Abstract
Background
Catheter ablation (CA) is a highly effective therapy for the treatment of paroxysmal atrial fibrillation (AF) when compared with antiarrhythmic drug therapy (ADT). No randomized studies have compared the two strategies in persistent AF. The present randomized trial aimed to compare the effectiveness of CA vs. ADT in treating persistent AF.
Methods and results
Patients with persistent AF were randomly assigned to CA or ADT (excluding patients with long-standing persistent AF). Primary endpoint at 12-month follow-up was defined as any episode of AF or atrial flutter lasting >24 h that occurred after a 3-month blanking period. Secondary endpoints were any atrial tachyarrhythmia lasting >30 s, hospitalization, and electrical cardioversion. In total, 146 patients were included (aged 55 ± 9 years, 77% male). The ADT group received class Ic (43.8%) or class III drugs (56.3%). In an intention-to-treat analysis, 69 of 98 patients (70.4%) in the CA group and 21 of 48 patients (43.7%) in the ADT group were free of the primary endpoint (P = 0.002), implying an absolute risk difference of 26.6% (95% CI 10.0–43.3) in favour of CA. The proportion of patients free of any recurrence (>30 s) was higher in the CA group than in the ADT group (60.2 vs. 29.2%; P < 0.001) and cardioversion was less frequent (34.7 vs. 50%, respectively; P = 0.018).
Conclusion
Catheter ablation is superior to medical therapy for the maintenance of sinus rhythm in patients with persistent AF at 12-month follow-up.
Clinical Trial Registration Information
NCT00863213 (http://clinicaltrials.gov/ct2/show/NCT00863213).
Keywords: Atrial fibrillation, Catheter ablation, Antiarrhythmic drug, Atrial flutter, Clinical trial
See page 482 for the editorial comment on this article (doi:10.1093/eurheartj/eht504)
Introduction
Catheter ablation (CA) is an effective therapy for the treatment of symptomatic paroxysmal atrial fibrillation (AF) when medical therapy fails1,2 and is considered a Class I indication under current guidelines (level of evidence A).3,4 However, its use in patients with persistent AF remains controversial. The evidence in favour of ablation derives from non-randomized trials and is weak (Class IIa indication; level of evidence B).3,4 Long-term CA success in patients with non-paroxysmal AF is largely dependent on patient characteristics, and most studies consider persistent and long-standing persistent AF together.5,6 The present study is the first randomized, controlled trial comparing the effectiveness of rhythm control of CA vs. antiarrhythmic drug therapy (ADT) in patients with symptomatic persistent AF, specifically excluding long-standing persistent AF and patients with advanced remodelling stage (left atrial diameter> 50 mm).
Methods
Study population
Patients with symptomatic persistent AF7 (>7 or <7 days requiring electrical or pharmacological cardioversion) refractory to at least one class I or class III antiarrhythmic drug were recruited. Exclusion criteria were age <18 or >70 years, long-standing persistent AF (>1 year of continuous AF), first episode of AF, hyper- or hypothyroidism, hypertrophic cardiomyopathy, implanted pacemaker or defibrillator, moderate or severe mitral disease or mitral prosthesis, left ventricular ejection fraction <30%, left atrial diameter >50 mm, prior ablation procedure, contraindication for oral anticoagulation, left atrial thrombus, active infection or sepsis, pregnancy, unstable angina, acute myocardial infarction during previous 3 months, life expectation <12 months, current participation in another clinical trial, mental disease or inability to give informed consent, or disease contraindicating ablation or ADT.
Study design
The study aimed to compare the effectiveness and safety of using CA or ADT to maintain sinus rhythm at 12-month follow-up. The present multicentre study (eight sites) was an open, parallel-groups, randomized trial. All recruiting centres have arrhythmia units with extensive experience in AF ablation procedures. Recruited patients were randomly assigned to either ablation (CA group) or medical therapy (ADT group) according to a 2:1 blocked randomization list stratified by centre. All patients gave written informed consent before enrolment. The study protocol was approved by the Ethics Committee of each participating centre.
Interventions
Ablation procedure
Pre- and postprocedural oral anticoagulation (international normalized ratio between 2 and 3) was required for at least 1 month before and after CA. Antiarrhythmic drugs were discontinued ≥5 half-life periods (or ≥1 week for amiodarone) before ablation; antiarrhythmics were re-initiated immediately after CA for the 3-month blanking period.
Transoesophageal echocardiography was performed in all patients before CA to exclude the presence of left atrial thrombus. After transseptal puncture to gain LA access, a bolus of heparin was administered (5000–6000 IU, according to patient weight), followed by additional boluses to maintain an activated clotting time of 250–300 s. A 3D map was constructed using an electroanatomic mapping system (CARTO, Biosense Webster, Diamond Bar, CA, USA or NAVX, St Jude Corporation, St Paul, MN, USA). Computed tomography or magnetic resonance images were integrated into the navigation system to improve LA anatomic reconstruction. Wide encircling pulmonary vein ablation was performed using radiofrequency energy (cooled-tip catheter) assisted by a circular multipolar catheter. The endpoint was the absence or dissociation of a local electrogram inside the entire surrounded region together with exit block by pacing within the pulmonary vein ostia. Additional ablation lines or ablation of complex fractioned electrograms were performed according to each hospital's protocol. When lines at the roof of the left atrium (connecting both superior pulmonary veins) or at the mitral isthmus (mitral annulus to the ostium of the left inferior pulmonary vein) were deployed, complete bidirectional conduction block was required. The endpoint for complex fractionated atrial electrogram ablation was the complete abatement of potentials at these sites.
Antiarrhythmic drugs
Patients were treated depending on physician's choice and according to current guidelines.3 Discontinuation of the antiarrhythmic treatment was not required before inclusion in the ADT group. Class III drugs (amiodarone) were recommended for patients with structural cardiomyopathy and class Ic (flecainide) plus diltiazem or β-blockers otherwise. There was not predefined protocol on the use of ADT during the blanking period.
Follow-up
Patient follow-up at 1, 3, 6, and 12 months after the initiation of the assigned intervention included 12-lead ECG. A 24-h Holter monitor was performed at 6 and 12 months of follow-up.
Study outcomes
The primary outcome was defined as episode of AF or flutter lasting >24 h or requiring cardioversion after a 3-month blanking period. The primary endpoint was assessed by an independent endpoint committee, which evaluated the episodes based on the information received. In cases where the Holter recording of AF lasted <24 h, symptoms and other relevant information were taken into consideration. Predefined secondary outcomes included any recurrence of AF or flutter lasting at least 30 s after the 3-month blanking period, hospitalization related to arrhythmia, electrical or pharmacological cardioversion, therapeutic crossover (reassigned to a therapy different from that randomized), AV node ablation, re-ablation (ablation group only), health-related quality of life (QoL) as measured by the AF-QoL questionnaire8 (total score and physical, psychological, and sexual dimension scores) at baseline and 6 and 12 months, and complications. Events were established by consensus of two experienced electrophysiologists, not participating in the study, independent from any institution involved in the study, and blinded to the treatment allocation. Adverse events were examined by an independent external event committee of cardiologists.
Statistical analysis
The study was sized to provide 80% power to show the superiority of ablation over drug therapy in a test comparing the primary outcome, with a two-sided 0.05 alpha-level, assuming probabilities of 0.7 (ablation) and 0.5 (drug therapy), and a 2:1 allocation ratio. The planned sample size was 208 patients (139 in the CA group and 69 in the ADT group). The analysis was conducted by intention-to-treat, assuming the worst case (i.e. ‘failure’) when data were not available. A per-protocol analysis was also performed with patients in whom the primary endpoint was assessed.
The pre-specified primary (confirmatory) analysis was an unadjusted comparison of the primary outcome by a χ2 test. Secondary analysis of the primary endpoint was performed using logistic regression to adjust by centre and to explore a possible intervention-by-centre interaction. Comparison of time-to-event analysis was performed by a log-rank test and Kaplan–Meier estimates by intervention group. Analysis of covariance was used to analyse the AF-QoL scores8 at 6 and 12 months, including the baseline values as a covariate and the treatment group as fixed effect. Other secondary outcomes were analysed by the χ2, Cochrane–Armitage trend, or Fisher exact test as appropriate. All tests were two-sided, and considered significant if P < 0.05. Data were described as mean (SD), or n (%) as appropriate. All analyses were performed with SAS version 9.2 for Windows.
Results
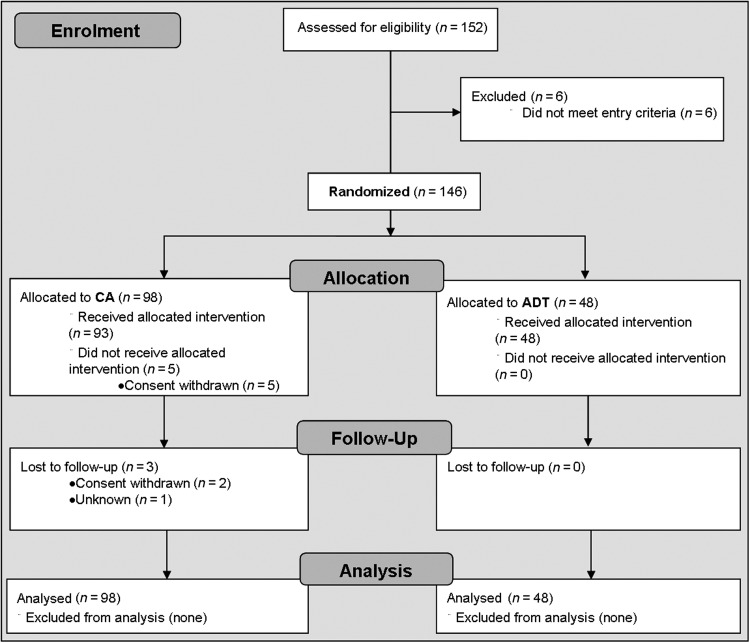
From May 2009 through November 2011, 146 patients were randomly assigned to CA (98 patients) or ADT (48 patients) (Figure 1). The study was terminated before reaching the planned sample size due to lower than expected recruitment rate; all data were blinded at the time of the study discontinuation. Baseline characteristics are summarized in Table 1.
Figure 1.
Study design flowchart (CONSORT flow diagram). CA, catheter ablation; ADT, antiarrhythmic drug therapy.
Table 1.
Baseline characteristics of the study patients according to assignment to catheter ablation or antiarrhythmic drug therapy
| Characteristic | Ablation (n = 98) | Drug therapy (n = 48) |
|---|---|---|
| Age (year) | 55 (9) | 55 (9) |
| Male sex, n (%) | 76 (77.5) | 37 (77.0) |
| Systolic blood pressure (mmHg) | 126 (16) | 128 (15) |
| Diastolic blood pressure (mmHg) | 79 (11) | 82 (11) |
| Medical history, n (%) | ||
| HBP | 46 (46.9) | 19 (39.5) |
| Sleep apnoea/hypoapnoea syndrome | 10 (10.2) | 8 (16.6) |
| TIA | 1 (1.0) | 1 (2.1) |
| CVA | 3 (3.1) | 1 (2.1) |
| Peripheral embolism | 3 (3.1) | 1 (2.1) |
| Ischaemic cardiopathy | 3 (3.1) | 1 (2.1) |
| Left atrial size (mm) | 41.3 (4.6) | 42.7 (5.1) |
| Left ventricular ejection fraction (%) | 61.1 (8.8) | 60.8 (9.7) |
| NYHA functional class, n (%) | ||
| I | 73 (74.5) | 39 (81.2) |
| II | 22 (22.4) | 9 (18.8) |
| III | 3 (3.1) | – |
Data are means (SD) or n (%).
HBP, high blood pressure; TIA, transient ischaemic accident; CVA, cerebrovascular accident; NYHA, New York Heart Association.
In the CA group, 93 patients (94.9%) underwent the ablation procedure. Eight patients (8.2%) underwent a second ablation due to recurrence of AF (five patients) or atypical atrial flutter (three patients). The ablation procedure included wide, encircling pulmonary vein lesions in all patients, roof line in 23 (23.4%), mitral isthmus line in 3 (3.1%), and ablation of complex fractionated electrograms in 8 (8.1%). Non-pulmonary vein foci were targeted in eight patients (8.1%) and cavotricuspid isthmus in two patients (2%). The mean procedural duration was 216 ± 68 min, the mean radiofrequency time was 33 ± 15 min, and the mean fluoroscopy time was 48 ± 27 min. Fifty-seven (58.2%) patients underwent the ablation procedure in AF; 15 (26.3%) converted to SR during radiofrequency applications; and 42 (73.7%) were cardioverted to check for bidirectional block.
At the time of randomization to the CA arm, 35 (35.7%) patients were under amiodarone: 30 (85.7%) remained under the same treatment, 4 (11.4%) changed to class Ic drugs, and 1 (2.9%) changed to another class III drug.
All patients in the ADT group received the assigned intervention with a mean of 1.3 ± 0.7 drugs per patient. At the time of randomization, 22 (45.8%) patients were under amiodarone, of whom 59.1% remained under the same treatment, 31.8% changed to class Ic drugs, and 9.1% changed to another class III drug. At the beginning of follow-up, 21 patients (43.8%) were receiving class IC and 27 patients (56.3%) class III drugs.
Primary outcome
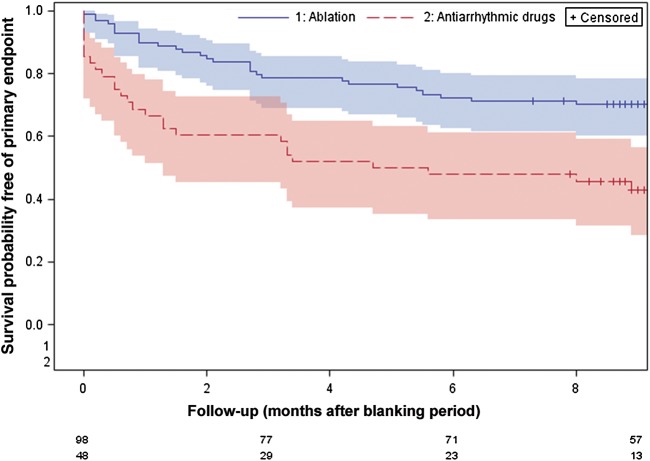
In an intention-to-treat analysis, the proportion of patients free of sustained episodes of AF at 12 months (primary endpoint) was 70.4% in the CA group and 43.7% in the ADT group (P = 0.002), implying an absolute risk difference of 26.6% (95% CI 10.0–43.3) favouring the ablation group. The adjusted, logistic regression analysis showed a significant treatment effect (Wald χ2 = 9.7; df = 1; P = 0.002), with an estimated odds ratio of 3.28 (95% CI 1.5–6.9) favouring the ablation group. No evidence of a treatment-by-site interaction, or of a site effect after removing the interaction term, was found (Wald χ2 = 8.6; df = 7; P = 0.285). Compared with the ADT group, the CA group showed higher probability of remaining free of sustained AF recurrence or flutter (log-rank χ2= 12.4; df = 1; P < 0.001) (Figure 2). All patients completed the follow-up except three patients in the CA arm, all of which reached the primary endpoint before the loss to follow-up. In a per-protocol analysis, 72.8% of the CA group and 43.8% of the ADT group were free of sustained episodes of AF (P < 0.001).
Figure 2.
Survival curves for the primary endpoint.
Secondary outcomes
In an intention-to-treat analysis, the proportion of patients free of any recurrence of AF or flutter (lasting >30 s) at the end of follow-up was higher in the CA than that in the ADT group (60.2 vs. 29.2%; P < 0.001), with 31% absolute risk difference (95% CI 14.9–47.1). The need for cardioversion was higher in the ADT group than that in the CA group (50 vs. 34.7%; P = 0.018). During the blanking period, the ADT group had a higher rate of recurrences compared with the CA group (54.2 vs. 29.6%, respectively; P = 0.004), without significant differences in the rate of cardioversions (33.3 vs. 23.5%, respectively; P = 0.206). Of those patients in the ADT group with recurrences during the blanking period, 65.4% were treated with class III antiarrhythmics and 34.6% were under class Ic. All patients treated with class III drugs remained under this therapy after the recurrence, whereas 33.3% of patients treated with class Ic changed to class III and 33.3% increased the dose of the same drug. Just 13 patients (13.3%) in the CA group and 7 (14.6%) in the ADT group recurred only during the blanking period. In the CA arm, early recurrences (blanking period) implied an increased risk for the occurrence of the primary endpoint (OR 5.30; 95% CI 2.05–13.69; P = 0.002).
At the end of follow-up, 23 patients (47.9%) in the ADT group underwent CA after reaching the primary endpoint (four required two procedures). None of the patients in the ADT group underwent CA before reaching the primary endpoint (0% of crossovers). In the CA arm, 35.7% of patients received ADT before reaching the primary endpoint (crossovers) due to symptoms of paroxysmal AF or undocumented palpitations. Hospitalizations due to arrhythmia recurrences were similar in both study groups (Table 2). There were no statistically significant differences between arms on the AF-QoL scores (Table 3).
Table 2.
Secondary outcomes
| Outcome | Ablation (n = 98) | Drug therapy (n = 48) |
|---|---|---|
| Free of any recurrence of AF or flutter (confirmed during >30 s) | 59 (60.2) | 14 (29.2)a,*** |
| Crossovers | 35 (35.7) | 0 (0)a,*** |
| Cardioversions | ||
| None | 64 (65.3) | 24 (50.0)b,* |
| 1 | 22 (22.4) | 10 (20.8) |
| 2 or more | 12 (12.2) | 14 (29.2) |
| Hospitalizations related to arrhythmia | 2 (2.0) | 3 (6.25)c |
AF, atrial flutter.
aχ2 test.
bCochrane–Armitage test.
cFisher's exact test.
*P < 0.05, **P < 0.01, ***P < 0.001
Table 3.
AF-QoL scores
| Ablation | Drug therapy | Difference (95% CI) | P-value* | |
|---|---|---|---|---|
| Global score | ||||
| Baselinea | 42.0 | 49.3 | ||
| 6 monthsb | 53.7 | 48.2 | 5.5 (−2.3 to 13.4) | 0.164 |
| 12 monthsb | 56.8 | 53.0 | 3.8 (−5.2 to 12.8) | 0.410 |
| Physical domain | ||||
| Baselinea | 44.1 | 48.7 | ||
| 6 monthsb | 54.1 | 49.7 | 4.4 (−4.8 to 13.6) | 0.343 |
| 12 monthsb | 56.2 | 55.6 | 0.6 (−9.8 to 11.0) | 0.912 |
| Psychological domain | ||||
| Baselinea | 34.8 | 45.7 | ||
| 6 monthsb | 51.8 | 44.2 | 7.6 (−0.8 to 16.0) | 0.076 |
| 12 monthsb | 54.8 | 48.9 | 5.8 (−4.1 to 15.7) | 0.247 |
| Sexual domain | ||||
| Baselinea | 53.9 | 60.8 | ||
| 6 monthsb | 57.0 | 51.9 | 5.1 (−5.4 to 15.6) | 0.336 |
| 12 monthsb | 63.7 | 55.4 | 8.4 (−2.1 to 18.8) | 0.117 |
aObserved means.
bBaseline-adjusted (least-squares) means
*ANCOVA analysis.
Adverse events
In the CA group, 98 procedures were performed and 6 (6.1%) had periprocedural complications: 2 pericarditis, 1 pericardial effusion and 3 minor vascular access complications that did not require intervention. During follow-up, 1 patient under oral anticoagulation had spontaneous renal haematoma and 1 patient had symptomatic pulmonary vein stenosis requiring stenting. The ADT group had one flecainide intoxication and one minor vascular access complication (4.2% of patients). No deaths, transient ischaemic events, or strokes were documented in either group.
Discussion
The present study is the first randomized trial comparing the effectiveness for rhythm control of CA vs. ADT in patients with persistent AF. Ablation is a well-established treatment for symptomatic drug-refractory paroxysmal AF, supported by a consistent body of evidence.1,2,9,10 Moreover, a recent publication suggests the potential benefit of CA as first-line therapy in this population.11 Due to the lack of randomized, controlled trials, there is no consensus on the role of CA in treating persistent AF.
Several studies have shown that CA is helpful in maintaining sinus rhythm and relieving symptoms in patients with non-paroxysmal AF.12–14 Although different ablation techniques have been tested and compared, the heterogeneity of the patient population and approach used affects potential comparisons.15 On the other hand, only one randomized trial to date has compared the effectiveness of CA and ADT in non-paroxysmal AF patients.13 The study by Oral et al. reported superiority of CA over ADT in patients with chronic AF (defined as continuous AF during at least 6 months), in which the mean AF duration before the intervention was ∼4 years. At 12-month follow-up, the recurrence rate of AF episodes lasting >30 s was 26 and 42% in the CA and ADT arms, respectively (intention-to-treat analysis). Although these results are encouraging, under current guidelines3,4 most patients included in that study had long-standing persistent AF and the results may not apply to patients with persistent AF. In a series including patients with paroxysmal and persistent AF, Stabile et al.16 demonstrated better rhythm control in the group of CA plus ADT when compared with ADT alone; nonetheless, the study was not designed to prove the effectiveness of CA alone. Moreover, data comparing these two strategies in patients with paroxysmal vs. persistent AF are not available, preventing extrapolation of the results to the present population. The present study demonstrated that CA was significantly more effective than ADT in maintaining sinus rhythm in patients with persistent AF, reducing the recurrence of sustained episodes (>24 h) of AF by 47.4% and the occurrence of any episode (>30 s) by 51.5%. Furthermore, CA had an acceptable safety profile according to the latest international registries.17
Previous studies have reported various ablation approaches to treat persistent and long-standing persistent AF, mostly by substrate modification.7,15,18–20 However, randomized data supporting the use of a specific strategy are not available.15 The present study applied a common approach of wide pulmonary vein encirclement and additional substrate modification was left to the operator's criteria. The present study was not designed to compare techniques; further randomized trials are needed to establish the best approach in this particular population. However, most patients received only antral PV ablation, with a relatively high success rate when compared with previously reported results in persistent AF,15 probably due to the strict selection criteria of the present trial, that excluded patients with long-standing persistent AF, severe cardiomyopathies, and patients with advanced atrial remodelling.
Compared with ADT, CA reduced sustained recurrences (>24 h) and electrical cardioversions, suggesting a better clinical outcome in the ablation group. On the other hand, recurrences of any tachyarrhythmia lasting at least 30 s were also lower in the ablation group, showing that not only sustained episodes but also short, paroxysmal episodes were less frequent with ablation therapy. The lack of any crossover to CA by patients in the ADT group highlights the differences between the arms in the clinical profile of recurrences. Recurrences in the ADT group were mostly due to persistent episodes (ablation after primary endpoint); in the CA group, patients received ADT to relieve symptoms of paroxysmal AF or even undocumented palpitations (ADT without reaching primary endpoint).
Monitoring during follow-up included 24-h Holter and ECG at each visit and whenever the patient had symptoms. We are aware that this monitoring strategy might be insufficient and might have underestimated the recurrence rate (mostly affecting the secondary endpoint or >30 s of AF); however, the overestimation of the success rate would have affected the outcome of both groups. For this reason we consider it unlikely that the choice of endpoint would substantially affect the difference between the groups.
Although AF type (paroxysmal, persistent, or long-standing persistent) has predictive value for AF ablation outcome,21–24 other clinical and remodelling factors may be better independent predictors.23,24 In the present study, the results of CA in persistent AF were comparable with those in paroxysmal AF reported in the literature.5 This is probably the result of strict patient selection criteria, which recruited a relatively young population with persistent AF (excluding long-standing persistent AF) and a relatively low remodelling stage (LA diameter <5 cm).
Procedural complications
Despite improvements in the procedural technique and increased effectiveness, safety remains a major concern when referring a patient for ablation. In the present trial, CA showed an acceptable safety profile, comparable with the latest international registries.17,25 Remarkably, neither deaths nor strokes occurred. Most ablation-related complications occurred periprocedurally. Only one case of pulmonary vein stenosis required stenting. Long-term ADT is associated with increased risk of adverse events.9 In the present study, a very low rate of complication was observed in this group.
Quality of life
Catheter ablation improves symptoms and QoL in patients with symptomatic AF and reduces arrhythmia recurrence.12,13,26 However, we did not find significant differences between study arms in the improvement of QoL. Although this finding might reflect a similar impact on QoL of both treatment strategies, the lack of statistical significance is likely due to insufficient statistical power for this secondary endpoint. Further studies are needed to evaluate the benefits on QoL of both treatments in patients with persistent AF.
Study limitations
The study was terminated before reaching the planned sample size due to a lower than expected recruitment rate. The main limitation for recruitment was related to conducting the study in ablation centres, where patients were specifically referred for AF ablation. This could have established a bias against participation among the centre's more symptomatic patients, who might well prefer prompt ablation to the possibility of being randomized to a drug therapy. However, the difference between groups in the primary endpoint was higher than assumed in the sample size calculation, which likely compensated for the loss of statistical power in the sample size. Remarkably, the evaluation of primary and secondary endpoints was performed by an external committee, blinded to treatment allocation. The superiority of CA over ADT is evaluated at relatively short term. Thus, whether these results would persist after the first year is unknown. The study aimed to be pragmatic; therefore, the cardiac rhythm monitoring during follow-up was performed with the current standard of care in our environment: 12-lead ECG at 1-, 3-, 6-, and 12-month follow-up and whenever patients reported symptoms, and 24-h Holter at 6 and 12 months. The purpose of the study was not to establish the absolute effectiveness of CA, but to compare it with ADT. Therefore, more intense monitoring could perhaps have identified a higher rate of recurrences; in any case, it would have equally affected both arms and would not have had a substantial impact on the difference between them. It is unlikely that sustained episodes (>24 h) remained undetected because the primary endpoint required episodes lasting at least 24 h. The primary endpoint was chosen by the steering committee as more appropriate in the context of patients with persistent AF than the recommended >30 s of AF, given the cardiac monitoring strategy applied during the follow-up. We recognize the novelty of the concept, but it was considered more robust given limited monitoring. This endpoint could lead to an overestimation of the positive results; however, we aimed to evaluate differences between groups instead of assessing the absolute efficacy of the treatment. Additionally, significant differences were found between treatments in the secondary endpoint (>30 s), validating to some extent the selection of the primary endpoint.
Conclusion
Catheter ablation is superior to medical therapy as a strategy for maintenance of sinus rhythm in patients with persistent AF at 12-month follow-up.
Funding
The study was supported by an unrestricted grant from Medtronic and Biosense Webster. F.B. was supported by a grant from Hospital Clínic (premi de Fi de Residència Emili Letang).
Conflict of interest: none declared.
Supplementary Material
Acknowledgements
We thank Neus Portella and Elaine Lilly, PhD, for editorial assistance and Dr Salvador Bergoñón for assistance with the coding and for quality control of the statistical analysis.
References
- 1.Jais P, Cauchemez B, Macle L, Daoud E, Khairy P, Subbiah R, Hocini M, Extramiana F, Sacher F, Bordachar P, Klein G, Weerasooriya R, Clementy J, Haissaguerre M. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008;118:2498–2505. doi: 10.1161/CIRCULATIONAHA.108.772582. doi:10.1161/CIRCULATIONAHA.108.772582. [DOI] [PubMed] [Google Scholar]
- 2.Pappone C, Augello G, Sala S, Gugliotta F, Vicedomini G, Gulletta S, Paglino G, Mazzone P, Sora N, Greiss I, Santagostino A, LiVolsi L, Pappone N, Radinovic A, Manguso F, Santinelli V. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF study. J Am Coll Cardiol. 2006;48:2340–2347. doi: 10.1016/j.jacc.2006.08.037. doi:10.1016/j.jacc.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 3.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P. 2012 Focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Eur Heart J. 2012;33:2719–2747. doi: 10.1093/eurheartj/ehs253. doi:10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 4.Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey JY, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann LS. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2011;57:e101. doi: 10.1016/j.jacc.2010.09.013. doi:10.1016/j.jacc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Ganesan AN, Shipp NJ, Brooks AG, Kuklik P, Lau DH, Lim HS, Sullivan T, Roberts-Thomson KC, Sanders P. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2:e004549. doi: 10.1161/JAHA.112.004549. doi:10.1161/JAHA.112.004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao TF, Tsao HM, Lin YJ, Tsai CF, Lin WS, Chang SL, Lo LW, Hu YF, Tuan TC, Suenari K, Li CH, Hartono B, Chang HY, Ambrose K, Wu TJ, Chen SA. Clinical outcome of catheter ablation in patients with nonparoxysmal atrial fibrillation: results of 3-year follow-up. Circ Arrhythm Electrophysiol. 2012;5:514–520. doi: 10.1161/CIRCEP.111.968032. doi:10.1161/CIRCEP.111.968032. [DOI] [PubMed] [Google Scholar]
- 7.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012;14:528–606. doi: 10.1093/europace/eus027. doi:10.1093/europace/eus027. [DOI] [PubMed] [Google Scholar]
- 8.Arribas F, Ormaetxe JM, Peinado R, Perulero N, Ramirez P, Badia X. Validation of the AF-QoL, a disease-specific quality of life questionnaire for patients with atrial fibrillation. Europace. 2010;12:364–370. doi: 10.1093/europace/eup421. doi:10.1093/europace/eup421. [DOI] [PubMed] [Google Scholar]
- 9.Calkins H, Reynolds MR, Spector P, Sondhi M, Xu Y, Martin A, Williams CJ, Sledge I. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol. 2009;2:349–361. doi: 10.1161/CIRCEP.108.824789. doi:10.1161/CIRCEP.108.824789. [DOI] [PubMed] [Google Scholar]
- 10.Wazni OM, Marrouche NF, Martin DO, Verma A, Bhargava M, Saliba W, Bash D, Schweikert R, Brachmann J, Gunther J, Gutleben K, Pisano E, Potenza D, Fanelli R, Raviele A, Themistoclakis S, Rossillo A, Bonso A, Natale A. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation—a randomized trial. JAMA. 2005;293:2634–2640. doi: 10.1001/jama.293.21.2634. doi:10.1001/jama.293.21.2634. [DOI] [PubMed] [Google Scholar]
- 11.Cosedis Nielsen J, Johannessen A, Raatikainen P, Hindricks G, Walfridsson H, Kongstad O, Pehrson S, Englund A, Hartikainen J, Mortensen LS, Hansen PS. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367:1587–1595. doi: 10.1056/NEJMoa1113566. doi:10.1056/NEJMoa1113566. [DOI] [PubMed] [Google Scholar]
- 12.Wokhlu A, Monahan KH, Hodge DO, Asirvatham SJ, Friedman PA, Munger TM, Bradley DJ, Bluhm CM, Haroldson JM, Packer DL. Long-term quality of life after ablation of atrial fibrillation the impact of recurrence, symptom relief, and placebo effect. J Am Coll Cardiol. 2010;55:2308–2316. doi: 10.1016/j.jacc.2010.01.040. doi:10.1016/j.jacc.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 13.Oral H, Pappone C, Chugh A, Good E, Bogun F, Pelosi F, Jr, Bates ER, Lehmann MH, Vicedomini G, Augello G, Agricola E, Sala S, Santinelli V, Morady F. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006;354:934–941. doi: 10.1056/NEJMoa050955. doi:10.1056/NEJMoa050955. [DOI] [PubMed] [Google Scholar]
- 14.Pappone C, Rosanio S, Augello G, Gallus G, Vicedomini G, Mazzone P, Gulletta S, Gugliotta F, Pappone A, Santinelli V, Tortoriello V, Sala S, Zangrillo A, Crescenzi G, Benussi S, Alfieri O. Mortality, morbidity, and quality of life after circumferential pulmonary vein ablation for atrial fibrillation: outcomes from a controlled nonrandomized long-term study. J Am Coll Cardiol. 2003;42:185–197. doi: 10.1016/s0735-1097(03)00577-1. doi:10.1016/S0735-1097(03)00577-1. [DOI] [PubMed] [Google Scholar]
- 15.Brooks AG, Stiles MK, Laborderie J, Lau DH, Kuklik P, Shipp NJ, Hsu LF, Sanders P. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm. 2010;7:835–846. doi: 10.1016/j.hrthm.2010.01.017. doi:10.1016/j.hrthm.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Stabile G, Bertaglia E, Senatore G, De Simone A, Zoppo F, Donnici G, Turco P, Pascotto P, Fazzari M, Vitale DF. Catheter ablation treatment in patients with drug-refractory atrial fibrillation: a prospective, multi-centre, randomized, controlled study (catheter ablation for the cure of atrial fibrillation study) Eur Heart J. 2006;27:216–221. doi: 10.1093/eurheartj/ehi583. doi:10.1093/eurheartj/ehi583. [DOI] [PubMed] [Google Scholar]
- 17.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, Skanes A, Ambrogi F, Biganzoli E. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32–38. doi: 10.1161/CIRCEP.109.859116. doi:10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 18.Dixit S, Marchlinski FE, Lin D, Callans DJ, Bala R, Riley MP, Garcia FC, Hutchinson MD, Ratcliffe SJ, Cooper JM, Verdino RJ, Patel VV, Zado ES, Cash NR, Killian T, Tomson TT, Gerstenfeld EP. Randomized ablation strategies for the treatment of persistent atrial fibrillation: RASTA study. Circ Arrhythm Electrophysiol. 2012;5:287–294. doi: 10.1161/CIRCEP.111.966226. doi:10.1161/CIRCEP.111.966226. [DOI] [PubMed] [Google Scholar]
- 19.Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, Khunnawat C, Ngarmukos T. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044–2053. doi: 10.1016/j.jacc.2003.12.054. doi:10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 20.Haissaguerre M, Hocini M, Sanders P, Sacher F, Rotter M, Takahashi Y, Rostock T, Hsu LF, Bordachar P, Reuter S, Roudaut R, Clementy J, Jais P. Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J Cardiovasc Electrophysiol. 2005;16:1138–1147. doi: 10.1111/j.1540-8167.2005.00308.x. doi:10.1111/j.1540-8167.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- 21.Themistoclakis S, Schweikert RA, Saliba WI, Bonso A, Rossillo A, Bader G, Wazni O, Burkhardt DJ, Raviele A, Natale A. Clinical predictors and relationship between early and late atrial tachyarrhythmias after pulmonary vein antrum isolation. Heart Rhythm. 2008;5:679–685. doi: 10.1016/j.hrthm.2008.01.031. doi:10.1016/j.hrthm.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 22.Balk EM, Garlitski AC, Alsheikh-Ali AA, Terasawa T, Chung M, Ip S. Predictors of atrial fibrillation recurrence after radiofrequency catheter ablation: a systematic review. J Cardiovasc Electrophysiol. 2010;21:1208–1216. doi: 10.1111/j.1540-8167.2010.01798.x. doi:10.1111/j.1540-8167.2010.01798.x. [DOI] [PubMed] [Google Scholar]
- 23.Berruezo A, Tamborero D, Mont L, Benito B, Tolosana JM, Sitges M, Vidal B, Arriagada G, Mendez F, Matiello M, Molina I, Brugada J. Pre-procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur Heart J. 2007;28:836–841. doi: 10.1093/eurheartj/ehm027. doi:10.1093/eurheartj/ehm027. [DOI] [PubMed] [Google Scholar]
- 24.Bisbal F, Guiu E, Calvo N, Marin D, Berruezo A, Arbelo E, Ortiz-Pérez J, de Caralt TM, Tolosana JM, Borràs R, Sitges M, Brugada J, Mont L. Left atrial sphericity: a new method to assess atrial remodeling. Impact on the outcome of atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2013;24:752–759. doi: 10.1111/jce.12116. doi:10.1111/jce.12116. [DOI] [PubMed] [Google Scholar]
- 25.Arbelo E, Brugada J, Hindricks G, Maggioni A, Tavazzi L, Vardas P, Anselme F, Inama G, Jais P, Kalarus Z, Kautzner J, Lewalter T, Mairesse G, Perez-Villacastin J, Riahi S, Taborsky M, Theodorakis G, Trines S. ESC-eurobservational research programme: the atrial fibrillation ablation pilot study, conducted by the European Heart Rhythm Association. Europace. 2012;14:1094–1103. doi: 10.1093/europace/eus153. doi:10.1093/europace/eus153. [DOI] [PubMed] [Google Scholar]
- 26.Reynolds MR, Walczak J, White SA, Cohen DJ, Wilber DJ. Improvements in symptoms and quality of life in patients with paroxysmal atrial fibrillation treated with radiofrequency catheter ablation versus antiarrhythmic drugs. Circ Cardiovasc Qual Outcomes. 2010;3:615–623. doi: 10.1161/CIRCOUTCOMES.110.957563. doi:10.1161/CIRCOUTCOMES.110.957563. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.