EVs and EVs isolated from the maternal peripheral circulation have been found to modulate T-cell function, possibly attenuating immune responses to the fetus [28]. EVs are also reported to play a role in immunological tolerance. IEC derived EVs, isolated from serum after antigen feeding, are capable of inducing antigen specific tolerance [21]. Moreover, EVs released from intestinal mucosal cells suppress activation of liver NKT cells via transport of mucus-derived PGE2, potentially identifying a pathway for induction of immune tolerance towards intestinal-related antigens [29]. Based on the described immunosuppressive and tolerance inducing effects of EVs we hypothesize that EVs in intestinal tissue play a role in the maintenance of intestinal homeostasis by modulating host-microbe responses. Until now, EVs were thought to modify the cellular responses of the recipient cells. Our observations in this study suggest that EVs play a role in host-microbe responses mainly through their interaction with specific MAMPs or LAB preventing or enhancing subsequent cellular responses.
Materials and Methods
Bacterial Fermentation and Enumeration
Lactobacillus strains (L. rhamnosus NutRes 1, L. plantarum NutRes 8 and L. caseï CNCM I-1518) and Bifidobacterium strains (B. longum NutRes 266, B. breve NutRes 200 and B. animalis DN173010) were grown at 37°C in a 400 ml reactor containing MRS broth (Oxoid, Badhoevedorp, The Netherlands) supplemented with 0.5 g/l L-cysteine for Bifidobacteria. The pH was maintained at 6.5 by addition of NaOH. To ensure anaerobic conditions the headspace was flushed with N2 or a gas mixture consisting of 5% H2, 5% CO2 and 90% N2 for Bifidobacteria. Bacteria were harvested in the early stationary phase, washed in PBS and stored with glycerol 20% (w/v), in aliquots at −80°C. Cell counts were determined by plating serial dilutions (CFU) and fluorescent microscopy by staining with DAPI.
Cell Lines and Reagents
Ultrapure lipopolysaccharide (LPS) derived from E. coli K12 and flagellin, FLA-ST (both from Invivogen, Toulouse, France) were used at the indicated concentrations. Synthetic bacterial lipopeptides Pam3CSK4, Pam2CKS4, FSL-1 (all from EMC microcollections, Tübingen, Germany) and purified LTA from S. aureus (Invivogen, Toulouse, France) were used at the indicated concentrations. Monocyte cell line THP-1 Blue-CD14 containing the NFκB reporter pNiFty2-SEAP, HEK293 TLR2-TLR6 stable transfectants and HEK293 TLR null control cells were purchased from Invivogen, Toulouse, France. HEK293 TLR2-TLR6 transfectants were stably transfected with the NFκB reporter plasmid pNiFty2-Luc (Invivogen, Toulouse, France). Cells were maintained in RPMI-1640 medium supplemented 10% FBS and the appropriate antibiotics according to the manufacturer’s protocols.
Serum Fractionation
Mouse sera collected from TLR2 knockout mice and wild types were generously provided by Shahla Abdollahi-Roodsaz (Radboud UMC, Nijmegen, The Netherlands). Human plasma was obtained after buffy coat separation using Leucosep tubes as described in the section on the differentiation of dendritic cells. Heat-inactivated human serum AB and fetal bovine serum were purchased from Lonza, Verviers, Belgium and Fisher Scientific, Landsmeer, The Netherlands respectively. 0.22 µm filtered serum was mixed with ExoQuick Exosome Precipitation Solution (System Biosciences) at a ratio of 4∶1 (serum: ExoQuick) and incubated at 4°C for 30mins and subsequently spun down. Depleted serum was transferred to a new tube. The EV-rich pellet was carefully rinsed with PBS and reconstituted in the original volume with the appropriate medium. Serum fractions were used 5% (v:v) or at the indicated concentrations.
Differentiation of Dendritic Cells
Human primary peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats obtained from healthy blood donors at the Sanquin Bloodbank, Nijmegen, The Netherlands. The mononuclear cell fraction was obtained by density centrifugation of blood diluted 1∶1 in PBS using Leucosep tubes (Greiner, Alphen a/d rijn, The Netherlands) according to the manufacturer’s instructions. CD14 MACS isolation beads were used to positively select monocytes. Monocytes were cultured in RPMI-1640 medium supplemented with 10% FBS, 10 ng/ml IL-4 and 5 ng/ml GM-CSF (both cytokines and magnetic beads obtained from Miltenyi Biotec, Leiden, The Netherlands). Cells were seeded at 1.5×106 cells/well in 6-wells plates and medium was refreshed every other day. After 6 days cells were harvested, washed and resuspended in culture medium. DC purity (CD209 positive, CD14 negative) was checked by flow cytometry.
Bacterial Co-culture and TLR Transfectants, DC Stimulation
A total of 2×105 DCs were added to 24-wells plates and incubated with either culture medium (RPMI-1640, 2% FBS, 150 µg/ml gentamycin) (negative control), or with B. breve or L. rhamnosus (all 2×106 microorganisms/well) at a ratio of 10∶1 (bacteria: DC) for 16 hours at 37°C without serum or with the indicated serum fractions. DC phagocytosis was inhibited by first pre-treating DCs with 10 µg/ml cytochalasine D (Sigma Aldrich) for 30 mins at 37°C. In a separate experiment, 1×105 DCs were added to 96 well flat-bottom plates and incubated with either culture medium (RPMI-1640, 1% FBS, 150 µg/ml gentamycin) (negative control), or with B. breve or L. rhamnosus (all 1×106 microorganisms/well) at a ratio of 10∶1 (bacteria: DC) for 16 hours at 37°C without serum or with the indicated serum fractions. TLR2 activity was inhibited by preincubating cells with 5 µg/ml TLR2 antibodies (clone T2.5, Invivogen, Toulouse, France) or mouse IgG1 isotype control antibodies (clone PPV-06, Bio-Connect, Huissen, The Netherlands) for 30 minutes before the addition of bacteria. 3×105 THP-1 cells/well were seeded in a U-bottom 96-wellsplate and stimulated with the different TLR2 ligands, at the indicated concentrations, in medium or the indicated serum fractions for 16 hours at 37°C. HEK293 TLR2-TLR6 transfectants were seeded in culture medium (DMEM, 10% FBS) at 3×104 cells/well in flat-bottom 96-wells plates and allowed to adhere overnight. Next day cells were washed and co-incubated with B. breve at a ratio of 15∶1 (bacteria: cell) with serum-free medium (DMEM, 150 µg/ml gentamycin) or medium with sera at the indicated percentages.
Bacterial Aggregation and Phagocytosis
To assess bacterial aggregation, 2.5×106 microorganims/well were seeded in flat bottom black 96-wells clear-bottom plates (Corning, Amsterdam, The Netherlands) with serum-free medium (DMEM, 150 µg/ml gentamycin) or medium supplemented with 5% of the different serum fractions for 16 hours. Digital pictures of the wells were obtained using an Olympus SC30 CMOS camera (Olympus, Zoeterwoude, The Netherlands). Images were subsequently processed and analyzed using the public domain program ImageJ, calculating the area covered by objects. To assess bacterial phagocytosis, B. breve and L. rhamnosus were labeled with the amine-reactive dye pHrodo Red, SE (Life Technologies, Bleiswijk, The Netherlands) in 900 µl of 0.1M sodium bicarbonate, pH 9.2 for 1 hour at room temperature. Labeled bacteria were extensively washed and labeling efficiency was checked using a flow cytometer at pH 4. 2.5×106 Labeled bacteria were co-incubated with 1×105 DCs seeded in U-bottom 96-wells plates in medium (RPMI1640, 150 µg/ml gentamycin) supplemented with the different serum fractions. DCs were collected after 3 hours and analyzed for fluorescence using flow cytometery. DC capacity for phagocytosis was measured using pHrodo Red Dextran 10Kd (Life Technologies, Bleiswijk, The Netherlands). 1×105 DCs were seeded in 96-well flat bottom plates with PBS or PBS supplemented with 5% of the different serum fractions. After 2 hours of incubation, DCs were supplemented with 30 µg/ml pHrodo Red Dextran and incubated for an additional hour after which cells were collected and measured using flow cytometry.
Cytokine, Luciferase and SEAP Measurements
DC supernatants were analyzed for IL-6 and TNFα (both from R&D Systems, Abingdon, UK) release using ELISA according to the manufacturer’s instructions. HEK293 TLR2-TLR6 transfectants were analyzed for luciferase content via addition of 1 volume of the luciferase substrate: BriteLite (Perkin Elmer, Groningen, The Netherlands) after which Luminescence was measured. THP-1 supernatants were analyzed for secreted embryonic alkaline phosphatase (SEAP) activity using QUANTI-Blue (Invivogen, Toulouse, France) after which OD was measured using a spectrophotometer.
Statistics
All data was analyzed with GraphPad Prism 4.1. All results are presented as means ± SEM (DC experiments) or means ± SD. Statistical analysis were performed with the use of unpaired two-tailed Students t-test where p<0.05 was considered statistical significant.
Results
Serum Inhibits Bacterial-induced TLR2/6 Activation
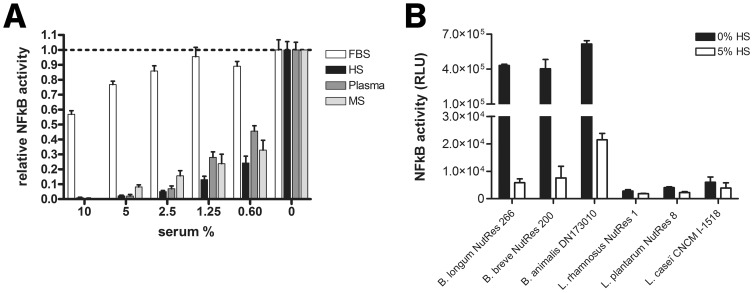
To assess the effect of sera on B. breve NutRes 200 induced TLR2/6 activation, TLR2 - TLR6 transfected HEK cells were stimulated overnight in the presence or absence of fetal bovine serum (FBS), normal human serum (HS), human plasma (plasma) or mouse serum (MS). Relative to conditions without serum, sera from different species dose-dependently inhibited TLR2/6 activation, with FBS being the least potent ( Figure 1 a). To study if this effect was species specific, the human serum capacity to inhibit TLR2/6 activity of three additional Bifidobacterium species and three Lactobacillus species was determined. In agreement with previous findings [30], Bifidobacterium species induced TLR2/6 activity in contrast to Lactobacillus species ( Figure 1 b). The presence of 5% human serum almost completely abolished TLR2/6 activity of Bifidobacteria regardless of species, indicating that human serum does not discriminate between species regarding its inhibitory effect on TLR2 activity. HEK293 TLR null control cells stimulated under similar conditions remained negative for NFκB activation, showing that cellular activation is critically dependent on the presence of the transfected TLR2– TLR6 construct (data not shown).
Figure 1. Bacterial induced TLR2/6 activation.
TLR2/6 expressing HEK transfectants were co-incubated with different strains of bifidobacteria or lactobacilli. (A) Dose response experiment indicating the effects of the different supplements (FBS = fetal bovine serum, HS = human serum, plasma = human plasma, MS = mouse serum) on TLR2/6 activity. TLR2/6 expressing cells were stimulated with B. breve NutRes 200 in a ratio of 15∶1 (bacteria:cell) for 16H. NFκB activity measured as Relative Light Units (RLU) was determined using a Luciferase reporting system as described in materials and methods. Relative NFκB activity was determined calculating the ratios between medium activity and the activity in the different serum fractions. (B) TLR2/6 activity induced by 3 Bifidobacteria and 3 Lactobacilli strains. TLR2/6-expressing cells were stimulated at a ratio of 15∶1 (bacteria:cell) in serum-free or 5% human serum (HS) containing medium. NFκB activity measured as RLU was determined as described above.
HS-EVs Differentially Modulate MAMP-induced TLR2 Responses
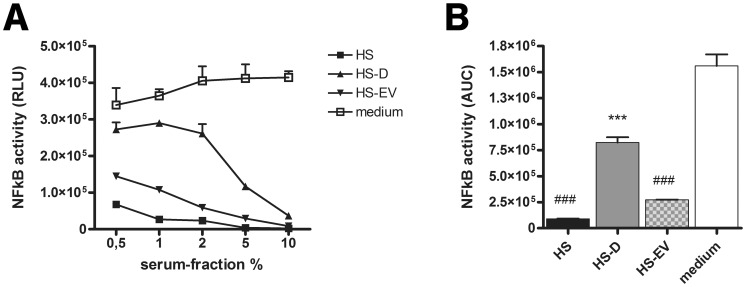
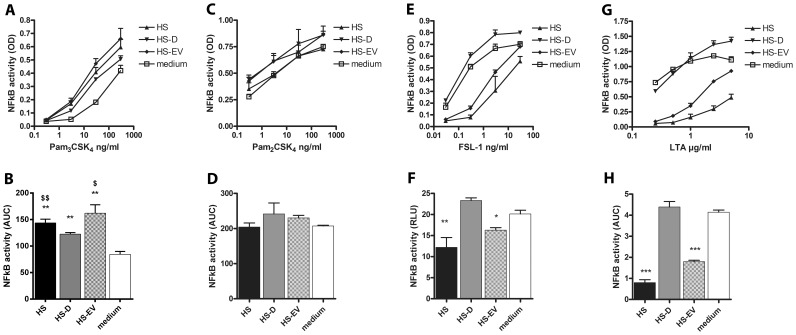
To specify the inhibitory effect of HS on B. breve NutRes 200 induced TLR2/6 activity, HS was depleted for extracellular vesicles (EV). The different fractions, HS, EV-depleted HS (HS-D) and the EV-enriched fraction (HS-EV) were tested for their relative capacity to inhibit TLR2/6 activity ( Figure 2 a). Depletion of EVs from HS significantly improved TLR2/6 activity when compared to HS. Medium reconstituted with HS-EVs significantly reduced TLR2/6 activity when compared to HS-D or medium control, suggesting that EVs are the principle factor contributing to the inhibitory capacity of HS ( Figure 2 b). Similar results were obtained upon using FBS serum fractions instead of HS (data not shown). TLR2 needs to form heterodimers with TLR1 or TLR6 in order to initiate signal transduction following activation. To research whether the inhibitory effect of HS and HS-EVs affects activation of TLR2/1 and TLR2/6 heterodimers similarly, THP-1 reporter cells were stimulated with the specific ligands Pam3CSK4 (TLR2/1), Pam2CSK4 (TLR2) or FSL-1 and LTA (TLR2/6) [31]–[33]. Unexpectedly, addition of HS, HS-D or HS-EV differently affected TLR2 ligand induced THP-1 activation ( Figure 3 a,c,e,g). Compared to medium, serum fractions HS, HS-D and HS-EV significantly enhanced THP-1 activation following Pam3CSK4 ligation. Moreover, depletion of EVs significantly reduced THP-1 activation compared to HS and HS-EVs ( Figure 3 b). No differences between HS fractions were measured upon Pam2CSK4 ligation ( Figure 3 d). Both HS and HS-EVs significantly reduced THP-1 activation following FSL-1 and LTA ligation, which could be rescued upon EV depletion ( Figure 3 f,h). Additionally, to investigate whether or not other surface TLRs were equally affected, THP-1 reporter cells were stimulated with LPS (TLR4) or flagellin (TLR5). LPS induced NFκB activity was enhanced by all serum fractions in contrast to flagellin induced activity where intact serum but not the serum fractions showed a significant inhibition. Moreover, depletion of EVs significantly reduced LPS induced THP-1 activity compared to HS and HS-EVs (Figure S1). Overall the data show that, depending on the TLR-ligand, EVs display an enhancing effect (Pam3CSK4, LPS) or an inhibiting effect (FSL-1, LTA) or no effect (Pam2CSK4, flagellin). To test whether the effect of EVs is not generically suppressive or enhancive irrespective of TLR signaling, HEK293 TLR null control cells or TLR2/6 transfectants were stimulated with TNFα in the presence of the different HS fractions (data not shown). No differences between TNFα induced NFκB activity could be observed when HS fractions were compared to medium, indicating that EVs selectively modulate TLR induced NFκB activity.
Figure 2. EVs inhibit Bifidobacterium breve induced TLR2/6 activation.
TLR2/6-expressing HEK transfectants were stimulated with B. breve NutRes 200 in a ratio of 1∶15 for 16H. (A) Dose response experiment indicating the effects of the different human serum fractions on TLR2/6 activity (HS = intact human serum, HS-D = EV depleted human serum, HS-EV = human serum EVs in medium). NFκB activity was determined as described in Figure 1. (B) Data presented in Figure 2a were used to calculate Area Under the Curve (AUC) values. HS and HS-EVs dose-dependently inhibited TLR2/6 activity. Hash-signs indicate a significant difference (##P<0.01) compared to medium. EV depletion dose dependently rescued TLR2/6 activity. Asterisks indicate a significant difference (**P<0.01) compared to HS-D.
Figure 3. EVs differentially affect synthetic ligand-induced TLR2 activation.
THP-1 reporter cells were incubated with synthetic TLR2 ligands addressing different TLR2 heterodimers in serum free medium, or medium supplemented with 5% of the indicated serum fractions (HS = intact human serum, HS-D = EV depleted human serum, HS-EV = human serum EVs in medium). (A, C, E, G) Dose response experiments indicating the effects of respectively Pam3CSK4, Pam2CSK4, FSL-1 or LTA stimulation on THP-1 activation. NFκB activity measured as OD values was determined using an alkaline phosphatase reporting system as described in materials and methods. (B, D, F, H) Data presented in Figure 3A,C,E,G were used to calculate Area Under the Curve (AUC) values. (B) Pam3CSK4 induced TLR2/1 activity was increased in medium supplemented with serum fractions compared to serum-free medium, depletion of EVs reduced TLR2/1 activation compared to HS ($$P<0.01) and HS-D ($P<0.05). (D) No modulatory effect by serum fractions were observed on Pam2SK4 stimulation. (E,F) HS and HS-EVs significantly inhibited FSL-1 and LTA induced TLR2/6 activity compared to medium (***P<0.001, **P<0.01, *P<0.05).
HS-EVs Inhibition of TLR2/6 Responses is Independent of EV TLR2 Expression
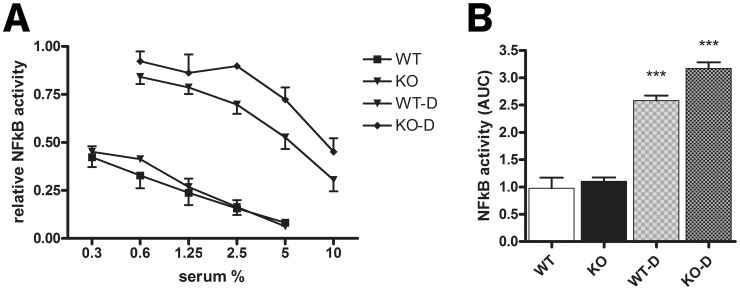
Proteomic analysis of breast milk derived EVs previously indicated the presence of TLR2 [26]. This finding led us to consider whether TLR2 expression on serum derived EVs might be responsible for the observed inhibitory effect. To that end, HEK TLR2/6 cells were stimulated with B. breve NutRes 200 in the presence of serum and EV depleted serum from TLR2−/− deficient (KO) and wild type (WT) mice and their inhibitory effect compared to medium control ( Figure 4 a). KO or WT serum was equally effective in inhibiting TLR2/6 activation indicating that EV-TLR2 expression does not play a role in the observed effects. EV depletion of WT as well as KO serum significantly increased TLR2/6 activity ( Figure 4 b).
Figure 4. EV-TLR2 expression is not required for the suppressive effect of EVs.
TLR2/6 expressing HEK transfectants were stimulated with B. breve NutRes 200 in a ratio of 1∶15 for 16H. (A) Dose response experiment indicating the effects of the different mouse serum fractions on TLR2/6 activity (WT = wild type, WT-D = EV depleted wild type serum, KO = TLR2 deficient mouse serum, KO-D = EVs depleted TLR2 deficient mouse serum in medium. NFκB activity was determined as described in Figure 1. Relative activity was determined calculating the ratios between medium activity and the activity in the different serum fractions. (B) Data presented in Figure 4a were used to calculate Area Under the Curve (AUC) values. EV depletion of both WT and KO sera significantly increased TLR2/6 activity (***P<0.001).
HS-EVs are Involved in Bacterial Aggregation
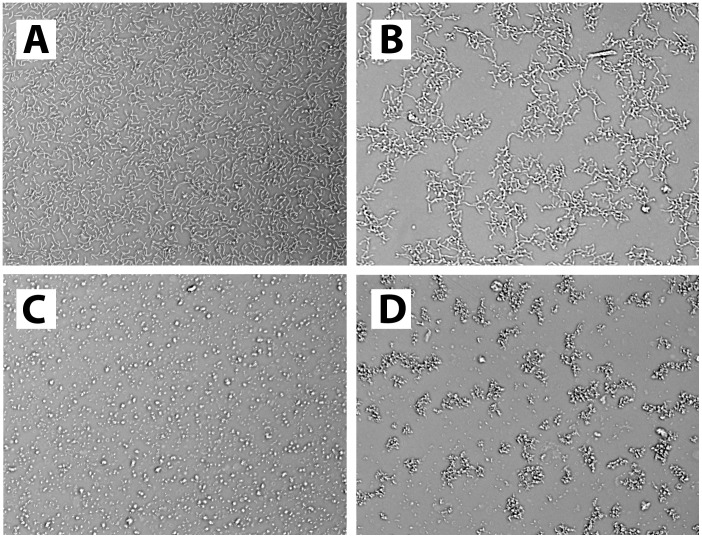
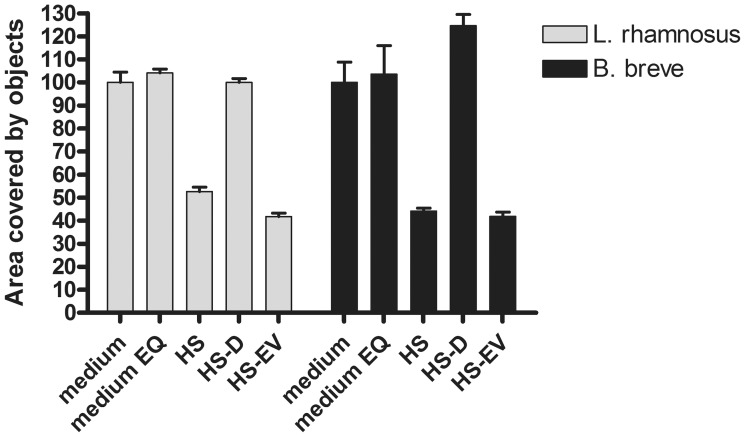
The reported presence of PRRs (including but not limited to TLRs) on EVs in combination with the important role of PRRs in host-microbe interactions led us to consider that EVs might interact directly with bacteria which could lead to bacterial aggregation. To that end, pure cultures of B. breve NutRes 200, and L. rhamnosus NutRes 1 were incubated with serum fractions HS, HS-D, HS-EVs or medium without serum. After overnight incubation cultures were inspected by microscopy. Bacterial aggregation could be detected in cultures supplemented with HS or HS-EVs but not HS-D or plain medium. No differences could be observed in bacterial aggregation between B. breve or L. rhamnosus ( Figure 5 ). Calculation of the relative area covered by objects (see materials and methods) confirms the initial observation, indicating an ∼ 50% decrease in the area covered by bacteria due to bacterial aggregation ( Figure 6 ).
Figure 5. EV mediated bacterial aggregation.
Picture A and C respectively represent a typical example of L. rhamnosus NutRes 1 and B. breve Nutres 200 co-incubated with medium, medium with ExoQuick or medium depleted for EVs. Picture B and D respectively represent a typical example of L. rhamnosus NutRes 1 and B. breve Nutres 200 co-incubated with HS or HS-EVs.
Figure 6. EVs induce bacterial aggregation.
B. breve NutRes 200 and L. rhamnosus NutRes 1 were seeded in flat-bottom 96-wells plates at 2.5×106 bacteria/well in medium, medium with ExoQuick (EQ)(control), HS, HS-D or HS-EV and incubated at 37°C. After 16H cultures were analyzed for aggregation using microscopy and pictures were taken. Pictures were digitally processed and analyzed using ImageJ software, calculating the area covered by objects. Intact serum as well as HS-EVs induced bacterial aggregation, reducing the area covered by objects by approximately 50%. Aggregation was not observed upon EV depletion. No differences between strains could be observed.
HS-EV are Involved in Bacterial Phagocytosis
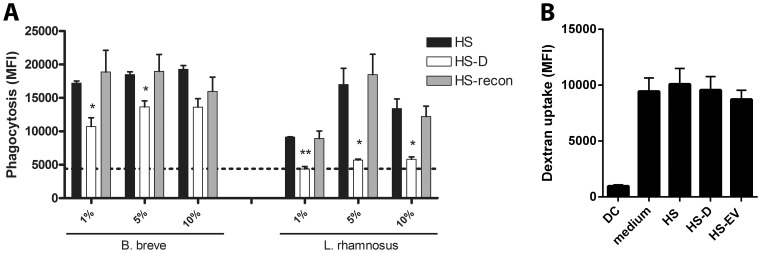
To gain further insight into the relevance of HS-EV interaction with bacteria, we examined whether or not HS-EVs would impact bacterial phagocytosis by DCs. Depletion of EVs from HS resulted in a significantly impaired phagocytosis of both B. breve NutRes 200 and L. rhamnosus NutRes 1 compared to intact HS. Reconstitution of HS-D with HS-EVs (HS-recon) normalized the phagocytic response when compared to intact HS ( Figure 7 a). Depletion of EVs from HS almost completely abolished L. rhamnosus NutRes 1 phagocytosis while having a minimal effect on B. breve NutRes 200 phagocytosis. To help discriminate effects on DC phagocytic activity from effects on bacterial interactions, DC capacity to phagocytose labeled dextran was measured. No differences in phagocytic capacity could be measured upon supplementation with the different serum fractions indicating that EVs do not interfere with the capacity of DCs to take up particles ( Figure 7 b).
Figure 7. EVs enhance DC phagocytosis of bacteria.
1×105 DCs were co-incubated with 2.5×106 pHrodo-labeled B. breve NutRes 200, L. rhamnosus NutRes 1 or pHrodo Red Dextran as a control for DC phagocytic capacity at 37°C. (A) Medium was supplemented with increasing concentrations of HS, HS-D or HS-D reconstituted with the serum EV fraction (HS-recon). After 3H, DCs were collected and analyzed for fluorescence using flow cytometery. Degree of phagocytosis is determined as an increase in mean fluorescence index (MFI). EV depletion significantly reduced B. breve phagocytosis at 1 and 5% supplementation compared to HS (*P<0.05). Phagocytosis of L. rhamnosus was reduced to background level (dotted line) upon EV depletion at 1%, 5% and 10% supplementation compared to HS (**P<0.01, *P<0.05, *P<0.05, respectively). Reconstitution of HS-D with EVs normalized the phagocytic response compared to HS. Data are represented as mean ± SEM n = 2. Experiment was repeated at least twice with similar results. (B) Medium was supplemented with 5% of the indicated serum fractions and 30 µg/ml pHrodo Red Dextran. After 1 hour DCs were harvested and MFI determined. Medium as well as all serum fractions increased dextran uptake compared to DCs alone (DC). No differences in dextran uptake could be measured between medium and the different serum fractions. Data are represented as mean ± SEM n = 4.
HS-EVs Modulate DC Cytokine Responses Strain-dependently
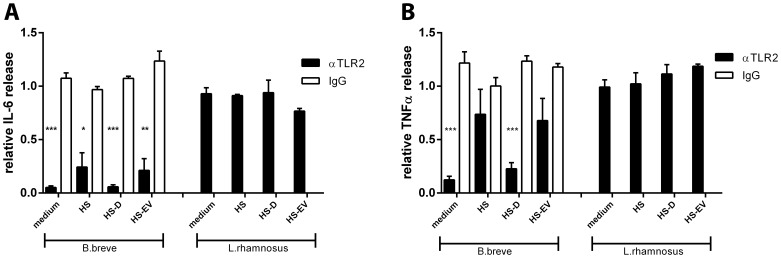
To further substantiate our findings of HS-EV induced inhibition of TLR2 activity and their contribution to DC phagocytosis, HS-EVs were examined for their effect on DC cytokine release after bacterial challenge. Additionally, to zoom in on the specific effect of HS-EVs on bacterial-induced DC signaling from surface receptors, we performed DC-bacteria co-culture experiments in the presence cytochalasine D (cytD), a known inhibitor of phagocytosis. To establish a role for TLR2 in the interaction of DCs with B. breve NutRes 200 and L. rhamnosus NutRes 1, DCs were pre-treated with antibodies directed against TLR2 or an isotype control. Blocking TLR2 significantly inhibited B. breve induced IL-6 release, independent of the presence of the different serum fractions. TLR2 inhibition had no significant effect on L. rhamnosus induced IL-6 release ( Figure 8 a). Blocking of TLR2 inhibited B. breve-induced TNFα release in the presence of medium or HS-D but not intact serum or HS-EVs, indicating a minor role for TLR2 in the B. breve induced TNFα release when incubated in the presence of HS or HS-EVs. TLR2 inhibition had no significant effect on L. rhamnosus induced TNFα release ( Figure 8 b). Next, the different serum fractions were analyzed for their potential to modulate bacterial induced DC IL-6 and TNFα release. Depletion of EVs from HS (HS-D) significantly increased IL-6, but not TNFα release. Treatment with cytD led to a reduction of both IL-6 and TNFα release, which could be rescued upon EV depletion ( Figure 9 a,b). L. rhamnosus NutRes 1 exposed DCs release IL-6 and TNFα and depletion of EVs led to a significant reduction in both IL-6 and TNFα cytokines. Treatment with cytD completely abolished DC cytokine production ( Figure 9 c,d). Taken together, the data suggests that DC proinflammatory cytokine release upon challenge by B. breve is dependent on the TLR2 inhibitory effect of HS-EV in contrast to cytokine release upon challenge by L. rhamnosus which is largely dependent on the HS-EV mediated increase in phagocytosis.
Figure 8. B. breve but not L. rhamnosus ligate DC-expressed TLR2.
1×105 DCs were co-incubated with 1×106 B. breve NutRes 200 or L. rhamnosus NutRes 1 at 37°C in medium, HS, HS-D or HS-EVs. TLR2 activity was inhibited by preincubating cells with a specific anti TLR2 antibody or isotype control. After 16H, supernatants were collected and analyzed for IL-6 and TNFα release. Relative cytokine levels were calculated according to the ratio between responses at serum-free medium and serum fraction supplemented medium. (A) Blocking TLR2 significantly inhibited DC IL-6 release after ligation by B. breve but not L. rhamnosus irrespective of the serum fractions. (B) Blocking TLR2 significantly inhibited DC TNFα release after ligation of B. breve in the presence of medium and HS-D but not intact HS or HS-EVs. DC TNFα release in response to ligation of L. rhamnosus was not affected. Data are represented as mean ± SEM n = 4 (***P<0.001)(**P<0.01)(*P<0.05).
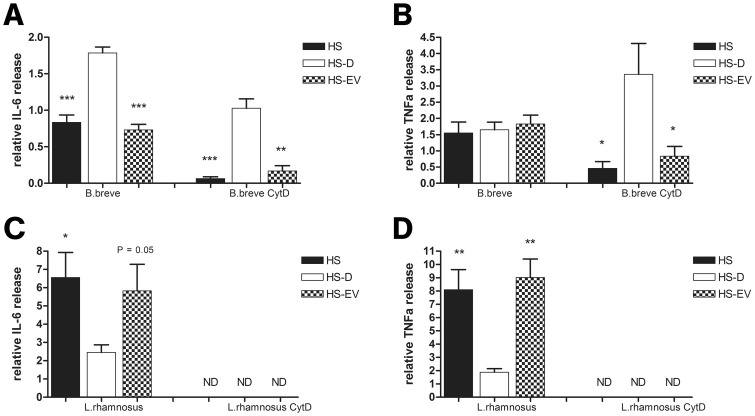
Figure 9. EVs differentially modulate bacterial induced DC cytokine release.
2×105 DCs were co-incubated with 2×106 B. breve NutRes 200 or L. rhamnosus NutRes 1 at 37°C in medium, HS, HS-D or HS-EVs. After 16H, supernatants were collected and analyzed for IL-6 and TNFα release. In another set of similar experiments, DCs were first pretreated with 10 µg/ml cytochalasine D, blocking bacterial phagocytosis. Relative cytokine levels were calculated according to the ratio between responses at serum-free medium and serum fraction supplemented medium. (A) HS and HS-EVs significantly inhibit B. Breve NutRes 200 induced DC IL-6 release compared to HS-D (***P<0.001)(**P<0.01). TNFα release was not affected but upon blocking phagocytosis a significant different TNFα release between HS, HS-EVs and HS-D could be measured (B) (*P<0.05). L. rhamnosus NutRes 1 stimulated DCs release significantly more IL-6 (C) and TNFα (D) in the presence of HS or HS-EVs compared to HS-D. Blocking L. rhamnosus NutRes 1 phagocytosis inhibited DC IL-6 and TNFα release below detection level (ND). Data are represented as mean ± SEM n = 4.
Discussion
Studies on the involvement of TLRs in immune recognition of microbes have helped us to understand how cells, especially DCs and IECs, sense and differentiate between harmless and harmful microbes. However, since microbes share MAMPs, how exactly immune cells differentiate between closely related bacteria is still largely unknown but important in the understanding of immunostimulatory versus immunosuppressive effects of LAB. We and others have previously shown a divergent role for TLR2 in the sensing of bifidobacteria and lactobacilli [17], [30]. Here, we corroborate previous findings demonstrating that specific bifidobacteria, in contrast to lactobacilli, ligate TLR2 and additionally show that TLR2 activation is dependent on the presence of serum. We further show that specifically the EV fraction of serum inhibits TLR2 activation, and that TLR2 activation could be rescued upon EV depletion. Differential effects of the presence of EVs were seen upon analyzing LAB-induced DC proinflammatory cytokine release. Presence of serum enhanced Lactobacillus rhamnosus, but not Bifidobacterium breve, induced DC proinflammatory cytokine release which was dependent on the presence of EVs. When DC phagocytosis was inhibited, B. breve -induced DC IL-6 and TNFα release were impaired in the presence of serum, attributable to the presence of EVs. Blocking L. rhamnosus phagocytosis completely inhibited DC cytokine release. This suggests that, in accordance with data on TLR2 inhibition, DC sense B. breve, but not L. rhamnosus, upon initial contact via membrane-expressed TLR2. Moreover, analogous to the data on HEK TLR2/6 cells, EVs inhibit subsequent B. breve induced TLR2 activation. Several types of interaction between EVs and recipient cells are suggested, such as adhesion of EVs to cellular surfaces through ligand-receptor interactions [19]. For instance, blocking antibodies for various integrins, adhesion molecules or tetraspanins significantly reduced DC EV capture [34]. Next to their function in cell-cell and cell-matrix interactions, integrins play a role in phagocytic processes including the clearance of microbes [35]. Additionally, querying a database of molecular data identified in the different subclasses of EVs (Vesiclepedia [36]) indicated the presence of EV-expressed PRRs (i.e. LPS-binding protein, CD14, scavenger receptors) associated with bacterial attachment to host-cells [37]. We therefore hypothesized that EV expression of PRRs (including but not limited to integrins) might mediate microbial attachment. In support of this hypothesis, intestinal epithelium luminal released EVs have been shown to directly bind the surface of Cryptosporidium parvum sporozoites which was mediated via unindentified specific molecules on the surface of both C. parvum sporozoites and epithelial cells [38]. Our observations on bacterial aggregation suggest that EV indeed interact with LAB and that this interaction results in LAB aggregation, since serum depleted for EVs was not able to do so. Interaction of EVs with bacterial surfaces might additionally expand the repertoire of microbial expressed molecules with host PRR-ligands providing additional molecules for recipient cells to interact with and subsequently influence phagocytic processes. Indeed, our data indicate that phagocytosis of both LAB strains was dependent on the presence of EVs. Interestingly, depletion of EVs only mildly reduced B. breve phagocytosis, in contrast to L. rhamnosus where phagocytosis was almost completely inhibited. TLRs are not phagocytic receptors by themselves, however, TLR-activation contributes to phagocytic processes [39]. Although we do not provide direct proof, we can hypothesize that the absence of surface TLR triggering by L. rhamnosus might be compensated by the EV-interaction with the bacterial surface prompting phagocytic uptake. LAB phagocytosis leads to microbial breakdown and the subsequent intracellular PRR ligation by cell wall products and bacterial DNA which, together with surface TLR2 signaling, contribute to DC cytokine release [17]. The increased DC proinflammatory cytokine response towards L. rhamnosus in the presence of EVs can therefore be explained by the EV-mediated increased phagocytosis. The biological relevance of these findings might be two-fold. On the one hand, EV modulation of LAB TLR2 activity and phagocytosis has an impact on the capacity of LAB to modulate subsequent immune responses. On the other hand, EVs in general might contribute to gut immune homeostasis by reducing TLR induced inflammatory responses and increasing microbial clearance [40], [41]. However, whether or not the serum-EV fraction is representative for the EVs present within the intestinal tissue remains to be determined.
Another key point from this study is the differential actions of EVs on specific TLR ligands. Using ligands specifically addressing the different heterodimers of TLR2, TLR4 or TLR5 we made the surprising observation that EVs specifically increase TLR2/1 and TLR4 activation, while having a suppressive effect on TLR2/6 activity or no effect on TLR5 activity. EVs mediate the traffic of a wide variety of lipids, proteins, mRNAs and microRNAs that are important to its biological function [19]. Especially microRNAs have been show to specifically target TLR activity of recipient cells [42]. TLR2 is thought to heterodimerize with TLR1 or TLR6 to broaden the ligand repertoire but upon activation share a similar signal transduction pathway [43]. Since serum and EVs inhibit TLR2/6 activity, while having a stimulatory effect on TLR2/1 and TLR4 activity, it seems unlikely that EV mediated miRNA interference is responsible for the observed effects.
EVs reportedly express TLR2 which might function in analogy to soluble TLR2 (sTLR2) by binding to ligands or via interference on cellular TLR2 heterodimerization ultimately inhibiting subsequent TLR2 activation [26], [44]. However, serum derived from TLR2 knockout animals or wild types was equally effective in reducing TLR2 activation which could be rescued upon EV depletion, ruling out EV-TLR2 expression as a potential mechanism of action.
FSL-1 is similar in molecular make-up to Pam2CSK4 apart from having a different peptide chain [33], yet our data show that FSL-1 activity is inhibited in contrast to Pam2CSK4. Similar findings were previously published where activity of a bacterial-derived LP from Mycobacterium tuberculosis was found to be suppressed by the presence of serum in contrast to Pam3CSK4 which is identical in molecular make-up apart from the peptide sequence [45]. In agreement with our data, the effectiveness of serum to inhibit TLR2 responses seems to be dependent on the non-acyl part of the ligands. Although the lipid acyl chains of LPs are critically important for TLR2 heterodimer discrimination and activation [32], the molecular make-up of the peptide chain has been shown to impact TLR2 immunological function [46]. How exactly the peptide moiety of LPs contributes to TLR2 activity is still unclear, but a clue might be derived from the reported interactions of TLR2 with additional PRRs [37]. For instance, CD14 as well as the scavenger receptor CD36 function as TLR2 co-receptor and have been researched in more detail regarding their role in TLR2 biological activity [47], [48]. CD36 does not directly interact with TLR2, but has been reported to bind TLR2 ligands having a negative charge like LTA and FSL-1, in contrast to positively charged ligands like Pam2CKS4 and Pam3CSK4 [49]. In contrast to CD36, CD14 directly interacts with the fatty acid portion of triacylated ligands, independent of the peptide moiety [50]. Since EVs are reported to express such PRRs [19], [26], a possible explanation for the reported findings might be that EV-expressed PRRs specifically scavenge LPs, possibly based on their acyl chain make-up or charge of their non-fatty acid part and thereby preventing interaction with surface expressed TLR2. This interdependency has not been researched in detail thus far but a systematically approach in synthesizing bacterial cell wall mimetics with different acyl chains and peptide moieties (LP) or carbohydrate moieties (LTA) would increase understanding of the principles of EV-LP interaction.
TLR activation can be viewed as a double-edged sword. It is critical for host-defense against microbes, but is also linked to inflammatory and autoimmune diseases via their activation by endogenous molecules [15], [51]. TLR responses need to be tightly controlled as prolonged and excessive activation of TLRs can lead to deleterious inflammation and tissue injury detrimental to the host. TLR signaling can be regulated through modification of intracellular pathways following activation, or via interference of TLR ligation by soluble factors and decoy receptors [40], [52]. Although we cannot rule out a possible contribution of miRNA species to the observed effects, our data suggests that the observed effects described are not on the level of host-cell transcription but rather on the level of EV-microbe or EV-specific MAMP interaction which subsequently prevents or enhances host cell surface TLR-activation while at the same time contributes to the clearance of microbes. The biological relevance of EVs enhancing or inhibiting specific TLR responses needs to be addressed in future studies. Determination of the origin of these specific EVs and the receptors involved may lead to new therapeutics for the prevention and/or treatment of inflammatory diseases.
Lastly, potential probiotic strains can be pre-screened in-vitro for their immunological potential. Cytokine production following co-culture of LABs with either peripheral blood mononuclear cells or DCs in the presence of FCS allows for ranking of LAB strains according to their pro or anti-inflammatory profile [53]–[55]. However, the value of in-vitro immunoassays as selection criteria for the use of probiotics in human studies remains to be determined [56]. Our data indicate that the predictive value of these screening strategies could be improved by doing experiments in the appropriate serum environment as LAB induced TLR2 activity was only slightly inhibited in the commonly used FCS as compared to mouse or human serum.
In summary, we present experimental evidence highlighting a previous unrecognized role of EVs in modulation of host-microbe responses. Using specific ligands, surface TLR responses were differentially influenced by the presence of EVs. We showed that TLR2 responses were differentially modulated by serum-derived EVs critically important in the response to and recognition of LAB derived cell wall ligands. Additionally, we provide evidence that EVs interact with LAB surfaces and induce bacterial aggregation while at the same time contributing to LAB phagocytosis, mechanisms known to be involved in host-defense responses by facilitation of bacterial clearance. Overall, as EVs can be found in any tissue or bodily fluid, our findings contribute to a better understanding of host-microbe responses important in intestinal homeostasis and unraveling the mechanism of action of probiotics.
Supporting Information
EVs differentially effect ligand induced TLR activation. THP-1 reporter cells were incubated with specific ligands addressing TLR4 and TLR5 in serum free medium, or medium supplemented with 5% of the indicated serum fractions (HS = intact human serum, HS-D = EV depleted human serum, HS-EV = human serum EVs in medium). (A, C) Dose response experiments indicating the effects of respectively LPS or flagellin stimulation on THP-1 activation. NFκB activity measured as OD values was determined using an alkaline phosphatase reporting system as described in materials and methods. Data presented in Figure 3A,C were used to calculate Area Under the Curve (AUC) values. (B) LPS induced TLR4 activity was increased in medium supplemented with serum fractions compared to serum-free medium, depletion of EVs reduced TLR4 activation compared to HS ($$P<0.01) and HS-D ($$$P<0.001). (D) HS significantly reduced flagellin induced TLR5 activation in contrast to HS-D or HS-EV which showed no effect. Data is represented as mean ± SD (***P<0.001, **P<0.01, *P<0.05).
(DOCX)
Funding Statement
This study was funded by Nutricia Research, in collaboration with the Utrecht University. The following authors are paid by Nutricia Research: JvB, LR, NK, JG, APV.
References
- 1. Chow J, Lee SM, Shen Y, Khosravi A, Mazmanian SK (2010) Host-bacterial symbiosis in health and disease. Adv Immunol 107: 243–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sansonetti PJ, Medzhitov R (2009) Learning tolerance while fighting ignorance. Cell 138: 416–420. [DOI] [PubMed] [Google Scholar]
- 3. Honda K, Littman DR (2012) The microbiome in infectious disease and inflammation. Annu Rev Immunol 30: 759–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sartor RB (2005) Probiotic therapy of intestinal inflammation and infections. Curr Opin Gastroenterol 21: 44–50. [PubMed] [Google Scholar]
- 5. Kalliomaki M, Salminen S, Poussa T, Isolauri E (2007) Probiotics during the first 7 years of life: a cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J Allergy Clin Immunol 119: 1019–1021. [DOI] [PubMed] [Google Scholar]
- 6. Ivanov, II, Honda K (2012) Intestinal commensal microbes as immune modulators. Cell Host Microbe 12: 496–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mennigen R, Bruewer M (2009) Effect of probiotics on intestinal barrier function. Ann N Y Acad Sci 1165: 183–189. [DOI] [PubMed] [Google Scholar]
- 8. Smelt MJ, de Haan BJ, Bron PA, van Swam I, Meijerink M, et al. (2012) L. plantarum, L. salivarius, and L. lactis attenuate Th2 responses and increase Treg frequencies in healthy mice in a strain dependent manner. PLoS One 7: e47244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jeon SG, Kayama H, Ueda Y, Takahashi T, Asahara T, et al. (2012) Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathog 8: e1002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gomez-Llorente C, Munoz S, Gil A (2010) Role of Toll-like receptors in the development of immunotolerance mediated by probiotics. Proc Nutr Soc 69: 381–389. [DOI] [PubMed] [Google Scholar]
- 11. Artis D (2008) Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol 8: 411–420. [DOI] [PubMed] [Google Scholar]
- 12. Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, et al. (2001) Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2: 361–367. [DOI] [PubMed] [Google Scholar]
- 13. Pickard JM, Chervonsky AV (2010) Sampling of the intestinal microbiota by epithelial M cells. Curr Gastroenterol Rep 12: 331–339. [DOI] [PubMed] [Google Scholar]
- 14. Shale M, Schiering C, Powrie F (2013) CD4(+) T-cell subsets in intestinal inflammation. Immunol Rev 252: 164–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11: 373–384. [DOI] [PubMed] [Google Scholar]
- 16. Lebeer S, Vanderleyden J, De Keersmaecker SC (2010) Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol 8: 171–184. [DOI] [PubMed] [Google Scholar]
- 17. Zeuthen LH, Fink LN, Frokiaer H (2008) Toll-like receptor 2 and nucleotide-binding oligomerization domain-2 play divergent roles in the recognition of gut-derived lactobacilli and bifidobacteria in dendritic cells. Immunology 124: 489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R (2012) Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev 64: 676–705. [DOI] [PubMed] [Google Scholar]
- 19. Thery C, Ostrowski M, Segura E (2009) Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 9: 581–593. [DOI] [PubMed] [Google Scholar]
- 20.Gould SJ, Raposo G (2013) As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles 2. [DOI] [PMC free article] [PubMed]
- 21. Karlsson M, Lundin S, Dahlgren U, Kahu H, Pettersson I, et al. (2001) “Tolerosomes” are produced by intestinal epithelial cells. Eur J Immunol 31: 2892–2900. [DOI] [PubMed] [Google Scholar]
- 22. Johansson SM, Admyre C, Scheynius A, Gabrielsson S (2008) Different types of in vitro generated human monocyte-derived dendritic cells release exosomes with distinct phenotypes. Immunology 123: 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C (2005) Exosomal-like vesicles are present in human blood plasma. Int Immunol 17: 879–887. [DOI] [PubMed] [Google Scholar]
- 24. Taylor DD, Akyol S, Gercel-Taylor C (2006) Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol 176: 1534–1542. [DOI] [PubMed] [Google Scholar]
- 25. Admyre C, Grunewald J, Thyberg J, Gripenback S, Tornling G, et al. (2003) Exosomes with major histocompatibility complex class II and co-stimulatory molecules are present in human BAL fluid. Eur Respir J 22: 578–583. [DOI] [PubMed] [Google Scholar]
- 26. Admyre C, Johansson SM, Qazi KR, Filen JJ, Lahesmaa R, et al. (2007) Exosomes with immune modulatory features are present in human breast milk. J Immunol 179: 1969–1978. [DOI] [PubMed] [Google Scholar]
- 27. El Andaloussi S, Mager I, Breakefield XO, Wood MJ (2013) Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 12: 347–357. [DOI] [PubMed] [Google Scholar]
- 28. Sabapatha A, Gercel-Taylor C, Taylor DD (2006) Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am J Reprod Immunol 56: 345–355. [DOI] [PubMed] [Google Scholar]
- 29. Deng ZB, Zhuang X, Ju S, Xiang X, Mu J, et al. (2013) Exosome-like nanoparticles from intestinal mucosal cells carry prostaglandin E2 and suppress activation of liver NKT cells. J Immunol 190: 3579–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Plantinga TS, van Maren WW, van Bergenhenegouwen J, Hameetman M, Nierkens S, et al. (2011) Differential Toll-like receptor recognition and induction of cytokine profile by Bifidobacterium breve and Lactobacillus strains of probiotics. Clin Vaccine Immunol 18: 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Buwitt-Beckmann U, Heine H, Wiesmuller KH, Jung G, Brock R, et al. (2006) TLR1- and TLR6-independent recognition of bacterial lipopeptides. J Biol Chem 281: 9049–9057. [DOI] [PubMed] [Google Scholar]
- 32. Buwitt-Beckmann U, Heine H, Wiesmuller KH, Jung G, Brock R, et al. (2005) Lipopeptide structure determines TLR2 dependent cell activation level. FEBS J 272: 6354–6364. [DOI] [PubMed] [Google Scholar]
- 33. Okusawa T, Fujita M, Nakamura J, Into T, Yasuda M, et al. (2004) Relationship between structures and biological activities of mycoplasmal diacylated lipopeptides and their recognition by toll-like receptors 2 and 6. Infect Immun 72: 1657–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, et al. (2004) Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 104: 3257–3266. [DOI] [PubMed] [Google Scholar]
- 35. Dupuy AG, Caron E (2008) Integrin-dependent phagocytosis: spreading from microadhesion to new concepts. J Cell Sci 121: 1773–1783. [DOI] [PubMed] [Google Scholar]
- 36. Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, et al. (2012) Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol 10: e1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Bergenhenegouwen J, Plantinga TS, Joosten LA, Netea MG, Folkerts G, et al. (2013) TLR2 & Co: a critical analysis of the complex interactions between TLR2 and coreceptors. J Leukoc Biol. [DOI] [PubMed]
- 38. Hu G, Gong AY, Roth AL, Huang BQ, Ward HD, et al. (2013) Release of luminal exosomes contributes to TLR4-mediated epithelial antimicrobial defense. PLoS Pathog 9: e1003261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Underhill DM, Ozinsky A (2002) Phagocytosis of microbes: complexity in action. Annu Rev Immunol 20: 825–852. [DOI] [PubMed] [Google Scholar]
- 40. Shibolet O, Podolsky DK (2007) TLRs in the Gut. IV. Negative regulation of Toll-like receptors and intestinal homeostasis: addition by subtraction. Am J Physiol Gastrointest Liver Physiol 292: G1469–1473. [DOI] [PubMed] [Google Scholar]
- 41. Duerkop BA, Vaishnava S, Hooper LV (2009) Immune responses to the microbiota at the intestinal mucosal surface. Immunity 31: 368–376. [DOI] [PubMed] [Google Scholar]
- 42. Quinn SR, O’Neill LA (2011) A trio of microRNAs that control Toll-like receptor signalling. Int Immunol 23: 421–425. [DOI] [PubMed] [Google Scholar]
- 43. Farhat K, Riekenberg S, Heine H, Debarry J, Lang R, et al. (2008) Heterodimerization of TLR2 with TLR1 or TLR6 expands the ligand spectrum but does not lead to differential signaling. J Leukoc Biol 83: 692–701. [DOI] [PubMed] [Google Scholar]
- 44. LeBouder E, Rey-Nores JE, Rushmere NK, Grigorov M, Lawn SD, et al. (2003) Soluble forms of Toll-like receptor (TLR)2 capable of modulating TLR2 signaling are present in human plasma and breast milk. J Immunol 171: 6680–6689. [DOI] [PubMed] [Google Scholar]
- 45. Schromm AB, Reiling N, Howe J, Wiesmuller KH, Roessle M, et al. (2010) Influence of serum on the immune recognition of a synthetic lipopeptide mimetic of the 19-kDa lipoprotein from Mycobacterium tuberculosis. Innate Immun 16: 213–225. [DOI] [PubMed] [Google Scholar]
- 46.Azuma M, Sawahata R, Akao Y, Ebihara T, Yamazaki S, et al. (2010) The peptide sequence of diacyl lipopeptides determines dendritic cell TLR2-mediated NK activation. PLoS One 5. [DOI] [PMC free article] [PubMed]
- 47. Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, et al. (2005) CD36 is a sensor of diacylglycerides. Nature 433: 523–527. [DOI] [PubMed] [Google Scholar]
- 48. Ranoa DR, Kelley SL, Tapping RI (2013) Human lipopolysaccharide-binding protein (LBP) and CD14 independently deliver triacylated lipoproteins to Toll-like receptor 1 (TLR1) and TLR2 and enhance formation of the ternary signaling complex. J Biol Chem 288: 9729–9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jimenez-Dalmaroni MJ, Xiao N, Corper AL, Verdino P, Ainge GD, et al. (2009) Soluble CD36 ectodomain binds negatively charged diacylglycerol ligands and acts as a co-receptor for TLR2. PLoS One 4: e7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nakata T, Yasuda M, Fujita M, Kataoka H, Kiura K, et al. (2006) CD14 directly binds to triacylated lipopeptides and facilitates recognition of the lipopeptides by the receptor complex of Toll-like receptors 2 and 1 without binding to the complex. Cell Microbiol 8: 1899–1909. [DOI] [PubMed] [Google Scholar]
- 51. Chen GY, Nunez G (2010) Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 10: 826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liew FY, Xu D, Brint EK, O’Neill LA (2005) Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol 5: 446–458. [DOI] [PubMed] [Google Scholar]
- 53. Foligne B, Nutten S, Grangette C, Dennin V, Goudercourt D, et al. (2007) Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J Gastroenterol 13: 236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gad M, Ravn P, Soborg DA, Lund-Jensen K, Ouwehand AC, et al. (2011) Regulation of the IL-10/IL-12 axis in human dendritic cells with probiotic bacteria. FEMS Immunol Med Microbiol 63: 93–107. [DOI] [PubMed] [Google Scholar]
- 55. Timmerman HM, Niers LE, Ridwan BU, Koning CJ, Mulder L, et al. (2007) Design of a multispecies probiotic mixture to prevent infectious complications in critically ill patients. Clin Nutr 26: 450–459. [DOI] [PubMed] [Google Scholar]
- 56. Meijerink M, Mercenier A, Wells JM (2013) Challenges in translational research on probiotic lactobacilli: from in vitro assays to clinical trials. Benef Microbes 4: 83–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
EVs differentially effect ligand induced TLR activation. THP-1 reporter cells were incubated with specific ligands addressing TLR4 and TLR5 in serum free medium, or medium supplemented with 5% of the indicated serum fractions (HS = intact human serum, HS-D = EV depleted human serum, HS-EV = human serum EVs in medium). (A, C) Dose response experiments indicating the effects of respectively LPS or flagellin stimulation on THP-1 activation. NFκB activity measured as OD values was determined using an alkaline phosphatase reporting system as described in materials and methods. Data presented in Figure 3A,C were used to calculate Area Under the Curve (AUC) values. (B) LPS induced TLR4 activity was increased in medium supplemented with serum fractions compared to serum-free medium, depletion of EVs reduced TLR4 activation compared to HS ($$P<0.01) and HS-D ($$$P<0.001). (D) HS significantly reduced flagellin induced TLR5 activation in contrast to HS-D or HS-EV which showed no effect. Data is represented as mean ± SD (***P<0.001, **P<0.01, *P<0.05).
(DOCX)