Abstract
Elegant tools are available for the genetic analysis of neural stem cell lineages in Drosophila, but a methodology for purifying stem cells and their differentiated progeny for transcriptome analysis is currently missing. Previous attempts to overcome this problem either involved using RNA isolated from whole larval brain tissue or co-transcriptional in vivo mRNA tagging. As both methods have limited cell type specificity, we developed a protocol for the isolation of Drosophila neural stem cells (neuroblasts, NBs) and their differentiated sibling cells by FACS. We dissected larval brains from fly strains expressing GFP under the control of a NB lineage-specific GAL4 line. Upon dissociation, we made use of differences in GFP intensity and cell size to separate NBs and neurons. The resulting cell populations are over 98% pure and can readily be used for live imaging or gene expression analysis. Our method is optimized for neural stem cells, but it can also be applied to other Drosophila cell types. Primary cell suspensions and sorted cell populations can be obtained within 1 d; material for deep-sequencing library preparation can be obtained within 4 d.
Keywords: Neural stem cells, FACS isolation, mRNA-sequencing, Drosophila
INTRODUCTION
Stem cells are the basis for the development of every multicellular organism and are involved in the maintenance of virtually all adult tissues. To carry out their function, stem cells have to remain in an undifferentiated state and maintain their ‘stemness’ properties over a series of cell divisions, while simultaneously generating developmentally restricted progeny. Drosophila larval neural progenitor cells (NBs) have proven to be an ideal model system1-3 to investigate how the balance between stem cell identity and differentiation is sustained, and how failures in restricting the proliferation potential of stem cells can lead to the formation of tumors.
NBs undergo repeated rounds of asymmetric cell division, the products of which are a larger cell that maintains stem cell identity and a smaller cell that will undergo neuronal differentiation. To date two different populations of NBs in the larval central brain and ventral nerve cord can be distinguished based on lineage and gene expression pattern4-6: ~ 4007,8 asymmetrically dividing type I NBs produce a self-renewing NB and a ganglion mother cell (GMC) that divides once more to generate two postmitotic neurons or glial cells. The 16 type II NBs per brain also divide asymmetrically, but give rise to an intermediate neuronal progenitor (INP) instead of a GMC. INPs undergo a series of maturation steps and then divide asymmetrically for a limited number of times generating GMCs and ultimately neurons or glial cells4-6.
Drosophila genetics allows for easy manipulation and investigation of larval NB lineages, but the complete set of factors controlling NB self-renewal and lineage progression is not known. Identification of genes specifically expressed in larval NBs and their various lineage intermediates would be a first step towards discovering these factors. Hence, various methodologies were employed to generate NB specific transcriptome data. For example, gene expression in wild-type brains was compared to brains from mutants affecting asymmetric cell division, which causes the formation of brain tumors highly enriched for NBs9. Indeed, this method identified a number of NB specific genes. However, the approach is problematic, as the mutant NBs have lost the ability to undergo differentiation and they divide in an uncontrolled manner; therefore, they resemble wild-type NBs only to some extend. In addition, both mutant and wild-type larval brains contain a heterogeneous mixture of cell types (NBs, embryonic and larval neurons and glial cells), masking the true expression pattern of genes expressed in NBs and neurons. A second technique, thio-uracil (TU)-tagging, utilizes specific labeling of newly synthesized mRNAs with 4-thio-uracil (4TU) by cell type specific expression of uracil phosphoribosyltransferase (UPRT), an enzyme needed in order to incorporate 4TU into mRNA10. TU-labeling of mRNAs allows their subsequent purification and analysis by deep sequencing. The drawback of this method is the equal inheritance of UPRT by both NB daughters, resulting in labeling of mRNAs also in the differentiating population.
Clearly, the simplest way to identify NB transcriptomes would be to isolate large numbers of these cells and their differentiating progeny, followed by analysis of gene expression levels. Different cell populations can be separated either by magnetic bead sorting11, or by FACS, see e.g.12-15. Typically, these approaches use the expression of a transgene, usually mCD8::GFP, under the control of a cell type specific promoter. Cells expressing the transgene are captured by magnetic beads or are FACS sorted on the basis of their fluorescent signal. NBs and neurons, however, can not be purified this way, because the fluorescent marker protein will be inherited by both NB daughter cells and therefore mark all cells within the NB lineage.
To circumvent these problems, we developed a protocol for FACS isolation of larval NBs and neurons that uses both differences in cell size and in levels of GFP expression to separate both cell types from the same larval brain16. Our protocol relies on a sufficient, yet gentle dissociation of larval brains into single cells. NBs in this cell suspension are alive and maintain lineage characteristics of wild-type larval NBs16 indicating that our method preserves the endogenous cell type specific transcription pattern well. We applied this method to determine the first complete expression profiles of NBs and neurons using 76-base paired-end Illumina Solexa sequencing16.
Overview
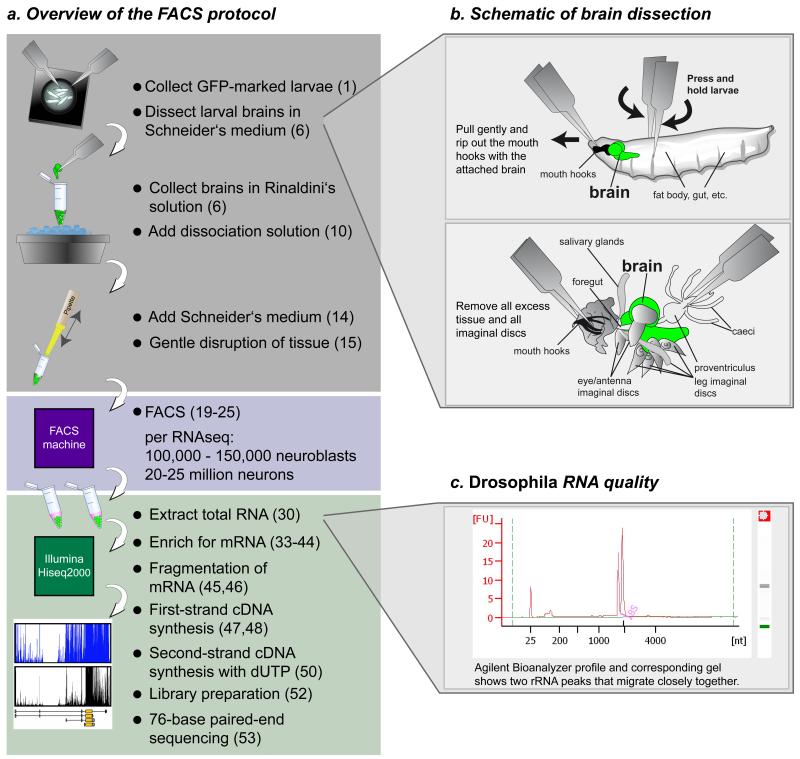
This protocol is designed to obtain pure populations of cells from Drosophila melanogaster for downstream applications such as live imaging, expression analysis or western blotting. The protocol is easy and straightforward (Fig. 1a), and allows the purification of pure cell populations within half a day. The first step is the dissection of larvae (Fig. 1b). The timing of this step depends on the dissection skills of the experimenter and should not exceed 60 min, because of risk of tissue degeneration. The brains are then enzymatically and mechanically disrupted, which takes another 60 minutes. After this 2-h procedure, the cells are subjected to FACS, which, depending on the number of dissected larval brains, takes up to 2 h (for 200 brains). We reproducibly sort around ~120 – 160 NBs and 18,000 – 25,000 neurons per larval brain. To o-tain high quality RNA sequencing data without the need for excessive amplification cycles, the experimenter needs to purify 100,000 – 150,000 NBs and ~20 – 25 million neurons. NBs are directly sorted into TRIzol LS to preserve RNA. Neurons are sorted in FACS flow in a 1.5 mL tube, and spun down immediately after the tube is filled. After removal of the FACS flow, TRIzol LS is added and up to five tubes of sorted neurons are combined. Several sorts (NBs and neurons) are collected and stored at −80 °C until further processing. Depending on the application and the cell numbers required, this may take several days. After TRIzol LS purification, RNA quality is measured on an Agilent Bioanalyzer (Fig. 1c). After enrichment for poly(A)+-RNA, the mRNA is fragmented and subjected to first-strand cDNA synthesis. During second-strand synthesis, dUTP is used to specifically label second-strand cDNA. Second-strand cDNA is digested during library preparation for Illumina Solexa sequencing to preserve strand-specific transcript information. Preparation of double stranded cDNA takes 4 d. Libraries from NBs and neurons are sequenced by 76-base paired-end sequencing on a Hiseq2000 machine, adding another 11 d to the protocol. In total, at least 4 weeks have to be allocated to obtain transcriptome information for the cell type of interest, including setting up fly crosses for FACS.
Figure 1.
Schematic overview of the FACS purification protocol for transcriptome analysis of larval neuroblasts (NBs) and neurons, and dissection of larval brains. (a–c) Larvae of the appropriate developmental stage expressing GFP under the control of a neuroblast-specific Gal4 line are collected. Larval brains are dissected in Schneider’s medium (a,b), and collected in ice-cold Rinaldini’s solution. The tissue is enzymatically digested and gently disrupted by pipetting. The resulting cell suspension is subjected to FACS to separate NBs and neurons. The sorted cells are kept in TRIzol LS reagent to preserve the RNA. After RNA extraction, RNA quality is measured on an Agilent Bioanalyzer (c), the samples undergo enrichment for poly(A) + RNA, are fragmented, and then first- and second-strand cDNA syntheses are performed. Finally, after library preparation, samples are subjected to transcriptome analysis by paired-end mRNA sequencing. Corresponding PROCEDURE steps are indicated in parentheses. (b) For the dissection of larval brains, hold the larva with two forceps, one at the anterior end and one in the middle of the body (upper panel). Gently rip out the mouth hooks with the attached brain, imaginal discs, salivary glands and gut (lower panel). Remove all excess tissue and transfer the isolated brain with forceps into the tube with Rinaldini’s solution on ice. (c) Assurance of quality and quantity of the extracted RNA using an Agilent Bioanalyzer with the Agilent RNA 6000 nano kit. A typical Drosophila RNA profile is shown with its corresponding gel. Note that 28S rRNA is not visible, as this rRNA breaks into two similar-sized fragments that migrate close to the 18S rRNA band27. It is important that only the two main rRNA bands are visible, with no background bands from degraded RNA.
Other applications of the protocol and future uses
A vast array of cell type–specific driver lines are available for Drosophila, and therefore many cell types can be unambiguously defined using additional parameters such as cell size or granularity. Steps that are most likely to require tissue and cell type–specific optimization include cell type-specific labeling and the dissociation protocol, although the latter can easily be optimized by modifying the amounts of enzyme and incubation times.
To demonstrate the broad applicability of our method, we used our protocol to purify Drosophila adult intestinal stem cells (ISCs; R.C., unpublished data). We used the ISC-specific escargot-Gal4 driver line to label these cells with GFP17,18. Initial experiments revealed that the concentration of enzymes had to be increased in order to dissociate midgut tissue. We were able to separate a specific population of cells with high GFP signal and could show that these are probably ISCs. Nevertheless, further optimization of the method for this application and quality control experiments are indispensible.
In addition, our protocol can be combined with other Drosophila methodologies and thereby applied to the study of a variety of different biological questions. For example, it can be used to determine NB transcriptomes at various developmental stages (e.g., embryonic or pupal NBs) or under specific conditions (e.g., starvation, hormones and drugs). In addition, FACS can be combined with RNA interference (RNAi) to determine transcriptional changes upon loss of specific genes. Furthermore, the TARGET system19 can be used to initiate GFP expression at specific time points to analyze transcriptional changes in a time-resolved manner. In combination with NB cell culture, for example, this might allow one to follow transcriptional changes in GMCs at various time points after mitosis. As the TARGET system can easily be combined with RNAi, the transcriptional changes occurring in NBs or their differentiating siblings upon inactivation of specific genes could be analyzed in a time-specific manner.
Limitations and problems of the method
FACS relies on single cells passing a detection unit and their subsequent sorting according to, in our case, GFP expression and cell size. The biggest limitation of our approach is the histology of the larval brain, as it contains only very few NBs that give rise to many neurons and glia cells. Therefore, even if we were able to bulk-isolate many larval brains (as is possible for imaginal discs20,21), FACS sorting of NBs would remain the rate-limiting step in our protocol. During FACS, the cells need to be cooled to 4 °C to keep NBs from dividing, which decreases their survival and probably affects gene expression profiles.
Sorting according to cell size and GFP expression levels will be problematic if specific labeling of the cell type of interest and its progeny is not possible. This can be either because the transgene is also expressed in an unwanted subset of cells with similar characteristics, e.g., granularity and size, or because the stem cell and its progeny do not differ in any of the aforementioned features.
Complete dissociation of tissue is a prerequisite condition for FACS. Sorting of pure cell populations will not be possible if the tissue of interest cannot be properly dissociated. In general, enzymatic digestion, mechanical stress, different temperatures and removing the cells from their cellular context could potentially affect gene expression, signaling (e.g., Notch signaling) and cell survival. However, in our case, we could show that NBs maintain their lineage characteristics after dissociation and FACS16.
Experimental design
Our protocol relies on sorting of GFP-labeled cells that can be separated on the basis of their size and strength of GFP expression. Exclusive labeling of stem cells using transgenic reporters, such as Gal4-driven GFP, is often not possible owning to the combination of a lack of driver lines that are exclusively expressed in the stem cells, and the perdurance of GFP (and thus its inheritance into the more differentiated daughter cells).
Labeling of a cell population
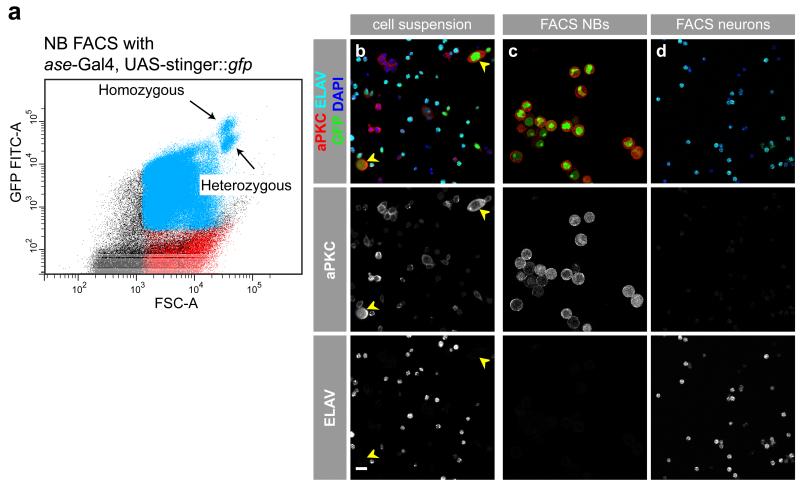
The larval brain is mostly made up of small neurons, medium-sized GMCs, INPs and NBs in the optic lobes, and of large NBs and glia cells. In addition to differences in cell size, subtypes of cells can be labeled with a fluorescent protein, for example, GFP. For this, either the UAS/Gal4 system in combination with a cell type-specific driver line or a line expressing GFP under the control of a cell type-specific promoter can be used. We decided to use the asense (ase)-Gal4 (ref. 22) line that is only expressed in type I but not in type II NB lineages or glia cells. We nevertheless checked for the presence of glia cells, but could not detect any Repo-positive cells after FACS16. We were also able to sort type II NBs, of which there are only 16 per brain, using the type II-specific driver lines worniu-Gal4, ase-Gal80 (ref. 23) or Pointed-Gal4 (ref. 24; data not shown). Therefore, in principle, more cell type-restricted Gal4 lines could be used to allow comparisons between different NB subpopulations. For GFP labeling of cells, we chose a strong nuclear GFP, called Stinger::GFP (stable insulated nuclear eGFP vectors25). This results in stronger resolution of the GFP signal, which gets weaker in differentiating daughter cells owing to reduced driver line expression and GFP degradation. We have also tested membrane-bound GFP (mCD8::GFP) to label cells. Compared with Stinger::GFP, this results in a much weaker GFP signal and less well-defined cell populations in the FACS plot. Even though the mCD8::GFP marker can be used to isolate highly enriched NB and neuron populations, we recommend Stinger::GFP for the initial establishment of the protocol. When Stinger::GFP is used, our method is sensitive enough to even distinguish homozygous from heterozygous GAL4/GFP-positive NBs, as shown in the FACS plot of Figure 2a.
Figure 2.
Pure populations of NBs and neurons can be separated by FACS. (a) By using a Gal4 fly strain harboring both heterozygous and homozygous Gal4 and UAS-Stinger::gfp, we were able to separate two NB populations when plotting the strength of the GFP signal (GFP FITC-A) to the size of the cells (FSC-A). Both populations are of equal cell size but are different in the strength of the GFP signal, as heterozygous insertions result in weaker GFP signals than homozygous insertions. (b-d) Confocal images of representative cell cultures before and after sorting by FACS. (b) Images of a primary (i.e., unsorted) cell suspension stained with the NBs marker aPKC in red, the neuronal marker ELAV in cyan, the DNA marker DAPI in blue and the Stinger::GFP driven by asense-Gal4 in green. Large NBs in a primary cell culture are marked with yellow arrowheads and show aPKC expression (b, middle) but no ELAV (b, bottom) expression. (c) Sorted NBs are positive for aPKC (c, middle) but not for ELAV (c, bottom). (d) Sorted neurons are negative for the NB marker aPKC (d, middle) but positive for the neuronal marker ELAV (d, bottom). Scale bar, 10 μm.
Material collection
Once transgenic lines are established, and the specificity of fluorescent protein expression in the cell types of choice is confirmed, biological material can be collected. For our protocol, we collect crawling third-instar larvae, 5 d after a 24-h period of egg laying, from two or three bottles. Larvae are washed in PBS and 70% (vol/vol) ethanol immediately after collection to remove residual food. Larval brains are dissected (Fig. 1b) for a maximum of 1 h and kept in Rinaldini’s solution on ice. Dissecting for longer times can decrease the number of viable NBs in the culture.
Dissociation
It is crucial to the success of the FACS sorting that tissues are sufficiently dissociated into single cells, but that the cells are not harmed by overly harsh or long treatment. Dissociation of larval brains with a combination of papain and collagenase I for 1 h at 30 °C has proven to be a good balance between sufficient dissociation, yield and survival of cells during FACS. For other applications, such as live imaging, a shorter incubation time of only 30 min might be sufficient, as the remaining neuron clusters are not problematic for imaging dividing NBs. Therefore, for different applications and tissues, incubation times and combinations of enzymes may need to be optimized.
Amount of material
The required amount of material to be collected depends on the downstream application of choice. For cell staining or live imaging, ~7,000 NBs will suffice, and this number can be obtained from 50 larval brains. In any case, we strongly recommend that live imaging and staining of sorted cells be used to evaluate the identity and viability of cells. To obtain enough cells for RNA extraction and subsequent mRNA sequencing, we routinely dissect ~150 larvae (in 1 h) per FACS round and conduct two consecutive sorts per day. We combine up to six FACS experiments to provide 300–500 ng of total RNA, which yields ~10 ng of double-stranded cDNA, from which libraries are prepared for deep sequencing. In such a large-scale preparation, the time devoted to the experiment and access time to a FACS machine can become the limiting parameters. For each sorting, 1 h of dissection time, 1 h of dissociation time and 2 h of FACS-sorting time need to be allocated. Therefore, sorting 36,000–48,000 type I NBs per day (from two experiments with ~300 larval brains) is conveniently possible using our methodology. Neurons, however, are highly abundant and can be sorted in essentially unlimited quantities (tens of millions).
MATERIALS
REAGENTS
D. melanogaster larvae, 5 d old, reared at 25 °C, e.g., asense-Gal4 (ref. 22)
PBS buffer (see REAGENT SETUP)
Ethanol (Roth, cat. no. P076)
Rinaldini’s solution (see REAGENT SETUP)
NaCl (Sigma-Aldrich, cat. no. S3014)
KCl (Sigma-Aldrich, cat. no. P9541)
NaH2PO4 (Sigma-Aldrich, cat. no. S9638)
Na2HPO4 (Sigma-Aldrich, cat. no. S3264)
NaHCO3 (Sigma-Aldrich, cat. no. S5761)
Glucose (Sigma-Aldrich, cat. no. G0350500)
Schneider’s culture medium (Gibco, cat. no. 21720024)
Penicillin-Streptomycin solution (Sigma-Aldrich, cat. no. P4458)
Insulin solution from bovine pancreas (Sigma-Aldrich, cat. no. I0516)
L-Glutamine (Sigma-Aldrich, cat. no. G7513)
L-Glutathione (Sigma-Aldrich, cat. no. G6013)
FBS, heat inactivated (Sigma-Aldrich, cat. no. F4135)
Collagenase Type I (Sigma-Aldrich, cat. no. C2674)
Papain (Sigma-Aldrich, cat. no. P4762)
FACSFlow sheath fluid (BD Bioscience, cat. no. 342003) Poly-D-lysin-hydobromide (Sigma-Aldrich, cat. no. P6282)
TRIzol LS reagent (Life technologies, cat. no. 10296-028)
!CAUTION TRIzol is highly toxic and should only be used in the fume hood. Gloves and lab coat should be worn when working with TRIzol.
Chloroform/Trichlormethan (Roth, cat. no. 3313.4)
Isopropanol (Roth, cat. no. CP41)
RNase free water (Life technologies, cat. no. AM9932)
Diethylpyrocarbonate (DEPC) treated water (Life technologies, cat. no. 750023) Glycoblue (Life technologies, cat. no. AM9515)
Agilent RNA 6000 Nano Kit (Agilent Technologies, cat. no. 5067-1511)
Agilent RNA 6000 Pico Kit (Agilent Technologies, cat. no. 5067-1513)
Dynabeads mRNA purification Kit (Life technologies, cat. no. 61006)
Fragmentation buffer 5× (see REAGENT SETUP)
Tris base, adjusted to pH 8.2 (Sigma-Aldrich, cat. no. T1503)
Potassium acetate (Sigma-Aldrich, cat. no. P1190)
Magensium acetate (Sigma-Aldrich, cat. no. M5661)
Sodium Acetate 3 M, pH 5.5 (Life technologies, cat. no. AM9740)
Random hexamer primers 3 μg/μL (Life technologies, cat. no. 48190-011)
dNTP Mix, 10 mM (Life technologies, cat. no. 18427-013)
SuperScript® II Reverse Transcriptase (Life technologies, cat. no. 18064-014)
SuperScript® III Reverse Transcriptase, in a set containing 5× first-strand buffer and 0.1 M DTT (Life technologies, cat. no. 18080-044)
iQ SYBR Green Supermix (Bio-Rad, cat. no. 170-8882)
RNaseOUT™ (Life technologies, cat. no. 10777-019)
Second-Strand Buffer (Life technologies, cat. no. 10812-014)
dUTP (Fermentas, cat. no. R0133)
dATP (Fermentas, cat. no. R0141)
dCTP (Fermentas, cat. no. R0151)
dGTP (Fermentas, cat. no. R0161)
DNA Polymerase I (Escherichia coli) (Life technologies, cat. no. 18010-017)
Ribonuclease H (Life technologies, cat. no. 18021-014)
E. coli DNA Ligase (Life technologies, cat. no. 18052-019)
MinElute Reaction Cleanup Kit (QIAGEN, cat. no. 28204)
Quant-iT Picogreen dsDNA Assay Kit (Life technologies, cat. no. P7589)
NEBNext DNA sample Prep Reagent kits (New England Biolabs, cat. no. E6040)
EQUIPMENT
Foreceps Dumont Nr.5 (Fine Science Tools, cat. no. 11252-23)
Stereomicrosope SteREO Discovery.V8 (Zeiss)
Glass-bottom dishes, 35mm (MatTek Corporation, cat. no. P35G-0-14-C)
Steriflip (Millipore, cat. no. SCGP00525)
Thermo mixer compact (Eppendorf)
Cell strainer, 35 μm (BD Bioscience Falcon, cat. no. 352235)
FACSAriaIII, cell sorter (BD Bioscience)
Low-binding reagent tubes, 1.5 mL (Life technologies, cat. no. AM12450)
Phase-lock heavy gel tubes, 2 mL (5 PRIME, cat. no. 2302830)
Agilent 2100 Bioanalyzer (Agilent Technologies)
Magnetic rack (for example DynaMag™-2, Life technologies, cat. no. 12321D)
NanoDrop 3300 fluorospectrophotometer (Thermo Scientific)
Genome Analyzer IIX (GAIIX; Illumina)
HiSeq 2000 sequence analyzer (Illumina)
REAGENT SETUP
PBS (1×) To prepare 1× PBS dilute a 20× stock solution in distilled H2O and adjust the pH to 7.4. For the 20× stock solution, mix 151.94 g NaCl, 19.88g Na2HPO4 and 8.28g NaH2PO4 in 1 liter distilled H2O.
Rinaldini’s solution To prepare 1× Rinaldini’s solution mix 800 mg NaCl, 20 mg KCl, 5 mg NaH2PO4, 100 mg NaHCO3, 100 mg Glucose in 100 ml distilled H2O and filter sterilize using the Millipore Steriflip. Rinaldini’s solution can be prepared as a 10× solution and stored at 4 °C for at least 3 months.
Complete Schneider’s culture medium To prepare the culture medium mix 5 mL heat inactivated fetal bovine serum, 0.1 mL Insulin, 1 mL PenStrep, 5 mL L-Glutamine, 0.4 mL L-Glutathione and 37.85 mL Schneider’s medium. Filter-sterilize the medium using the Millipore Steriflip. This solution can be stored at 4 °C for 1 week for FACS, and for 3 d for live imaging.
CRITICAL We realized that individual lots of Schneider’s Medium differ in quality. Some batches can cause problems for the cells and batches need to be tested individually.
Enzyme solutions To prepare collagenase I and papain solutions, dissolve enzymes at a final concentration of 20 mg/mL in distilled H2O. Prepare single-use aliquots and store them at −20 °C for at least 2 months.
CRITICAL Avoid repeated freeze/thaw cycles, allow a maximum of one freeze/thaw cycle.
Dissociation solution For this buffer combine 450 μL of complete Schneider’s culture medium with 25 μL of collagenase I and 25 μL of papain.
CRITICAL This solution can not be stored and always has to be freshly prepared.
Fragmentation buffer (5×) Combine Tris (pH 8.2) at a final concentration of 200 mM, KOAc at 500 mM and MgOAc at 150 mM in DEPC treated water, and filter-sterilize the buffer using the Millipore Steriflip. Fragmentation buffer can be stored at room temperature for at least 1 year.
EQUIPMENT SETUP
BD AriaIII cell sorter
We used an AriaIII cell sorter equipped with a Coherent Sapphire Solid State 13mW laser, but in principle any sorter equipped with a 488 nm laser can be used. Sample station and collection module should be cooled to 4 °C during the entire duration of the sort. To ensure survival of cells, a 100 μm nozzle tip at a sheath pressure of 20 p.s.i. is recommended.
Coating of glass-bottom dishes
Glass-bottom dishes are washed once with freshly prepared 70% (vol/vol) ethanol and left to dry in a laminar flow hood. Poly-D-lysine at a final concentration of 0.01 % is added to the dishes, and then the dishes are left in the laminar flow hood for 30 min. After removal of the poly-D-lysine, the dishes are washed twice with distilled water and dried under UV-light.
PROCEDURE
Dissociation of larval brains Timing 2 h 15 min
1 Collect larvae of appropriate developmental time and genotype into a glass dish in 1 mL of PBS.
2 Wash the larvae once in 1 mL of PBS.
3 Wash the larvae in 1 mL 70% (vol/vol) of ethanol for 1 minute to remove all remaining food.
4 Remove ethanol and add Schneider’s culture medium.
5 Fill a fresh 1.5 ml low binding reagent tube with cold Rinaldini’s solution.
6 Dissect larval brains with forceps under the stereomicroscope and directly transfer each brain into the tube with Rinaldini’s solution (Fig. 1b).
CRITICAL Do not dissect for more than 1 h total to ensure tissue integrity of the dissected brains.
7 Prepare the dissociation solution.
8 After all the brains have sunken to the bottom of the tube, carefully remove the Rinalidini’s solution and wash the brains once by adding 500 μL of Rinaldini’s solution. For washing, take up 200 μL of liquid and expel it with force, causing the brains to stir up.
CRITICAL When you are washing and mixing, avoid sucking up brains into the pipette tip as they will stick to the plastic, thereby causing loss of material.
CRITICAL Visually check wheter all the brains have sunken to the bottom. Do not let the brains dry out while removing the solution - leave a small amount to cover them.
9 Remove the Rinaldindi’s solution and all material that was transferred into the tube during dissection and does not sink to the bottom of the tube (for example the remaining salivary glands).
10 Add the dissociation solution to the brains and stir the brains up by pipetting in order to allow for complete mixing, again avoiding taking up brains into the pipette tip. Leave the tube for 1 h at 30 °C in a Thermomixer. Mix the content twice gently during incubation by taking up 200 μL of dissociation buffer and expelling it with force.
11 Remove the dissociation solution slowly without disrupting the brains at the bottom of the tube.
12 Wash the brain samples carefully twice with 500 μL Rinaldini’s solution.
CRITICAL In all washing steps, add and remove the solution very slowly, so that no brains are stirred up. If brains were stirred up, let them settle down at the bottom of the tube on ice before continuing the washing step.
13 Wash the samples carefully twice with 500 μL Schneider’s culture medium.
14 After removing the medium from step 13, add 200 μL of fresh Schneider’s culture medium.
15 Disrupt the tissue by using a 200 μL pipette tip and pipette the brains and solution up and down, if possible without foaming, 10-20 times or until the solution appears homogenous.
? TROUBLESHOOTING
16 Filter the cell suspension through a 30 μm mesh 5 mL FACS tube.
17 Depending on the number of dissociated brains, dilute the cell suspension with more Schneider’s medium. We typically add Schneider’s medium until the final amount adds up to ~10 μL of medium per dissected larval brain.
18 Different downstream applications can be chosen at this point. For immunohistochemistry, place primary (i.e., unsorted) cell suspension onto a coated (poly-D-lysine) dish and proceed with standard staining protocols26. A typical primary cell suspension stained for the NB marker aPKC and the neuronal marker ELAV is shown in Fig. 2b-d.
Sorting of NBs and neurons by FACS Timing maximum up to 2 h
CRITICAL When you are proceeding with FACS, place the cell solution on ice and start sorting within the next 5-10 min.
CRITICAL The amount of medium in which the cells are suspended determines how fast the cells can be sorted. A solution that is too diluted can be sorted with maximum speed (flow rate of 5 on AriaIII), but the event rate might be low, thereby prolonging the sorting time and decreasing the survival rate of the cells. If the solution is too concentrated, the event rate can be high even at low sorting speeds, thus decreasing the sorting efficiency and leading to a lower yield of cells.
19 Before sorting, resuspend the cells by flicking the tube several times before placing it into the refrigerated sample station.
CRITICAL Turn on sample agitation at 200-300 r.p.m. to ensure a homogeneous suspension of cells and to avoid sinking of cells to the bottom of the FACS tube.
20 Run the cell sample in a FACS machine. Record at least 100,000 events to gain initial information on all cell populations present.
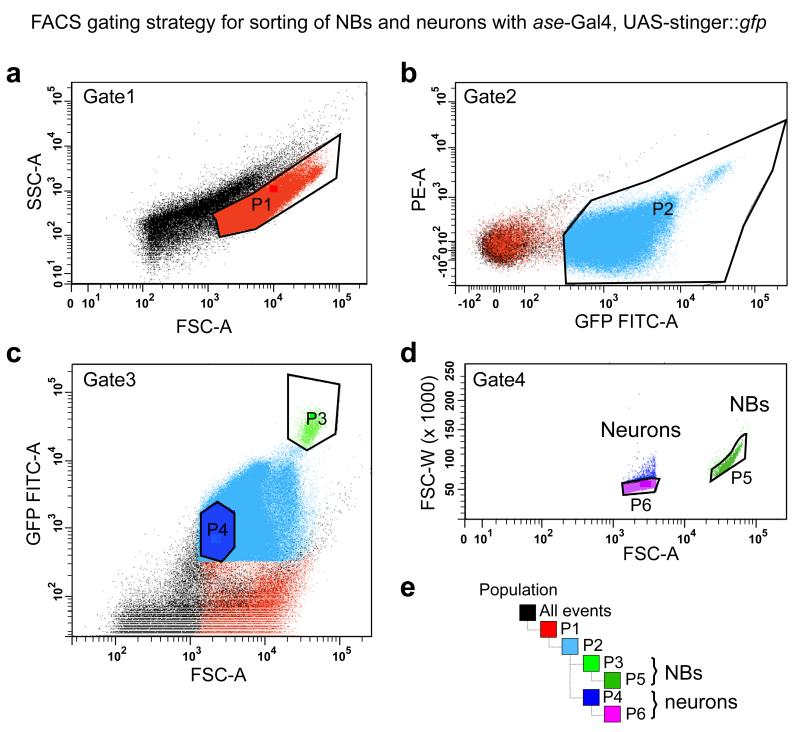
CRITICAL Depending on your tissue composition and cell size, axis scaling and the number of sorted events have to be adjusted. NBs are large cells and very rare compared with other neural cell types; therefore, we use logarithmic scaling and record a large number of events to be able to see a defined NB population (Fig. 3).
Figure 3.
Representative flow cytometry data to illustrate the gating strategy for FACS purification of larval NBs and neurons. (a) To discriminate between healthy and damaged or dying cells, side scatter (SSC-A, log) and forward scatter (FSC-A, log) are plotted. Higher SSC-A signals indicate increased granularity and therefore likely dying cells. Thus, one should define the sorting gate as the population with the lower SSC-A signal (P1). (b) To gate for GFP-positive cells, plot the signal from the excitation of cells with the 488-nm laser (GFP FITC-A, log) against the signal from the PE laser (PE-A, log). The population with the higher GFP FITC-A signal is chosen for sorting (P2). (c) Plotting the GFP signal against FSC-A reveals a population of bright and large cells (NBs, P3), and smaller cells with a weaker signal (neurons, P4). (d) Exclude cell clusters by sorting only events with a low FSC width (FSC-W) versus area (FSC-A) signal (P5, NBs; P6, neurons). (e) Gating hierarchy.
21 Plot side scatter (SSC, log) and forward scatter (FSC, log). Two populations of the same size with different SSC signals should appear. The population with the higher SSC signal probably contains damaged or dying cells; therefore, the sorting gate is defined as the population with the lower SSC signal (Fig. 3a).
22 Plotting the signal from the excitation of cells with the 488-nm laser [GFP FITC-A (area), log] and the phycoerythrin laser (PE-A, log) should separate auto-fluorescent (low GFP FITC-A signal) from GFP (high GFP FITC-A signal) expressing cells (Fig. 3b).
23 A separate population of cells should be visible by plotting FSC-A versus GFP FITC-A. NBs are large and strongly GFP positive cells (Fig. 3c). Neurons are small and dimmer, and because of to the much larger numbers of neurons per brain this population is easier to determine when less events are plotted. Thus, for a better resolution of the neuron population we recommend to plot only 10 % of the total recorded events.
24 Cell clusters are excluded by plotting the area of the FSC signal versus its width (FSC-W, log). Events with a stronger FSC-W signal are excluded. Owing to the large size of NBs, the linearity of the scale is not maintained and the population appears bent (Fig. 3d).
25 Sort the cells with an event rate of a maximum of 6,(000 events per second, which ensures good sort efficiency (>80 %).
26 (Optional) Place a subset of the sorted NBs and neurons onto coated glass-bottom dishes to assess the identity and purity of the sorted cells by immunofluorescence. Typical cell cultures with sorted NBs and sorted neurons stained with the NB marker aPKC and the neuronal marker ELAV are shown in Fig. 2b-d. Owing to the high reproducibility between sortings and the low numbers of NBs obtained, this step was not performed in every experiment. Initially the populations of interest were gated and quality control experiments like expression analysis of known specific markers by quantitative PCR (qPCR) and immunohistochemistry were performed, to ensure that the sorted populations are reproducibly pure 16.
? TROUBLESHOOTING
27 Sort NBs into cooled 1.5 mL RNase-free tubes containing 750 μL of TRIzol LS. Sort the neurons into empty 1.5 mL tubes, with 400,000 events per tube.
CRITICAL At low-pressure setting, the volume of one drop containing one event is 3 nL. Therefore, a maximum number of 83,000 cells can be added to 750 μL of TRIzol, and a maximum of 500,000 events can be sorted in an empty 1.5 mL tube. We recommend, however, not filling up the tube to its full capacity to ensure easy handling in the following steps.
28 Centrifuge the tubes containing neurons in FACS flow at 0.9×g for 15 min at 4 °C directly after each tube is filled. Remove all but ~50 μL of the FACS flow and add TRIzol LS. Up to five tubes filled with neurons can be combined in 750 μL TRIzol LS.
29 Fill up the tube containing NBs in TRIzol LS up to 1 mL with DEPC treated water.
PAUSE POINT Cells in TRIzol LS can be stored at −80 °C for several months or until enough material is collected for the desired downstream application.
Generation of double-stranded cDNA for Solexa sequencing library preparation
Timing 4d
30 Isolate total RNA from TRIzol according to the manufacturer’s instructions, adjusted for a small amount of RNA. Use 2-ml phase-lock heavy gel tubes to ensure less genomic DNA carryover when removing the aqueous phase. Dissolve the RNA in 5 μl of DEPC-treated water in an RNase-free low-binding tube.
PAUSE POINT Extracted RNA can be stored at −80 °C for several weeks.
31 Combine up to five sortings, or add DEPC treated water to a total of 25 μL.
32 Asses the quality and quantity of the extracted RNA on an Agilent Bioanalyzer using the Agilent RNA 6000 Nano Kit. Note that the machine cannot assign an RNA integrity number, as Drosophila 28S ribosomal RNA breaks into two similar-sized fragments that migrate close to the 18S rRNA band27. Visually ensure that only these two main rRNA bands are visible with early no background bands from degraded RNA. (Fig. 1c)
? TROUBLESHOOTING
33 Heat ~300-500 ng of total RNA from step 31 to 65 °C to disrupt secondary structures; place the tubes on ice.
34 To enrich for mRNA, aliquot 50 μL of well-suspended Dynabeads oligo(dT)25 into a fresh RNase-free, low-binding tube.
35 Wash beads twice with 50 μL binding buffer. After each washing, place the tube containing the magnetic beads onto the appropriate magnetic rack until all beads have migrated to the wall of the tube and the supernatant can be removed.
36 Resuspend the beads in 25 μL of binding buffer, add the 25 μL of total RNA from step 33 and rotate the tube for 5 min at room temperature. All of the mRNA will be bound to the oligo(dT)-coated beads.
CRITICAL Owing to the small volume and high surface tension, flick the tube with your finger once every minute to make sure that the solution is mixed well.
37 Remove the supernatant and wash the beads twice with 50 μL washing buffer B (included in the Dynabeads purification kit).
38 Remove the washing buffer B and elute the mRNA by adding 10 μL 10 mM Tris-HCl and heating to 80 °C for 2 min.
39 Separate the beads with the magnetic rack and transfer the supernatant containing the poly(A)+-enriched RNA into a fresh RNase-free, low-binding tube containing 40 μL of binding buffer; heat to 65 °C for 5 min, and then place it on ice. Keep the first tubes with the used beads for a second round of purification.
40 Wash the used beads twice with 50 μL of washing buffer B.
41 Add the poly(A)+-enriched RNA solution from step 39 (50 μl) to the beads from step 40 and incubate the mixture again at room temperature for 5 min while rotating the tube.
42 Remove the supernatant and wash the beads twice with 50 μL of washing buffer B.
43 Remove the washing buffer B and elute the mRNA from the beads by adding 10 μL 10 mM Tris-HCl and incubating it at 80 °C for 2 min.
44 Separate the beads with the magnetic rack and transfer the supernatant into a fresh RNase-free, low-binding tube.
CRITICAL Check the depletion of rRNA from the poly(A)+-enriched RNA by qPCR with rRNA-specific primer pairs.
? TROUBLESHOOTING
BOX 1. VERIFICATION OF rRNA DEPLETION BY qPCR.
Timing 5h
1. Take a 1/10 volume of the poly(A) + -enriched RNA, as well as an untreated total RNA sample, and generate first-strand cDNA using SuperScript III with random hexamer primers according to the manufacturer’s instructions.
2. Dilute the cDNA at least tenfold so as not to inhibit the subsequent qPCR reaction.
3. Set up the qPCR reaction using SYBR green according to the manufacturer’s instructions with the following Drosophila rRNA primers and actin 5C primers as a housekeeping gene control.
| rRNA | Forward primer | Reverse primer |
|---|---|---|
| 18S rRNA | TAGACCGAGAGGTCCGGGTA | CAAAGGGCAGGGACGTAATCAA |
| 5.8S rRNA | CGATGAAGAACGCAGCAAACTG | CATGGACTGCGATATGCGTTCA |
| 28S rRNA left | CCTGATGGAAGACCGAAACAGT | CTGAGGGAAACTTCGGAAGGAA |
| 28S rRNA right | GAGTTTGACTGGGGCGGTAC | GCTTTTGCTGTCCCTGTGTGTA |
| 5S rRNA | ACGACCATACCACGCTGAATAC | GCGGTCCCCCATCTAAGTAC |
| Actin 5C | CCCCATTGAGCACGGTATCG | CACGCAGCTCATTGTAGAAGGT |
-
4. We routinely use the following one-step PCR protocol:
1 × 95 °C 3 min
40 × 95 °C 10 s
62 °C 1 min (plate read)
At the end of this PCR protocol, we add the following steps to generate a melting curve, which allows us to evaluate the quality of the PCR products, as primer dimers and other PCR artifacts have a lower melting temperature:
1 × 95 °C 10 s
62 °C 1 min
62 °C, inclines of 0.5 °C up to 95 °C 5 s (plate read after each incline)
5. Normalize the data using actin 5C expression and calculate the fold change of rRNA depletion by comparing it with the rRNA expression in the untreated sample.
6. Expect at least an 80% reduction of rRNA concentration in the mRNA-enriched samples. With high sequencing depth, low levels of remaining rRNA will still allow for sufficient sequencing of large numbers of cDNA reads.
45 For mRNA fragmentation, add 10 μL of fragmentation buffer and 30 μL of DEPC-treated water to the purified mRNA from step 44. After incubation at 94 °C for exactly 2.5 min, precipitate RNA with 5 μL (1/10 volume) of 3 M sodium acetate, 125 μL (2.5 volume) of 100 % (vol/vol) ethanol and 1 μL of glycogen overnight at −20 °C.
46 Pellet the fragmented mRNA by centrifugation at maximum speed for 1 h at 4 °C and wash it once with 500 μl of ice-cold 80% (vol/vol) ethanol and once with 100% (vol/vol) ethanol. Air-dry the pellet for 10 min and dissolve it in 10 μl of DEPC-treated water. Analyze the fragment sizes on an Agilent Bioanalyzer using the Agilent RNA 6000 pico kit. The peak of fragment sizes should range from 200 to 300 nt..
CRITICAL Do not let the RNA air-dry for too long, because solubility decreases with increased drying time.
? TROUBLESHOOTING
47 For first-strand cDNA synthesis, add 1 μL of random primers, 1.5 μL of dNTPs and 3.5 μL of H2O; heat up the mixture to 65 °C for 5 min and cool it on ice for at least 1 min.
48 Add 6 μL of 5× first-strand buffer, 3 μL of 1M DTT, 1.5 μL of RNAseOUT, 1.5 μL of Superscript II enzyme and 4 μL of H2O; incubate the mixture for 5 min at 25 °C and for 50 min 42 °C, and then inactivate the enzyme at 70 °C for 15 min.
49 Precipitate the first-strand cDNA with 3 μL of NaCL (4 M) and 90 μL of 96 % (vol/vol) ethanol at −20 °C for at least 2 h or overnight. After centrifugation at maximum speed at 4 °C for 25 min, remove the ethanol and wash once with 80 % (vol/vol) ethanol. Air-dry the pellet and dissolve it in 54 μL of H2O.
PAUSE POINT First-strand cDNA samples can be stored at −80 °C for at least 1 year. 50 For second-strand cDNA synthesis, add 20 μL of 5× second-strand buffer, 1 μL of random primers, 0.2 μL of dATP, 0.2 μL of dCTP, 0.2 μL of dGTP, 0.2 μL of dUTP, 2.66 μL of DNA polymerase I, 0.66 μL E. coli DNA ligase and 0.66 μL RNase H to the first-strand cDNA; incubate the misture at 16 °C for 2 h.
CRITICAL To be able to obtain strand specificity, second-strand cDNA is labeled with dUTP instead of dTTP.
51 Purify the double-stranded cDNA using the MinElute reaction cleanup kit, and then quantify it using the Quant-iT Picogreen dsDNA assay in a NanoDrop 3300 fluorospectrophotometer.
Library preparation Timing 11 d
52 Prepare the library for deep sequencing using a protocol from Illumina and the NEBNext DNA sample prep reagent kit. Digest dUTP-containing second-strand cDNA after size selection of adapter-ligated libraries but before PCR amplification of the adapter-modified fragments.
53 Perform 76-base paired-end sequencing GAIIX or Hiseq2000 machines.
? TROUBLESHOOTING
Troubleshooting advice can be found in Table 1.
Table 1. Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 15 | Tissue pieces in cell suspension |
Insufficient enzymatic digestion |
Increase enzyme concentration or incubation time. Do not exceed 2 mg mL−1 of final concentration of enzyme, or 1.5 h of incubation |
| Prepare fresh enzyme stocks | |||
| Do not freeze and thaw enzymes more than twice |
|||
| Try different enzyme combination (e.g., trypsin or elastase) |
|||
| 15 | Cells die after dissociation |
Tissue is damaged during dissection procedure |
Reduce the period of dissection to maximum 45 min |
| Enzymatic treatment is damaging tissue |
Reduce time of enzymatic treatment (e.g. 30 min) |
||
| Reduce the concentration of enzymes, or use either papain or collagenase I only |
|||
| Reduce incubation temperature (i.e. incubate at room temperature) |
|||
| Mechanical disruption is damaging tissue |
Try to be as gentle as possible (avoid foaming); do not use a pipette tip smaller than 200 μL and do not pipette the tissue up and down more than 30 times |
||
| 26 | Fewer cells than expected during FACS (expected yield 50-70% of total NB numbers) |
Insufficient enzymatic digestion/ cells die after dissociation |
See troubleshooting guidance for step 15 |
| Incorrect FACS instrument setting |
Reduce pressure to a low pressure, maximum of 20 p.s.i. |
||
| Use 100 μm nozzle | |||
| 32 | Insufficient amounts of RNA isolated; at least 300-500 ng of total RNA is required per RNA-seq analysis |
Not enough material was sorted during FACS |
Increase number of dissected larva for each FACS round Increase number of FACS rounds to collect more cells to isolate RNA from. |
| 32 | Low RNA yield, denatured RNA, or DNA contamination in RNA Prep |
Multiple steps during RNA isolation can be causal |
We suggest following the advice given in the TRIzol LS manual |
| Adapt the RNA isolation protocol to a small amount of material or change the method of RNA isolation. For example, use the RNeasy micro kit from Qiagen (cat. no. 74004), which includes an optional on column DNase digest step (RNase-free DNase set, Qiagen cat. no. 79254) |
|||
| 44 | Insufficient mRNA enrichment |
mRNA isolation protocol does not work well |
Do not exceed the manufacturer’s recommended amount of total RNA for enrichment of mRNA with Dynabeads, but also do not use <100 ng of total RNA for mRNA purification |
| Chose a different protocol to enrich for mRNA (for example the RiboZero Magnetic Kit from Epicentre (cat. no. MRZH116)) |
|||
| 46 | Wrong mRNA fragment sizes |
Incubation with fragmentation buffer |
Incubation with fragmentation buffer is either too long or too short. Set up a time-course experiment with different incubation times at 94 °C to optimize fragment size, and check the sizes on an Agilent Bioanalyzer with the Agilent RNA 6000 Pico Kit |
TIMING
Steps 1-18, dissociation of larval brains: 2h 15 min
Steps 19-29, sorting of NBs and neurons by FACS: ~2 h
Steps 30-51, generation of cell type-specific double-stranded cDNA for Solexa-sequencing library preparation: 4 d (day 1: RNA isolation, overnight; day 2: mRNA enrichment and fragmentation of mRNA; day 3: first-strand cDNA synthesis; day 4: second-strand cDNA synthesis and purification of double-stranded cDNA)
Steps 52 and 53, library preparation: 11 d (day 1: end repair and adapter ligation; day 2: size selection of libraries, UDGase treatment, PCR enrichment of fragments and validation of libraries on a Bioanalyzer; days 3-11: sequencing)
Box 1, verification of rRNA depletion by qPCR: 5 h
ANTICIPATED RESULTS
Dissociation of larval brains (Steps 1-18)
The successful generation of a primary cell suspension is crucial for the procedure. It depends on a finely tuned interplay of the dissection procedure, time of enzymatic treatment and mechanical disruption of the tissue. Larval dissection needs to be practiced, because a shorter dissection time increases the cell survival rate and helps preserve in vivo gene expression patterns. Depending on the specificity of the fluorescent labeling of the cells of interest, the precision of dissection may vary. In our case, ase-Gal4 is also expressed in imaginal discs, and therefore we usually remove all attached discs and residual parts of the gut. We routinely monitor the quality of preparations by cell type-specific antibody staining and live imaging on a spinning disc microscope, monitoring several rounds of asymmetric divisions of both NBs and their direct progeny, the GMC16.
Sorting of NBs and neurons by FACS (Steps 19-29)
Depending on the downstream application, we suggest adapting the duration of FACS sorting. If cells are used for live imaging or immunohistochemistry staining, we sort ~7,000 NBs, which should not take longer than 20-30 min. We directly sort the cells into complete Schneider’s medium on poly-d-lysine-coated cell culture dishes and let them settle for 1-1.5 h. For transcriptome or qPCR analysis, we sort NBs directly into reaction tubes containing TRIzol LS reagent.
Quality assessment of cell type-specific deep-sequencing data (Step 53)
After obtaining cell type-specific deep-sequencing data, the reads should be checked with FastQC (Babraham Bioinformatics, http://www.bioinformatics.babraham.ac.uk/), which is an important tool for quickly assessing the quality of the raw sequencing data. FastQC checks for general biases, for example, in sequence quality or sequence content per base. To estimate the probability of incorrect base calling, the PHRED score can be used. This score should be above 30, which would indicate a probability of 1 in 1,000 that a base is assigned incorrectly.
It is possible that the quality of the sequence reads is good, but that the sequencing depth is insufficent, and therefore the information content is inadequate. One reason could be incorrect fragment size distribution, which is assessed by aligning the fragments uniquely to the transcriptome. If fragments are too short, it is nucleotides from the adapters that are sequenced. In case of paired-end sequencing, too short fragments result in sequencing the same part of the fragment twice.
Another reason for reduced mRNA-sequencing depth could be contamination with, and thus sequencing of, RNA species other than poly(A) + RNA. This can also affect downstream analyses, as computing of large data sets is time- and resource-consuming. To assess rRNA contamination, the sequencing reads are aligned to all known rRNA species (using RefSeq), with the fraction of rRNA reads being a good indicator for the quality of the mRNA purification. Thus, bioinformatics analyses should preferably be done on reads that align only to mRNA sequences, with all other non-poly(A)+ -containing RNA species being masked.
Finally, we also recommend manual inspection of the data in a genome browser. This helps visualize alternatively spliced genes, expression levels of transcripts and their different isoforms, genomic or intronic contamination or lack of strandedness.
ACKNOWLEDGMENTS
We thank G. Stengl, T. Lendl and N. Corsini for FACS, P. Pasierbek for imaging support, T.R. Burkard for bioinformatics analyses, and all members of the Knoblich group for discussion and suggestions. We are grateful to the Campus Science Support Facilities Next Generation Sequencing Unit for performing library preparation and next-generation sequencing. C.B. is supported by an EMBO Long Term Fellowship. Work in J.A.K.’s laboratory is supported by the Austrian Academy of Sciences, the Austrian Science Fund, the EU FP7 network EuroSystems and an advanced grant from the European Research Council.
Footnotes
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests..
REFERENCES
- 1.Chia W, Somers WG, Wang H. Drosophila neuroblast asymmetric divisions: cell cycle regulators, asymmetric protein localization, and tumorigenesis. J Cell Biol. 2008;180:267–272. doi: 10.1083/jcb.200708159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doe CQ. Neural stem cells: balancing self-renewal with differentiation. Development. 2008;135:1575–1587. doi: 10.1242/dev.014977. [DOI] [PubMed] [Google Scholar]
- 3.Homem CC, Knoblich JA. Drosophila neuroblasts: a model for stem cell biology. Development. 2012;139:4297–4310. doi: 10.1242/dev.080515. [DOI] [PubMed] [Google Scholar]
- 4.Bello BC, Izergina N, Caussinus E, Reichert H. Amplification of neural stem cell proliferation by intermediate progenitor cells in Drosophila brain development. Neural Develop. 2008;3:5. doi: 10.1186/1749-8104-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boone JQ, Doe CQ. Identification of Drosophila type II neuroblast lineages containing transit amplifying ganglion mother cells. Dev Neurobiol. 2008;68:1185–1195. doi: 10.1002/dneu.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowman SK, et al. The tumor suppressors Brat and Numb regulate transit-amplifying neuroblast lineages in Drosophila. Dev Cell. 2008;14:535–546. doi: 10.1016/j.devcel.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito K, Hotta Y. Proliferation pattern of postembryonic neuroblasts in the brain of Drosophila melanogaster. Dev Biol. 1992;149:134–148. doi: 10.1016/0012-1606(92)90270-q. [DOI] [PubMed] [Google Scholar]
- 8.Truman JW, Bate M. Spatial and temporal patterns of neurogenesis in the central nervous system of Drosophila melanogaster. Dev Biol. 1988;125:145–157. doi: 10.1016/0012-1606(88)90067-x. [DOI] [PubMed] [Google Scholar]
- 9.Carney TD, et al. Functional genomics identifies neural stem cell sub-type expression profiles and genes regulating neuroblast homeostasis. Dev Biol. 2012;361:137–146. doi: 10.1016/j.ydbio.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller MR, Robinson KJ, Cleary MD, Doe CQ. TU-tagging: cell type-specific RNA isolation from intact complex tissues. Nat Methods. 2009;6(6):439–41. doi: 10.1038/nmeth.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Starz-Gaiano M, Bridges T, Montell D. Purification of specific cell populations from Drosophila tissues by magnetic bead sorting, for use in gene expression profiling. Protocol Exchange. 2008 doi:10.1038/nprot.2008.28. [Google Scholar]
- 12.Cumberledge S, Krasnow MA. Preparation and analysis of pure cell populations from Drosophila. Methods Cell Biol. 1994;44:143–159. doi: 10.1016/s0091-679x(08)60911-5. [DOI] [PubMed] [Google Scholar]
- 13.Kai T, Williams D, Spradling AC. The expression profile of purified Drosophila germline stem cells. Dev Biol. 2005;283:486–502. doi: 10.1016/j.ydbio.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 14.Tirouvanziam R, Davidson CJ, Lipsick JS, Herzenberg LA. Fluorescence-activated cell sorting (FACS) of Drosophila hemocytes reveals important functional similarities to mammalian leukocytes. Proc Natl Acad Sci U S A. 2004;101:2912–2917. doi: 10.1073/pnas.0308734101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de la Cruz AF, Edgar BA. Flow cytometric analysis of Drosophila cells. Methods Mol Biol. 2008;420:373–389. doi: 10.1007/978-1-59745-583-1_24. [DOI] [PubMed] [Google Scholar]
- 16.Berger C, et al. FACS purification and transcriptome analysis of Drosophila neural stem cells reveals a role for Klumpfuss in self-renewal. Cell Rep. 2012;2:407–418. doi: 10.1016/j.celrep.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goulas S, Conder R, Knoblich JA. The par complex and integrins direct asymmetric cell division in adult intestinal stem cells. Cell Stem Cell. 2012;11:529–540. doi: 10.1016/j.stem.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- 19.McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE. 2004;2004:pl6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- 20.Fristrom JW, Mitchell HK. The preparative isolation of imaginal discs from larvae of Drosophila melanogaster. J Cell Biol. 1965;27:445–448. doi: 10.1083/jcb.27.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zweidler A, Cohen LH. Large-scale isolation and fractionation of organs of Drosophila melanogaster larvae. J Cell Biol. 1971;51:240–248. doi: 10.1083/jcb.51.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu S, et al. Gradients of the Drosophila Chinmo BTB-Zinc Finger Protein Govern Neuronal Temporal Identity. Cell. 2006;127:409–422. doi: 10.1016/j.cell.2006.08.045. [DOI] [PubMed] [Google Scholar]
- 23.Neumuller RA, et al. Genome-wide analysis of self-renewal in Drosophila neural stem cells by transgenic RNAi. Cell Stem Cell. 2011;8:580–593. doi: 10.1016/j.stem.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu S, Barshow S, Wildonger J, Jan LY, Jan YN. Ets transcription factor Pointed promotes the generation of intermediate neural progenitors in Drosophila larval brains. Proc Natl Acad Sci U S A. 2011;108:20615–20620. doi: 10.1073/pnas.1118595109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barolo S, Carver LA, Posakony JW. GFP and beta-galactosidase transformation vectors for promoter/enhancer analysis in Drosophila. Biotechniques. 2000;29:726, 728, 730–732. doi: 10.2144/00294bm10. [DOI] [PubMed] [Google Scholar]
- 26.Ceron J, Tejedor FJ, Moya F. A primary cell culture of Drosophila postembryonic larval neuroblasts to study cell cycle and asymmetric division. Eur J Cell Biol. 2006;85:567–575. doi: 10.1016/j.ejcb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Winnebeck EC, Millar CD, Warman GR. Why does insect RNA look degraded? J Insect Sci. 2010;10:159. doi: 10.1673/031.010.14119. [DOI] [PMC free article] [PubMed] [Google Scholar]