SUMMARY
Aim
The Severe Impairment Battery (SIB), a reliable cognitive measure for evaluating treatment response in advanced Alzheimer's disease (AD), takes approximately 20 minutes to administer. A recently derived 8-item version of the SIB—the SIB-8—which takes about 3 minutes to administer, may represent a more convenient tool for use in clinical practice. The current analyses further explored the SIB-8 scale with respect to its validity and sensitivity.
Methods
A post hoc analysis was performed using data from a 24-week trial of donepezil 23 mg/day and 10 mg/day in > 1400 patients with moderate to severe AD (baseline Mini-Mental State Examination [MMSE] score 0–20). Treatment effects on cognition (patterns of score change) were assessed using the full SIB and SIB-8 in the total study population and subgroups based on concomitant memantine use and baseline MMSE. Internal consistency/agreement and correlations between the SIB and SIB-8 and other clinical end points were evaluated.
Results
Assessment of score changes from baseline to Week 24 with donepezil (23 mg/day or 10 mg/day) demonstrated comparable patterns of change when using the SIB-8 and the full SIB, despite inherent differences in the total score ranges for the two scales. Internal consistency/agreement between the full SIB and SIB-8 was good (Cronbach's alphas: 0.77– 0.95). SIB-8 scores reliably correlated with SIB total scores (r = 0.859, baseline; r = 0.900, Week 24; p < 0.0001), as well as MMSE scores (r = 0.7163, baseline; r = 0.7963, Week 24; p < 0.0001). Scores on both SIB scales were moderately associated with functional measures at baseline and Week 24.
Conclusions
In this post hoc analysis, similar treatment effects were measured by the full SIB and the SIB-8. Very good internal consistency/agreement and strong correlations between the SIB and the more rapid and convenient SIB-8 indicate that the SIB-8 may be a useful and efficient clinical proxy for the full SIB in evaluating treatment response in patients with advanced AD.
Introduction
The Severe Impairment Battery (SIB) scale was created over two decades ago, primarily to overcome floor effects that had limited the utility of tools previously used to measure cognitive changes in patients with moderate or severe Alzheimer's disease (AD) enrolled in clinical trials [1]. The full SIB scale consists of 40 items organized into nine subscales reflecting aspects of cognition that are sensitive to change over time in the later stages of AD, including social interaction, orientation, visual perception, construction, language, memory, praxis, attention and orienting to name [1]. The SIB, which takes approximately 20 minutes to administer, has been shown to be a valid and reliable measure of cognition as AD progresses through the advanced stages, [1, 2] and is now a standard assessment tool in clinical trials studying patients with moderate or severe AD [3-7].
Through its ability to measure cognition in patients who were previously considered “untestable,” [1] the SIB has reinforced evidence indicating that patients with more advanced AD do have meaningful cognitive capacities that can be maintained or improved by treatment [8]. Nevertheless, in practice, patients with moderate or severe AD may continue to present a challenge to the busy health care professional who is striving to gauge the appropriateness and effectiveness of therapy. Indeed, in the moderate and severe stages of AD, there is a significant loss of recent memory and expressive language skills, often accompanied by the inability to perform many instrumental or basic activities of daily living (ADLs) [9], which can make patient assessment challenging. However, although there are a number of barriers to overcome in ensuring that these patients are assessed and managed or treated appropriately, patients in the more advanced stages of AD can respond to continued therapy, and the benefits of treatment should be recognized in this patient population. Setting expectations of treatment response among patients and caregivers may also help to reinforce the need to treat in this patient population. Moreover, since the number of patients with advanced AD is increasing toward unprecedented levels, it is essential that physicians are equipped with the necessary tools to assist in managing these patients and assessing their response to treatment over time.
The reality of time limitations in clinical practice, and the availability of effective symptomatic treatment for moderate and severe AD generated a need for a measurement tool that was as accurate as the SIB, but more efficient in the clinical setting. To address this need, a database of patients with severe AD (Mini Mental State Examination [MMSE] 1–12, inclusive) enrolled in four studies of donepezil was examined to identify SIB items that are sensitive to change over time [10]. After examining loading factors of the various cognitive domains and items, eight items from the full SIB were found to be sensitive to change with treatment and relatively easy to administer (Table 1). These results led to the creation of the SIB-8 scale, which takes approximately 3 minutes to administer and which correlates well with the full SIB [10]. This tool has the potential to monitor change due to disease progression and treatment, and to help practitioners manage and evaluate patients in the later stages of AD; therefore, a closer study of its suitability as an efficient measure of cognitive change in clinical settings is appropriate and could be accomplished through analysis of existing data from newly completed clinical trials.
Table 1.
| Item | Domain |
|---|---|
| Month | Orientation |
| Months of year | Language |
| Write name | Language |
| Sentence | Memory |
| Fluency | Language |
| Confrontational naming—spoon | Language |
| Using spoon | Praxis |
| Digit span | Attention |
Items derived from analysis of patients with severe AD (MMSE 1-12). Each item has a minimum score of 0 and a maximum score of 2; scores range from 0 to 16.
SIB-8, Severe Impairment Battery, 8-item version; MMSE, Mini Mental State Examination
A recent randomised, double-blind trial involving more than 1400 patients compared the standard dose of the acetylcholinesterase inhibitor donepezil (10 mg/day) with a high-dose formulation (23 mg/day) [11]. All patients in the study had moderate or severe AD, defined as a baseline MMSE score between 0 and 20 (inclusive); more than 70% of patients had a baseline MMSE between 0 and 16, and 36% were already taking adjunctive memantine therapy. The study included two co-primary end points: change in cognition at Week 24, as measured by scores on the full SIB, and change in global function at Week 24, as measured by the Clinician's Interview-Based Impression of Change plus caregiver input (CIBIC-plus). The trial's secondary efficacy measures included the MMSE and the severe version of the Alzheimer's Disease Cooperative Study-Activities of Daily Living scale (ADCS-ADL-sev).
Due to the variety of traditional clinical assessments utilised, and the large size of the donepezil trial, further examination of the cognitive features of AD and their relationships with other measures of AD status is possible. In particular, the study database provides an opportunity to further examine the clinical utility of the SIB-8. As such, the current post hoc exploratory analysis of data from this trial was performed to further assess the validity of the SIB-8 in a population of patients with moderate or severe AD, particularly in relation to its ability to track treatment effects in a comparable manner to the full SIB, and to explore the relationships between the full SIB and SIB-8 scales and other measurements of AD severity (e.g., MMSE and available functional scales).
Methods
Detailed methods for the original clinical trial have been fully described [11]. In brief, this was a 24-week, double-blind trial in patients with moderate or severe AD (baseline MMSE 0–20, inclusive) who had been receiving a stable dose of donepezil 10 mg/day for at least 3 months. Patients were randomized 2:1 to receive the high-dose formulation of donepezil, 23 mg/day, or to continue taking the existing 10-mg/day dose. Concomitant use of memantine was allowed if the patient had been taking a stable dose of ≤ 20 mg/day for at least 12 weeks prior to the screening visit and provided the patient agreed to maintain their memantine dosage throughout the trial.
Post hoc exploratory analysis
The post hoc analysis of the SIB-8 was divided into three parts: an analysis of treatment effect, an internal consistency/agreement analysis and a correlation analysis between outcome measures. All analyses were performed on the intent-to-treat (ITT) population, defined as those patients who received ≥ 1 dose of study medication and in whom either [a] SIB data was available for baseline and ≥ 1 postbaseline visit or [b] Clinician's Interview-Based Impression of Severity plus caregiver input [CIBIS-plus]/CIBIC-plus data was available for baseline and ≥ 1 postbaseline visit.
Analysis of treatment effect
Scores on the eight individual items included in the SIB-8 (Table 1) were extracted from the full SIB data and summed; SIB-8 scores range from 0 to 16. Changes from baseline to Week 24 in SIB total scores and SIB-8 scores for all patients treated with either donepezil 23 mg/day or 10 mg/day (pooled group data) were examined using an analysis of covariance model with terms for baseline score, country and treatment. The least squares (LS) or adjusted mean score change was calculated, using the last observation carried forward (LOCF) method to account for missing data, and the pattern of score change evaluated for the SIB-8 and the full SIB in order to compare the respective sensitivity to treatment effects. Evaluations were performed for the full study population and in subgroups of patients based on concomitant use of memantine and on greater cognitive impairment at baseline (MMSE 0–16).
Consistency/agreement analysis
Analyses were performed to evaluate the internal consistency and agreement between SIB total and SIB-8 scores, i.e., to evaluate the reliability of using the SIB-8 as an alternative measure for the full SIB. Cronbach's coefficient alphas were calculated for baseline, Week 24 and change from baseline scores at Week 24; an alpha between 0.7 and 0.95 is considered acceptable [12].
Correlation analysis
Relationships between the SIB total score and SIB-8 scores were evaluated. Additionally, relationships were examined between SIB total and SIB-8 scores and scores on the other measure of cognition (MMSE) and measures of global function (CIBIS-plus and CIBIC-plus) and daily functioning (ADCS-ADL-sev). The ADCS-ADL-sev scale was analysed both for the total score and for two subscale scores reflecting basic and instrumental ADL abilities. Pearson correlation coefficients were calculated for baseline, Week 24 and change from baseline scores at Week 24. As higher scores on the SIB, SIB-8, MMSE and ADCS-ADL-sev indicate less impairment, correlation coefficients between these measures are positive; since higher scores on the CIBIS-plus/CIBIC-plus indicate greater impairment, correlation coefficients for relationships between SIB or SIB-8 scores and CIBIS-plus/CIBIC-plus scores are negative.
Results
Patients
In total, 1371 patients comprised the full ITT population: 909 receiving donepezil 23 mg/day and 462 receiving donepezil 10 mg/day. As described previously, demographics and clinical characteristics were comparable in both treatment groups and no clinically relevant differences were seen between the treatment groups in relation to baseline efficacy scores [11, 13]. Moreover, baseline SIB-8 scores were also comparable between the patients receiving donepezil 23 mg/day (mean [SD]: 10.4 [3.4]) and patients receiving 10 mg/day (mean [SD]: 10.6 [3.4]).
Treatment effects analysis
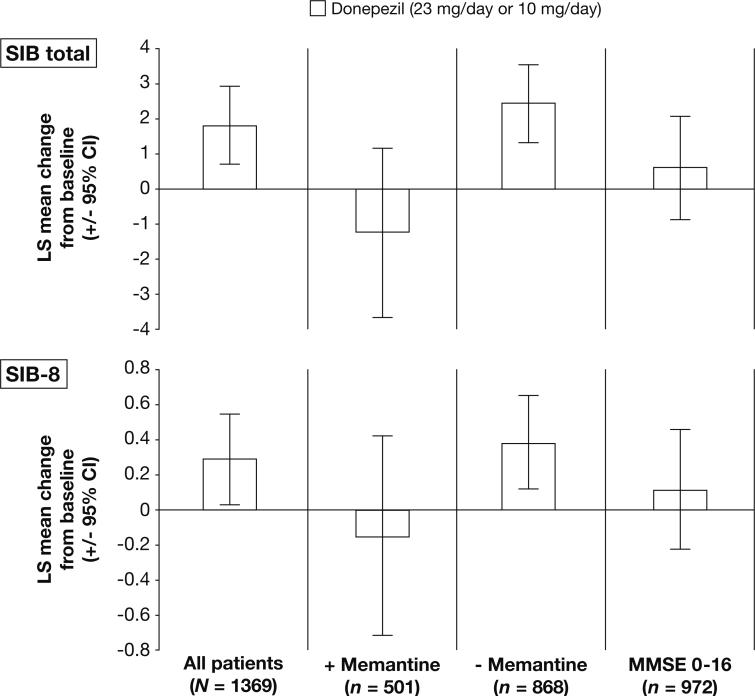
Despite the inherent differences in the total score ranges for the two versions of the SIB, the pattern of change in score for each population/subgroup assessed was generally similar when using SIB total and SIB-8 scores (Figure 1).
Figure 1.
Patterns of score change (LS mean; LOCF) from baseline to Week 24 among patients receiving donepezil therapy as measured using the SIB total and SIB-8 scales
Consistency/agreement analysis
The consistency and agreement between the full SIB and SIB-8 was very good. Cronbach's coefficient alphas were greater than 0.90 both at baseline and Week 24 and greater than 0.75 for change from baseline at Week 24 (Table 2).
Table 2.
Cronbach's coefficient alpha values for SIB total scores and SIB-8 scores (full ITT population)
| SIB measurement | Cronbach's coefficient alpha |
|---|---|
| Baseline | 0.9240 |
| Week 24 | 0.9474 |
| Change from baseline | 0.7736 |
SIB, Severe Impairment Battery; SIB-8, Severe Impairment Battery, 8-item version; ITT, intent-to-treat
Correlation analysis
SIB vs. SIB-8
SIB-8 scores were strongly correlated with SIB total scores. Pearson correlation coefficients were greater than 0.85 both at baseline and Week 24 (Table 3); the Pearson's r value for score change between the two SIB versions at Week 24 was also moderately high (r = 0.64).
Table 3.
Correlations between SIB total scores and SIB-8 scores (full ITT population)*
| SIB-8 | |||
|---|---|---|---|
| Baseline | Week 24 | Change from baseline | |
| SIB total | |||
| Baseline | 0.8587 | ||
| Week 24 | 0.9004 | ||
| Change from baseline | 0.6417 | ||
Ns for individual correlation coefficients ranged from 1369 to 1371. All correlations were statistically significant (p < 0.0001).
SIB, Severe Impairment Battery; SIB-8, Severe Impairment Battery, 8-item version; ITT, intent-to-treat
SIB and SIB-8 vs. other measures
The overall pattern of correlation with other measures used in the study was similar for the full SIB and the SIB-8 scales. At baseline, SIB total and SIB-8 scores were strongly correlated with MMSE scores and moderately correlated with ADCS-ADL-sev (total and subscale scores) and CIBIS-plus scores (Table 4).
Table 4.
Correlations between SIB total and SIB-8 scores and scores on the ADCS-ADL-sev (total, basic, and instrumental), CIBIS-plus/CIBIC-plus, and MMSE (full ITT population)*
| SIB total | SIB-8 | |||||
|---|---|---|---|---|---|---|
| Baseline | Week 24 | Change from baseline | Baseline | Week 24 | Change from baseline | |
| ADCS-ADL-sev total | ||||||
| Baseline | 0.5681 | 0.5105 | ||||
| Week 24 | 0.6398 | 0.6031 | ||||
| Change from baseline | 0.3318 | 0.2420 | ||||
| ADCS-ADL-sev basic | ||||||
| Baseline | 0.5523 | 0.4972 | ||||
| Week 24 | 0.6138 | 0.5683 | ||||
| Change from baseline | 0.3101 | 0.2036 | ||||
| ADCS-ADL-sev instrumental | ||||||
| Baseline | 0.5127 | 0.4643 | ||||
| Week 24 | 0.5990 | 0.5705 | ||||
| Change from baseline | 0.2764 | 0.2139 | ||||
| CIBIS-plus/CIBIC-plus | ||||||
| Baseline (CIBIS-plus) | −0.5286 | −0.5075 | ||||
| Week 24 (CIBIC-plus) | −0.2153 | −0.2548 | ||||
| Change from baseline (CIBIC-plus) | −0.4026 | −0.2706 | ||||
| MMSE | ||||||
| Baseline | 0.6999 | 0.7163 | ||||
| Week 24 | 0.7437 | 0.7963 | ||||
| Change from baseline | 0.4645 | 0.3629 | ||||
Ns for individual correlation coefficients ranged from 1363 to 1371. All correlations were statistically significant (p < 0.0001).
SIB, Severe Impairment Battery; SIB-8, Severe Impairment Battery, 8-item version; ADCS-ADL-sev, Alzheimer's Disease Cooperative Study-Activities of Daily Living scale, severe version; CIBIS-plus, Clinician's Interview-Based Impression of Severity plus caregiver input; CIBIC-plus, Clinician's Interview-Based Impression of Change plus caregiver input; MMSE, Mini Mental State Examination; ITT, intent-to-treat
Correlations between SIB total and SIB-8 scores and scores on the MMSE and ADCS-ADL-sev (total and subscale scores) were marginally but consistently stronger at Week 24 than at baseline. In contrast, correlations between SIB total and SIB-8 scores and CIBIC-plus scores at Week 24 were weaker than seen with CIBIS-plus scores at baseline (Table 4).
Assessment of correlations between change from baseline for SIB total and SIB-8 scores and change from baseline in the other measures were of weak to moderate strength, with MMSE showing the strongest correlation with the two versions of the SIB (Table 4). Change from baseline in SIB total and SIB-8 scores correlated weakly with baseline MMSE, CIBIS-plus or ADCS-ADL-sev (total and subscales) scores.
Discussion
This post hoc analysis of data from the study of patients receiving donepezil 23 mg/day or donepezil 10 mg/day demonstrated that similar patterns of score change were observed when using the SIB-8 and the full SIB, suggesting that the two scales possess a comparable sensitivity to treatment effects despite inherent differences in the total score ranges. Moreover, our results also showed Cronbach's alphas of 0.77 to 0.95 and Pearson's r values of 0.64 to 0.90, providing evidence that the strength of consistency and correlation between the full SIB and the SIB-8 is high. Since the SIB-8 is brief and easy to administer, these results suggest a potential utility for the abridged scale as a clinical proxy for the full SIB in clinical practice.
Given that the present study addresses change on the SIB-8 in patients receiving one of two doses of donepezil, the question of SIB-8 change in untreated AD is a relevant issue for evaluating the clinical impact of donepezil on patient response when using this tool. To best evaluate the measurement characteristics of the SIB-8 in an untreated AD cohort and to provide a theoretical reference for the clinical meaningfulness of changes on the SIB-8 following donepezil treatment, SIB-8 scores (n = 104) were derived from the original Alzheimer's Disease Cooperative Study (ADCS) Instrument Trial, [2, 14] and evaluated for progression over time using an ANCOVA with baseline SIB-8 as a covariate. As expected, SIB-8 performances decrease over time; a statistically significant decline of about one point over 6 months (mean = −0.86 or 5% of the SIB-8 range; p < 0.006) and more than two points over one year (mean = −2.27 or 14% of the SIB-8 range; p < 0.0001). In the present analysis, SIB-8 scores increases by around 0.3 points over 6 months following donepezil therapy. However, prospective studies including a placebo arm would be required to determine the true clinical impact of donepezil treatment vs. no treatment as measured by the SIB-8.
Correlations between SIB-8 scores and SIB total scores were strong in the current analysis; the Pearson correlation coefficient between the two instruments was 0.86 at baseline and 0.90 at Week 24 (which accounts for 74% and 81% of the variance, respectively). The correlation between change from baseline in the full SIB and SIB-8 was weaker than seen at baseline or Week 24, but remained moderately strong (r = 0.64). Overall, these findings are comparable to the results reported in the original evaluation of the SIB-8 in patients with severe AD [10]. The similarity with the previous report on the SIB-8 provides further evidence to support the validity of the technique used to derive the SIB-8 from the full SIB and once again indicates that the SIB-8 may be a useful method of assessing cognition and tracking individual patients with moderate or severe AD over time. Furthermore, the 3-minute SIB-8 test is quick, easy to administer, and simple to explain to both patients and caregivers. Therefore, it has the potential to be an efficient new tool for the practising physician. However, prospective studies are needed to formally validate the SIB-8 with respect to sensitivity to longitudinal decline in cognition. Indeed, evidence for the validity of the SIB-8 would be greatly enhanced by the use of this scale as a primary measure in clinical studies, as opposed to being extrapolated from the full SIB. Use of a derived SIB-8 in the current study may limit the interpretation of our results. Moreover, further analysis of the SIB-8 scale in patients treated with other acetylcholinesterase inhibitors or memantine will be necessary to fully establish its utility in measuring treatment-related changes in cognition.
Correlations between SIB total and SIB-8 scores and scores on the MMSE were strong at both baseline and Week 24, a finding that is also supportive of previously published studies [2, 10, 15]. Furthermore, moderate baseline correlations were seen between the measures of cognition (SIB total and SIB-8 scores) and measures of both daily functioning (ADCS-ADL-sev total and subscale scores) and global function (CIBIS-plus), lending weight to previous reports suggesting that impairments in cognition and function are somewhat interrelated in the moderate to severe AD population [16, 17]. Correlations between the two SIB scales and the functional measures in terms of the changes in score from baseline to Week 24 were only of weak to moderate strength, which is perhaps not surprising considering that changes in ADCS-ADL-sev and CIBIC-plus scores in this study were small [11]. Furthermore, a previous factor analysis of the nine SIB domains, in relation to other measures of autonomy/dependency of functioning, showed that the constructional praxis domain was related to dependency with 20% shared variance, but that the main cognitive domains of the SIB were not related to dependency [17]. Hence, the SIB vs. ADL associations in the present analyses might be considered rather robust. Finally, the weak correlations observed between the change in SIB and SIB-8 scores at Week 24 and baseline ADCS-ADL-sev or CIBIS-plus scores suggest that baseline functional impairment does not impact a patient's potential to show cognitive response, and that even patients with impaired ADLs/function can realise clinically meaningful cognitive benefits with treatment.
Conclusion
In this exploratory post hoc analysis, similar treatment effect outcomes were observed when using the SIB-8 and the full SIB and the two scales showed a high level of consistency and agreement. The analyses also showed strong correlations between SIB-8 and SIB total scores at baseline and Week 24 and moderate correlations for change from baseline in SIB-8 and SIB total scores, further suggesting that the SIB-8 scale may represent an objective, accurate and clinically useful tool for practising physicians in the measurement of cognitive status and treatment effects in patients with advanced AD. Furthermore, changes on the SIB-8 may also serve as a clinical indicator of changes in other domains of interest in patients with AD, such as ADLs. Additional prospective studies utilizing the SIB-8 scale as a primary measure are, however, required to fully determine the applicability of the SIB-8 scale in clinical practice.
What's known?
The SIB-8 scale is a brief instrument (taking ~3 minutes to administer) for patients with severe AD that is sensitive to change and able to detect treatment response. The SIB-8, which was developed using pooled data from 4 donepezil clinical trials in subjects with severe AD, correlates well with the parent scale (the full SIB) and with another measure of cognition, the MMSE.
What's new?
Using data from a large single-study population, the current analysis supports previous findings by providing evidence of good internal consistency/agreement and strong correlation between the full SIB and the more rapid and convenient SIB-8. Additionally, analyses provide novel data on correlations between the full SIB and SIB-8 and measures of global function and ADL, which may assist in our understanding of the relationships between different measures of AD status and symptomatic treatment response.
Acknowledgements
The authors would like to thank the ADCS group for allowing access to their historical dataset in relation to the preparation of the current manuscript (NIH grant 1-UO1 AG10483). The authors would also like to thank Zane Bai (formerly of Eisai Inc.) and Liang Liu (Eisai Inc.) for additional post hoc analysis and critical review of the manuscript. The described analyses derive from a phase 3 clinical study (ClinicalTrials.gov identifier: NCT00478205) sponsored by Eisai Inc. During the development of this manuscript, editorial assistance was provided by Richard Daniel, PhD, at PAREXEL Inc., and was funded by Eisai Inc. and Pfizer Inc.
Footnotes
Disclosures:
FAS: Has no financial interests to report; has served on a Data Safety Monitoring Committee for Pfizer for which the University of Kentucky received reimbursement (<$4,000).
JS: Currently receives royalties from the original SIB on which this reduced scale is based. JS has been paid as a consultant to Eisai Inc./Pfizer Inc.
SHF: Has served as a paid scientific consultant to Pfizer related to donepezil and to several investigational compounds (less than $10,000/year; there was no payment for participation in this article). His institution has received grant/contract support from Pfizer for the conduct of clinical trials for dementia and to support educational programs. He has also served as a scientific consultant to other companies marketing, developing or contributing to the development of drugs for cognition, including Accera, Baxter, Bristol-Myers Squibb, Eisai, Elan, Eli Lilly, Janssen AI, MedAvante, Merck, Merz, Neuronix, Novartis, Takeda and United Biosource; and his institution has received grant/contract support for clinical trials from Baxter, Bristol Myers-Squibb, Eisai, Eli Lilly, Janssen AI, Merck and Roche. He also has stock options from Accera, Intellect Neurosciences, MedAvante, and Neuronix, and stock from Lexicon.
JM: Employee of Pfizer Inc.
YS: Former employee of Eisai Inc.
Author contributions
FAS: Helped conceive/design the described analyses, participated in the data analysis, and assisted in the drafting, editing, and interpretation of the manuscript.
JS: Developed the original SIB scale, helped conceive/design the described analyses, participated in the data analysis, and assisted in the drafting, editing, and interpretation of the manuscript.
SHF: Helped conceive/design the described analyses, participated in the data analysis, and assisted in the drafting, editing, and interpretation of the manuscript.
JM: Helped conceive/design the described analyses, participated in the data analysis, and assisted in the drafting, editing, and interpretation of the manuscript.
YS: Statistically assisted in the planning and performance of the post hoc analyses and assisted in the drafting, editing, and interpretation of the manuscript.
All authors read and approved the final version of the manuscript.
References
- 1.Saxton J, Swihart AA. Neuropsychological assessment of the severely impaired elderly patient. Clin Geriatr Med. 1989 Aug;5(3):531–43. [PubMed] [Google Scholar]
- 2.Schmitt FA, Ashford W, Ernesto C, Saxton J, Schneider LS, Clark CM, et al. The severe impairment battery: concurrent validity and the assessment of longitudinal change in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S51–S56. [PubMed] [Google Scholar]
- 3.Black SE, Doody R, Li H, McRae T, Jambor KM, Xu Y, et al. Donepezil preserves cognition and global function in patients with severe Alzheimer disease. Neurology. 2007 Jul 31;69(5):459–69. doi: 10.1212/01.wnl.0000266627.96040.5a. [DOI] [PubMed] [Google Scholar]
- 4.Emre M, Mecocci P, Stender K. Pooled analyses on cognitive effects of memantine in patients with moderate to severe Alzheimer's disease. J Alzheimers Dis. 2008 Jun;14(2):193–9. doi: 10.3233/jad-2008-14207. [DOI] [PubMed] [Google Scholar]
- 5.Homma A, Imai Y, Tago H, Asada T, Shigeta M, Iwamoto T, et al. Donepezil treatment of patients with severe Alzheimer's disease in a Japanese population: results from a 24-week, double-blind, placebo-controlled, randomized trial. Dement Geriatr Cogn Disord. 2008;25(5):399–407. doi: 10.1159/000122961. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt FA, Wichems CH. A systematic review of assessment and treatment of moderate to severe Alzheimer's disease. Prim Care Companion J Clin Psychiatry. 2006;8(3):158–9. doi: 10.4088/pcc.v08n0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winblad B, Kilander L, Eriksson S, Minthon L, Batsman S, Wetterholm AL, et al. Donepezil in patients with severe Alzheimer's disease: double-blind, parallel-group, placebo-controlled study. Lancet. 2006 Apr 1;367(9516):1057–65. doi: 10.1016/S0140-6736(06)68350-5. [DOI] [PubMed] [Google Scholar]
- 8.Ferris S, Ihl R, Robert P, Winblad B, Gatz G, Tennigkeit F, et al. Treatment effects of Memantine on language in moderate to severe Alzheimer's disease patients. Alzheimers Dement. 2009 Sep;5(5):369–74. doi: 10.1016/j.jalz.2009.05.604. [DOI] [PubMed] [Google Scholar]
- 9.Galasko D, Schmitt F, Thomas R, Jin S, Bennett D. Detailed assessment of activities of daily living in moderate to severe Alzheimer's disease. J Int Neuropsychol Soc. 2005 Jul;11(4):446–53. doi: 10.1017/s1355617705050502. [DOI] [PubMed] [Google Scholar]
- 10.Schmitt FA, Saxton JA, Xu Y, McRae T, Sun Y, Richardson S, et al. A brief instrument to assess treatment response in the patient with advanced Alzheimer disease. Alzheimer Dis Assoc Disord. 2009 Oct;23(4):377–83. doi: 10.1097/WAD.0b013e3181ac9cc1. [DOI] [PubMed] [Google Scholar]
- 11.Farlow MR, Salloway S, Tariot PN, Yardley J, Moline ML, Wang Q, et al. Effectiveness and tolerability of high-dose (23 mg/d) versus standard-dose (10 mg/d) donepezil in moderate to severe Alzheimer's disease: A 24-week, randomized, double-blind study. Clin Ther. 2010 Jul;32(7):1234–51. doi: 10.1016/j.clinthera.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Streiner DL, Norman GR. Health measurement scales: A practical guide to their development and use. Oxford University Press; Oxford: 2003. [Google Scholar]
- 13.Ferris SH, Schmitt FA, Saxton J, Richardson S, Mackell J, Sun Y, et al. Analyzing the impact of 23 mg/day donepezil on language dysfunction in moderate to severe Alzheimer's disease. Alzheimers Res Ther. 2011;3(3):22. doi: 10.1186/alzrt84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferris SH, Mackell JA, Mohs R, Schneider LS, Galasko D, Whitehouse PJ, et al. A multicenter evaluation of new treatment efficacy instruments for Alzheimer's disease clinical trials: overview and general results. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S1–12. [PubMed] [Google Scholar]
- 15.Panisset M, Roudier M, Saxton J, Boller F. Severe impairment battery. A neuropsychological test for severely demented patients. Arch Neurol. 1994 Jan;51(1):41–5. doi: 10.1001/archneur.1994.00540130067012. [DOI] [PubMed] [Google Scholar]
- 16.Feldman HH, Van BB, Kavanagh SM, Torfs KE. Cognition, function, and caregiving time patterns in patients with mild-to-moderate Alzheimer disease: a 12-month analysis. Alzheimer Dis Assoc Disord. 2005 Jan;19(1):29–36. doi: 10.1097/01.wad.0000157065.43282.bc. [DOI] [PubMed] [Google Scholar]
- 17.Pelissier C, Roudier M, Boller F. Factorial validation of the Severe Impairment Battery for patients with Alzheimer's disease. A pilot study. Dement Geriatr Cogn Disord. 2002;13(2):95–100. doi: 10.1159/000048640. [DOI] [PubMed] [Google Scholar]