Abstract
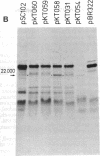
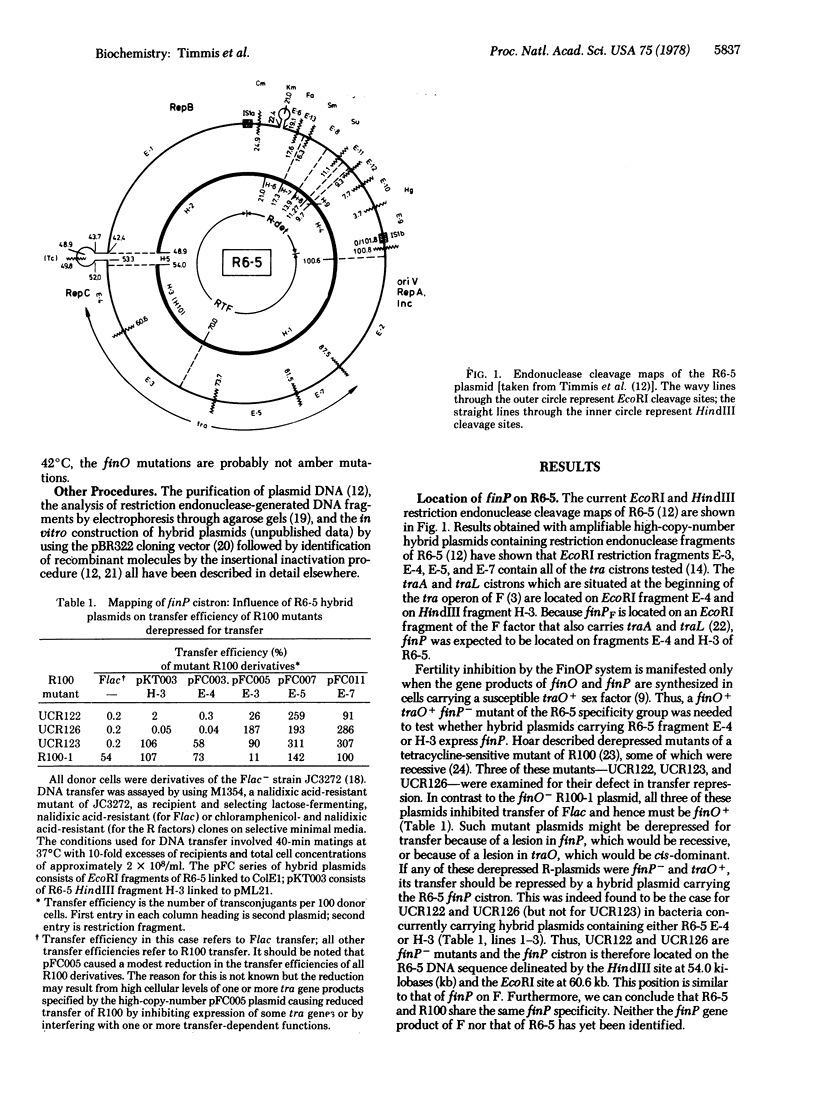
The locations of the fertility inhibition genes finO and finP of the F-like conjugative multiple antibiotic-resistance plasmid R6-5 have been determined. As found previously for that of the fertility plasmid F, the finP gene of R6-5 is located close to the origin of DNA transfer, oriT, and to the promoter-proximal segment of the tra operon. Thus, finP is close to the site of action of the FinOP fertility inhibition system. In contrast, the finO gene is located on the other side of the tra operon, greater than 35 kilobases from the finP gene; finO is very close to the origin of vegetative replication, oriV, and to cistrons encoding functions involved in autonomous plasmid replication and plasmid incompatibility. A 4.5-kilobase fragment of R6-5 DNA containing the finO gene has been cloned on the high-copy amplifiable vector plasmid pBR322. This hybrid plasmid, designated pKTO31, causes severe repression of conjugal transfer of plasmid F, indicating the production of high cellular levels of finO protein. Two independent finO mutant derivatives were obtained after mutagenesis of the pKTO31 plasmid. Comparison of proteins synthesized by minicells carrying finO- mutant plasmids with those carrying various finO+ plasmids enables the finO gene product to be tentatively identified as a 22,000-dalton protein.
Keywords: gene cloning, replication region, conjugation, minicells, in vitro mutagenesis
Full text
PDF
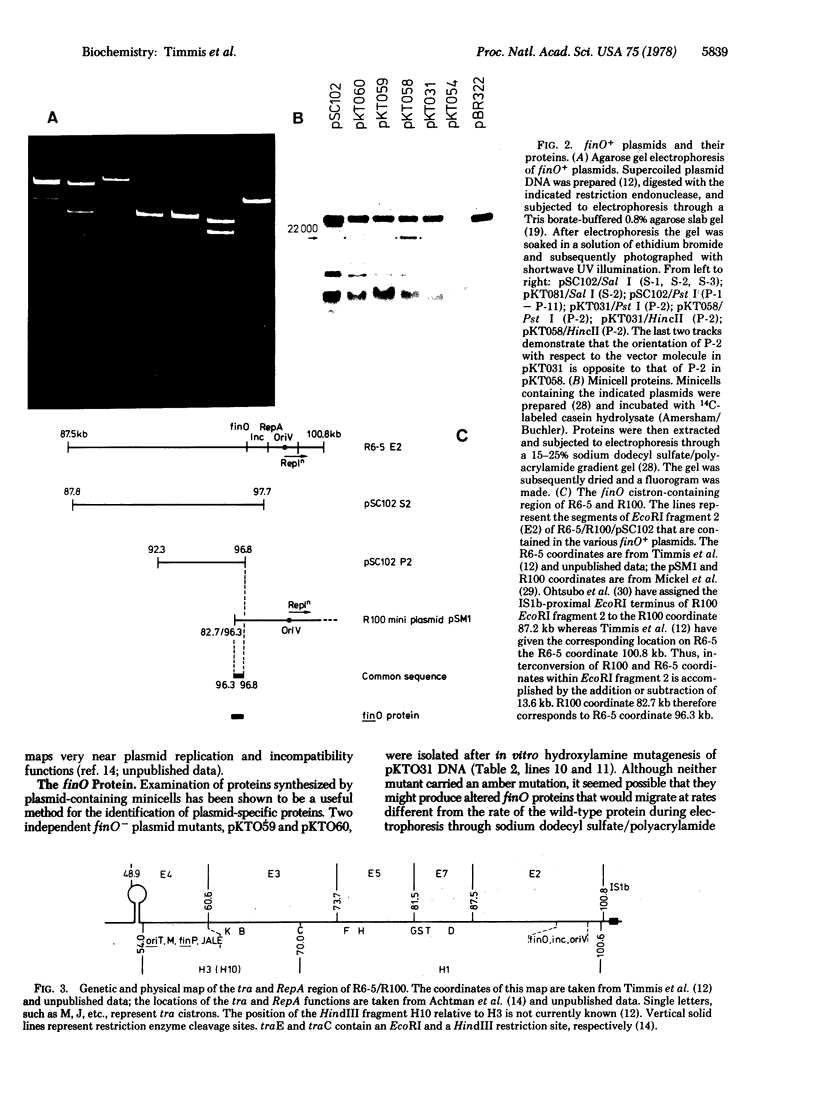

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Kusećek B., Timmis K. N. Tra cistrons and proteins encoded by the Escherichia coli antibiotic resistance plasmid R6-5. Mol Gen Genet. 1978 Jul 11;163(2):169–179. doi: 10.1007/BF00267407. [DOI] [PubMed] [Google Scholar]
- Achtman M., Skurray R. A., Thompson R., Helmuth R., Hall S., Beutin L., Clark A. J. Assignment of tra cistrons to EcoRI fragments of F sex factor DNA. J Bacteriol. 1978 Mar;133(3):1383–1392. doi: 10.1128/jb.133.3.1383-1392.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Willetts N., Clark A. J. Beginning a genetic analysis of conjugational transfer determined by the F factor in Escherichia coli by isolation and characterization of transfer-deficient mutants. J Bacteriol. 1971 May;106(2):529–538. doi: 10.1128/jb.106.2.529-538.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Boyer H. W., Helling R. B. Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3240–3244. doi: 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan D. J., Willetts N. S. Two classes of Flac mutants insensitive to transfer inhibition by an F-like R factor. Mol Gen Genet. 1971;111(3):256–264. doi: 10.1007/BF00433110. [DOI] [PubMed] [Google Scholar]
- Finnegan D., Willetts N. The site of action of the F transfer inhibitor. Mol Gen Genet. 1973 Dec 31;127(4):307–316. doi: 10.1007/BF00267101. [DOI] [PubMed] [Google Scholar]
- Helmuth R., Achtman M. Operon structure of DNA transfer cistrons on the F sex factor. Nature. 1975 Oct 23;257(5528):652–656. doi: 10.1038/257652a0. [DOI] [PubMed] [Google Scholar]
- Hoar D. I. Fertility regulation in F-like resistance transfer factors. J Bacteriol. 1970 Mar;101(3):916–920. doi: 10.1128/jb.101.3.916-920.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoar D. I. Genetic evidence for subunits in the fertility repressor produced by F-like R factors. J Bacteriol. 1971 Oct;108(1):582–583. doi: 10.1128/jb.108.1.582-583.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Smith H. R., Anderson E. S. Mutagenesis of plasmid DNA with hydroxylamine: isolation of mutants of multi-copy plasmids. Mol Gen Genet. 1976 Apr 23;145(1):101–108. doi: 10.1007/BF00331564. [DOI] [PubMed] [Google Scholar]
- Kennedy N., Beutin L., Achtman M., Skurray R., Rahmsdorf U., Herrlich P. Conjugation proteins encoded by the F sex factor. Nature. 1977 Dec 15;270(5638):580–585. doi: 10.1038/270580a0. [DOI] [PubMed] [Google Scholar]
- Meynell E., Meynell G. G., Datta N. Phylogenetic relationships of drug-resistance factors and other transmissible bacterial plasmids. Bacteriol Rev. 1968 Mar;32(1):55–83. doi: 10.1128/br.32.1.55-83.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickel S., Bauer W. Isolation, by tetracycline selection, of small plasmids derived from R-factor R12 in Escherichia coli K-12. J Bacteriol. 1976 Jul;127(1):644–655. doi: 10.1128/jb.127.1.644-655.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickel S., Ohtsubo E., Bauer W. Heteroduplex mapping of small plasmids derived from R-factor R12: in vivo recombination occurs at IS1 insertion sequences. Gene. 1977;2(3-4):193–210. doi: 10.1016/0378-1119(77)90017-8. [DOI] [PubMed] [Google Scholar]
- Ohtsubo E., Feingold J., Ohtsubo H., Mickel S., Bauer W. Unidirectional replication in Escherichia coli of three small plasmids derived from R factor R12. Plasmid. 1977 Nov;1(1):8–18. doi: 10.1016/0147-619x(77)90004-x. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Cohen S. N., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. II. Structure of drug resistance (R) factors and F factors. J Mol Biol. 1973 Apr 5;75(2):235–255. doi: 10.1016/0022-2836(73)90018-1. [DOI] [PubMed] [Google Scholar]
- Smith J. D., Barnett L., Brenner S., Russell R. L. More mutant tyrosine transfer ribonucleic acids. J Mol Biol. 1970 Nov 28;54(1):1–14. doi: 10.1016/0022-2836(70)90442-0. [DOI] [PubMed] [Google Scholar]
- Timmis K. N., Andrés I., Slocombe P. M. Plasmid incompatibility: cloning analysis of an incFII determinant of R6-5. Nature. 1978 May 4;273(5657):27–32. doi: 10.1038/273027a0. [DOI] [PubMed] [Google Scholar]
- Timmis K. N., Cabello F., Cohen S. N. Cloning and characterization of EcoRI and HindIII restriction endonuclease-generated fragments of antibiotic resistance plasmids R6-5 and R6. Mol Gen Genet. 1978 Jun 14;162(2):121–137. doi: 10.1007/BF00267869. [DOI] [PubMed] [Google Scholar]
- Timmis K., Cabello F., Cohen S. N. Cloning, isolation, and characterization of replication regions of complex plasmid genomes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2242–2246. doi: 10.1073/pnas.72.6.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis K., Cabello F., Cohen S. N. Utilization of two distinct modes of replication by a hybrid plasmid constructed in vitro from separate replicons. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4556–4560. doi: 10.1073/pnas.71.11.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S. The kinetics of inhibition of Flac transfer by R100 in E. coli. Mol Gen Genet. 1974 Mar 14;129(2):123–130. doi: 10.1007/BF00268626. [DOI] [PubMed] [Google Scholar]
- Willetts N., Maule J., McIntire S. The genetic locations of traO, finP and tra-4 on the E. coli K12 sex factor F. Genet Res. 1975 Dec;26(3):255–263. doi: 10.1017/s0016672300016050. [DOI] [PubMed] [Google Scholar]
- Willetts N. The transcriptional control of fertility in F-like plasmids. J Mol Biol. 1977 May 5;112(1):141–148. doi: 10.1016/s0022-2836(77)80161-7. [DOI] [PubMed] [Google Scholar]