Abstract
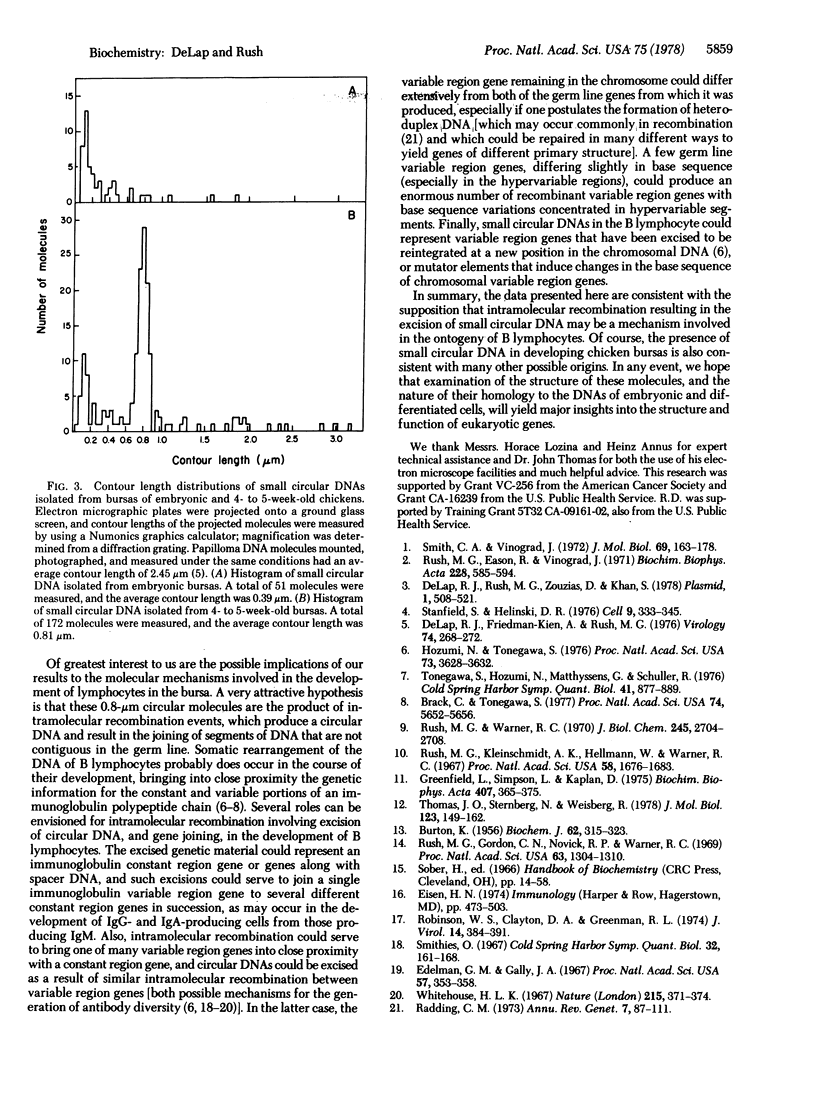
Small circular DNAs ranging in contour length from 0.06 to 3.5 micrometers have been isolated from bursas of 19-day chicken embryos and 4- to 5-week-old chickens. Small circular DNA is present in bursas of 19-day embryos at approximately 0.2 molecules per cell and is very heterogeneous, lacking distinct size classes; most molecules have contour lengths of less than 0.04 micrometers. In contrast, small circular DNA is present in bursas of 4- to 5-week old chickens at about 4 molecules per cell, and although this DNA is still heterogeneous, it contains a major distinct class of molecules 0.8 micrometers in size. These small circular DNAs may be products of developmental gene rearrangements occurring in the chromosomal DNA of lymphocytes in the bursa.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack C., Tonegawa S. Variable and constant parts of the immunoglobulin light chain gene of a mouse myeloma cell are 1250 nontranslated bases apart. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5652–5656. doi: 10.1073/pnas.74.12.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLap R. J., Rush M. G., Zouzias D., Khan S. Isolation and preliminary characterization of the small circular DNA present in African green monkey kidney (BSC-1) cells. Plasmid. 1978 Sep;1(4):508–521. doi: 10.1016/0147-619x(78)90008-2. [DOI] [PubMed] [Google Scholar]
- Delap R. D., Friedman-klen A., Rush M. G. The absence of human papilloma viral DNA sequences in condylomata acuminata. Virology. 1976 Oct 1;74(1):268–272. doi: 10.1016/0042-6822(76)90155-0. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Gally J. A. Somatic recombination of duplicated genes: an hypothesis on the origin of antibody diversity. Proc Natl Acad Sci U S A. 1967 Feb;57(2):353–358. doi: 10.1073/pnas.57.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield L., Simpson L., Kaplan D. Conversion of closed circular DNA molecules to single-nicked molecules by digestion with DNAase I in the presence of ethidium bromide. Biochim Biophys Acta. 1975 Oct 15;407(3):365–375. doi: 10.1016/0005-2787(75)90104-5. [DOI] [PubMed] [Google Scholar]
- Hozumi N., Tonegawa S. Evidence for somatic rearrangement of immunoglobulin genes coding for variable and constant regions. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3628–3632. doi: 10.1073/pnas.73.10.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radding C. M. Molecular mechanisms in genetic recombination. Annu Rev Genet. 1973;7:87–111. doi: 10.1146/annurev.ge.07.120173.000511. [DOI] [PubMed] [Google Scholar]
- Robinson W. S., Clayton D. A., Greenman R. L. DNA of a human hepatitis B virus candidate. J Virol. 1974 Aug;14(2):384–391. doi: 10.1128/jvi.14.2.384-391.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush M. G., Eason R., Vinograd J. Identification and properties of complex forms of SV40 DNA isolated from SV40-infected African Green monkey (BSC-1) cells. Biochim Biophys Acta. 1971 Feb 11;228(3):585–594. doi: 10.1016/0005-2787(71)90723-4. [DOI] [PubMed] [Google Scholar]
- Rush M. G., Gordon C. N., Novick R. P., Warner R. C. Penicillinase plasmid DNA from Staphylococcus aureus. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1304–1310. doi: 10.1073/pnas.63.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush M. G., Kleinschmidt A. K., Hellmann W., Warner R. C. Multiple-length rings in preparations of phi-X174 replicative form. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1676–1683. doi: 10.1073/pnas.58.4.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush M. G., Warner R. C. Alkali denaturation of covalently closed circular duplex deoxyribonucleic acid. J Biol Chem. 1970 May 25;245(10):2704–2708. [PubMed] [Google Scholar]
- Smith C. A., Vinograd J. Small polydisperse circular DNA of HeLa cells. J Mol Biol. 1972 Aug 21;69(2):163–178. doi: 10.1016/0022-2836(72)90222-7. [DOI] [PubMed] [Google Scholar]
- Stanfield S., Helinski D. R. Small circular DNA in Drosophila melanogaster. Cell. 1976 Oct;9(2):333–345. doi: 10.1016/0092-8674(76)90123-9. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Sternberg N., Weisberg R. Altered arrangement of the DNA in injection-defective lambda bacteriophage. J Mol Biol. 1978 Aug 5;123(2):149–161. doi: 10.1016/0022-2836(78)90318-2. [DOI] [PubMed] [Google Scholar]
- Tonegawa S., Hozumi N., Matthyssens G., Schuller R. Somatic changes in the content and context of immunoglobulin genes. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):877–889. doi: 10.1101/sqb.1977.041.01.097. [DOI] [PubMed] [Google Scholar]
- Whitehouse H. L. Cross over model of antibody variability. Nature. 1967 Jul 22;215(5099):371–374. doi: 10.1038/215371a0. [DOI] [PubMed] [Google Scholar]




