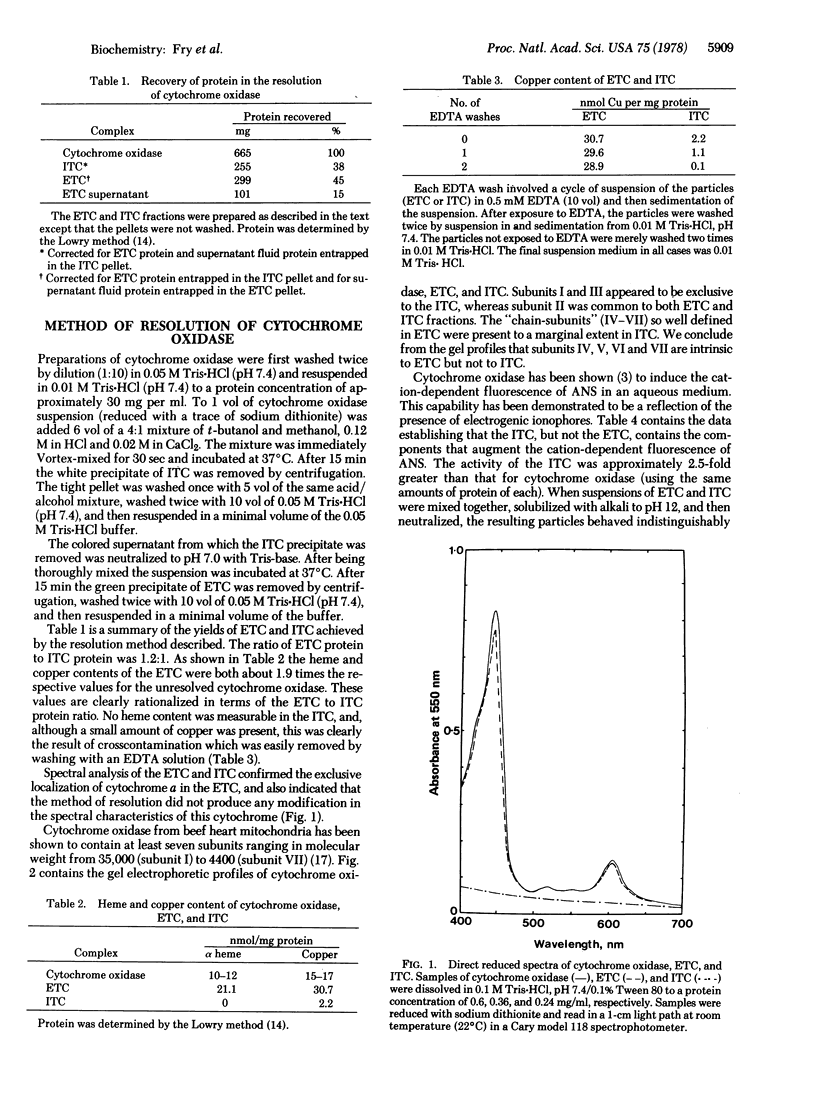
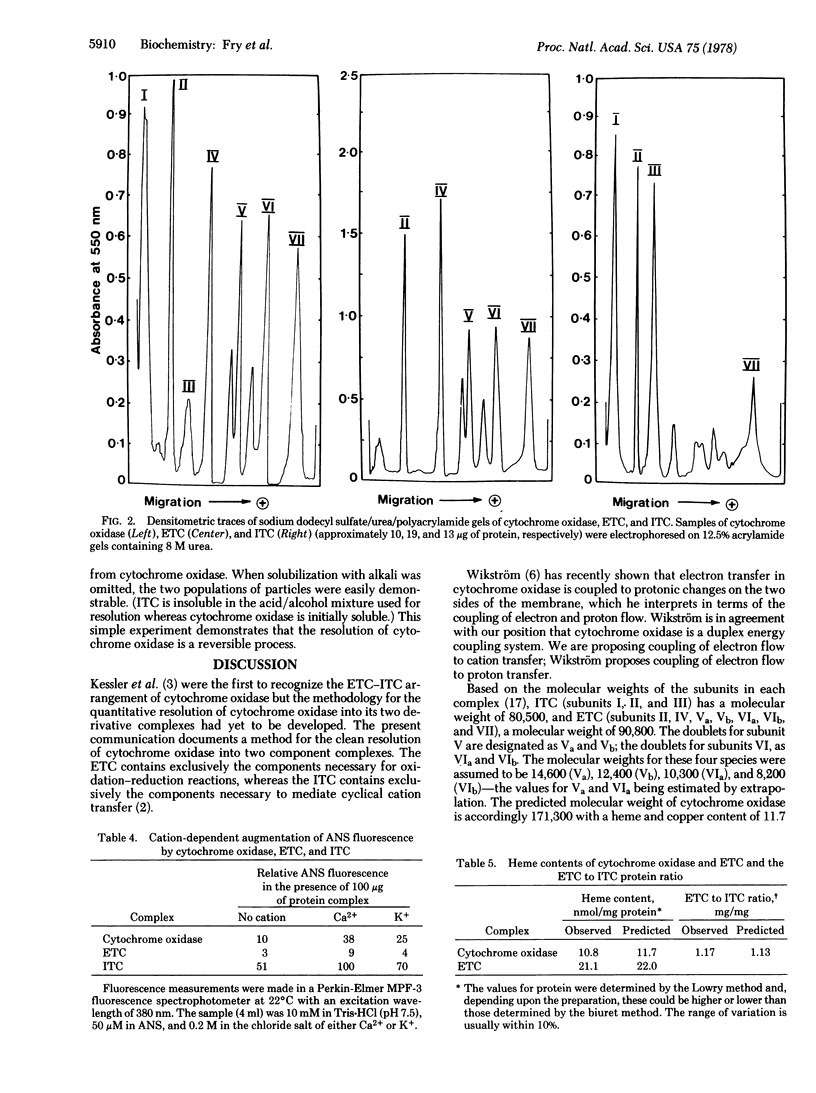
Abstract
Cytochrome c oxidase (ferrocytochrome c: oxygen oxidoreductase, EC 1.9.3.1) has been resolved into a pair of complexes of unequal molecular weight. The larger complex (electron transfer complex) contains exclusively the oxidation-reduction proteins characteristic of cytochrome oxidase; the smaller complex (ion transfer complex) shows exclusively the capability for cation-dependent induction of the fluorescence of 8-anilino-1-naphthalenesulfonic acid--a capability demonstrable in preparations of cytochrome oxidase. The duplex nature of cytochrome oxidase has important implications for the mechanism of energy coupling.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BASFORD R. E., TISDALE H. D., GLENN J. L., GREEN D. E. Studies on the terminal electron transport system. VII. Further studies on the succinic dehydrogenase complex. Biochim Biophys Acta. 1957 Apr;24(1):107–115. doi: 10.1016/0006-3002(57)90152-x. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Hayashi H. The polypeptide composition of cytochrome oxidase from beef heart mitochondria. FEBS Lett. 1972 Oct 1;26(1):261–263. doi: 10.1016/0014-5793(72)80587-8. [DOI] [PubMed] [Google Scholar]
- Downer N. W., Robinson N. C. Characterization of a seventh different subunit of beef heart cytochrome c oxidase. Similarities between the beef heart enzyme and that from other species. Biochemistry. 1976 Jun 29;15(13):2930–2936. doi: 10.1021/bi00658a036. [DOI] [PubMed] [Google Scholar]
- FELSENFELD G. The determination of cuprous ion in copper proteins. Arch Biochem Biophys. 1960 Apr;87:247–251. doi: 10.1016/0003-9861(60)90168-5. [DOI] [PubMed] [Google Scholar]
- FOWLER L. R., RICHARDSON S. H., HATEFI Y. A rapid method for the preparation of highly purified cytochrome oxidase. Biochim Biophys Acta. 1962 Oct 8;64:170–173. doi: 10.1016/0006-3002(62)90770-9. [DOI] [PubMed] [Google Scholar]
- Feinstein M. B., Felsenfeld H. The detection of ionophorous antibiotic-cation complexes in water with fluorescent probes. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2037–2041. doi: 10.1073/pnas.68.9.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry M., Green D. E. Resolution of complex III of the mitochondrial electron transfer chain into two component complexes. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5377–5380. doi: 10.1073/pnas.75.11.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. E., Blondin G. A. Molecular mechanism of mitochondrial energy coupling. Bioscience. 1978 Jan;28(1):18–24. [PubMed] [Google Scholar]
- Hinkle P. C., Kim J. J., Racker E. Ion transport and respiratory control in vesicles formed from cytochrome oxidase and phospholipids. J Biol Chem. 1972 Feb 25;247(4):1338–1339. [PubMed] [Google Scholar]
- Hunter D. R., Capaldi R. A. Respiratory control in cytochrome oxidase. Biochem Biophys Res Commun. 1974 Feb 4;56(3):623–628. doi: 10.1016/0006-291x(74)90650-0. [DOI] [PubMed] [Google Scholar]
- Hunter D. R., Haworth R. A., Southard J. H. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J Biol Chem. 1976 Aug 25;251(16):5069–5077. [PubMed] [Google Scholar]
- Kessler R. J., Blondin G. A., Vande Zander H., Haworth R. A., Green D. E. Coupling in cytochrome c oxidase. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3662–3666. doi: 10.1073/pnas.74.9.3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Phan S. H., Mahler H. R. Studies on cytochrome oxidase. Partial resolution of enzymes containing seven or six subunits, from yeast and beef heart, respectively. J Biol Chem. 1976 Jan 25;251(2):257–269. [PubMed] [Google Scholar]
- Phan S. H., Mahler H. R. Studies on cytochrome oxidase. Preliminary characterization of an enzyme containing only four subunits. J Biol Chem. 1976 Jan 25;251(2):270–276. [PubMed] [Google Scholar]
- RAWLINSON W. A., HALE J. H. Prosthetic groups of the cytochromes present in Corynebacterium diphtheriae with especial reference to cytochrome a. Biochem J. 1949;45(3):247-55, pl. doi: 10.1042/bj0450247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater E. C. Mechanism of oxidative phosphorylation. Annu Rev Biochem. 1977;46:1015–1026. doi: 10.1146/annurev.bi.46.070177.005055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]



