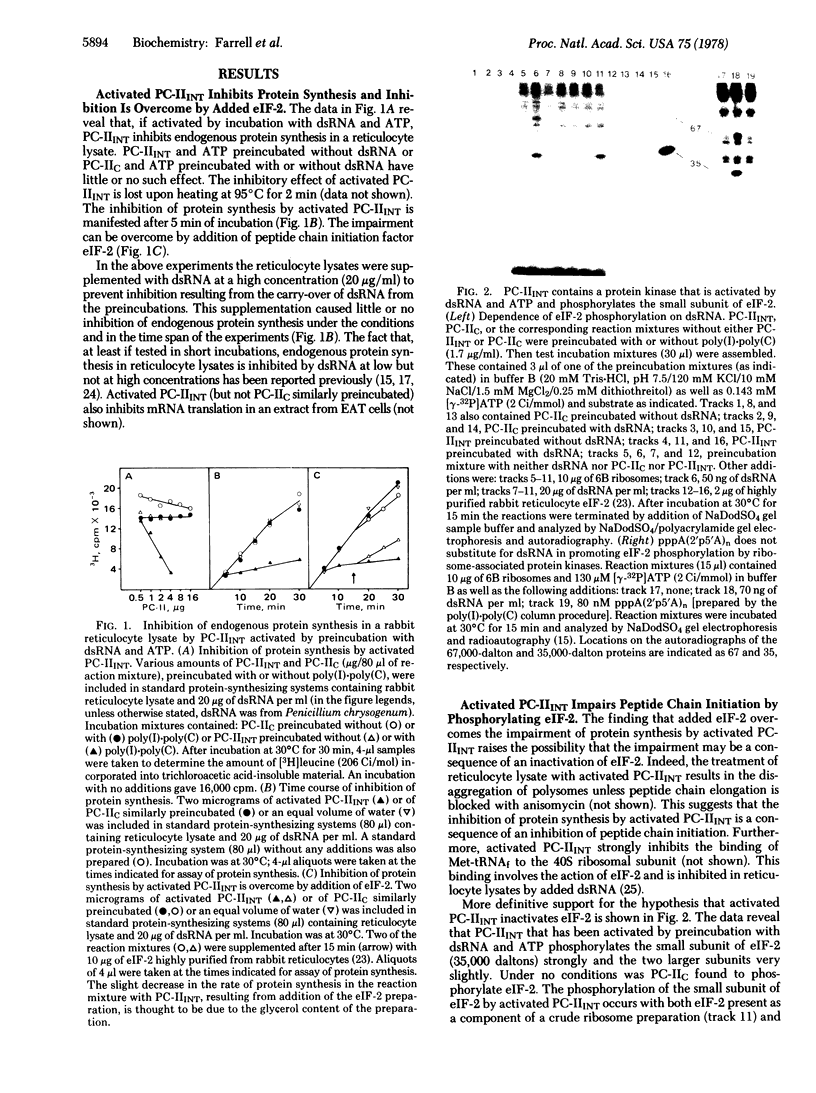
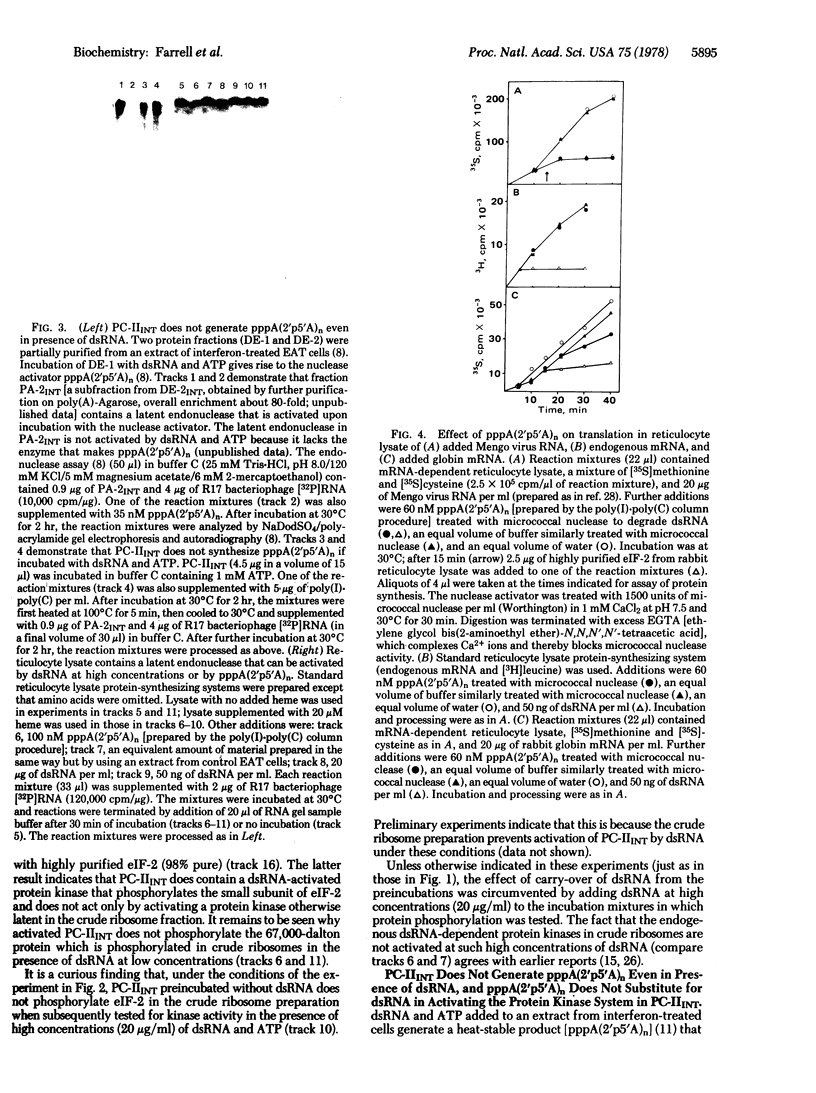
Abstract
Double-stranded RNA inhibits protein synthesis in at least two ways. It activates a protein kinase that blocks peptide chain initiation by phosphorylating the peptide chain initiation factor eIF-2 and also activates an endonuclease that inactivates different mRNAs at different rates. The protein kinase and the endonuclease have been partially purified from interferon-treated Ehrlich ascites tumor cells. The 2',5'-oligoadenylates [pppA(2'p5'A)n], found found earlier to be mediators in the activation of the endonuclease by double-stranded RNA, are not mediators in the activation of the protein kinase by double-stranded RNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baglioni C., Minks M. A., Maroney P. A. Interferon action may be mediated by activation of a nuclease by pppA2'p5'A2'p5'A. Nature. 1978 Jun 22;273(5664):684–687. doi: 10.1038/273684a0. [DOI] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Oligonucleotide inhibitor of protein synthesis made in extracts of interferon-treated chick embryo cells: comparison with the mouse low molecular weight inhibitor. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1167–1171. doi: 10.1073/pnas.75.3.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. E., Lebleu B., Kawakita M., Shaila S., Sen G. C., Lengyel P. Increased endonuclease activity in an extract from mouse Ehrlich ascites tumor cells which had been treated with a partially purified interferon preparation: dependence of double-stranded RNA;. Biochem Biophys Res Commun. 1976 Mar 8;69(1):114–122. doi: 10.1016/s0006-291x(76)80280-x. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., Williams B. R. Inhibition of cell-free protein synthesis by pppA2'p5'A2'p5'A: a novel oligonucleotide synthesized by interferon-treated L cell extracts. Cell. 1978 Mar;13(3):565–572. doi: 10.1016/0092-8674(78)90329-x. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Farrell P. J. Extracts of interferon-treated cells can inhibit reticulocyte lysate protein synthesis. Biochem Biophys Res Commun. 1977 Jul 11;77(1):124–131. doi: 10.1016/s0006-291x(77)80173-3. [DOI] [PubMed] [Google Scholar]
- Darnbrough C., Legon S., Hunt T., Jackson R. J. Initiation of protein synthesis: evidence for messenger RNA-independent binding of methionyl-transfer RNA to the 40 S ribosomal subunit. J Mol Biol. 1973 May 25;76(3):379–403. doi: 10.1016/0022-2836(73)90511-1. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Kerr I. M. Synthesis of an oligonucleotide inhibitor of protein synthesis in rabbit reticulocyte lysates analogous to that formed in extracts from interferon-treated cells. Eur J Biochem. 1978 Mar;84(1):149–159. doi: 10.1111/j.1432-1033.1978.tb12151.x. [DOI] [PubMed] [Google Scholar]
- Hunter T., Hunt T., Jackson R. J., Robertson H. D. The characteristics of inhibition of protein synthesis by double-stranded ribonucleic acid in reticulocyte lysates. J Biol Chem. 1975 Jan 25;250(2):409–417. [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E., Ball L. A. Increased sensitivity of cell-free protein synthesis to double-stranded RNA after interferon treatment. Nature. 1974 Jul 5;250(461):57–59. doi: 10.1038/250057a0. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E., Hovanessian A. G. Nature of inhibitor of cell-free protein synthesis formed in response to interferon and double-stranded RNA. Nature. 1977 Aug 11;268(5620):540–542. doi: 10.1038/268540a0. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E. pppA2'p5'A2'p5'A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):256–260. doi: 10.1073/pnas.75.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebleu B., Sen G. C., Shaila S., Cabrer B., Lengyel P. Interferon, double-stranded RNA, and protein phosphorylation. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3107–3111. doi: 10.1073/pnas.73.9.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz J. R., Baglioni C. Inhibition of protein synthesis by double-stranded RNA and phosphorylation of initiation factor, eIF-2. J Biol Chem. 1978 Jun 25;253(12):4219–4223. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ranu R. S., London I. M., Das A., Dasgupta A., Majumdar A., Ralston R., Roy R., Gupta N. K. Regulation of protein synthesis in rabbit reticulocyte lysates by the heme-regulated protein kinase: inhibition of interaction of Met-tRNAfMet binding factor with another initiation factor in formation of Met-tRNAfMet.40S ribosomal subunit complexes. Proc Natl Acad Sci U S A. 1978 Feb;75(2):745–749. doi: 10.1073/pnas.75.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Sen G. C., Brown G. E., Lebleu B., Kawakita M., Cabrer B., Slattery E., Lengyel P. Interferon, double-stranded RNA and RNA degradation. Characteristics of an endonuclease activity. Eur J Biochem. 1977 Oct 3;79(2):565–577. doi: 10.1111/j.1432-1033.1977.tb11841.x. [DOI] [PubMed] [Google Scholar]
- Ratner L., Wiegand R. C., Farrell P. J., Sen G. C., Cabrer B., Lengyel P. Interferon, double-stranded RNA and RNA degradation. Fractionation of the endonucleaseINT system into two macromolecular components; role of a small molecule in nuclease activation. Biochem Biophys Res Commun. 1978 Apr 14;81(3):947–954. doi: 10.1016/0006-291x(78)91443-2. [DOI] [PubMed] [Google Scholar]
- Roberts W. K., Hovanessian A., Brown R. E., Clemens M. J., Kerr I. M. Interferon-mediated protein kinase and low-molecular-weight inhibitor of protein synthesis. Nature. 1976 Dec 2;264(5585):477–480. doi: 10.1038/264477a0. [DOI] [PubMed] [Google Scholar]
- Safer B., Anderson W. F., Merrick W. C. Purification and physical properties of homogeneous initiation factor MP from rabbit reticulocytes. J Biol Chem. 1975 Dec 10;250(23):9067–9075. [PubMed] [Google Scholar]
- Sen G. C., Desrosiers R., Ratner L., Shaila S., Brown G. E., Lebleu B., Slattery E., Kawakita M., Cabrer B., Taira H. Messenger RNA methylation, translation and degradation in extracts of interferon-treated cells. Tex Rep Biol Med. 1977;35:221–229. [PubMed] [Google Scholar]
- Sen G. C., Gupta S. L., Brown G. E., Lebleu B., Rebello M. A., Lengyel P. Interferon treatment of Ehrlich ascites tumor cells: effects on exogenous mRNA translation and tRNA inactivation in the cell extract. J Virol. 1975 Jan;17(1):191–203. doi: 10.1128/jvi.17.1.191-203.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen G. C., Lebleu B., Brown G. E., Kawakita M., Slattery E., Lengyel P. Interferon, double-stranded RNA and mRNA degradation. Nature. 1976 Nov 25;264(5584):370–373. doi: 10.1038/264370a0. [DOI] [PubMed] [Google Scholar]
- Sen G. C., Taira H., Lengyel P. Interferon, double-stranded RNA, and protein phosphorylation. Characteristics of a double-stranded RNA-activated protein kinase system partially purified from interferon treated Ehrlich ascites tumor cells. J Biol Chem. 1978 Sep 10;253(17):5915–5921. [PubMed] [Google Scholar]
- Zilberstein A., Federman P., Shulman L., Revel M. Specific phosphorylation in vitro of a protein associated with ribosomes of interferon-treated mouse L cells. FEBS Lett. 1976 Sep 15;68(1):119–124. doi: 10.1016/0014-5793(76)80418-8. [DOI] [PubMed] [Google Scholar]
- de Haro C., Ochoa S. Mode of action of the hemin-controlled inhibitor of protein synthesis: studies with factors from rabbit reticulocytes. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2713–2716. doi: 10.1073/pnas.75.6.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]