Abstract
Helicobacter pylori has been causally linked to a number of diseases, including peptic ulcer disease, gastric adenocarcinoma, mucosa-associated lymphoid tissue lymphoma, and dyspepsia. It is the most prevalent bacterial pathogen in humans, and while the overall prevalence in the United States is about 30 %, the distribution is heterogeneous amongst different ethnic groups. Recent immigrants from high prevalence areas such as Korea, Japan, and China bear an increased burden of its disease and complications. There is clear evidence that treatment of H. pylori resolves peptic ulcer disease, and increasing evidence for protection against development of gastric adenocarcinoma. However, H. pylori treatment failure is common and alternative regimens may be necessary. The following case-based review will highlight these issues, including the epidemiology of H. pylori in the immigrant population, an approach to dyspepsia, and the role of H. pylori in gastric adenocarcinoma.
KEY WORDS: Helicobacter pylori, immigrant, peptic ulcer, gastric adenocarcinoma, dyspepsia
BACKGROUND
In 2005, the Nobel Prize was awarded to Marshall and Warren for their landmark discovery that Helicobacter pylori fulfilled Koch’s postulate for peptic ulcer disease.1 They challenged the prevailing dogma of peptic ulcer disease and established the important causal role of chronic infection and inflammation on the development of disease, leading to additional discoveries of H. pylori as a causative agent of both gastric adenocarcinoma and mucosa-associated lymphoid tissue (MALT) lymphoma.2–4 In addition, H. pylori has been causally implicated in a multitude of other conditions, such as iron deficiency anemia,5 immune thrombocytopenic purpura,6 gastroesophageal reflux disease,7 and functional dyspepsia.8
H. pylori infection is the most common chronic bacterial pathogen in humans, infecting approximately 50 % of the global population,9 and an estimated 30–40 % of the US population.10 Indeed, discovery of H. pylori infection has completely altered the epidemiology and clinical management of upper gastrointestinal tract diseases over the last two decades. This is important throughout the developed world, but it is particularly important to understand how this relates to immigrants from high-risk areas, since this population bears an increased burden of disease and its complications. The purpose of this case-based review is to highlight major clinical and epidemiologic studies on H. pylori infection for primary care providers, help identify high-risk individuals, review the indications for H. pylori treatment, and manage resistant or recurrent disease, particularly important during management of complex peptic ulcer disease and gastric adenocarcinoma.
Case
A 53-year-old Korean man presents to your clinic complaining of gnawing, mid-epigastric pain, which started about 4 weeks ago. He tried some over-the-counter antacids with minimal relief and he now seeks your advice.
EPIDEMIOLOGY OF H. PYLORI IN THE UNITED STATES
Data from the National Health and Nutrition Examination Survey from 1999 to 2000 estimates the overall prevalence of H. pylori infection in the United States to be 30.7 %.11 The prevalence has been decreasing over time (Fig. 1).12 However, the distribution is heterogeneous and different ethnic groups are disparately affected. For example, Mexican Americans (64 %) and non-black Hispanics (52 %) have higher rates of seropositivity compared to non-Hispanic whites (21 %).11 Furthermore, providers should be aware that recent Asian immigrants have much higher rates of H. pylori seropositivity compared to whites. High prevalence countries include China (58 %), Japan (39 %), Korea (60 %), Vietnam (75 %), India (79 %) and Thailand (57 %).13 The risk of H. pylori in immigrants appears to decrease with each successive generation born in the United States.14
Figure 1.
H. pylori prevalence at age 20 by birth cohort.12
H. PYLORI AND DYSPEPSIA
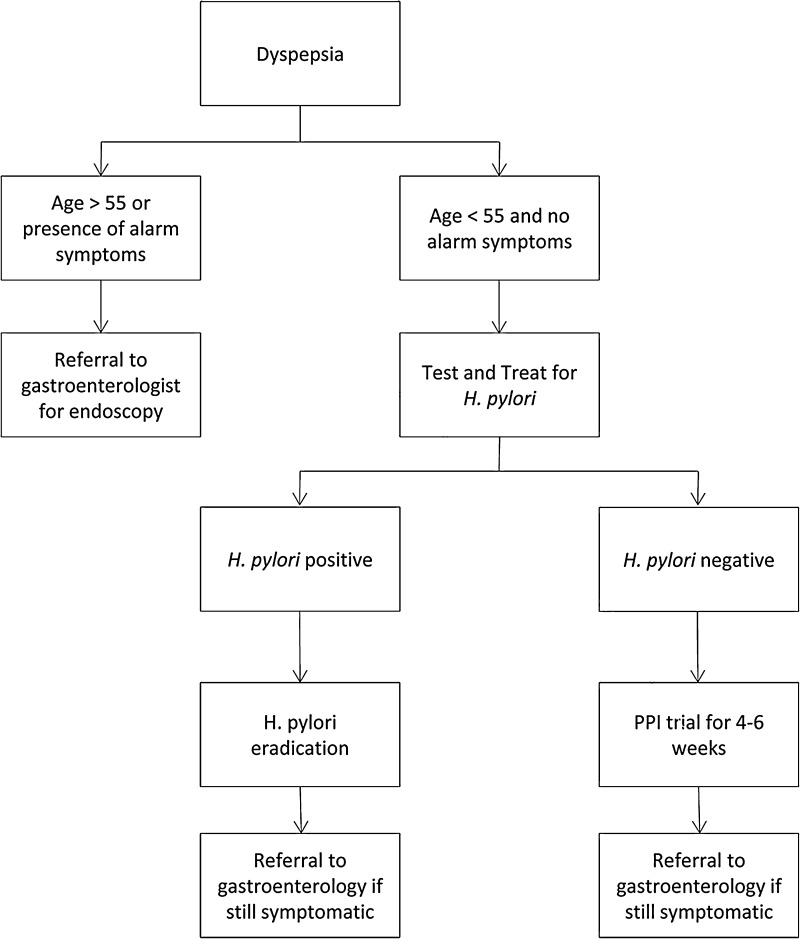
Dyspepsia is defined as pain or discomfort in the upper abdomen, and should be distinguished from the constellation of symptoms that define gastroesophageal reflux disease (GERD). Dyspepsia is common, occurring in about 25 % of the US population. The history and physical should focus on identifying culprit medications such as nonsteroidal anti-inflammatory drugs (NSAIDs). According to guidelines by the American Gastroenterological Association (AGA), patients should be stratified into two groups: the high-risk group includes individuals >55 years old or younger individuals with alarm symptoms, while the low-risk group includes individuals <55 years old and without any alarm symptoms.15 Alarm signs or symptoms include family history of gastric cancer, weight loss, dysphagia/odynophagia, gastrointestinal bleeding, unexplained anemia, or jaundice. It should be noted, however, that multiple studies demonstrate the poor positive predictive value of alarm symptoms.16–18 Patients in the latter category (< 55 years old, no alarm symptoms) should be tested and treated for H. pylori infection, while patients in the high-risk category should be referred directly to a gastroenterologist for an endoscopic evaluation to exclude malignancy (Fig. 2).
Figure 2.
Management of Dyspepsia. Adapted from the AGA Medical Position Statement on Dyspepsia.15 Alarm signs or symptoms include family history of gastric cancer, weight loss, dysphagia/odynophagia, gastrointestinal bleeding, unexplained anemia, or jaundice.
This “test and treat” method has been validated in a randomized controlled study of 294 Canadian low-risk patients with at least moderate dyspepsia (cannot be ignored but does not influence daily activities) and a diagnosis of active H. pylori infection confirmed by urea breath test. Treatment with triple therapy was significantly more effective than placebo in achieving symptom resolution at 1 year, defined as minimal to no dyspepsia (54 % vs. 39 %; P = 0.02, number needed to treat [NNT] = 6.7).19 Much of the benefit derived from H. pylori eradication likely occurs because of treatment of peptic ulcer disease, because in a cohort comprised of non-ulcer dyspepsia (negative by endoscopy), the benefit of H. pylori eradication reported by a meta-analysis of 12 randomized controlled trials of 2,903 patients was much more limited (relative risk [RR] = 0.91; 95 % CI 0.86–0.95, NNT = 12).8 Therefore, patients in the high-risk group (> 55 or with alarm symptoms) or those that have failed the “test and treat” approach should be referred to a gastroenterologist for esophagogastroduodenoscopy.
H. PYLORI AND PEPTIC ULCER DISEASE
H. pylori infection is the major cause of peptic ulcer disease (PUD), which overlaps with symptoms of dyspepsia as discussed above. The burden of this disease in the US has dramatically decreased with H. pylori eradication and the advent of proton pump inhibitors; however, failure to eradicate H. pylori and the prevalent use of NSAIDs require attention. The risk of PUD is significantly higher among NSAID users with H. pylori compared to NSAID users without H. pylori. (OR = 3.53; 95 % CI 2.16–5.75).20
H. pylori eradication is vital for ulcer healing in the absence of proton pump inhibitors. In a meta-analysis of 24 trials and 2,102 patients, ulcer healing rates in H. pylori-eradicated patients were significantly higher compared with patients with persistent infection (98 % vs. 57.5 % for duodenal ulcers and 97.1 % vs. 60.9 % for gastric ulcers).21 Current expert guidelines recommend H. pylori screening and treatment prior to initiating long-term NSAID therapy in patients with a history of dyspepsia or peptic ulcer disease.22 A recent prospective study showed that the risk of ulcer bleeding in patients with a history of bleeding ulcers taking low dose aspirin after H. pylori eradication was similar to an average risk cohort on aspirin ( incidence rate ratio [IRR] 1.47; 95 % CI 0.75–3.38).23
Case Continued
You obtain a thorough history and physical, and the patient does not report any red flag symptoms. You decide to test him for H. pylori and treat if he is positive. What are the available diagnostic tests?
TESTING FOR H. PYLORI
Three non-endoscopic tests for H. pylori infection are available: the serology test, the urea breath test (UBT), and the fecal antigen test (FAT) (Table 1). The serology for immunoglobulin G (IgG) is inexpensive and widely available. It has a sensitivity of 85 % and a specificity of 80 %.24 However, it has a low positive predictive value in low prevalence areas (< 20 %), limiting its usefulness in this setting.25 Furthermore, since it is an antibody test, it cannot distinguish between active and ongoing infection from previous infection; therefore, it cannot be used to confirm eradication. The UBT has a 95 % sensitivity and specificity for active H. pylori infection.26,27 To perform the test, the patient drinks urea containing nonradioactive 13C or radioactive 14C, which is broken down by the intrinsic urease activity in H. pylori, releasing labeled CO2 gas, which can then be measured in an expired breath. The UBT is the most reliable means of confirming eradication of H. pylori.28 Because the test relies on the intrinsic urease activity of active H. pylori, the test performance is decreased in the presence of proton pump inhibitors (PPI), bismuth-containing agents, and antibiotics. As such, it is recommended that antibiotics be held for 28 days and PPI/bismuth agents for 7–14 days prior to the test.29,30 The UBT is the most expensive of the available non-endoscopic tests. Lastly, the fecal antigen test (FAT) identifies H. pylori antigen in the stool by an enzyme-linked immunoassay with either a monoclonal or polyclonal antibody. A meta-analysis of 89 studies showed that the test has a pooled sensitivity and specificity of 91 % and 93 %, respectively.31 The performance characteristics of FAT is therefore similar to UBT.28 Although the FAT is easy to perform, the need to handle and transport the stool may be unappealing for patients. Similar to the UBT, both antibiotics and PPI use affect the sensitivity of the FAT. PPI decreases the availability of H. pylori antigens in the stool through direct antibacterial effects.32 For our case patient, the H. pylori blood test is preferable, given its low cost and because the patient has not been previously treated for H. pylori. Endoscopic biopsy with histology is typically the gold standard against which the non-endoscopic tests are evaluated.
Table 1.
Tests for H. pylori
| Test | Advantages | Disadvantages | Sensitivity/Specificity | CPT | Cost |
|---|---|---|---|---|---|
| Serology for IgG | Inexpensive, widely available | Low PPV in low prevalence areas. Cannot be used for confirming eradication. | 85 %/80 %24 | 86677 | $19.95 |
| Fecal antigen test* | Excellent test characteristics. Can be used to confirm eradication. | Need for patient to handle/transport stool sample. | 91 %/93 %31 | 87338 | $19.77 |
| Urea breath test* | Excellent test characteristics. Can be used to confirm eradication. | Most expensive of the available tests. | 95 %/95 %26 | 78267+78268 for 14C 83013+83014 for 13C |
$103.40 $103.40 |
*PPI/bismuth agents should be held 7–14 days prior to the test
CPT Current Procedural Terminology code; PPV positive predictive value
2013 Cost data from http://www.cms.hhs.gov/ClinicalLabFeeSched (last accessed 8/27/13)
Case Continued
You send the serology test, which returns positive for H. pylori. You treat him with standard triple therapy, yet he is still symptomatic 2 months after treatment. At that point, you refer him to a gastroenterologist. Esophagogastroduodenoscopy reveals gastric adenocarcinoma with evidence of active H. pylori infection. He subsequently undergoes a subtotal gastrectomy without evidence of lymph node involvement. He sees you in clinic for follow-up. He asks about H. pylori, its role in gastric cancer, and whether or not he should be treated for it, even after having a subtotal gastrectomy.
H. PYLORI MEDIATED CANCER PROGRESSION
Chronic H. pylori infection is instrumental in the development of gastric cancer.33,34 Indeed, H. pylori is the only bacteria that is classified as a carcinogen by the World Health Organization.35 Epidemiological studies estimate the increased risk of gastric cancer in H. pylori-infected patients to be approximately 20-fold.36 A prospective cohort study of 1,526 Japanese individuals reported that over an average follow-up period of 7.8 years, 2.9 % of H. pylori infected individuals developed gastric adenocarcinoma compared to 0 % of uninfected individuals.2 A stepwise carcinogenic pathway, known as the Correa pathway involving H. pylori, has been proposed.37,38 In the proposed pathway, chronic H. pylori infection leads to chronic active gastritis manifested by inflammatory infiltrates including neutrophils, lymphocytes and plasma cells.39 The chronic inflammation then leads to the destruction of parietal and chief cells, resulting in achlorhydria and atrophic gastritis. It has been postulated that the loss of parietal cells, cells responsible for secreting signals that normally modulate growth and differentiation of the gastric mucosa, results in the proliferation of undifferentiated gastric progenitor cells and ultimately intestinal metaplasia. Intestinal metaplasia is a premalignant lesion that may evolve into dysplasia and carcinoma.40
H. pylori-mediated carcinogenesis may be accelerated in the context of host and environmental factors, which include family history of gastric adenocarcinoma, atrophic gastritis, specific host genetic polymorphisms, intake of nitrosoamine compounds (found in cured meats and fish) and high salt diets.41–43 For example, patients with H. pylori infection and a family history of gastric adenocarcinoma have a three-fold increase in the incidence of gastric adenocarcinoma.44
EPIDEMIOLOGY OF GASTRIC ADENOCARCINOMA IN THE UNITED STATES
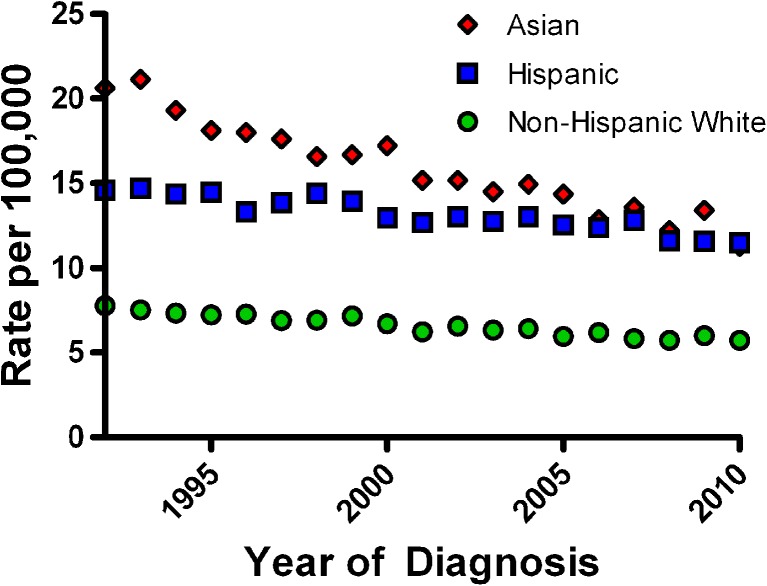
Gastric adenocarcinoma is the fourth most common malignancy in the world and remains the second most common cause of cancer-related deaths worldwide, accounting for over 700,000 deaths annually.45,46 The highest-risk areas (more than 20 per 100,000) include East Asian countries such as China, Japan, and Korea, while intermediate risk countries include Taiwan, Malaysia, and Singapore. India, Australia, New Zealand, and Thailand are considered low risk countries (less than 10 per 100,000).47 While the overall incidence and death from gastric adenocarcinoma in the United States is considered low, the burden of disease rests predominantly with recent immigrants from high-risk countries (Fig. 3). For example, Korean men living in Los Angeles have an age-adjusted standardized incidence rate of 40 per 100,000 for gastric cancer, which is comparable to the highest rates reported in the world.48 This high gastric cancer incidence rate approximates that of colorectal cancer. In addition, epidemiologic data from the United States also demonstrate a higher incidence of gastric cancer in Hispanics (IRR 1.75; 95 % CI 1.70–1.81) and blacks (IRR 2.35; 95 % CI 2.25–2.45), compared to non-Hispanic whites.45,49 To date, there is no consensus regarding screening for gastric cancer in the United States, but physicians should be especially aware of the risk in immigrants from these high-risk areas.50 Consensus guidelines do not recommend population-based H. pylori screening in the United States, given the overall low rate of mortality from gastric cancer. However, H. pylori screening is recommended by the Asia-Pacific Gastric Cancer Group for gastric cancer prevention in patients from countries with high background rates of gastric adenocarcinoma, such as China, Japan, and Korea.48
Figure 3.
Age-adjusted gastric adenocarcinoma incidence rates by race. Adapted from the Surveillance Epidemiology and End Results (SEER) website http://seer.cancer.gov/faststats/ (last accessed 8/27/13).
ROLE OF H. PYLORI ERADICATION IN THE PREVENTION OF GASTRIC ADENOCARCINOMA
Multiple studies underscore H. pylori eradication for patients who have undergone resection for gastric adenocarcinoma. In a Japanese prospective cohort study of 132 patients with gastric adenocarcinoma treated with endoscopic resection, 65 patients underwent H. pylori eradication compared with 67 untreated controls. At 3 years, 9 % of the untreated group developed a recurrent or an incident gastric adenocarcinoma compared with none in the eradication group.51 Another study of 544 patients diagnosed with early gastric adenocarcinoma treated with endoscopic resection randomized to H. pylori eradication demonstrated that H. pylori eradication was associated with a 65 % lower incidence of metachronous gastric adenocarcinoma at 3 years.52,53 These compelling data support secondary prevention of gastric adenocarcinoma by eradicating H. pylori, which is the recommendation for the patient in this case.
Case Continued
You explain to the patient that H. pylori and host factors contribute to the development of gastric cancer. Given his positive biopsy for H. pylori (and the fact that he had already failed standard triple therapy), you treat him with bismuth subsalicylate 525 mg QID, metronidazole 250 mg QID, tetracycline 500 mg QID, and omeprazole 20 mg BID for 10 days for secondary prevention of gastric cancer.54 You intend to confirm eradication of H. pylori and retreat if necessary.
The patient’s son, a 21-year-old man, comes to see you in clinic. His father told him of the predisposition to developing gastric cancer among family members and he is concerned that he too may develop gastric cancer. He wants to know if there is anything that he can do to prevent this from happening.
PRIMARY PREVENTION OF GASTRIC ADENOCARCINOMA
Primary prevention of gastric adenocarcinoma through antibiotic treatment of H. pylori infection appears to modestly alter the natural history of gastric adenocarcinoma.55 A meta-analysis of seven randomized trials encompassing 6,695 patients suggested that H. pylori eradication reduced the incidence of gastric adenocarcinoma by 35 % (an absolute risk reduction of 0.6 %).56 Consequently, the Asia-Pacific consensus guideline gave a grade A recommendation for H. pylori screening and eradication in high-risk populations.57,58 High-risk populations include recent immigrants from prevalent areas such as China, Korea, and Japan. Furthermore, the Lejondal H. pylori-Gastric Cancer Task Force recommends H. pylori screening and treatment for first-degree relatives of gastric cancer patients, given their increased risk of cancer.59
Case Continued
Given the 21-year-old son’s family history of gastric cancer, you decide to screen him for H. pylori with a serology test, which comes back positive. The patient asks you what treatments are available.
WHAT SHOULD BE USED FOR PRIMARY THERAPY?
Treatment recommendations for H. pylori infection include: 1. Clarithromycin based triple therapy (a proton pump inhibitor, clarithromycin, and either amoxicillin or metronidazole) for 14 days or 2. Bismuth based quadruple therapy (a proton pump inhibitor, bismuth, metronidazole, and tetracycline) for 10–14 days.28 Studies have shown that the eradication rates with both of these regimens range from 70 % to 85 %.60–63
Case Continued
The patient sees you in follow-up, at which time you decide to confirm eradication with a fecal antigen test, which comes back positive. The patient asks you why this happened and what the next steps are.
RESISTANCE
US-based antibiotic treatment trials for H. pylori estimate clarithromycin resistance between 10.6 % and 12 % and metronidazole resistance between 21.6 % and 39 %64; indeed, exposure to macrolides or metronidazole is significantly associated with resistance to these agents.65 Furthermore, the number of clarithromycin resistant isolates appears to be increasing.66 This may explain why the eradication rate of clarithromycin-based triple therapy, the dominant H. pylori regimen, has decreased over the years.67 For this reason, either bismuth-based quadruple therapy or sequential therapy (5 days of twice daily PPI and amoxicillin followed by 5 days of twice daily proton pump inhibitor (PPI), clarithromycin, and tinidazole) is recommended as first-line therapy in areas with high clarithromycin resistance.22,68 A recent randomized trial from Taiwan demonstrated that 10 days of sequential therapy was superior to 14 days of standard triple therapy for H. pylori eradication (90.7 % vs. 82.3 %; P = 0.003; NNT to reduce one failure = 12). Furthermore, eradication rates were higher with sequential therapy, even in strains that were susceptible to both clarithromycin and metronidazole.69
When a patient has failed initial clarithromycin-based triple therapy, bismuth-based quadruple therapy is a commonly recommended salvage therapy.22 As a second line therapy, bismuth-based quadruple therapy is approximately 68 % efficacious or in other words, a 32 % failure rate.54,70,71 Other promising alternatives include standard sequential therapy and levofloxacin-based triple or sequential therapy.72,73 A meta-analysis of four randomized controlled trials found that levofloxacin-based triple therapy (levofloxacin, amoxicillin, PPI) had a higher eradication rate compared to bismuth-based quadruple therapy in patients with persistent H. pylori infection (87 % vs. 60 %, NNT = 3.7).74 Table 2 summarizes the major treatment regimens. Other combinations using different medications have been proposed, but their efficacy as salvage therapy is rather limited. This includes a rifaximin plus levofloxacin-based regimen or quadruple therapy with rifabutin as salvage therapy.75,76
Table 2.
Therapies for Treatment of H. Pylori
| “Common name” | Regimen | Duration | Eradication rates |
|---|---|---|---|
| Triple therapy | Clarithromycin 500 mg PO BID Amoxicillin 1 g PO BID (metronidazole 500 mg PO BID if penicillin allergic) Standard dose PPI BID |
14 days | First line: 70–85%61,63 |
| Quadruple therapy | Bismuth subsalicylate 525 mg PO QID Metronidazole 250 mg PO QID Tetracycline 500 mg PO QID Ranitidine 150 mg PO BID or standard dose PPI QD to BID |
10–14 days | First line: 70–90%63,89
Second line: 70–75.8%54,70,71 |
| Sequential therapy | PPI + amoxicillin 1 g PO BID × 5 days Followed by PPI, clarithromycin 500 mg PO BID, tinidazole 500 mg PO BID × 5 days |
10 days | First line: 77.8–94.4%90 |
| Levofloxacin-based therapy | Levofloxacin 500 mg PO BID, amoxicillin 1 g PO BID, standard dose PPI | 10 days | Second line: 70–94%74 |
CONFIRMATORY TESTING FOR H. PYLORI ERADICATION
Treatment failure in H. pylori is common, and the consequence of failure is high in several diseases. As mentioned, H. pylori is causally implicated in the pathogenesis of gastric adenocarcinoma, MALT lymphoma, and peptic ulcer disease. In the case of peptic ulcer disease, H. pylori treatment failure leads to recurrent duodenal ulcer and gastric ulcer in 59 % and 69 % of individuals after 6 months, respectively.77 As such, confirmatory testing to establish eradication is important in these diseases. This should be done when the patient has been off PPI therapy for 2–4 weeks and off all H. pylori therapy for 1 month.28
COMPLIANCE AND H. PYLORI ERADICATION
For those failing multiple regimens, it is imperative to reinforce compliance with therapy and work closely with the patient to achieve success. It has been shown that approximately10% of patients fail to take more than 60 % of their H. pylori medications and that 30 % take less than 90 % of their medications.78 The most commonly cited reason for medication noncompliance is gastrointestinal intolerance. This has implications with regards to H. pylori eradication rates. Historical data demonstrated that patients who took greater than 60 % of the prescribed medications had a 96 % eradication rate compared to 69 % for those who took less than 60 %.79 In a contemporary randomized study evaluating three treatment arms involving 1,463 patients with H. pylori infection from Latin America, the 1-year eradication rate was significantly associated with adherence to medications (defined as taking ≥ 80 % of drugs).80
Case Continued
For second-line therapy, you prescribe a course of bismuth-based quadruple therapy. Following completion of therapy, the patient reports that his symptoms are markedly improved. A follow-up fecal antigen test confirms eradication.
COST-EFFECTIVENESS OF H. PYLORI SCREENING AND TREATMENT
Historical estimates of the annual cost of peptic ulcer disease in the United States are in the range of $6 billion.81 A randomized, controlled trial from 1998 showed that H. pylori eradication for duodenal ulcers saved $547 per patient after 1 year compared to PPI therapy alone.82 Retrospective data from a large managed care organization showed that patients with dyspepsia had 54 % higher costs compared to controls, and costs in the initial diagnostic period were $483 greater per person.83 A Markov model published in 2002 demonstrated that population screening for H. pylori was cost neutral when balanced with long-term treatment for dyspepsia, and could be cost-effective if mortality from PUD and gastric adenocarcinoma was decreased by 10 %.84 A randomized controlled trial involving over 1,000 patients in the United Kingdom was published in 2005, evaluating cost effectiveness of population screening (H. pylori prevalence was 28 %). The patients were randomized to either a screen and eradicate program or placebo and were followed for 10 years. At 10 years, there was a statistically significant cost savings of $117 per person for dyspepsia-related resource usage compared to placebo.85 Data from 2004 estimates the total cost of gastric cancer in the United States to be $1.9 billion.86 Although recommendations are mixed, a recent systematic review including 12 studies on population screening for H. pylori for gastric cancer prevention found that screening was cost-effective, across a wide range of H. pylori prevalence ranges.87 A decision analysis in a population where the prevalence of H. pylori was 13 %, and incorporating all H. pylori related diseases, concluded that the incremental cost per patient in the screening group was $26.88
CONCLUSION
H. pylori is the most common bacterial pathogen worldwide and has been causally implicated in peptic ulcer disease, gastric cancer, MALT lymphoma, and ITP. Identifying H. pylori infection in immigrants from high-risk areas such as Korea, China, and Japan is important, since this population bears an increased burden of disease, and its complications and successful eradication can positively affect the natural history of many H. pylori-associated complications.
Acknowledgements
This work was supported by the National Institutes of Health NCI K23 CA157929.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
REFERENCES
- 1.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1(8390):1311–1315. doi: 10.1016/S0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 2.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345(11):784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology. 1998;115(3):642–648. doi: 10.1016/S0016-5085(98)70143-X. [DOI] [PubMed] [Google Scholar]
- 4.Wotherspoon AC, Diss TC, Pan LX, Schmid C, Kerr-Muir MG, Lea SH, et al. Primary low-grade B-cell lymphoma of the conjunctiva: a mucosa-associated lymphoid tissue type lymphoma. Histopathology. 1993;23(5):417–424. doi: 10.1111/j.1365-2559.1993.tb00489.x. [DOI] [PubMed] [Google Scholar]
- 5.DuBois S, Kearney DJ. Iron-deficiency anemia and Helicobacter pylori infection: a review of the evidence. Am J Gastroenterol. 2005;100(2):453–459. doi: 10.1111/j.1572-0241.2005.30252.x. [DOI] [PubMed] [Google Scholar]
- 6.Emilia G, Luppi M, Zucchini P, Morselli M, Potenza L, Forghieri F, et al. Helicobacter pylori infection and chronic immune thrombocytopenic purpura: long-term results of bacterium eradication and association with bacterium virulence profiles. Blood. 2007;110(12):3833–3841. doi: 10.1182/blood-2006-12-063222. [DOI] [PubMed] [Google Scholar]
- 7.Raghunath A, Hungin AP, Wooff D, Childs S. Prevalence of Helicobacter pylori in patients with gastro-oesophageal reflux disease: systematic review. BMJ. 2003;326(7392):737. doi: 10.1136/bmj.326.7392.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moayyedi P, Deeks J, Talley NJ, Delaney B, Forman D. An update of the Cochrane systematic review of Helicobacter pylori eradication therapy in nonulcer dyspepsia: resolving the discrepancy between systematic reviews. Am J Gastroenterol. 2003;98(12):2621–2626. doi: 10.1111/j.1572-0241.2003.08724.x. [DOI] [PubMed] [Google Scholar]
- 9.Pounder RE, Ng D. The prevalence of Helicobacter pylori infection in different countries. Aliment Pharmacol Ther. 1995;9(Suppl 2):33–39. [PubMed] [Google Scholar]
- 10.Everhart JE. Recent developments in the epidemiology of Helicobacter pylori. Gastroenterol Clin N Am. 2000;29(3):559–578. doi: 10.1016/S0889-8553(05)70130-8. [DOI] [PubMed] [Google Scholar]
- 11.Grad YH, Lipsitch M, Aiello AE. Secular trends in Helicobacter pylori seroprevalence in adults in the United States: evidence for sustained race/ethnic disparities. Am J Epidemiol. 2012;175(1):54–59. doi: 10.1093/aje/kwr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh JM, Hur C, Schrag D, Kuntz KM, Ezzati M, Stout N, et al. Contribution of H. pylori and smoking trends to US incidence of intestinal-type noncardia gastric adenocarcinoma: a microsimulation model. PLoS Med. 2013;10(5):e1001451. doi: 10.1371/journal.pmed.1001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fock KM, Ang TL. Epidemiology of Helicobacter pylori infection and gastric cancer in Asia. J Gastroenterol Hepatol. 2010;25(3):479–486. doi: 10.1111/j.1440-1746.2009.06188.x. [DOI] [PubMed] [Google Scholar]
- 14.Tsai CJ, Perry S, Sanchez L, Parsonnet J. Helicobacter pylori infection in different generations of Hispanics in the San Francisco Bay Area. Am J Epidemiol. 2005;162(4):351–357. doi: 10.1093/aje/kwi207. [DOI] [PubMed] [Google Scholar]
- 15.Talley NJ. American Gastroenterological Association medical position statement: evaluation of dyspepsia. Gastroenterology. 2005;129(5):1753–1755. doi: 10.1053/j.gastro.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Hansen JM, Bytzer P.Value of the unaided clinical diagnosis in dyspeptic patients in primary care. Am J Gastroenterol. 2001;96(5):1417–21. [DOI] [PubMed]
- 17.Hammer J, Eslick GD, Howell SC, Altiparmak E, Talley NJ. Diagnostic yield of alarm features in irritable bowel syndrome and functional dyspepsia. Gut. 2004;53(5):666–672. doi: 10.1136/gut.2003.021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heikkinen M, Pikkarainen P, Takala J, Rasanen H, Julkunen R. Etiology of dyspepsia: four hundred unselected consecutive patients in general practice. Scand J Gastroenterol. 1995;30(6):519–523. doi: 10.3109/00365529509089783. [DOI] [PubMed] [Google Scholar]
- 19.Chiba N, Van Zanten SJ, Sinclair P, Ferguson RA, Escobedo S, Grace E. Treating Helicobacter pylori infection in primary care patients with uninvestigated dyspepsia: the Canadian adult dyspepsia empiric treatment-Helicobacter pylori positive (CADET-Hp) randomised controlled trial. BMJ (Clin Res ed) 2002;324(7344):1012–1016. doi: 10.1136/bmj.324.7344.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: a meta-analysis. Lancet. 2002;359(9300):14–22. doi: 10.1016/S0140-6736(02)07273-2. [DOI] [PubMed] [Google Scholar]
- 21.Leodolter A, Kulig M, Brasch H, Meyer-Sabellek W, Willich SN, Malfertheiner P. A meta-analysis comparing eradication, healing and relapse rates in patients with Helicobacter pylori-associated gastric or duodenal ulcer. Aliment Pharmacol Ther. 2001;15(12):1949–1958. doi: 10.1046/j.1365-2036.2001.01109.x. [DOI] [PubMed] [Google Scholar]
- 22.Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, et al. Management of Helicobacter pylori infection--the Maastricht IV/Florence Consensus Report. Gut. 2012;61(5):646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 23.Chan FK, Ching JY, Suen BY, Tse YK, Wu JC, Sung JJ. Effects of Helicobacter pylori infection on long-term risk of peptic ulcer bleeding in low-dose aspirin users. Gastroenterology. 2013;144(3):528–535. doi: 10.1053/j.gastro.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 24.Loy CT, Irwig LM, Katelaris PH, Talley NJ. Do commercial serological kits for Helicobacter pylori infection differ in accuracy? A meta-analysis. Am J Gastroenterol. 1996;91(6):1138–1144. [PubMed] [Google Scholar]
- 25.Nurgalieva ZZ, Graham DY. Pearls and pitfalls of assessing Helicobacter pylori status. Dig Liver Dis. 2003;35(6):375–377. doi: 10.1016/S1590-8658(03)00166-X. [DOI] [PubMed] [Google Scholar]
- 26.Chey WD. Accurate diagnosis of Helicobacter pylori. 14C-urea breath test. Gastroenterol Clin N Am. 2000;29(4):895–902. doi: 10.1016/S0889-8553(05)70157-6. [DOI] [PubMed] [Google Scholar]
- 27.Peura DA, Pambianco DJ, Dye KR, Lind C, Frierson HF, Hoffman SR, et al. Microdose 14C-urea breath test offers diagnosis of Helicobacter pylori in 10 minutes. Am J Gastroenterol. 1996;91(2):233–238. [PubMed] [Google Scholar]
- 28.Chey WD, Wong BC. Practice parameters committee of the American College of G. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102(8):1808–1825. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 29.Chey WD, Woods M, Scheiman JM, Nostrant TT, DelValle J. Lansoprazole and ranitidine affect the accuracy of the 14C-urea breath test by a pH-dependent mechanism. Am J Gastroenterol. 1997;92(3):446–450. [PubMed] [Google Scholar]
- 30.Stermer E, Levy N, Tabak M, Neeman I. Lanzoprazole and ranitidine affect the accuracy of the 14C-urea breath test by a pH-dependent mechanism. Am J Gastroenterol. 1997;92(9):1575–1576. [PubMed] [Google Scholar]
- 31.Gisbert JP, Pajares JM. Stool antigen test for the diagnosis of Helicobacter pylori infection: a systematic review. Helicobacter. 2004;9(4):347–368. doi: 10.1111/j.1083-4389.2004.00235.x. [DOI] [PubMed] [Google Scholar]
- 32.Bravo LE, Realpe JL, Campo C, Mera R, Correa P. Effects of acid suppression and bismuth medications on the performance of diagnostic tests for Helicobacter pylori infection. Am J Gastroenterol. 1999;94(9):2380–2383. doi: 10.1111/j.1572-0241.1999.01361.x. [DOI] [PubMed] [Google Scholar]
- 33.Hansen S, Melby KK, Aase S, Jellum E, Vollset SE. Helicobacter pylori infection and risk of cardia cancer and non-cardia gastric cancer. A nested case–control study. Scand J Gastroenterol. 1999;34(4):353–360. doi: 10.1080/003655299750026353. [DOI] [PubMed] [Google Scholar]
- 34.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 35.International Agency for Research on Cancer. Schistosomes, liver flukes and Helicobacter pylori. IARC working group on the evaluation of carcinogenic risks to humans. Lyon, 7–14 June 1994. IARC monographs on the evaluation of carcinogenic risks to humans/World Health Organization, International Agency for Research on Cancer. 1994;61:1–241. [PMC free article] [PubMed]
- 36.Brenner H, Arndt V, Stegmaier C, Ziegler H, Rothenbacher D. Is Helicobacter pylori infection a necessary condition for noncardia gastric cancer? Am J Epidemiol. 2004;159(3):252–258. doi: 10.1093/aje/kwh039. [DOI] [PubMed] [Google Scholar]
- 37.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process–First American cancer society award lecture on cancer epidemiology and prevention. Cancer Res. 1992;52(24):6735–6740. [PubMed] [Google Scholar]
- 38.Kuipers EJ, Uyterlinde AM, Pena AS, Roosendaal R, Pals G, Nelis GF, et al. Long-term sequelae of Helicobacter pylori gastritis. Lancet. 1995;345(8964):1525–1528. doi: 10.1016/S0140-6736(95)91084-0. [DOI] [PubMed] [Google Scholar]
- 39.Yakirevich E, Resnick MB. Pathology of gastric cancer and its precursor lesions. Gastroenterol Clin North Am. 2013;42(2):261–284. doi: 10.1016/j.gtc.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Investigat. 2007;117(1):60–69. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404(6776):398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 42.El-Omar EM, Oien K, Murray LS, El-Nujumi A, Wirz A, Gillen D, et al. Increased prevalence of precancerous changes in relatives of gastric cancer patients: critical role of H. pylori. Gastroenterology. 2000;118(1):22–30. doi: 10.1016/S0016-5085(00)70410-0. [DOI] [PubMed] [Google Scholar]
- 43.Joossens JV, Hill MJ, Elliott P, Stamler R, Lesaffre E, Dyer A, et al. Dietary salt, nitrate and stomach cancer mortality in 24 countries. European Cancer Prevention (ECP) and the INTERSALT Cooperative Research Group. Int J Epidemiol. 1996;25(3):494–504. doi: 10.1093/ije/25.3.494. [DOI] [PubMed] [Google Scholar]
- 44.Chen MJ, Wu DC, Ko YC, Chiou YY. Personal history and family history as a predictor of gastric cardiac adenocarcinoma risk: a case–control study in Taiwan. Am J Gastroenterol. 2004;99(7):1250–1257. doi: 10.1111/j.1572-0241.2004.30872.x. [DOI] [PubMed] [Google Scholar]
- 45.Lau M, Le A, El-Serag HB. Noncardia gastric adenocarcinoma remains an important and deadly cancer in the United States: secular trends in incidence and survival. Am J Gastroenterol. 2006;101(11):2485–2492. doi: 10.1111/j.1572-0241.2006.00778.x. [DOI] [PubMed] [Google Scholar]
- 46.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer. J Int du Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 47.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 48.Talley NJ, Fock KM, Moayyedi P. Gastric Cancer Consensus conference recommends Helicobacter pylori screening and treatment in asymptomatic persons from high-risk populations to prevent gastric cancer. Am J Gastroenterol. 2008;103(3):510–514. doi: 10.1111/j.1572-0241.2008.01819.x. [DOI] [PubMed] [Google Scholar]
- 49.Howe HL, Wu X, Ries LA, Cokkinides V, Ahmed F, Jemal A, et al. Annual report to the nation on the status of cancer, 1975–2003, featuring cancer among U.S. Hispanic/Latino populations. Cancer. 2006;107(8):1711–1742. doi: 10.1002/cncr.22193. [DOI] [PubMed] [Google Scholar]
- 50.Hirota WK, Zuckerman MJ, Adler DG, Davila RE, Egan J, Leighton JA, et al. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63(4):570–580. doi: 10.1016/j.gie.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 51.Uemura N, Mukai T, Okamoto S, Yamaguchi S, Mashiba H, Taniyama K, et al. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiol Biomark Prev. 1997;6(8):639–642. [PubMed] [Google Scholar]
- 52.Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372(9636):392–397. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 53.Kato M, Asaka M. Recent knowledge of the relationship between Helicobacter pylori and gastric cancer and recent progress of gastroendoscopic diagnosis and treatment for gastric cancer. Jpn J Clin Oncol. 2010;40(9):828–837. doi: 10.1093/jjco/hyq119. [DOI] [PubMed] [Google Scholar]
- 54.Rokkas T, Sechopoulos P, Robotis I, Margantinis G, Pistiolas D. Cumulative H. pylori eradication rates in clinical practice by adopting first and second-line regimens proposed by the Maastricht III consensus and a third-line empirical regimen. Am J Gastroenterol. 2009;104(1):21–25. doi: 10.1038/ajg.2008.87. [DOI] [PubMed] [Google Scholar]
- 55.Zhou L, Sung JJ, Lin S, Jin Z, Ding S, Huang X, et al. A five-year follow-up study on the pathological changes of gastric mucosa after H. pylori eradication. Chin Med J. 2003;116(1):11–14. [PubMed] [Google Scholar]
- 56.Fuccio L, Zagari RM, Eusebi LH, Laterza L, Cennamo V, Ceroni L, et al. Meta-analysis: can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med. 2009;151(2):121–128. doi: 10.7326/0003-4819-151-2-200907210-00009. [DOI] [PubMed] [Google Scholar]
- 57.Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ, et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009;24(10):1587–1600. doi: 10.1111/j.1440-1746.2009.05982.x. [DOI] [PubMed] [Google Scholar]
- 58.Fock KM, Talley N, Moayyedi P, Hunt R, Azuma T, Sugano K, et al. Asia-Pacific consensus guidelines on gastric cancer prevention. J Gastroenterol Hepatol. 2008;23(3):351–365. doi: 10.1111/j.1440-1746.2008.05314.x. [DOI] [PubMed] [Google Scholar]
- 59.Malfertheiner P, Sipponen P, Naumann M, Moayyedi P, Megraud F, Xiao SD, et al. Helicobacter pylori eradication has the potential to prevent gastric cancer: a state-of-the-art critique. Am J Gastroenterol. 2005;100(9):2100–2115. doi: 10.1111/j.1572-0241.2005.41688.x. [DOI] [PubMed] [Google Scholar]
- 60.Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007;12(4):275–278. doi: 10.1111/j.1523-5378.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 61.Katelaris PH, Forbes GM, Talley NJ, Crotty B. A randomized comparison of quadruple and triple therapies for Helicobacter pylori eradication: The QUADRATE Study. Gastroenterology. 2002;123(6):1763–1769. doi: 10.1053/gast.2002.37051. [DOI] [PubMed] [Google Scholar]
- 62.Luther J, Higgins PD, Schoenfeld PS, Moayyedi P, Vakil N, Chey WD. Empiric quadruple vs. triple therapy for primary treatment of Helicobacter pylori infection: Systematic review and meta-analysis of efficacy and tolerability. Am J Gastroenterol. 2010;105(1):65–73. doi: 10.1038/ajg.2009.508. [DOI] [PubMed] [Google Scholar]
- 63.Gene E, Calvet X, Azagra R, Gisbert JP. Triple vs quadruple therapy for treating Helicobacter pylori infection: an updated meta-analysis. Aliment Pharmacol Ther. 2003;18(5):543–544. doi: 10.1046/j.1365-2036.2003.t01-1-01712.x. [DOI] [PubMed] [Google Scholar]
- 64.Osato MS, Reddy R, Reddy SG, Penland RL, Malaty HM, Graham DY. Pattern of primary resistance of Helicobacter pylori to metronidazole or clarithromycin in the United States. Arch Intern Med. 2001;161(9):1217–1220. doi: 10.1001/archinte.161.9.1217. [DOI] [PubMed] [Google Scholar]
- 65.McMahon BJ, Hennessy TW, Bensler JM, Bruden DL, Parkinson AJ, Morris JM, et al. The relationship among previous antimicrobial use, antimicrobial resistance, and treatment outcomes for Helicobacter pylori infections. Ann Intern Med. 2003;139(6):463–469. doi: 10.7326/0003-4819-139-6-200309160-00008. [DOI] [PubMed] [Google Scholar]
- 66.Fischbach LA, Goodman KJ, Feldman M, Aragaki C. Sources of variation of Helicobacter pylori treatment success in adults worldwide: a meta-analysis. Int J Epidemiol. 2002;31(1):128–139. doi: 10.1093/ije/31.1.128. [DOI] [PubMed] [Google Scholar]
- 67.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59(8):1143–1153. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 68.Vaira D, Zullo A, Vakil N, Gatta L, Ricci C, Perna F, et al. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a randomized trial. Ann Intern Med. 2007;146(8):556–563. doi: 10.7326/0003-4819-146-8-200704170-00006. [DOI] [PubMed] [Google Scholar]
- 69.Liou JM, Chen CC, Chen MJ, Chen CC, Chang CY, Fang YJ, et al. Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet. 2013;381(9862):205–213. doi: 10.1016/S0140-6736(12)61579-7. [DOI] [PubMed] [Google Scholar]
- 70.Hojo M, Miwa H, Nagahara A, Sato N. Pooled analysis on the efficacy of the second-line treatment regimens for Helicobacter pylori infection. Scand J Gastroenterol. 2001;36(7):690–700. doi: 10.1080/003655201300191941. [DOI] [PubMed] [Google Scholar]
- 71.Gisbert JP, Gisbert JL, Marcos S, Jimenez-Alonso I, Moreno-Otero R, Pajares JM. Empirical rescue therapy after Helicobacter pylori treatment failure: a 10-year single-centre study of 500 patients. Aliment Pharmacol Ther. 2008;27(4):346–354. doi: 10.1111/j.1365-2036.2007.03573.x. [DOI] [PubMed] [Google Scholar]
- 72.Basu PP, Rayapudi K, Pacana T, Shah NJ, Krishnaswamy N, Flynn M. A randomized study comparing levofloxacin, omeprazole, nitazoxanide, and doxycycline versus triple therapy for the eradication of Helicobacter pylori. Am J Gastroenterol. 2011;106(11):1970–1975. doi: 10.1038/ajg.2011.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Romano M, Cuomo A, Gravina AG, Miranda A, Iovene MR, Tiso A, et al. Empirical levofloxacin-containing versus clarithromycin-containing sequential therapy for Helicobacter pylori eradication: a randomised trial. Gut. 2010;59(11):1465–1470. doi: 10.1136/gut.2010.215350. [DOI] [PubMed] [Google Scholar]
- 74.Saad RJ, Schoenfeld P, Kim HM, Chey WD. Levofloxacin-based triple therapy versus bismuth-based quadruple therapy for persistent Helicobacter pylori infection: a meta-analysis. Am J Gastroenterol. 2006;101(3):488–496. doi: 10.1111/j.1572-0241.2006.00637.x. [DOI] [PubMed] [Google Scholar]
- 75.Tay CY, Windsor HM, Thirriot F, Lu W, Conway C, Perkins TT, et al. Helicobacter pylori eradication in Western Australia using novel quadruple therapy combinations. Aliment Pharmacol Ther. 2012;36(11–12):1076–1083. doi: 10.1111/apt.12089. [DOI] [PubMed] [Google Scholar]
- 76.Yun SP, Seon HG, Ok CS, Yoo KH, Kang MK, Kim WH, et al. Rifaximin plus levofloxacin-based rescue regimen for the eradication of Helicobacter pylori. Gut Liver. 2012;6(4):452–456. doi: 10.5009/gnl.2012.6.4.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hopkins RJ, Girardi LS, Turney EA. Relationship between Helicobacter pylori eradication and reduced duodenal and gastric ulcer recurrence: a review. Gastroenterology. 1996;110(4):1244–1252. doi: 10.1053/gast.1996.v110.pm8613015. [DOI] [PubMed] [Google Scholar]
- 78.Lee M, Kemp JA, Canning A, Egan C, Tataronis G, Farraye FA. A randomized controlled trial of an enhanced patient compliance program for Helicobacter pylori therapy. Arch Intern Med. 1999;159(19):2312–2316. doi: 10.1001/archinte.159.19.2312. [DOI] [PubMed] [Google Scholar]
- 79.Graham DY, Lew GM, Malaty HM, Evans DG, Evans DJ, Jr, Klein PD, et al. Factors influencing the eradication of Helicobacter pylori with triple therapy. Gastroenterology. 1992;102(2):493–496. doi: 10.1016/0016-5085(92)90095-g. [DOI] [PubMed] [Google Scholar]
- 80.Morgan DR, Torres J, Sexton R, Herrero R, Salazar-Martinez E, Greenberg ER, et al. Risk of recurrent Helicobacter pylori infection 1 year after initial eradication therapy in 7 Latin American communities. JAMA. 2013;309(6):578–586. doi: 10.1001/jama.2013.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sonnenberg A, Everhart JE. Health impact of peptic ulcer in the United States. Am J Gastroenterol. 1997;92(4):614–620. [PubMed] [Google Scholar]
- 82.Sonnenberg A, Schwartz JS, Cutler AF, Vakil N, Bloom BS. Cost savings in duodenal ulcer therapy through Helicobacter pylori eradication compared with conventional therapies: results of a randomized, double-blind, multicenter trial. Gastrointestinal Utilization Trial Study Group. Arch Intern Med. 1998;158(8):852–860. doi: 10.1001/archinte.158.8.852. [DOI] [PubMed] [Google Scholar]
- 83.Mapel D, Roberts M, Overhiser A, Mason A. The epidemiology, diagnosis, and cost of dyspepsia and Helicobacter pylori gastritis: a case–control analysis in the Southwestern United States. Helicobacter. 2013;18(1):54–65. doi: 10.1111/j.1523-5378.2012.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mason J, Axon AT, Forman D, Duffett S, Drummond M, Crocombe W, et al. The cost-effectiveness of population Helicobacter pylori screening and treatment: a Markov model using economic data from a randomized controlled trial. Aliment Pharmacol Ther. 2002;16(3):559–568. doi: 10.1046/j.1365-2036.2002.01204.x. [DOI] [PubMed] [Google Scholar]
- 85.Ford AC, Forman D, Bailey AG, Axon AT, Moayyedi P. A community screening program for Helicobacter pylori saves money: 10-year follow-up of a randomized controlled trial. Gastroenterology. 2005;129(6):1910–1917. doi: 10.1053/j.gastro.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 86.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology. 2009;136(2):376–386. doi: 10.1053/j.gastro.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 87.Areia M, Carvalho R, Cadime AT, Rocha Goncalves F, Dinis-Ribeiro M. Screening for gastric cancer and surveillance of premalignant lesions: a systematic review of cost-effectiveness studies. Helicobacter. 2013 doi: 10.1111/hel.12050. [DOI] [PubMed] [Google Scholar]
- 88.Leivo T, Salomaa A, Kosunen TU, Tuominen R, Farkkila M, Linna M, et al. Cost-benefit analysis of Helicobacter pylori screening. Health policy. 2004;70(1):85–96. doi: 10.1016/j.healthpol.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 89.Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26(3):343–357. doi: 10.1111/j.1365-2036.2007.03386.x. [DOI] [PubMed] [Google Scholar]
- 90.Zullo A, De Francesco V, Hassan C, Morini S, Vaira D. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut. 2007;56(10):1353–1357. doi: 10.1136/gut.2007.125658. [DOI] [PMC free article] [PubMed] [Google Scholar]