Abstract
Organisms living outside the tropics measure the changes in the length of the day to adapt to seasonal changes in the environment. Animals that breed during spring and summer are called long-day breeders, while those that breed during fall are called short-day breeders. Although the influence of thyroid hormone in the regulation of seasonal reproduction has been known for several decades, its precise mechanism remained unknown. Recent studies revealed that the activation of thyroid hormone within the mediobasal hypothalamus plays a key role in this phenomenon. This localized activation of the thyroid hormone is controlled by thyrotropin (thyroid-stimulating hormone) secreted from the pars tuberalis of the pituitary gland. Although seasonal reproduction is a rate-limiting factor in animal production, genes involved in photoperiodic signal transduction pathway could emerge as potential targets to facilitate domestication.
Keywords: seasonal reproduction, mediobasal hypothalamus, ependymal cell, pars tuberalis, thyrotropin, thyroid hormone, iodothyronine deiodinase
Introduction
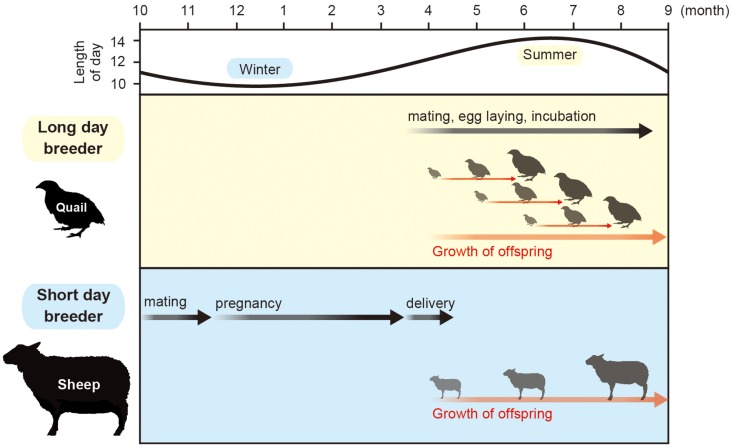
Orbiting of the earth around the sun causes changing seasons. To adapt to the seasonal changes in the environment, animals alter their physiology and behavior, which is characterized by the changes in growth, metabolism, immune function, reproductive activity, migration, hibernation, and molting. Most of the organisms use the changes in the length of the day (photoperiod) as a calendar, because temperature and precipitation varies throughout each year and are unreliable when compared with the length of the day. This phenomenon is called “photoperiodism” (1). Among the various seasonally regulated phenomena, the mechanism of seasonal reproduction has been extensively studied. Small mammals and birds breed during the spring and summer. Therefore, they are called long-day (LD) breeders. The gestation or incubation period of these animals last only a few weeks and their offspring are born during the spring and summer. In contrast, larger mammals, such as goats and sheep, breed during fall. Therefore, they are called short-day (SD) breeders. These animals have a gestation period of approximately 6 months. Therefore, their offspring are also born and raised during spring and summer. Accordingly, the offspring of both LD and SD breeders grow when the climate is moderate and food is abundant (Figure 1).
Figure 1.
Calendar of seasonal breeding animals. Most animals mate in a specific time of a year. Small animals with short gestation or incubation period mate in spring and summer, while large animals that have a 6-month gestation period mate in fall to give birth in spring.
Seasonal reproduction of vertebrate species is regulated by the hypothalamic–pituitary–gonadal (HPG) axis. The secretion of gonadotropin-releasing hormone (GnRH) from the hypothalamus induces the secretion of gonadotropins [luteinizing hormone (LH) and follicle-stimulating hormone (FSH)] from the anterior pituitary gland, which in turn activates gonadal activity. In other words, the HPG axis of seasonally breeding animals is only activated during the breeding season. Among the various vertebrate species, birds show the most dramatic changes in gonadal size (typically more than a 100-fold) (2). Therefore, birds have a highly sophisticated photoperiodic mechanism in comparison to other vertebrate species (3). In addition to the robust gonadal responses, most of the birds have very short breeding seasons, as the HPG axis is automatically switched off and their gonads start to regress even though the length of the day is still increasing. This phenomenon is known as photorefractoriness (4, 5). The length of the breeding season tends to be shorter in higher latitude due to the short benign season in higher latitude. Among mammals, hamsters and sheep are extensively studied, because they show dramatic photoperiodic responses. However, the magnitude of the seasonal gonadal development and regression is less robust in mammals than in birds, as their gonads change only by a few-folds.
Influence of Thyroid Hormone in the Seasonal Changes
It has been known for many decades that thyroid hormone is somehow involved in the regulation of seasonal reproductive function in various organisms including fish, birds, and mammals (2, 6, 7). In some species, thyroidectomy prevents the transition to reproductive state (i.e., seasonal testicular development and/or regression) (8–11), and thyroxine (T4) treatment mimics the effects of a long photoperiod (12–14). However, photo-stimulated gonadal maturation appears to have been largely unaffected by thyroidectomy in some species (2). Therefore, the reported effects of thyroidectomy on seasonal breeding are often contradictory and the role of T4 is thought to be permissive. Although the requirement of T4 for an appropriate response to photoperiod has been documented (15), the mechanism by which thyroid hormone regulates seasonal reproduction remained unknown for several decades.
Photoperiodic Changes in Type 2 and Type 3 Deiodinases Within the Hypothalamus
The Japanese quail (Coturnix japonica) is an excellent model for studying photoperiodism, because of its rapid and robust responses to changing photoperiods (3). Local illumination of the mediobasal hypothalamus (MBH) by radioluminous-painted beads induce testicular growth (16), and lesions of MBH blocks the photoperiodic response of LH secretion and gonadal development (17, 18). In addition, expression of c-Fos, a marker of neuronal activation, is induced in the MBH by LD stimulus (19). The MBH is therefore considered central for the seasonal reproduction in quail. By using differential subtractive hybridization analysis, LD-induction of type 2 deiodinase gene (DIO2) and LD-suppression of type 3 deiodinase gene (DIO3) were observed in the ependymal cells (also known as tanycytes) that line the ventrolateral walls of the third ventricle within the MBH [Ref. (20, 21), Figure 2]. DIO2 encodes the thyroid hormone-activating enzyme that converts the prohormone T4 to bioactive triiodothyronine (T3) (22), while DIO3 encodes thyroid hormone-inactivating enzyme that metabolizes T4 and T3 to inactive reverse T3 (rT3) and 3,3′-diiodothyronine (T2), respectively. The reciprocal switching of DIO2 and DIO3 appears to regulate the local thyroid hormone concentration precisely within the MBH. Moreover, T3 concentration within the MBH is about 10-fold higher under LD conditions than under SD conditions, even though plasma concentrations are similar to both photoperiods (20). The functional significance of this locally activated thyroid hormone has been demonstrated by pharmacological analyses. Intracerebroventricular (i.c.v.) infusion of T3 in SD conditions induced testicular development while infusion of a DIO2 inhibitor (iopanoic acid) in LD conditions attenuated testicular development (20). Photoperiodic regulation of DIO2 and/or DIO3 has also been confirmed in a number of other avian species, such as the tree sparrow (23), chicken (24), great tits (25), and canary (26). Similarly, photoperiodic regulation of thyroid hormone metabolism in the MBH has been confirmed in various mammalian species, including LD breeders like Siberian hamsters (27–30), Syrian hamsters (31, 32), rats (33, 34), mice (35), and SD-breeding goats (36) and sheep (37). Activation of thyroid hormone within the MBH decodes the LD information. Therefore, daily T3 subcutaneous injections induce testicular development (28) and chronic replacement of T3 in the hypothalamus prevents the onset of testicular regression (27) in LD-breeding Siberian hamsters. In contrast, in the SD breeders, LD-induced DIO2 appears to convert T4 to T3 to terminate the breeding season (37). In addition, LD stimulus induces the expression of DIO2, and T4 administration terminates the breeding season via a decrease in serum LH (38, 39).
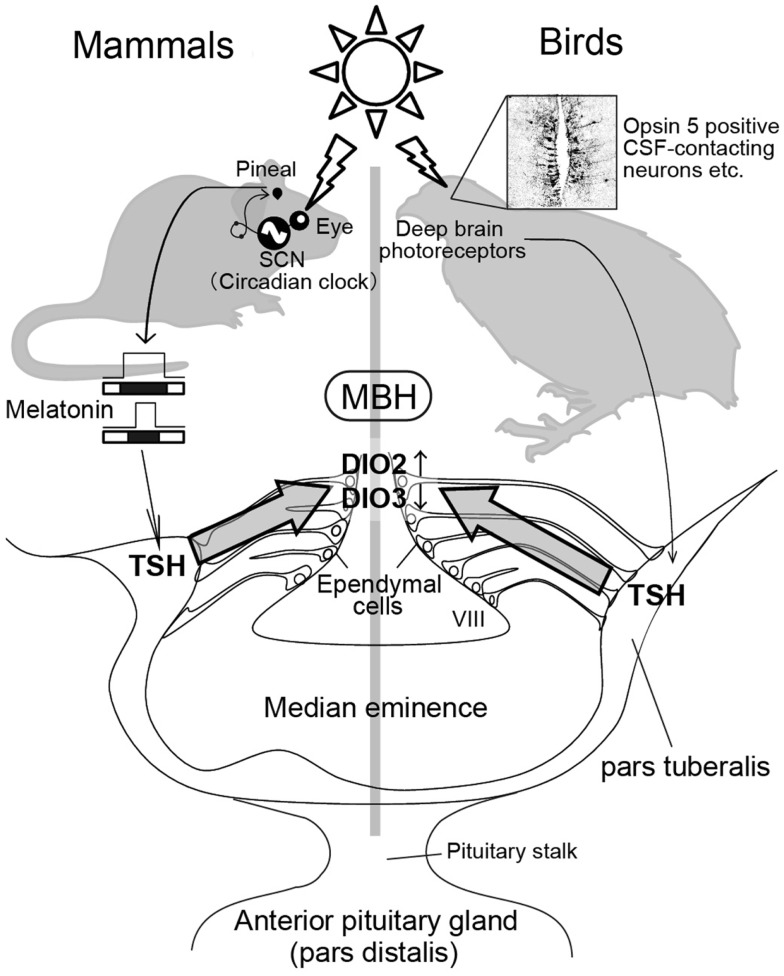
Figure 2.
Photoperiodic signal transduction pathway in mammals and birds. In mammals, light information is received by the eye and transmitted to the pineal gland via the circadian pacemaker, the suprachiasmatic nucleus (SCN). The duration of the pineal melatonin signal encodes the length of night and regulates TSH secretion in the pars tuberalis. The pars tuberalis TSH acts on TSH receptor expressed in the ependymal cells lining ventrolateral walls of the third ventricle (VIII) to induce DIO2 and reduce DIO3. Local thyroid hormone activation within the mediobasal hypothalamus (MBH) by DIO2/DIO3 switching plays a key role in the regulation of seasonal reproduction. In contrast, light information received by deep brain photoreceptors induces TSH secretion from the pars tuberalis in birds. Nevertheless, melatonin is not involved in the seasonal reproduction of birds. The schematic is a modified version of illustration published by Ikegami and Yoshimura (40).
Thyroid Hormone Transport to the Ependymal Cells
Due to their lipophilic nature, thyroid hormones are believed to traverse plasma membranes by passive diffusion. However, involvement of a membrane transport system for thyroid hormone has been reported recently and a mechanism that facilitates the transport of thyroid hormone into the ependymal cells was examined. Some members of the organic anion transporting polypeptide (Oatp) family have been shown to transport thyroid hormones in mammals (41, 42) and the involvement of a member of this family in transporting T4 into the quail brain has been investigated (43). Oatp1c1, which is expressed in the ependymal cells within the MBH, has been demonstrated to be a highly specific transporter of T4. In addition to Oatp1c1, another thyroid hormone transporter, monocarboxylate transporter 8 (MCT8), has been found in the ependymal cells within hamster MBH (29). Although MCT8 appears to be involved in the regulation of photoperiodism, its expression is upregulated under SD conditions, which does not require thyroid hormone.
Regulation of Hypothalamic Deiodinases by the Pars Tuberalis TSH
When quail are transferred from SD conditions to LD conditions, an increase in plasma gonadotropin (LH) is observed 22 h after the dawn of the first LD (3, 44, 45). As discussed previously, reciprocal switching of DIO2 and DIO3 plays a critical role in the regulation of seasonal reproduction in birds and mammals. In quail, the reciprocal switching of DIO2 and DIO3 precedes photoperiodic induction of gonadotropin release by roughly 4 h (21). Genome-wide gene expression analysis during the transition from SD conditions to LD conditions in Japanese quail (45) identified the induction of two genes 4 h prior to DIO2/DIO3 switching (i.e., 14 h after dawn) in the pars tuberalis of the pituitary gland. The pars tuberalis consists of thin layers of cells surrounding the median eminence (Figure 2). One of these genes encode the thyroid-stimulating hormone β subunit (TSHB) and the other encode the transcriptional co-activator eyes absent 3 (EYA3). Although EYA3 is a transcriptional co-activator, the expression sites of EYA3 and DIO2/DIO3 are different (i.e., EYA3 in the pars tuberalis and DIO2/DIO3 in the ependymal cells). Therefore, it appears that EYA3 is not involved in the regulation of DIO2/DIO3 switching. On the other hand, the expression of TSH receptor (TSHR) and binding of 125I-labeled thyroid-stimulating hormone (TSH) were observed in the ependymal cells where DIO2 and DIO3 are expressed. In addition to these, i.c.v. TSH administration induced DIO2 expression and reduced DIO3 expression in the ependymal cells even under SD conditions, while passive immunization against TSH attenuated LD-induction of DIO2 expression (45). The involvement of TSHR-Gsα-cAMP signaling pathway in this TSH regulation of DIO2 expression was demonstrated by the promoter analysis. Considering that the magnitude of testicular growth induced by i.c.v. TSH infusion was almost similar to that observed in birds exposed to LD stimulus, the LD-induced pars tuberalis TSH appears to be a major factor regulating the seasonal reproduction in birds.
In birds, eyes are not necessary for the regulation of seasonal reproduction because deep brain photoreceptors are involved in this process (46, 47). Although pineal organ is a photoreceptive organ in non-mammalian vertebrates (48, 49), pineal organ is not involved in the regulation of seasonal reproduction (50, 51). In contrast, local illumination of the septal region of the telencephalon or the MBH using radioluminous-painted beads caused testicular growth in quail, suggesting the existence of deep brain photoreceptors in these regions (16). Localization of several rhodopsin family proteins (rhodopsin; OPN4: melanopsin; OPN5: neuropsin and VA opsin: vertebrate ancient opsin) are reported in these brain regions and projections that link some of these photoreceptor cells to the pars tuberalis have also been reported (52–62). These photoreceptors are therefore thought to be involved in the seasonal regulation of reproduction in birds (Figure 2).
In a marked contrast to avian species, eyes are the only photoreceptive organ in mammalian species (63–69). Therefore, removal of the eyes abolishes the photoperiodic response (64, 68). Light information received by the eye is transmitted to the pineal gland through the suprachiasmatic nucleus (SCN), where the circadian pacemaker is localized (68, 70–74). The duration of night corresponds to the nocturnal secretion profile of melatonin, which plays a crucial role in the regulation of seasonal reproduction in mammalian species. For example, in both LD and SD breeders, pinealectomy abolishes seasonal responses, while melatonin administration restores them (68, 74, 75). Melatonin acts via melatonin receptors and there are two subtypes of melatonin receptors (MT1 and MT2) in mammals (76, 77). However, these melatonin receptors are not expressed in the ependymal cells where DIO2 and DIO3 are expressed (78, 79). The MT1 receptor is reportedly expressed in the thyrotroph cells of the pars tuberalis (80, 81). Therefore, pars tuberalis TSH likely mediates the influence of melatonin in the DIO2/DIO3 switching in mammalian species. Although it is generally considered that laboratory mice are non-seasonal breeders, many researchers noticed that mice do not breed well during the winter (e.g., small litter size) even though they are kept under standardized conditions. To determine whether pars tuberalis TSH mediates the influence of melatonin in the DIO2/DIO3 switching, laboratory mice were analyzed as experimental models. Two key enzymes, arylalkylamine N-acetyltransferase (AA-NAT) and hydroxyindole-O-methyltransferase (HIOMT) are involved in melatonin biosynthesis from serotonin (74). However, most inbred mice genetically lack the ability to produce these enzymes, resulting in minimal melatonin generation (82, 83). Therefore, it was predicted that melatonin-producing strains would have the capacity to respond to photoperiodic changes, while melatonin-deficient strains would be resilient to such changes. As expected, clear photoperiodic regulation of TSHB, DIO2, and DIO3 was observed in the melatonin-producing CBA strain, while such responses were not observed in the melatonin-deficient C57BL strain (35). In addition, daily intraperitoneal (i.p.) melatonin injections mimicked the effect of SD conditions on the expression of these genes (35). To test the involvement of the TSH–TSHR signaling pathway in the melatonin-mediated regulation of DIO2/DIO3 expression, the effects of melatonin administration were examined in TSHR-null mice (35). The TSHR-null mice failed to respond to melatonin administration. This result clearly suggested the involvement of a TSH–TSHR signaling pathway in the melatonin-mediated regulation of DIO2/DIO3 in mammals. In addition, the analysis of mice that lacked the MT1 and MT2 melatonin receptors revealed the involvement of MT1 melatonin receptors in this regulation (84). It is also interesting to note that TSH is involved in the LD-induction of DIO2 in SD-breeding sheep (37). Thus, pars tuberalis TSH appears to relay the seasonal information in both LD and SD-breeding animals and sensitize them for spring.
Thyroid Hormone Action within the Hypothalamus
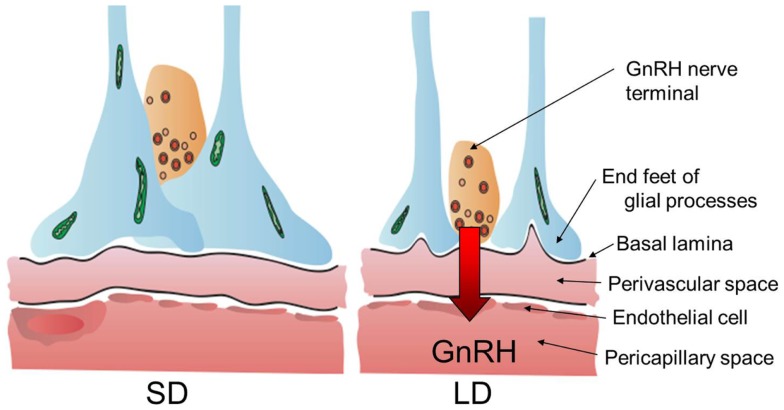
Thyroid hormone is involved in the development and plasticity of the central nervous system (22). The expression of thyroid hormone receptors (THRα, THRβ, and RXRα) in the median eminence suggested that the median eminence is the target site of action for the photo-induced increase in T3 in the quail MBH (20). To understand the action of thyroid hormone within the MBH, the ultrastructure of the median eminence was examined under SD and LD conditions using electron microscopy. Dynamic morphological changes were observed between the GnRH nerve terminals and glial endfeet within the median eminence (85). In SD conditions, many GnRH nerve terminals are encased by the endfeet of glial processes and do not contact the basal lamina, while many GnRH nerve terminals are in close proximity to the basal lamina under LD conditions (Figure 3). It has been proposed that the nerve terminals of hypothalamic neurons are required to directly contact the pericapillary space for the secretion of the hypothalamic neurohormone from the hypothalamus into the portal capillary (86). Morphological changes between the GnRH nerve terminals and endfeet of glial processes are observed in SD quail treated with T3 to stimulate testicular growth (87). Therefore, these morphological changes appear to regulate or modulate the seasonal GnRH secretion from the median eminence. It is also interesting to note that the seasonal plasticity within the GnRH system is reported in ewes (88).
Figure 3.
Neuro–glial interaction between GnRH nerve terminals and glial endfeet. Locally activated thyroid hormone within the MBH regulates neuro–glial interaction in the median eminence and these morphological changes appear to regulate or modulate seasonal GnRH secretion from the hypothalamus to portal capillary. The illustration has been modified from that published by Yoshimura (89).
Photoperiodic Signaling Pathway and Domestication
Seasonal reproduction is a rate-limiting factor for the animal procreation. The photoperiodic signaling pathway could also be a potential target that facilitates human-driven domestication process. As discussed previously, most laboratory mice lack the enzyme activity of melatonin biosynthesis pathway (82, 83, 90, 91). In addition, occurrence of selective sweeps was found at the TSHR locus in all domestic chickens (92). This observation suggests that the TSHR may be a domestication locus in chicken (92). Although we still do not know the correlation with domestication, it is interesting to note that photoperiodic regulation of DIO3 is absent in Syrian hamster (27). Thus, genes involved in the photoperiodic signaling pathway could emerge as useful targets for the domestication of wild animals.
Conclusion
Involvement of thyroid hormone in the regulation of seasonal reproduction has been suggested in the past several decades. Recent comparative studies clearly reveal that the local activation of thyroid hormone within the hypothalamus is a key factor in the regulation of seasonal reproduction in a number of mammalian and avian species. It is important to note that this mechanism is also conserved in fish (93) and is universal among various vertebrate species. Although thyroid hormone influences both LD and SD breeders, the mechanism that differentiates LD breeders from SD breeders remains unknown. Presumably, the responsiveness of pathways downstream of T3 activity (e.g., responsiveness of T3 target genes to LD-induced T3 etc.) differs in LD and SD breeders. The switching mechanism of LD breeder and SD breeder needs to be clarified in the future studies.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work is supported by the Funding Program for Next Generation World Leading Researchers (NEXT Program) initiated by the Council for Science and Technology Policy (CSTP) (LS055). WPI-ITbM is supported by World Premier International Research Center Initiative (WPI), MEXT, Japan.
References
- 1.Garner WW, Allard HA. Effect of the relative length of day and night and other factors of the environment on growth and reproduction in plants. J Agric Res (1920) 18:553–606 [Google Scholar]
- 2.Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. J Biol Rhythms (2001) 16:365–80 10.1177/074873001129002079 [DOI] [PubMed] [Google Scholar]
- 3.Follett BK, King VM, Meddle SL. Rhythms and photoperiodism in birds. In: Lumsden PJ, Miller AJ. editors. Biological Rhythms and Photoperiodism in Plants. Oxford: Biostatistics Scientific; (1998). p. 231–42 [Google Scholar]
- 4.Hahn TP, MacDougall-Shackleton SA. Adaptive specialization, conditional plasticity and phylogenetic history in the reproductive cue response systems of birds. Philos Trans R Soc Lond B Biol Sci (2008) 363:267–86 10.1098/rstb.2007.2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholls TJ, Goldsmith AR, Dawson A. Photorefractoriness in birds and comparison with mammals. Physiol Rev (1988) 68:133–76 [DOI] [PubMed] [Google Scholar]
- 6.Cyr DG, Eales JG. Interrelationships between thyroidal and reproductive endocrine systems in fish. Rev Fish Biol Fish (1996) 6:165–200 10.1007/BF00182342 [DOI] [Google Scholar]
- 7.Nicholls TJ, Follett BK, Goldsmith AR, Pearson H. Possible homologies between photorefractoriness in sheep and birds: the effect of thyroidectomy on the length of the ewe’s breeding season. Reprod Nutr Dev (1988) 28:375–85 10.1051/rnd:19880304 [DOI] [PubMed] [Google Scholar]
- 8.Moenter SM, Woodfill CJ, Karsch FJ. Role of the thyroid gland in seasonal reproduction: thyroidectomy blocks seasonal suppression of reproductive neuroendocrine activity in ewes. Endocrinology (1991) 128:1337–44 10.1210/endo-128-3-1337 [DOI] [PubMed] [Google Scholar]
- 9.Dawson A. Thyroidectomy progressively renders the reproductive system of starlings (Sturnus vulgaris) unresponsive to changes in daylength. J Endocrinol (1993) 139:51–5 10.1677/joe.0.1390051 [DOI] [PubMed] [Google Scholar]
- 10.Dawson A. Thyroidectomy of house sparrows (Passer domesticus) prevents photo-induced testicular growth but not the increased hypothalamic gonadotrophin-releasing hormone. Gen Comp Endocrinol (1998) 110:196–200 10.1006/gcen.1998.7065 [DOI] [PubMed] [Google Scholar]
- 11.Parkinson TJ, Follett BK. Thyroidectomy abolishes seasonal testicular cycles of Soay rams. Proc Biol Sci (1995) 259:1–6 10.1098/rspb.1995.0001 [DOI] [PubMed] [Google Scholar]
- 12.Follett BK, Nicholls TJ. Influences of thyroidectomy and thyroxine replacement on photoperiodically controlled reproduction in quail. J Endocrinol (1985) 107:211–21 10.1677/joe.0.1070211 [DOI] [PubMed] [Google Scholar]
- 13.Goldsmiths AR, Nicholls TJ. Thyroxine effects upon reproduction, prolactin secretion and plumage moult in thyroidectomised European starlings Sturnus vulgaris. Ornis Scand (1992) 23:398–404 10.2307/3676666 [DOI] [Google Scholar]
- 14.Wilson FE, Reinert BD. Thyroid hormone acts centrally to programme photostimulated male American tree sparrows (Spizella arborea) for vernal and autumnal components of seasonality. J Neuroendocrinol (2000) 12:87–95 10.1046/j.1365-2826.2000.00437.x [DOI] [PubMed] [Google Scholar]
- 15.Bentley GE. Photoperiodism and reproduction in birds. In: Nelson RJ, Denlinger DL, Somers DE. editors. Photoperiodism: The Biological Calendar. New York: Oxford University Press; (2010). p. 420–45 [Google Scholar]
- 16.Homma K, Ohta M, Sakakibara Y. Photoinducible phase of the Japanese quail detected by direct stimulation of the brain. In: Suda M, Hayaishi O, Nakagawa H. editors. Biological Rhythms and Their Central Mechanism. Amsterdam: Elsevier; (1979). p. 85–94 [Google Scholar]
- 17.Sharp PJ, Follett BK. The effect of hypothalamic lesions on gonadotrophin release in Japanese quail (Coturnix coturnix japonica). Neuroendocrinology (1969) 5:205–18 10.1159/000121861 [DOI] [PubMed] [Google Scholar]
- 18.Juss TS, Meddle SL, Servant RS, King VM. Melatonin and photoperiodic time measurement in Japanese quail (Coturnix coturnix japonica). Proc R Soc Lond B Biol Sci (1993) 254:21–8 10.1098/rspb.1993.0121 [DOI] [PubMed] [Google Scholar]
- 19.Meddle SL, Follett BK. Photoperiodically driven changes in Fos expression within the basal tuberal hypothalamus and median eminence of Japanese quail. J Neuroscience (1997) 17:8909–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshimura T, Yasuo S, Watanabe M, Iigo M, Yamamura T, Hirunagi K, et al. Light-induced hormone conversion of T4 to T3 regulates photoperiodic response of gonads in birds. Nature (2003) 426:178–81 10.1038/nature02117 [DOI] [PubMed] [Google Scholar]
- 21.Yasuo S, Watanabe M, Nakao N, Takagi T, Follett BK, Ebihara S, et al. The reciprocal switching of two thyroid hormone-activating and – inactivating enzyme genes is involved in the photoperiodic gonadal response of Japanese quail. Endocrinology (2005) 146:2551–4 10.1210/en.2005-0057 [DOI] [PubMed] [Google Scholar]
- 22.Bernal J. Action of thyroid hormone in brain. J Endocrinol Invest (2002) 25:268–88 [DOI] [PubMed] [Google Scholar]
- 23.Watanabe T, Yamamura T, Watanabe M, Yasuo S, Nakao N, Dawson A, et al. Hypothalamic expression of thyroid hormone-activating and -inactivating enzyme genes in relation to photorefractoriness in birds and mammals. Am J Physiol Regul Integr Comp Physiol (2007) 292:R568–72 10.1152/ajpregu.00521.2006 [DOI] [PubMed] [Google Scholar]
- 24.Ono H, Nakao N, Yamamura T, Kinoshita K, Mizutani M, Namikawa T, et al. Red jungle fowl (Gallus gallus) as a model for studying the molecular mechanism of seasonal reproduction. Anim Sci J (2009) 80:328–32 10.1111/j.1740-0929.2009.00628.x [DOI] [PubMed] [Google Scholar]
- 25.Perfito N, Jeong SY, Silverin B, Calisi RM, Bentley GE, Hau M. Anticipating spring: wild populations of great tits (Parus major) differ in expression of key genes for photoperiodic time measurement. PLoS One (2012) 7:e34997. 10.1371/journal.pone.0034997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevenson TJ, Ball GF. Disruption of neuropsin mRNA expression via RNA interference facilitates the photoinduced increase in thyrotropin-stimulating subunit β in birds. Eur J Neurosci (2012) 36:2859–65 10.1111/j.1460-9568.2012.08209.x [DOI] [PubMed] [Google Scholar]
- 27.Barrett P, Ebling FJ, Schuhler S, Wilson D, Ross AW, Warner A, et al. Hypothalamic thyroid hormone catabolism acts as a gatekeeper for the seasonal control of body weight and reproduction. Endocrinology (2007) 148:3608–17 10.1210/en.2007-0316 [DOI] [PubMed] [Google Scholar]
- 28.Freeman DA, Teubner BJ, Smith CD, Prendergast BJ. Exogenous T3 mimics long day lengths in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol (2007) 292:R2368–72 10.1152/ajpregu.00713.2006 [DOI] [PubMed] [Google Scholar]
- 29.Herwig A, Wilson D, Logie TJ, Boelen A, Morgan PJ, Mercer JG, et al. Photoperiod and acute energy deficits interact on components of the thyroid hormone system in hypothalamic tanycytes of the Siberian hamster. Am J Physiol Regul Integr Comp Physiol (2009) 296:R1307–15 10.1152/ajpregu.90755.2008 [DOI] [PubMed] [Google Scholar]
- 30.Watanabe M, Yasuo S, Watanabe T, Yamamura T, Nakao N, Ebihara S, et al. Photoperiodic regulation of type 2 deiodinase gene in Djungarian hamster: possible homologies between avian and mammalian photoperiodic regulation of reproduction. Endocrinology (2004) 145:1546–9 10.1210/en.2003-1593 [DOI] [PubMed] [Google Scholar]
- 31.Revel FG, Saboureau M, Pévet P, Mikkelsen JD, Simonneaux V. Melatonin regulates type 2 deiodinase gene expression in the Syrian hamster. Endocrinology (2006) 147:4680–7 10.1210/en.2006-0606 [DOI] [PubMed] [Google Scholar]
- 32.Yasuo S, Yoshimura T, Ebihara S, Korf HW. Temporal dynamics of type 2 deiodinase expression after melatonin injections in Syrian hamsters. Endocrinology (2007) 148:4385–92 10.1210/en.2007-0497 [DOI] [PubMed] [Google Scholar]
- 33.Ross AW, Helfer G, Russell L, Darras VM, Morgan PJ. Thyroid hormone signalling genes are regulated by photoperiod in the hypothalamus of F344 rats. PLoS One (2011) 6:e21351. 10.1371/journal.pone.0021351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yasuo S, Watanabe M, Iigo M, Nakamura TJ, Watanabe T, Takagi T, et al. Differential response of type 2 deiodinase gene expression to photoperiod between photoperiodic Fischer 344 and nonphotoperiodic Wistar rats. Am J Physiol Regul Integr Comp Physiol (2007) 292:R1315–9 10.1152/ajpregu.00396.2006 [DOI] [PubMed] [Google Scholar]
- 35.Ono H, Hoshino Y, Yasuo S, Watanabe M, Nakane Y, Murai A, et al. Involvement of thyrotropin in photoperiodic signal transduction in mice. Proc Natl Acad Sci USA (2008) 105:18238–42 10.1073/pnas.0808952105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yasuo S, Nakao N, Ohkura S, Iigo M, Hagiwara S, Goto A, et al. Long-day suppressed expression of type 2 deiodinase gene in the mediobasal hypothalamus of the Saanen goat, a short-day breeder: implication for seasonal window of thyroid hormone action on reproductive neuroendocrine axis. Endocrinology (2006) 147:432–40 10.1210/en.2005-0507 [DOI] [PubMed] [Google Scholar]
- 37.Hanon EA, Lincoln GA, Fustin JM, Dardente H, Masson-Pévet M, Morgan PJ, et al. Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr Biol (2008) 18:1147–52 10.1016/j.cub.2008.06.076 [DOI] [PubMed] [Google Scholar]
- 38.Anderson GM, Hardy SL, Valent M, Billings HJ, Connors JM, Goodman RL. Evidence that thyroid hormones act in the ventromedial preoptic area and the premammillary region of the brain to allow the termination of the breeding season in the ewe. Endocrinology (2003) 144:2892–901 10.1210/en.2003-0322 [DOI] [PubMed] [Google Scholar]
- 39.Billings HJ, Viguié C, Karsch FJ, Goodman RL, Connors JM, Anderson GM. Temporal requirements of thyroid hormones for seasonal changes in luteinizing hormone secretion. Endocrinology (2002) 143:2618–25 10.1210/en.143.7.2618 [DOI] [PubMed] [Google Scholar]
- 40.Ikegami K, Yoshimura T. Circadian clocks and the measurement of daylength in seasonal reproduction. Mol Cell Endocrinol (2012) 349:76–81 10.1016/j.mce.2011.06.040 [DOI] [PubMed] [Google Scholar]
- 41.Abe T, Suzuki T, Unno M, Tokui T, Ito S. Thyroid hormone transporters: recent advances. Trends Endocrinol Metab (2002) 13:215–20 10.1016/S1043-2760(02)00599-4 [DOI] [PubMed] [Google Scholar]
- 42.Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch (2004) 447:653–65 10.1007/s00424-003-1168-y [DOI] [PubMed] [Google Scholar]
- 43.Nakao N, Takagi T, Iigo M, Tsukamoto T, Yasuo S, Masuda T, et al. Possible involvement of organic anion transporting polypeptide 1c1 in the photoperiodic response of gonads in birds. Endocrinology (2006) 147:1067–73 10.1210/en.2005-1090 [DOI] [PubMed] [Google Scholar]
- 44.Nicholls TJ, Follett BK, Robinson JE. A photoperiodic response in gonadectomized Japanese quail exposed to a single long day. J Endocrinol (1983) 97:121–6 10.1677/joe.0.0970121 [DOI] [PubMed] [Google Scholar]
- 45.Nakao N, Ono H, Yamamura T, Anraku T, Takagi T, Higashi K, et al. Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature (2008) 452:317–22 10.1038/nature06738 [DOI] [PubMed] [Google Scholar]
- 46.Benoit J. Le role des yeux dans l’action stimulante de la lumiere sure le developpement testiulaire chez le canard. C R Soc Biol (Paris) (1935) 118:669–71 [Google Scholar]
- 47.Oliver J, Bayle JD. Brain photoreceptors for the photoinduced testicular response in birds. Experientia (1982) 38:1020–9 10.1007/BF01955346 [DOI] [PubMed] [Google Scholar]
- 48.Max M, McKinnon PJ, Seidenman KJ, Barrett RK, Applebury ML, Takahashi JS, et al. Pineal opsin: a nonvisual opsin expressed in chick pineal. Science (1995) 267:1502–6 10.1126/science.7878470 [DOI] [PubMed] [Google Scholar]
- 49.Okano T, Yoshizawa T, Fukada Y. Pinopsin is a chicken pineal photoreceptive molecule. Nature (1994) 372:94–7 10.1038/372094a0 [DOI] [PubMed] [Google Scholar]
- 50.Siopes TD, Wilson WO. Extraocular modification of photoreception in intact and pinealectomized coturnix. Poult Sci (1974) 53:2035–41 10.3382/ps.0532035 [DOI] [PubMed] [Google Scholar]
- 51.Menaker M, Roberts R, Elliott J, Underwood H. Extraretinal light perception in the sparrow. III. The eyes do not participate in photoperiodic photoreception. Proc Natl Acad Sci USA (1970) 67:320–5 10.1073/pnas.67.1.320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silver R, Witkovsky P, Horvath P, Alones V, Barnstable CJ, Lehman MN. Coexpression of opsin- and VIP-like-immunoreactivity in CSF-contacting neurons of the avian brain. Cell Tissue Res (1988) 253:189–98 10.1007/BF00221754 [DOI] [PubMed] [Google Scholar]
- 53.Wada Y, Okano T, Adachi A, Ebihara S, Fukada Y. Identification of rhodopsin in the pigeon deep brain. FEBS Lett (1998) 424:53–6 10.1016/S0014-5793(98)00138-0 [DOI] [PubMed] [Google Scholar]
- 54.Bailey MJ, Cassone VM. Melanopsin expression in the chick retina and pineal gland. Brain Res Mol Brain Res (2005) 134:345–8 10.1016/j.molbrainres.2004.11.003 [DOI] [PubMed] [Google Scholar]
- 55.Chaurasia SS, Rollag MD, Jiang G, Hayes WP, Haque R, Natesan A, et al. Molecular cloning, localization and circadian expression of chicken melanopsin (Opn4): differential regulation of expression in pineal and retinal cell types. J Neurochem (2005) 92:158–70 10.1111/j.1471-4159.2004.02874.x [DOI] [PubMed] [Google Scholar]
- 56.Kang SW, Leclerc B, Kosonsiriluk S, Mauro LJ, Iwasawa A, El Halawani ME. Melanopsin expression in dopamine-melatonin neurons of the premammillary nucleus of the hypothalamus and seasonal reproduction in birds. Neuroscience (2010) 170:200–13 10.1016/j.neuroscience.2010.06.082 [DOI] [PubMed] [Google Scholar]
- 57.Tomonari S, Takagi A, Akamatsu S, Noji S, Ohuchi H. A non-canonical photopigment, melanopsin, is expressed in the differentiating ganglion, horizontal, and bipolar cells of the chicken retina. Dev Dyn (2005) 234:783–90 10.1002/dvdy.20600 [DOI] [PubMed] [Google Scholar]
- 58.Tomonari S, Takagi A, Noji S, Ohuchi H. Expression pattern of the melanopsin-like (cOpn4m) and VA opsin-like genes in the developing chicken retina and neural tissues. Gene Expr Patterns (2007) 7:746–53 10.1016/j.modgep.2007.06.001 [DOI] [PubMed] [Google Scholar]
- 59.Davies WI, Turton M, Peirson SN, Follett BK, Halford S, Garcia-Fernandez JM, et al. Vertebrate ancient opsin photopigment spectra and the avian photoperiodic response. Biol Lett (2012) 8:291–4 10.1098/rsbl.2011.0864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Halford S, Pires SS, Turton M, Zheng L, Gonzalez-Menendez I, Davies WL, et al. VA opsin-based photoreceptors in the hypothalamus of birds. Curr Biol (2009) 19:1396–402 10.1016/j.cub.2009.06.066 [DOI] [PubMed] [Google Scholar]
- 61.Nakane Y, Ikegami K, Ono H, Yamamoto N, Yoshida S, Hirunagi K, et al. A mammalian neural tissue opsin (Opsin 5) is a deep brain photoreceptor in birds. Proc Natl Acad Sci USA (2010) 107:15264–8 10.1073/pnas.1006393107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamashita T, Ohuchi H, Tomonari S, Ikeda K, Sakai K, Shichida Y. Opn5 is a UV-sensitive bistable pigment that couples with Gi subtype of G protein. Proc Natl Acad Sci USA (2010) 107:22084–9 10.1073/pnas.1012498107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Groos GA, van der Kooy D. Functional absence of brain photoreceptors mediating entrainment of circadian rhythms in the adult rat. Experientia (1981) 37:71–2 10.1007/BF01965576 [DOI] [PubMed] [Google Scholar]
- 64.Legan SJ, Karsch FJ. Importance of retinal photoreceptors to the photoperiodic control of seasonal breeding in the ewe. Biol Reprod (1983) 29:316–25 10.1095/biolreprod29.2.316 [DOI] [PubMed] [Google Scholar]
- 65.Lockley SW, Skene DJ, Thapan K, English J, Ribeiro D, Haimov I, et al. Extraocular light exposure does not suppress plasma melatonin in humans. J Clin Endocrinol Metab (1998) 83:3369–72 10.1210/jc.83.9.3369 [DOI] [PubMed] [Google Scholar]
- 66.Meijer JH, Thio B, Albus H, Schaap J, Ruijs ACJ. Functional absence of extraocular photoreception in hamster circadian rhythms entrainment. Brain Res (1999) 831:337–9 10.1016/S0006-8993(99)01509-7 [DOI] [PubMed] [Google Scholar]
- 67.Nelson RJ, Zucker I. Absence of extraocular photoreception in diurnal and nocturnal rodents exposed to direct sunlight. Comp Biochem Physiol (1981) 69A:145–8 10.1016/0300-9629(81)90651-4 [DOI] [Google Scholar]
- 68.Reiter RJ. The pineal and its hormones in the control of reproduction in mammals. Endocr Rev (1980) 1:109–31 10.1210/edrv-1-2-109 [DOI] [PubMed] [Google Scholar]
- 69.Yamazaki S, Goto M, Menaker M. No evidence for extraocular photoreceptors in the circadian system of the Syrian hamster. J Biol Rhythms (1999) 14:197–201 10.1177/074873099129000605 [DOI] [PubMed] [Google Scholar]
- 70.Inouye ST, Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc Natl Acad Sci USA (1979) 76:5962–6 10.1073/pnas.76.11.5962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Klein DC, Moore RY, Reppert SM. Suprachiasmatic Nucleus: The Mind’s Clock. New York: Oxford University Press; (1991). [Google Scholar]
- 72.Lehman MN, Silver R, Gradstone WR, Kahn RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J Neurosci (1987) 7:1626–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science (1990) 247:975–8 10.1126/science.2305266 [DOI] [PubMed] [Google Scholar]
- 74.Arendt J. Melatonin and the Mammalian Pineal Gland. London: Chapman & Hall; (1995). [Google Scholar]
- 75.Hoffman RA, Reiter RJ. Pineal gland: influence on gonads of male hamsters. Science (1965) 148:1609–11 10.1126/science.148.3677.1609 [DOI] [PubMed] [Google Scholar]
- 76.Reppert SM, Weaver DR, Ebisawa T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron (1994) 13:1177–85 10.1016/0896-6273(94)90055-8 [DOI] [PubMed] [Google Scholar]
- 77.Reppert SM, Godson CG, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b-melatonin receptor. Proc Natl Acad Sci USA (1995) 92:8734–8 10.1073/pnas.92.19.8734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schuster C, Gauer F, Guerrero H, Lakhdar-Ghazal N, Pévet P, Masson-Pévet M. Photic regulation of mt1 melatonin receptors in the Siberian hamster pars tuberalis and suprachiasmatic nuclei: involvement of the circadian clock and intergeniculate leaflet. J Neuroendocrinol (2000) 12:207–16 10.1046/j.1365-2826.2000.00039.x [DOI] [PubMed] [Google Scholar]
- 79.Song CK, Bartness TJ. CNS sympathetic outflow neurons to white fat that express MEL receptors may mediate seasonal adiposity. Am J Physiol Regul Integr Comp Physiol (2001) 281:R666–72 [DOI] [PubMed] [Google Scholar]
- 80.Klosen P, Bienvenu C, Demarteau O, Dardente H, Guerrero H, Pévet P, et al. The mt1 melatonin receptor and RORb receptor are co-localized in specific TSH-immunoreactive cells in the pars tuberalis of the rat pituitary. J Histochem Cytochem (2002) 50:1647–57 10.1177/002215540205001209 [DOI] [PubMed] [Google Scholar]
- 81.Wittkowski W, Bergmann M, Hoffmann K, Pera F. Photoperiod-dependent changes in TSH-like immunoreactivity of cells in the hypophysial pars tuberalis of the Djungarian hamster, Phodopus sungorus. Cell Tissue Res (1988) 251:183–7 10.1007/BF00215463 [DOI] [PubMed] [Google Scholar]
- 82.Ebihara S, Marks T, Hudson DJ, Menaker M. Genetic control of melatonin synthesis in the pineal gland of the mouse. Science (1986) 231:491–3 10.1126/science.3941912 [DOI] [PubMed] [Google Scholar]
- 83.Goto M, Oshima I, Tomita T, Ebihara S. Melatonin content of the pineal gland in different mouse strains. J Pineal Res (1989) 7:195–204 10.1111/j.1600-079X.1989.tb00667.x [DOI] [PubMed] [Google Scholar]
- 84.Yasuo S, Yoshimura T, Ebihara S, Kolf HW. Melatonin transmits photoperiodic signals through the MT1 melatonin receptor. J Neurosci (2009) 29:2885–9 10.1523/JNEUROSCI.0145-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yamamura T, Hirunagi K, Ebihara S, Yoshimura T. Seasonal morphological changes in the neuro-glial interaction between gonadotropin-releasing hormone nerve terminals and glial endfeet in Japanese quail. Endocrinology (2004) 145:4264–7 10.1210/en.2004-0366 [DOI] [PubMed] [Google Scholar]
- 86.Prevot V, Croix D, Bouret S, Dutoit S, Tramu G, Stefano GB, et al. Definitive evidence for the existence of morphological plasticity in the external zone of the median eminence during the rat estrous cycle: implication of neuro-glio-endothelial interactions in gonadotropin-releasing hormone release. Neuroscience (1999) 94:809–19 10.1016/S0306-4522(99)00383-8 [DOI] [PubMed] [Google Scholar]
- 87.Yamamura T, Yasuo S, Hirunagi K, Ebihara S, Yoshimura T. T3 implantation mimics photoperiodically reduced encasement of nerve terminals by glial processes in the median eminence of Japanese quail. Cell Tissue Res (2006) 324:175–9 10.1007/s00441-005-0126-8 [DOI] [PubMed] [Google Scholar]
- 88.Jansen HT, Cutter C, Hardy S, Lehman MN, Goodman RL. Seasonal plasticity within the gonadotropin-releasing hormone (GnRH) system of the ewe: changes in identified GnRH inputs and glial association. Endocrinology (2003) 144:3663–76 10.1210/en.2002-0188 [DOI] [PubMed] [Google Scholar]
- 89.Yoshimura T. Molecular bases for seasonal reproduction in birds. J Poult Sci (2004) 41:251–8 10.1016/j.yfrne.2013.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kasahara T, Abe K, Mekada K, Yoshiki A, Kato T. Genetic variation of melatonin productivity in laboratory mice under domestication. Proc Natl Acad Sci USA (2010) 107:6412–7 10.1073/pnas.0914399107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shimomura K, Lowrey PL, Vitaterna MH, Buhr ED, Kumar V, Hanna P, et al. Genetic suppression of the circadian clock mutation by the melatonin biosynthesis pathway. Proc Natl Acad Sci USA (2010) 107:8399–403 10.1073/pnas.1004368107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rubin CJ, Zody MC, Eriksson J, Meadows JR, Sherwood E, Webster MT, et al. Whole-genome resequencing reveals loci under selection during chicken domestication. Nature (2010) 464:587–91 10.1038/nature08832 [DOI] [PubMed] [Google Scholar]
- 93.Nakane Y, Ikegami K, Iigo M, Ono H, Takeda K, Takahashi D, et al. The saccus vasculosus of fish is a sensor of seasonal changes in day length. Nat Commun (2013) 4:2108. 10.1038/ncomms3108 [DOI] [PubMed] [Google Scholar]