Highlights
-
•
We present a case of cervical large cell neuroendocrine carcinoma (LCNEC) and neoadjuvant chemotherapy with irinotecan plus cisplatin that was extremely effective.
-
•
Cervical LCNEC is uncommon and highly aggressive, and optimal therapy has yet to be determined.
-
•
This case suggests that neoadjuvant chemotherapy followed by radical hysterectomy could be a useful treatment option for bulky cervical LCNEC.
Keywords: Uterine cervix, Bulky cervical cancer, Neuroendocrine carcinoma, Neoadjuvant chemotherapy, Radical hysterectomy
Introduction
Large cell neuroendocrine carcinoma (LCNEC) is an uncommon histologic subtype of cervical cancer with a prevalence of approximately 0.087% to 0.9% (Wang et al., 2009), and is an extremely aggressive tumor with very poor prognosis. Knowledge of its distinct cytological and histological features is necessary for early diagnosis and provision of appropriate therapies. Multimodal treatments including surgery, chemotherapy and radiotherapy are needed. However, because of the low incidence, optimal therapy has yet to be determined, although neoadjuvant chemotherapy (NAC) appears useful as a therapeutic tool that may increase the resectability of these tumors.
Recently, the efficacy of chemotherapy with irinotecan hydrochloride, a topoisomerase I inhibitor, and cisplatin against metastatic small cell lung cancer and cervical LCNEC after non-curative surgery was reported (Noda et al., 2002; Tanimoto et al., 2012). NAC with irinotecan plus nedaplatin was reported to be effective for bulky cervical squamous cell carcinoma (SCC) (Yamaguchi et al., 2012). These reports led us to select NAC with irinotecan plus cisplatin followed by radical hysterectomy for bulky tumor of cervical LCNEC. This report describes the clinicopathological features of a case of cervical LCNEC showing marked therapeutic efficacy with NAC using irinotecan plus cisplatin.
Case report
A 51-year-old woman, gravida 3, para 3, presented with a history of vaginal bleeding over several months. She had no history of medical or surgical diseases. Gynecological examination revealed a bulky and protruding tumor of the cervix, with involvement of the anterior fornix of the vagina, but without parametrial invasion.
Magnetic resonance imaging (MRI) showed a 75 × 64 × 55-mm exophytic cervical mass with slight signal hyperintensity on T2-weighted imaging (T2WI), signal hyperintensity on diffusion-weighted imaging, slight enhancement after injection of gadolinium chelate, and evidence of invasion to the anterior fornix of the vagina, but no evidence of invasion to the parametrium (Fig. 1). Computed tomography (CT) and 18F-fluorodeoxyglucose positron emission tomography (FDG-PET)/CT showed no apparent lymph node involvement or metastatic disease. The tumor was classified as clinical stage IIA2 (FIGO 2008).
Fig. 1.
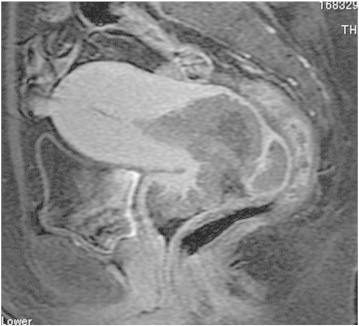
MRI at initial diagnosis shows an exophytic cervical mass with evidence of invasion to the anterior fornix of the vagina.
Levels of carcinoembryonic antigen (CEA) were elevated, but other tumor markers were within normal ranges: CEA, 13.0 ng/ml; carbohydrate antigen (CA) 125 12.5 U/ml; CA19-9, 11.82 U/ml; squamous cell carcinoma antigen (SCC), 0.7 ng/ml; neuron-specific enolase (NSE), 13.87 ng/ml; and progastrin releasing peptide (proGRP), 23.2 pg/ml. High-risk human papillomavirus (HPV) DNA testing yielded positive results by hybrid capture 2 assay.
Histological findings
Biopsy specimens showed a trabecular or solid growth pattern of the atypical cells, with extensive geographic necrosis. The neoplastic cells had hyperchromatic round to oval nuclei 3 to 5 times the size of lymphocytes and moderately eosinophilic cytoplasms. The tumor was mitotically active with a mitotic rate of more than 10 per 10 high-power fields (HPFs) (Fig. 2). Immunohistochemistry revealed moderately positive results for synaptophysin and focally positive results for chromogranin A. Consequently, this tumor was diagnosed as LCNEC. The tumor showed diffuse and strong immunoreactivity for p16, but was almost negative for p53 or CD56. HPV18 was identified by polymerase chain reaction (PCR).
Fig. 2.
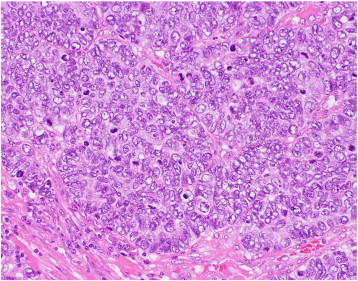
Histological findings of the biopsy specimen show tumor cells with hyperchromatic nuclei 3 to 5 times the size of lymphocytes, and a mitotic rate of more than 10 per 10 HPFs (H&E).
Treatments and follow-up
Previous studies have recommended chemotherapy than radiation therapy for LCNEC, because this tumor is more likely to have early distant hematogenous or lymphatic metastasis (Embry et al., 2011; Bermúdez et al., 2001). This bulky LCNEC led us to select chemotherapy prior to surgery for the purpose of complete resection. We selected combined therapy with irinotecan and cisplatin whose efficacy and safety for small cell lung cancer were reported by Noda et al. (2002). The patient underwent NAC with intravenous irinotecan (60 mg/m2; days 1, 8 and 15) and cisplatin (10 mg/m2; days 1–6). After two cycles of chemotherapy, MRI showed a marked reduction in tumor size, to 37 × 45 × 34 mm. Serum CEA level was also decreased to 0.7 ng/ml.
The patient then underwent radical hysterectomy, bilateral salpingo-oophorectomy and bilateral pelvic lymphadenectomy. Sectioned surfaces of the tumor were yellowish and necrotic (Fig. 3-a). Histologically, numerous foamy histiocytes were apparent in the tumor, probably attributable to chemotherapy-induced apoptosis of cancer cells. A few adenocarcinoma of the usual type coexisted with LCNEC, with stromal invasion to 10 mm in depth (Fig. 3-b, c). Involvements of the vaginal wall, parametrium and lymph nodes were not observed, but those of vascular and lymphatic spaces were observed. The surgical margins were negative. The postoperative diagnosis was LCNEC with adenocarcinoma, ypT1b1N0M0.
Fig. 3.
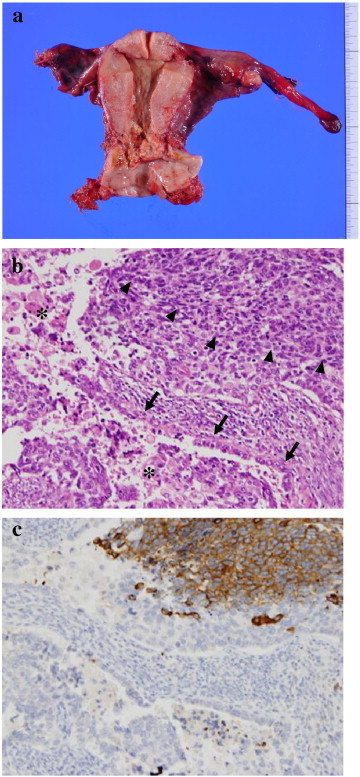
a) Macroscopic findings of the surgically removed uterus show a yellowish and necrotic tumor in the cervix. b) H&E. c) Immunohistochemical staining for synaptophysin. b) Histological findings of the surgical specimen show large cell neuroendocrine carcinoma (LCNEC) (arrow head) coexisting with a few adenocarcinoma of the usual type (arrow), and numerous histiocytes and apoptotic changes (asterisk). c) Tumor cells of LCNEC are positive for synaptophysin.
Postoperatively, the patient convalesced well and received four cycles of the same chemotherapy. The patient has received follow-up by pelvic examination, cytology and tumor marker at every two months, with CT scan at every six months. No evidence of recurrence has been detected as of 21 months postoperatively.
Discussion
Neuroendocrine tumors of the uterine cervix are classified into four categories: (typical) carcinoid; atypical carcinoid; LCNEC; and small cell carcinoma (SmCC) (Albores-Saavedra et al., 1997). LCNECs have frequently been histologically misdiagnosed, possibly due to the occasional coexistence of other histological types. Thirty-three percent of LCNECs were mixed type with SmCC, adenocarcinoma or SCC (Rekhi et al., 2013).
The growth of LCNEC shows an insular, trabecular or solid pattern, often with prominent peripheral palisading, rosette formation and geographical tumor necrosis. Tumor cells are large and display vesicular nuclei, prominent nucleoli, abundant cytoplasm, and eosinophilic intracytoplasmic granules. Mitoses are frequent (more than 10 per 10 HPFs). The diagnosis of LCNEC must be confirmed by an argyrophilic reaction or immunopositive results for chromogranin or synaptophysin (Albores-Saavedra et al., 1997; Gilks et al., 1997). However, positive rates of LCNEC were 87% for chromogranin, 56% for synaptophysin, and 88% for at least one of chromogranin, synaptophysin, CD56, or NSE (Rekhi et al., 2013).
LCNEC needs to be distinguished from atypical carcinoid, SmCC, poorly differentiated SCC and adenocarcinoma. LCNECs often contain a component of adenocarcinoma, SCC or SmCC (Rekhi et al., 2013); thus, an adequately sized biopsy is needed for accurate diagnosis. In terms of differential diagnosis, an atypical carcinoid shows a mitotic rate of less than 5 to 10 per 10 HPFs. SmCC shows small cells with scant cytoplasm. SmCC, poorly differentiated SCC and adenocarcinoma lack intracytoplasmic eosinophilic granules and conspicuous nucleoli.
In our case, HPV18 was detected by PCR, and intranuclear HPV signals were observed in both LCNEC and adenocarcinoma lesions by in situ hybridization (ISH). Overexpression of p16, which is regarded as a surrogate marker of HPV-induced cancer, was observed in both types of tumor cells. The presence of HPV has been demonstrated in most reported cases of LCNEC, ranging from 53% to 100% (Siriaunkgul et al., 2011), and associated with HPV16 and HPV18. Consequently, currently available HPV vaccines may enable us to eliminate this tumor.
Embry et al. reported median overall survival rates of patients with LCNEC as 19 months for stage I, 17 months for stage II, 3 months for stage III, and 1.5 months for stage IV, and 70% of all patients experienced disease relapses, with the most common sites of metastasis being the liver and lung (Embry et al., 2011). Higher rates of lymph node involvement of 40% to 86% have also been reported (Bermúdez et al., 2001). Despite multiple treatment modalities, 47% of patients with even stage I died of disease. Embry et al. confirmed that younger age, earlier FIGO stage, any surgery, specifically radical hysterectomy, chemotherapy at any point during initial treatment, or specifically platinum chemotherapy were associated with improved survival (Embry et al., 2011). Cervical neuroendocrine carcinoma can cause lymphatic or hematogenous metastases, even at an early stage, so chemotherapy is considered an extremely effective therapy (Bermúdez et al., 2001).
Bermúdez et al. suggested that a therapeutic strategy combining NAC, surgery and adjuvant chemotherapy could improve patient survival with cervical neuroendocrine carcinoma. In tumors larger than 4 cm in diameter, NAC was proposed for resectability, and consecutive surgery and postoperative chemotherapy were recommended, because distant recurrence is seen in 64% of cases. In tumors less than 4 cm in diameter, patients with unfavorable prognostic factors such as lymphovascular space involvement, perineural infiltration, or deep cervical invasion (> 10 mm) were recommended to receive adjuvant chemotherapy (Bermúdez et al., 2001).
The chemotherapy regimen including platinum has been considered most effective. Bermúdez et al. recommended BEP therapy (bleomycin, 15 mg/m2/day, days 1–3; etoposide, 100 mg/m2/day, days 1–3; platinum, 75 mg/m2/day, day 1, with 21-day intervals) (Bermúdez et al., 2001). Recently, Noda et al. have demonstrated that irinotecan plus cisplatin (irinotecan 60 mg/m2, days 1, 8, and 15; cisplatin 60 mg/m2, day 1, with 4-week cycles) was more effective than etoposide plus cisplatin for the treatment of metastatic small cell carcinoma of the lung (Noda et al., 2002). Tanimoto et al. reported the efficacy of postoperative irinotecan plus cisplatin (using the same regimen applied by Noda) for cervical LCNEC (Tanimoto et al., 2012).
In our case, two cycles of NAC using irinotecan plus cisplatin were extremely effective for bulky LCNEC tumor, and allowed complete resection by radical hysterectomy. Appearance of numerous bizarre giant cells with multiple nuclei or macronuclei on cytological examination after NAC, and the identification of numerous histiocytes and apoptotic changes on histological examination were considered as therapeutic effects.
This case suggests that NAC with irinotecan plus cisplatin followed by radical hysterectomy plus postoperative chemotherapy could be a useful treatment option for bulky tumor of cervical LCNEC. Further studies are necessary to obtain evidence of the most effective and suitable treatment modalities for cervical LCNEC.
Conflict of interest statement
The authors declare no conflict of interest.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Albores-Saavedra J., Gersell D., Gilks C.B., Henson D.E., Lindberg G., Santiago H. Terminology of endocrine tumors of the uterine cervix: results of a workshop sponsored by the College of American Pathologists and the National Cancer Institute. Arch. Pathol. Lab. Med. 1997;121:34–39. [PubMed] [Google Scholar]
- Bermúdez A., Vighi S., García A., Sardi J. Neuroendocrine cervical carcinoma: a diagnostic and therapeutic challenge. Gynecol. Oncol. 2001;82:32–39. doi: 10.1006/gyno.2001.6201. [DOI] [PubMed] [Google Scholar]
- Embry J.R., Kelly M.G., Post M.D., Spillman M.A. Large cell neuroendocrine carcinoma of the cervix: prognostic factors and survival advantage with platinum chemotherapy. Gynecol. Oncol. 2011;120:444–448. doi: 10.1016/j.ygyno.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Gilks C.B., Young R.H., Gersell D.J., Clement P.B. Large cell neuroendocrine carcinoma of the uterine cervix: a clinicopathologic study of 12 cases. Am. J. Surg. Pathol. 1997;21:905–914. doi: 10.1097/00000478-199708000-00004. [DOI] [PubMed] [Google Scholar]
- Noda K., Nishiwaki Y., Kawahara M., Negoro S., Sugiura T., Yokoyama A. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N. Engl. J. Med. 2002;346:85–91. doi: 10.1056/NEJMoa003034. [DOI] [PubMed] [Google Scholar]
- Rekhi B., Patil B., Deodhar K.K., Maheshwari A.A., Kerkar R., Gupta S. Spectrum of neuroendocrine carcinomas of the uterine cervix, including histopathologic features, terminology, immunohistochemical profile, and clinical outcomes in a series of 50 cases from a single institution in India. Ann. Diagn. Pathol. 2013;17:1–9. doi: 10.1016/j.anndiagpath.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Siriaunkgul S., Utaipat U., Settakorn J., Sukpan K., Srisomboon J., Khunamornpong S. HPV genotyping in neuroendocrine carcinoma of the uterine cervix in northern Thailand. Int. J. Gynaecol. Obstet. 2011;115:175–179. doi: 10.1016/j.ijgo.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Tanimoto H., Hamasaki A., Akimoto Y., Honda H., Takao Y., Okamoto K. A case of large cell neuroendocrine carcinoma (LCNEC) of the uterine cervix successfully treated by postoperative CPT-11 + CDDP chemotherapy after non-curative surgery. Gan To Kagaku Ryoho. 2012;39:1439–1441. [PubMed] [Google Scholar]
- Wang K.L., Wang T.Y., Huang Y.C., Lai J.C., Chang T.C., Yen M.S. Human papillomavirus type and clinical manifestation in seven cases of large-cell neuroendocrine cervical carcinoma. J. Formos. Med. Assoc. 2009;108:428–432. doi: 10.1016/S0929-6646(09)60088-7. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., Nishimura R., Yaegashi N., Kiguchi K., Sugiyama T., Kita T. Phase II study of neoadjuvant chemotherapy with irinotecan hydrochloride and nedaplatin followed by radical hysterectomy for bulky stage Ib2 to IIb, cervical squamous cell carcinoma: Japanese. Oncol. Rep. 2012;28:487–493. doi: 10.3892/or.2012.1814. [DOI] [PubMed] [Google Scholar]


