Abstract
Objective. To evaluate health-related quality of life (HRQOL) and corticosteroid use in patients with moderate to severely active SLE enrolled in two international, multicentre, randomized controlled trials of epratuzumab (ALLEVIATE-1 and -2) and a long-term extension study (SL0006).
Methods. Ninety ALLEVIATE patients (43% BILAG A, mean BILAG score 13.2) were randomized to receive 360 mg/m2 (n = 42) or 720 mg/m2 (n = 11) epratuzumab or placebo (n = 37), plus standard of care, in 12-week cycles. Corticosteroid use, patient and physician global assessments of disease activity (PtGA and PGA) and 36-item Medical Outcomes Survey Short Form (SF-36) results were recorded at baseline and every 4 weeks. Both trials were prematurely discontinued due to a drug supply interruption; patients followed for ≥6 months were analysed. Twenty-nine patients continued in SL0006, with interim analysis at a median exposure of 120 (range 13–184) weeks.
Results. At week 12, proportions of patients with a PGA ≥20% above baseline or with a PtGA improvement greater than or equal to the minimum clinically important difference were higher in the epratuzumab arms than the placebo arm. PGA and PtGA improvements were sustained but did not reach statistical significance. At week 24, mean cumulative corticosteroid doses with epratuzumab 360 and 720 mg/m2 were 1051 and 1973 mg less than placebo (P = 0.034 and 0.081, respectively). At week 48, SF-36 scores approached or exceeded US age- and gender-matched norms in five domains with the 360 mg/m2 treatment. Improvements were maintained in SL0006 over ∼2 years.
Conclusion. Epratuzumab treatment produced clinically meaningful and sustained improvements in PGA, PtGA and HRQOL and reductions in corticosteroid doses.
Keywords: epratuzumab, CD22, ALLEVIATE, lupus, SLE, HRQOL, SF-36, corticosteroids, clinical trial, monoclonal antibody
Introduction
SLE is a complex autoimmune disease characterized by the involvement of multiple organ systems and an unpredictable disease course [1–3]. Patient survival has improved over the past two decades, making outcome measures other than mortality increasingly important [4]. However, developing effective treatments for SLE has proved difficult [5]; patient-reported health-related quality of life (HRQOL) has improved little in recent years [2, 6]. HRQOL, including physical, social and emotional well-being, is recommended by OMERACT as a domain for assessment in SLE randomized controlled trials (RCTs) [7–9].
Patients with SLE report worse HRQOL than healthy controls [10, 11] or those with other chronic diseases, such as coronary artery disease [12]. A contributing factor is that currently available treatments are often associated with adverse effects, which often can be severe [13]. In the Lupus European Online survey, 2070 patients with SLE reported impairments in all domains of the Lupus Quality of Life questionnaire, including 82.5% with fatigue [14]. In another survey by the Lupus Foundation of America and Lupus Europe in 2009 in 914 self-identified lupus patients, 49.3% of respondents indicated they were satisfied or very satisfied with their current treatment regimen, with a majority reporting that side effects, especially those associated with the use of corticosteroids and/or immunosuppressives reduced their physical well-being (88.4%) and negatively impacted everyday activities (82.1%) [15].
In five RCTs, patients with SLE reported large decreases in HRQOL at baseline by the 36-item Medical Outcomes Survey Short Form questionnaire (SF-36). Lower scores across all domains were highly correlated with a history of renal disease, presence of anti-dsDNA antibodies, higher disease activity by Safety of Estrogens in Lupus Erythematosus National Assessment (SELENA) and/or SLEDAI scores, hypocomplementaemia, African American ethnicity and age [16, 17].
There is a clear need for new therapies for SLE. However, the complexity and heterogeneous manifestations of SLE, and its variable clinical course, pose challenges in the assessment of disease activity and clinical trial design [2, 3, 5, 6, 18]. One promising new therapeutic target is CD22, a 135-kDa transmembrane sialoglycoprotein that is differentially expressed during B cell differentiation and regulates B cell activation and interaction with T cells [19, 20]. Epratuzumab, a humanized monoclonal antibody targeting CD22, has shown evidence of therapeutic potential in SLE [21], presumably modulating proliferation and trafficking of activated B cells [22].
Two similarly designed, international, multicentre RCTs [ALLEVIATE-1 (SL0003) and ALLEVIATE-2 (SL0004)] were initiated in patients with moderate to severely active SLE and were prematurely discontinued due to interruption of the drug supply. Available data were pooled to increase the number of observations to enable preliminary analyses of efficacy, safety and HRQOL. The primary efficacy and safety results are described in a separate manuscript [23]. Patients at sites in the USA who participated in either RCT were allowed inclusion in an open-label, long-term safety study, SL0006. Here we present Patient and Physician Global Assessments of disease activity (PtGA and PGA, respectively), HRQOL and corticosteroid dosing data from the RCTs and extension.
Patients and methods
The ALLEVIATE and SL0006 trials were conducted in accordance with the International Conference on Harmonization E6 Note for Guidance on Good Clinical Practice (CPMP/ICH/135/95). Informed consents, reviewed and approved by independent ethics committees or institutional review boards from all sites, were signed by all patients for the ALLEVIATE and SL0006 trials.
ALLEVIATE RCTs
Patients
Patients were ≥18 years of age, with an ANA titre ≥1:40 (measured by enzyme immunoassay, with indirect fluorescent antibody confirmation for pattern) with four or more of the ACR revised classification criteria [24].
Patients in ALLEVIATE-1 had BILAG A disease activity in one or more organ systems, excluding renal or central neurological systems [25, 26]. Patients in ALLEVIATE-2 had BILAG B activity in two or more organ systems [25, 26], having received oral corticosteroids (prednisone 5–20 mg/day or equivalent) at stable doses for ≥4 weeks before study entry. Similarly they were to have received treatment with one or more immunosuppressive for ≥8 weeks (AZA, chlorambucil, LEF, MTX or MMF, but not ciclosporin or CYC) and/or antimalarials for ≥12 weeks, with stable doses for ≥4 weeks before study entry.
Patients were excluded for pregnancy, prior treatment with B cell–targeted therapies, a history of malignancy, an active infection, allergy to murine or human antibodies, receipt of experimental therapy or any therapy with human or murine antibodies within 3 months, thrombosis, spontaneous or induced abortion, stillbirth, live birth within 4 weeks or aPL plus a history of thromboembolic events. For ALLEVIATE-1, patients were also excluded if they had active severe central nervous system and/or renal disease (BILAG A). For ALLEVIATE-2, patients were excluded if they had a BILAG A score in any organ system.
Study design and treatment
The ALLEVIATE trials were international, multicentre, 48-week, phase II/III RCTs. The study designs were almost identical with regard to visit intervals, treatment cycles and scheduled assessments of safety, efficacy and pharmacokinetics. ALLEVIATE-1 was conducted at 16 sites in six countries (Belgium, Hungary, the Netherlands, Spain, the UK, and the USA) and ALLEVIATE-2 was conducted at 28 sites in six countries (Belgium, Italy, the Netherlands, Spain, the UK, and the USA). Patients in ALLEVIATE-1 were randomized to either standard of care (SOC) plus repeated administrations of 360 or 720 mg/m2 of epratuzumab or placebo (1:1:1). Patients in ALLEVIATE-2 were randomized to SOC plus repeated administrations of epratuzumab 360 mg/m2 or placebo (1:1). In both RCTs, epratuzumab or placebo were administered intravenously in 12-week cycles for up to 48 weeks (four infusions at weeks 0, 1, 2 and 3 for cycle 1; two infusions at weeks 0 and 1 for subsequent cycles).
At study entry, patients initiated a protocol-prescribed corticosteroid regimen and continued SOC without change. For ALLEVIATE-1, patients received a flare regimen of oral or i.v. corticosteroids (1 g methylprednisolone, 150 mg dexamethasone, or equivalent) administered three times in <1 week, followed by oral corticosteroids. The oral corticosteroid dose was selected by the investigator on an individual patient basis (0.5–0.8 mg/kg/day prednisone or equivalent, not exceeding 60 mg/day), with tapering from 4 weeks onwards as clinically indicated, and a goal of 7.5–10 mg/day prednisone (or equivalent) by weeks 20 and 24. In ALLEVIATE-2, patients increased their oral steroid dosage by 10 mg/day prednisone (or equivalent), again maintained for ≥4 weeks, with a goal of 5–7.5 mg/day prednisone (or equivalent) by weeks 20 and 24. No other means of corticosteroid administration were allowed in this study.
Recruitment started in the spring of 2005. Dosing and enrolment in both trials were prematurely discontinued on 1 September 2006 due to interruption of the drug supply. Patients were followed for ≥6 months. The primary endpoint was prospectively revised prior to unblinding, based on combined data from the two RCTs.
Clinical efficacy endpoints
Disease activity in each trial was measured every 4 weeks by BILAG and centrally graded by an independent, blinded reviewer [25, 26]. The pre-specified three-category primary efficacy endpoint in both RCTs was revised to the BILAG response at week 12 before the trials were unblinded. The BILAG response required all BILAG A scores at entry reduced to a B or lower, or both BILAG B scores at entry reduced to C or lower, with no new BILAG A and less than two new BILAG B scores in other organ systems; plus no new or increased use of corticosteroids and/or other immunosuppressants above baseline dose before week 12.
Secondary endpoints included PGA and PtGA, scored on a category scale of 1–5 (1 = very poor, 2 = poor, 3 = fair, 4 = good, 5 = very good), at 4-week intervals throughout the study. Post hoc analysis of the minimum clinically important difference (MCID) for the PtGA was defined as an improvement of ≥1 point (20%) on the 5-point Likert scale [27] and ≥5 points in SF-36 domain scores. The percentage of patients reporting such improvements by PtGA were compared with those considered ≥20% improved by PGA.
HRQOL assessments
HRQOL was evaluated by SF-36, which includes eight domains: physical functioning (PF), role physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role emotional (RE) and mental health (MH), scored from 0 to 100. Normalized and z-transformed domain scores are grouped into physical and mental component summary (PCS and MCS, respectively) scores. PCS positively weights PF, RP, BP, GH and VT, while MCS positively weights VT, SF, RE and MH [28]. The pre-specified endpoint evaluated was the percentage of patients reporting clinically meaningful improvements greater than or equal to MCID from baseline to week 48 in PCS (defined as changes of ≥2.5 points overall and changes of ≥5.0 points in eight domain scores of the SF-36); this was a planned endpoint, but was evaluated for the pooled ALLEVIATE population following termination of the studies. The MCID for no deterioration was defined as no worsening greater than −0.8 for PCS and MCS scores and none greater than −2.5 points for the eight domains [29]. Mean changes from baseline in the SF-36 domain scores are portrayed using Spydergram plots [30] compared with protocol-specific age- and gender-matched US normative scores [31].
Corticosteroid-sparing endpoints
The protocol-specified corticosteroid endpoint was attainment of dose-tapering criteria (7.5–10 mg/day for ALLEVIATE-1, 5–7.5 mg/day for ALLEVIATE-2) by week 20 and maintenance of these doses to week 24. Other endpoints included cumulative and median corticosteroid doses over time.
Statistical analyses
The originally planned sample sizes were not achieved and there was limited statistical power to detect treatment differences even with a combined study population. Statistical analyses of secondary endpoints were exploratory in nature and not adjusted for multiple testing, so P-values and CIs for secondary endpoints should be interpreted cautiously.
SL0006 open-label extension study
All ALLEVIATE patients at US sites were eligible for enrolment in SL0006, if in the investigator’s judgment, the patient had benefited from randomized treatment and there were no safety concerns that precluded receiving epratuzumab. The primary objective was to assess the long-term safety and efficacy of epratuzumab 360 mg/m2. All patients were assigned to receive this dose in 12-week maintenance cycles (two infusions, on weeks 0 and 1 of each cycle). Because of interruption of the drug supply, there was a median delay of 165 (range 1–400) days between completion of the ALLEVIATE studies and entry into the SL0006. Safety and efficacy assessments in the SL0006, similar to those in the ALLEVIATE RCTs, were performed at 4-week intervals [23, 32]. An interim analysis was conducted to obtain preliminary long-term safety and efficacy data, with a cut-off of 31 December 2009, representing a median 120 weeks of exposure (range 13–184).
Results
Patient characteristics
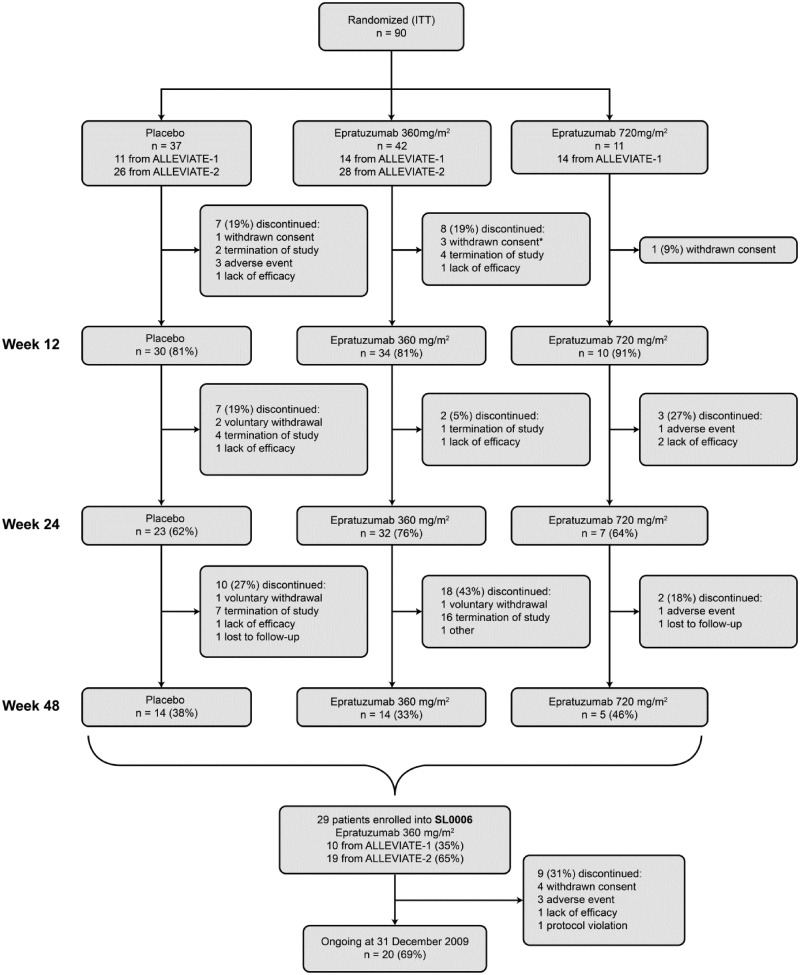
Ninety patients were randomized in the ALLEVIATE RCTs before the latter were prematurely terminated. Of these, 74 patients who received one cycle of therapy between weeks 0 and 3 (four infusions) were evaluated at week 12; 62 received two cycles and were evaluated at week 24 and 33 received three cycles and were evaluated at week 48 (Fig. 1). Median (range) epratuzumab exposure was 2920 (1413–7191) mg and 4341 (2103–7360) mg for the 360 and 720 mg/m2 arms, respectively. A total of 29 patients (17 who originally received epratuzumab 360 mg/m2, 4 who received 720 mg/m2 and 8 who received placebo) were subsequently enrolled into the SL0006. Patients in the SL0006 received a median of 11 infusion cycles of epratuzumab (minimum 2, maximum 14), representing a median 21 individual infusions per subject (minimum 4, maximum 28).
Fig. 1.
Patient disposition (ITT population) through ALLEVIATE and SL0006.
Patients who continued to week 12 received a total of 4 infusions (one treatment cycle), patients who continued to week 24 received a total of 8 infusions (two treatment cycles) and patients who continued to week 48 received a total of 12 infusions (three treatment cycles). *Two randomized but did not receive epratuzumab.
Treatment arms were balanced with respect to age, sex, ethnicity and weight (Table 1). As indicated by disease activity and SF-36 scores, these patients had a high burden of disease activity at baseline (Table 1).
Table 1.
Patient demographics and disease status at baseline in ALLEVIATE-1 and -2 (SL0003 and SL0004) and at study entry into SL0006
| ALLEVIATE |
SL0006 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n = 37) | Epratuzumab 360 mg/m2 (n = 42) | Epratuzumab 720 mg/m2 (n = 11) | Epratuzumab 360 mg/m2 (n = 29) | |||||
| Age, years | ||||||||
| Median (range) | 38.0 (18–58) | 39.0 (20–59) | 38.0 (21–52) | 39.0 (22–61) | ||||
| Gender, n (%) | ||||||||
| Male | 3 (8.1) | 1 (2.4) | 1 (9.1) | 3 (10.3) | ||||
| Female | 34 (91.9) | 41 (97.6) | 10 (90.9) | 26 (89.7) | ||||
| Ethnicity, n (%) | ||||||||
| Caucasian | 25 (67.6) | 27 (64.3) | 7 (63.6) | 23 (79.3) | ||||
| Black | 8 (21.6) | 7 (16.7) | 3 (27.3) | 3 (10.3) | ||||
| Asian | 1 (2.7) | 4 (9.5) | 1 (9.1) | 2 (6.9) | ||||
| Other | 3 (8.1) | 4 (9.5) | 0 (0.0) | 1 (3.4) | ||||
| Weight, mean (s.d.), kg | 67.8 (16.4) | 68.4 (17.9) | 71.1 (21.8) | 70.4 (17.5) | ||||
| Immunosuppressive, antimalarial and steroid use | ||||||||
| Immunosuppressive use, n (%) | 24 (65) | 28 (67) | 5 (46) | 29 (100) | ||||
| Antimalarial use, n (%) | 24 (65) | 31 (74) | 9 (82) | N/A | ||||
| Prednisone dose >25 mg/day, n (%) | 13 (35) | 18 (43) | 8 (73) | N/A | ||||
| Disease activity and HRQOL, mean (s.d.) | ||||||||
| PGA | 2.6 (0.60) | 2.7 (0.54) | 2.2 (0.60) | N/A | ||||
| PtGA | 2.8 (0.73) | 2.6 (0.66) | 1.8 (0.87) | N/A | ||||
| SF-36 PCS | 34.6 (8.36) | 36.5 (9.17) | 29.0 (8.59) | 31.8 (8.80) | ||||
| SF-36 MCS | 41.8 (9.35) | 43.9 (9.42) | 37.8 (12.60) | 42.2 (10.00) | ||||
| Total BILAGa | 13.2 (4.85) | 12.4 (4.01) | 16.3 (6.57) | 12.6 (3.50) | ||||
| Number of patients with at least one BILAG A, n (%) | 13 (35) | 15 (35.7) | 11 (100) | 10 (34.5) | ||||
| BILAG scores for each body system, n (%) | A | B | A | B | A | B | A | B |
| General | 0 (0) | 11 (30) | 1 (2) | 16 (38) | 1 (9) | 3 (27) | 0 (0) | 14 (48) |
| Mucocutaneous | 5 (14) | 26 (70) | 10 (24) | 26 (62) | 3 (27) | 3 (27) | 5 (17) | 19 (66) |
| Neurological | 0 (0) | 1 (3) | 0 (0) | 2 (5) | 1 (9) | 0 (0) | 1 (3) | 3 (10) |
| Musculoskeletal | 5 (14) | 24 (65) | 4 (10) | 29 (69) | 6 (55) | 3 (27) | 2 (7) | 23 (79) |
| CV and respiratory | 1 (8) | 6 (16) | 2 (5) | 3 (7) | 1 (9) | 1 (9) | 2 (7) | 2 (7) |
| Vasculitis | 2 (5) | 7 (19) | 0 (0) | 5 (12) | 1 (9) | 1 (9) | 0 (0) | 4 (14) |
| Renal | 1 (3) | 5 (14) | 0 (0) | 4 (10) | 0 (0) | 1 (9) | 0 (0) | 1 (3) |
| Haematological | 1 (3) | 3 (8) | 0 (0) | 7 (17) | 0 (0) | 1 (9) | 0 (0) | 1 (3) |
aMean total BILAG, where BILAG A = 9, BILAG B = 3, BILAG C = 1 and BILAG D/E = 0 [39]. N/A: not measured; CV: cardiovascular; HRQOL: health-related quality of life; MCS: mental component summary; PCS: physical component summary; PGA: physician global assessment; PtGA: patient global assessment; SF-36: 36-item Medical Outcomes Survey Short Form questionnaire.
Of the patients enrolled in the ALLEVIATE RCTs, 63% (n = 57) were receiving immunosuppressives, 71% (n = 64) antimalarials and 43% (n = 39) prednisone > 25 mg/day (Table 1). As might be expected with higher disease activity, more epratuzumab 720 mg/m2 patients were receiving >25 mg/day corticosteroids (73%, vs 43% of the 360 mg/m2 patients and 35% of placebo patients) as well as antimalarials (82%, vs 74% of the 360 mg/m2 patients and 65% of placebo patients).
Primary efficacy and safety endpoints
The primary efficacy and safety results are described in more detail in a separate manuscript [23]. There was no significant difference in BILAG responses at week 12. In the epratuzumab 360 mg/m2 arm, 44.1% (15/34) of patients were responders, vs 20.0% (2/10) in the 720 mg/m2 arm and 30.3% (9/30) in the placebo arm (P = 0.177) [23, 32]. The incidences of all adverse events (AEs), serious adverse events (SAEs), infusion-related AEs and infections were similar between the epratuzumab- and placebo-treated groups [23, 32].
PGA of disease activity
Baseline values are shown in Table 1. The proportions of patients perceived by the physician as improved by ≥20% in the PGA from baseline to week 12 were higher with epratuzumab [77% (26/34) for 360 mg/m2 and 80% (8/10) for 720 mg/m2] than placebo [60% (18/30)]. This difference was sustained throughout the ALLEVIATE studies, but did not achieve statistical significance.
PtGA of disease activity and HRQOL
Baseline values are shown in Table 1. The proportions of patients reporting PtGA improvements greater than or equal to the MCID from baseline to week 12 were higher with epratuzumab [68% (23/34) for 360 mg/m2 and 70% (7/10) for 720 mg/m2] than placebo [53% (16/30)]. Mean changes in disease activity with placebo occurred early and decreased over 24–48 weeks, whereas large clinically meaningful improvements were evident over 12–48 weeks with 720 mg/m2 and over 36–48 weeks with 360 mg/m2. These changes did not achieve statistical significance.
At baseline, mean SF-36 PCS scores were 2−3 s.d. lower than normative scores of 50 [31] and MCS scores were ≤1 s.d. less, indicative of the impact of active SLE on HRQOL. This was particularly pronounced among patients with BILAG A scores who received the 720 mg/m2 dose.
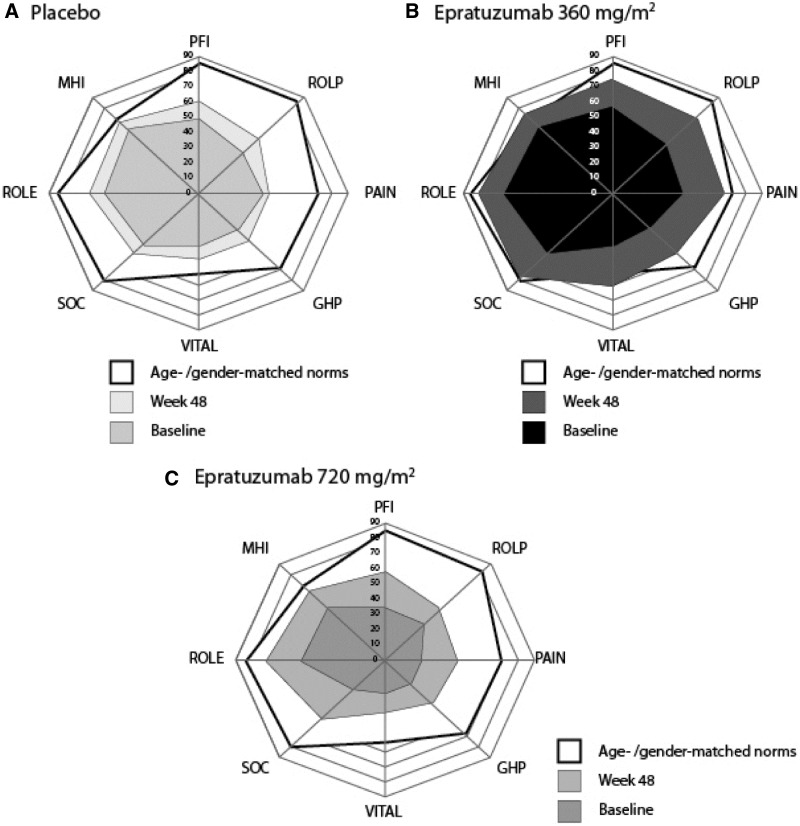
Mean scores for the eight domains of SF-36 at baseline and week 48 are shown in Fig. 2 and Table 2, compared with age- and gender-matched US norms as a benchmark. At week 48, mean SF-36 scores reported by patients receiving epratuzumab 360 mg/m2 approached or exceeded normative values in five domain scores: BP, SF, RE, MH and VT, with the largest improvements in VT, which exceeded normative values. Improvements were evident as well in the 720 mg/m2 group, but were less marked (Fig. 2 and Table 2).
Fig. 2.
Baseline and week 48 mean SF-36 domain scores in ALLEVIATE.
Spydergrams showing baseline and week 48 mean SF-36 domain scores vs age- and gender-matched norms for (A) placebo (n = 36 and n = 14, respectively), (B) epratuzumab 360 mg/m2 (n = 40 and n = 14) and (C) epratuzumab 720 mg/m2 (n = 10 and n = 4) in ALLEVIATE. Inner polygon (light blue) indicates baseline domain scores, outer polygon (yellow) indicates age- and gender-matched norms and intermediate polygon (dark blue) indicates mean changes at week 48. Physical domains are presented clockwise from the top and mental domains clockwise from the bottom. Physical domains are: PFI: physical function; ROLP: physical role; PAIN: bodily pain; GHP: general health. Mental domains are: VITAL: vitality; SOC: social function; ROLE: emotional role; MHI: mental health.
Table 2.
SF-36 domain scores vs age- and gender-matched norms at baseline and week 48 for all treatment groups in ALLEVIATE (intention-to-treat population)
| Physical domains |
Mental domains |
|||||||
|---|---|---|---|---|---|---|---|---|
| PF | RP | BP | GH | VT | SF | RE | MH | |
| Placebo | ||||||||
| Baseline | 49.6 (26.20) | 36.8 (23.05) | 38.2 (21.24) | 32.9 (17.51) | 34.8 (15.97) | 48.3 (22.08) | 58.6 (29.04) | 60.3 (17.89) |
| Week 48 | 61.4 (25.90) | 50.5 (31.81) | 41.6 (24.06) | 43.4 (22.74) | 43.8 (20.36) | 55.4 (20.64) | 66.7 (26.15) | 67.3 (14.95) |
| Epratuzumab 360 mg/m2 | ||||||||
| Baseline | 58.0 (28.03) | 45.8 (30.11) | 42.0 (21.87) | 32.8 (18.90) | 36.8 (19.87) | 56.3 (28.19) | 66.3 (27.13) | 62.0 (16.42) |
| Week 48 | 75.0 (29.74) | 71.9 (28.46) | 67.2 (21.68) | 54.1 (22.08) | 62.1 (18.26) | 79.5 (25.29) | 81.5 (21.48) | 73.9 (19.33) |
| Epratuzumab 720 mg/m2 | ||||||||
| Baseline | 35.0 (24.83) | 33.0 (29.86) | 22.1 (19.17) | 23.2 (17.07) | 22.7 (20.40) | 28.4 (28.00) | 51.5 (31.36) | 49.5 (24.34) |
| Week 48 | 57.5 (29.26) | 47.5 (33.25) | 43.6 (30.7) | 40.2 (26.63) | 34.4 (27.72) | 55.0 (31.37) | 73.3 (30.28) | 66.0 (19.81) |
| Age- and gender-matched norms [16] | 85.5 | 84.3 | 71.0 | 69.6 | 54.1 | 81.8 | 85.8 | 70.5 |
Data are mean (s.d.). PF: physical function; RP: physical role; BP: bodily pain; GH: general health; VT: vitality; SF: social function; RE: emotional role; MH: mental health.
Changes in corticosteroid medication
At week 24, more patients in the epratuzumab treatment groups met the corticosteroid-tapering criteria: 75.0% (n = 24) receiving 360 mg/m2, 100.0% (n = 6) receiving 720 mg/m2 and 56.5% (n = 13) receiving placebo (Table 3). In the combined ALLEVIATE trials, cumulative corticosteroid doses per patient over 24 weeks were lower in both epratuzumab groups than placebo, after adjustment for ethnicity, baseline immunosuppressive use and steroid flare regimen. Cumulative corticosteroid doses (i.e. least-square mean cumulative steroid dose from baseline until week 24) were 1051 mg less in the 360 mg/m2 group (P = 0.034, 95% CI −2018, −83) and 1973 mg less in the 720 mg/m2 treatment group (P = 0.081, 95% CI −4203, 256) than in the placebo group. At week 48, differences were not significant (Table 3).
Table 3.
Corticosteroid use and reductions in ALLEVIATE (intention to treat population)
| Placebo (n = 37) | Epratuzumab 360 mg/m2 (n = 42) | Epratuzumab 720 mg/m2 (n = 11) | |
|---|---|---|---|
| Baseline corticosteroid dose, median (range), mg/day | 20.0 (15.0–60.0) | 25.0 (10.0–60.0) | 46.0 (10.0–80.0) |
| Baseline–week 24 | n = 37 | n = 40 | n = 11 |
| Corticosteroid dose, median (range), mg/day | 9.64 (0–137.4) | 10.55 (0–24.6) | 13.51 (4.3–49.0) |
| Cumulative corticosteroid use, median (range), mg | 2533 (595–16585) | 2384 (1078–4985) | 4668 (1240–6960) |
| Cumulative corticosteroid use, mean (s.d.), mg | 3738 (3412) | 2786 (1195) | 4566 (1601) |
| Least-squares (LS) mean difference from placebo in cumulative corticosteroid use (95% CI), mg | −1051 (−2018, −83) | −1973 (−4203, 256) | |
| P-value (LS mean vs placebo) | 0.034* | 0.081 | |
| Week 24–48 | n = 31 | n = 34 | n = 9 |
| Corticosteroid dose, median (range), mg/day | 4.79 (−0.2 to 87.1) | 4.85 (−0.2 to 56.0) | 4.28 (−0.4 to 8.4) |
| Cumulative corticosteroid use, median (range), mg | 1268 (45–11120) | 1254 (55–6035) | 1358 (458–5020) |
| Cumulative corticosteroid use, mean (s.d.), mg | 2292 (2678) | 1670 (1578) | 1534 (1361) |
| LS mean difference from placebo in cumulative corticosteroid use (95% CI), mg | −675 (−1744, 395) | −652 (−2907, 1603) | |
| P-value (LS mean vs placebo) | 0.212 | 0.561 | |
| Patients who achieved corticosteroid-tapering criteriaa at week 24 | |||
| Assessed | 23 | 32 | 6 |
| Yes, n (%) | 13 (56.5) | 24 (75) | 6 (100) |
| No, n (%) | 10 (43.5) | 8 (25) | 0 (0) |
| Difference in proportion | 18.5 | 43.5 | |
| P-value | 0.25 | 0.072 | |
aCorticosteroid-tapering criteria: reduction in corticosteroids to ≤10 mg/day (ALLEVIATE-1) or ≤7.5 mg/day (ALLEVIATE-2) prednisone equivalents by week 24. *Statistically significant P-value from analysis of variance (ANOVA), adjusting for baseline factors.
SL0006 open-label extension study
Twenty-nine patients entered SL0006, having received placebo (n = 8) or 360 (n = 17) or 720 (n = 4) mg/m2 epratuzumab during the ALLEVIATE RCTs. In these 29 patients, the mean total numerical BILAG score at ALLEVIATE baseline was 12.6 and at entry into the SL0006 it was 8.4. At week 100, in 19 patients with available data, the mean BILAG score was 7.2. No new or unexpected AEs, SAEs, infusion-related AEs or infections were reported. Over a median (range) treatment duration of 120 weeks (range 13–184), all 29 patients reported at least one AE, with SAEs in 10 patients (35%) and 3 patients (10%) discontinuing because of AEs [23, 32].
Among the 29 patients who entered SL0006, the median (range) corticosteroid dose was 21.0 (10–80) mg/day at the baseline visit of the ALLEVIATE trials and 7.5 (0–30) mg/day at the SL0006 baseline visit. Most patients were receiving corticosteroids at the SL0006 screening (27/29, 93.1%): 17 (59%) patients at ≤7.5 mg/day, 11 (38%) at >7.5 to ≤20 mg/day and 1 (3%) at >20 to ≤30 mg/day. During the SL0006, 21 patients (77.8%) had reductions in dose and 11 (40.7%) discontinued corticosteroid treatment. Further tapering occurred during SL0006, to a median of 5.0 mg/day (0–85 mg/day; n = 28) at week 48 and 5.0 mg/day (0–40 mg/day; n = 19) at week 100. Improvements in HRQOL from the ALLEVIATE baseline were evident by entry into SL0006 in all SF-36 domains, and were maintained or further improved during the extension study following treatment with epratuzumab 360 mg/m2 (Table 4).
Table 4.
Mean SF-36 domain scores of the 29 patients that took part in SL0006 versus age- and gender-matched norms
| Physical domains |
Mental domains |
|||||||
|---|---|---|---|---|---|---|---|---|
| PF | RP | BP | GH | VT | SF | RE | MH | |
| ALLEVIATE baseline (n = 29) | 52.8 | 35.8 | 37.6 | 30.5 | 31.5 | 48.7 | 67.8 | 62.6 |
| SL0006 | ||||||||
| Screening (n = 27) | 58.5 | 47.0 | 44.3 | 37.0 | 34.5 | 53.2 | 71.3 | 64.3 |
| Week 48 (n = 28) | 63.4 | 55.1 | 52.6 | 43.4 | 40.0 | 59.8 | 70.2 | 66.1 |
| Week 100 (n = 19) | 66.3 | 55.3 | 47.6 | 41.6 | 39.8 | 65.1 | 71.5 | 66.1 |
| Age-/gender-matched norms | 85.5 | 84.3 | 71.0 | 69.6 | 54.1 | 81.8 | 85.8 | 70.5 |
Discussion
The results presented here are based on a pooled analysis of data from two interrupted RCTs, based on pre-specified HRQOL and corticosteroid-sparing endpoints. In this analysis, numeric differences between the epratuzumab arms and the placebo group in PGA, PtGA and SF-36 persisted throughout the study but did not achieve statistical significance. However, the magnitude of some of these changes suggests clinical relevance. For instance, in patients receiving epratuzumab 360 mg/m2, SF-36 scores at week 48 met or exceeded age- and gender-matched norms in five domains in those receiving epratuzumab 360 mg/m2, despite low baseline scores [31]. Improvements in SF-36 domain scores were maintained over the 2 years of follow-up in SL0006.
In addition, at week 24 in the two ALLEVIATE trials, more patients met the pre-specified corticosteroid dose reduction criterion in the epratuzumab groups than the placebo group. Differences in cumulative corticosteroid doses between epratuzumab 360 mg/m2 and placebo at week 24 were also statistically significant, which would be expected to reduce the adverse effect burden of treatment. Most patients also experienced further reductions in corticosteroid use during SL0006.
The outcomes of the analyses reported here are consistent with the primary efficacy and safety results of the ALLEVIATE RCTs [23, 32]. Of interest, given prior recognized discordance, patients and physicians reported similar numbers, with ≥20% improvement in disease activity. The correspondence between these measurements is notable: patient-reported outcomes are strongly predictive of subsequent morbidity and mortality [33], but frequently do not correlate well with physician assessments in SLE [6]. As evident in surveys of patients with SLE [15, 34–36], they focus more on functional limitations resulting from SLE and/or co-morbid conditions (such as fibromyalgia), whereas physicians focus on clinical and laboratory assessments [33, 37]. In surveys, patients also consistently report dissatisfaction with their treatment, in particular with adverse effects associated with corticosteroid use [15], ubiquitous in patients with moderate to severely active disease [38].
Although regulatory agencies encourage the use of patient-reported outcomes in RCTs, they are often not included in SLE trials, in contrast with other chronic rheumatic diseases [6, 33]. The value of the assessment of HRQOL, especially in view of the broad impact of active disease on physical, emotional and social functioning, is significant and supports inclusion of patient-reported outcomes alongside measures of disease activity and damage in clinical trials.
The endpoints reported in this article are based on a pooled analysis of two interrupted RCTs. The two RCT protocols were very similar, HRQOL and corticosteroid dosing endpoints reported here were pre-specified and the combined analysis was revised before unblinding of the studies. Interpretation of the results is limited by differing inclusion criteria, the small number of patients involved and the fact that statistical analyses of secondary endpoints were not adjusted for multiple testing. Results from the SL0006 extension must also be interpreted with caution, given that it was open label and that 8 of 29 patients had previously received placebo.
Nevertheless, in these analyses epratuzumab treatment was associated with large and clinically meaningful improvements in HRQOL and PtGA of disease activity over weeks 12–48 and clinically meaningful reductions in corticosteroid doses. These early improvements were maintained over 2 years of follow-up. Although most endpoints in these analyses did not achieve statistical significance, taken together with the primary efficacy and safety results, these data support continued development of epratuzumab as a treatment for patients with SLE. Phase 3 studies are under way in patients with moderate to severely active SLE.
Rheumatology key messages.
Epratuzumab treatment resulted in clinically meaningful improvements in health-related quality of life for patients with moderate to severe SLE.
Epratuzumab treatment reduced corticosteroid doses by clinically meaningful amounts in patients with moderate to severe SLE.
Epratuzumab treatment resulted in clinically meaningful improvements in physician global assessments in patients with moderate to severe SLE.
Acknowledgements
We thank Daphnee Pushparajah, UCB Pharma, for providing publication management support, and Enkeleida Nikaï, UCB Pharma, for helpful comments on the draft manuscript. The authors also thank all the investigators who participated in this study. Medical writing and editorial assistance in the development of this manuscript was provided by Niall Harrison and Liz Haygreen of Darwin Healthcare Communications and was supported by UCB Pharma.
Funding: The ALLEVIATE (SL0003/4) studies were partly funded by Immunomedics and UCB Pharma and the SL0006 was funded by UCB Pharma.
Disclosure statement: K.H. has been a consultant for UCB and HGS and is a member of a speakers’ bureau for Abbott and HGS. D.M.G. is an officer, shareholder and employee of Immunomedics. V.S. is a consultant for and received honoraria from UCB Pharma, Abbott Immunology, Amgen, Anthera, AstraZeneca, BMS, Genentech/Roche, GSK, Human Genome Sciences, Idera, Janssen, Lilly, Medimmune, Novartis Pharmaceuticals, Novo Nordisk, Orbimed, Pfizer, Rigel, Sanofi and Takeda. M.P. received research grants from and acted as a consultant for UCB Pharma. B.K. is an employee and shareholder of UCB Pharma. L.K. was an employee of UCB Pharma at the time the clinical trials and analysis was performed. C.G. has received research grants from Aspreva and consulting fees from Aspreva, Bristol-Myers Squibb, GSK, Genentech, Biogen IDEC, MedImmune, Merck Serono Pharmaceuticals, Roche, Immunomedics and UCB Pharma. D.J.W. has been paid consulting fees by Bristol-Myers Squibb, Genentech, Biogen IDEC, Human Genome Sciences, MedImmune, Novo Nordisk and UCB Pharma. W.A.W. is an employee and stockholder of Immunomedics. K.K. has been paid consulting fees by Bristol-Myers Squibb, Genentech, Biogen IDEC, Anthera, MedImmune, Novo Nordisk, Zymogenetics, Serono and UCB and received research grants from Genentech, Biogen IDEC, Cephalon, MedImmune, Novo Nordisk and UCB Pharma.
References
- 1.Ben-Menachem E. Review article: systemic lupus erythematosus: a review for anesthesiologists. Anesth Analg. 2010;111:665–76. doi: 10.1213/ANE.0b013e3181e8138e. [DOI] [PubMed] [Google Scholar]
- 2.Eisenberg R. Why can’t we find a new treatment for SLE? J Autoimmun. 2009;32:223–30. doi: 10.1016/j.jaut.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–39. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 4.Kiani AN, Petri M. Quality-of-life measurements versus disease activity in systemic lupus erythematosus. Curr Rheumatol Rep. 2010;12:250–8. doi: 10.1007/s11926-010-0114-1. [DOI] [PubMed] [Google Scholar]
- 5.Strand V, Chu A. Measuring outcomes in SLE clinical trials. Expert Rev Pharmacoecon Outcomes Res. 2011;11:455–68. doi: 10.1586/erp.11.38. [DOI] [PubMed] [Google Scholar]
- 6.Strand V. Lessons learned from clinical trials in SLE. Autoimmun Rev. 2007;6:209–14. doi: 10.1016/j.autrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Strand V, Gladman D, Isenberg D, et al. Outcome measures to be used in clinical trials in systemic lupus erythematosus. J Rheumatol. 1999;26:490–7. [PubMed] [Google Scholar]
- 8.Smolen JS, Strand V, Cardiel M, et al. Randomized clinical trials and longitudinal observational studies in systemic lupus erythematosus: consensus on a preliminary core set of outcome domains. J Rheumatol. 1999;26:504–7. [PubMed] [Google Scholar]
- 9.Strand V, Chu AD. Generic versus disease-specific measures of health-related quality of life in systemic lupus erythematosus. J Rheumatol. 2011;38:1821–3. doi: 10.3899/jrheum.110766. [DOI] [PubMed] [Google Scholar]
- 10.Abu-Shakra M, Mader R, Langevitz P, et al. Quality of life in systemic lupus erythematosus: a controlled study. J Rheumatol. 1999;26:306–9. [PubMed] [Google Scholar]
- 11.Stoll T, Gordon C, Seifert B, et al. Consistency and validity of patient administered assessment of quality of life by the MOS SF-36; its association with disease activity and damage in patients with systemic lupus erythematosus. J Rheumatol. 1997;24:1608–14. [PubMed] [Google Scholar]
- 12.Fortin PR, Abrahamowicz M, Neville C, et al. Impact of disease activity and cumulative damage on the health of lupus patients. Lupus. 1998;7:101–7. doi: 10.1191/096120398678919813. [DOI] [PubMed] [Google Scholar]
- 13.Elbirt D, Sthoeger D, Asher I, et al. The management of systemic lupus erythematosus: facts and controversies. Clin Dermatol. 2010;28:330–6. doi: 10.1016/j.clindermatol.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Schneider M, Gordon C, Lerstrom K, et al. Impact of lupus on fatigue, health-related quality of life and work productivity: results from the Lupus European Online (LEO) survey. Ann Rheum Dis. 2011;70(Suppl 3):425. [Google Scholar]
- 15.Lerstrom K, Crimmings M, Govoni M, et al. Disease symptoms and coping strategies in patient with lupus. Portugal: European Lupus Meeting, Porto; 2011. p. 145. [Google Scholar]
- 16.Strand V, Petri M, Buyon J, et al. Systemic lupus erythematosus (SLE) impacts all domains of health-related quality of life (HRQOL): baseline results from five randomized controlled trials (RCTs) Ann Rheum Dis. 2007;66:482. [Google Scholar]
- 17.Thumboo J, Strand V. Health-related quality of life in patients with systemic lupus erythematosus: an update. Ann Acad Med Singapore. 2007;36:115–22. [PubMed] [Google Scholar]
- 18.Gayed M, Gordon C. Novel treatments for systemic lupus erythematosus. Curr Opin Investig Drugs. 2010;11:1256–64. [PubMed] [Google Scholar]
- 19.Dorner T, Goldenberg DM. Targeting CD22 as a strategy for treating systemic autoimmune diseases. Ther Clin Risk Manag. 2007;3:953–9. [PMC free article] [PubMed] [Google Scholar]
- 20.Walker JA, Smith KG. CD22: an inhibitory enigma. Immunology. 2008;123:314–25. doi: 10.1111/j.1365-2567.2007.02752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorner T, Kaufmann J, Wegener WA, et al. Initial clinical trial of epratuzumab (humanized anti-CD22 antibody) for immunotherapy of systemic lupus erythematosus. Arthritis Res Ther. 2006;8:R74. doi: 10.1186/ar1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobi AM, Goldenberg DM, Hiepe F, et al. Differential effects of epratuzumab on peripheral blood B cells of patients with systemic lupus erythematosus versus normal controls. Ann Rheum Dis. 2008;67:450–7. doi: 10.1136/ard.2007.075762. [DOI] [PubMed] [Google Scholar]
- 23.Wallace DJ, Gordon C, Strand V, et al. Efficacy and safety of epratuzumab in patients with moderate/severe flaring systemic lupus erythematosus: results from two randomized, double-blind, placebo-controlled, multicenter studies (ALLEVIATE) and follow-up. Rheumatology. 2013;52:1313–22. doi: 10.1093/rheumatology/ket129. [DOI] [PubMed] [Google Scholar]
- 24.ACR. 1997 update of the 1982 American College of Rheumatology revised criteria for classification of systemic lupus erythematosus. http://www.rheumatology.org/practice/clinical/classification/SLE/1997_update_of_the_1982_acr_revised_criteria_for_classification_of_sle.pdf (30 October 2013, date last accessed) [Google Scholar]
- 25.Hay EM, Bacon PA, Gordon C, et al. The BILAG index: a reliable and valid instrument for measuring clinical disease activity in systemic lupus erythematosus. Q J Med. 1993;86:447–58. [PubMed] [Google Scholar]
- 26.Isenberg DA, Gordon C. From BILAG to BLIPS—disease activity assessment in lupus past, present and future. Lupus. 2000;9:651–4. doi: 10.1191/096120300672904669. [DOI] [PubMed] [Google Scholar]
- 27.Likert R. A technique for the measurement of attitudes. Arch Psychol. 1932;140:1–55. [Google Scholar]
- 28.Strand V, Smolen JS, van Vollenhoven RF, et al. Certolizumab pegol plus methotrexate provides broad relief from the burden of rheumatoid arthritis: analysis of patient-reported outcomes from the RAPID 2 trial. Ann Rheum Dis. 2011;70:996–1002. doi: 10.1136/ard.2010.143586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strand V, Crawford B. Improvement in health-related quality of life in patients with SLE following sustained reductions in anti-dsDNA antibodies. Expert Rev Pharmacoecon Outcomes Res. 2005;5:317–26. doi: 10.1586/14737167.5.3.317. [DOI] [PubMed] [Google Scholar]
- 30.Strand V, Crawford B, Singh J, et al. Use of ‘spydergrams’ to present and interpret SF-36 health-related quality of life data across rheumatic diseases. Ann Rheum Dis. 2009;68:1800–4. doi: 10.1136/ard.2009.115550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ware JE, Kosinski M. SF-36 Physical and Mental Health Summary scales: a manual for users of version 1. 2nd edn. Lincoln, RI: QualityMetric; 2001. [Google Scholar]
- 32.Petri M, Hobbs K, Gordon C, et al. Clinically meaningful improvements with epratuzumab (anti-CD22 mAb targeting B-cells) in patients (pts) with moderate/severe systemic lupus erythematosus (SLE) flares: results from 2 randomized controlled trials. Arthritis Rheum. 2008;57(Suppl):1087. [Google Scholar]
- 33.Jolly M. Pitfalls and opportunities in measuring patient outcomes in lupus. Curr Rheumatol Rep. 2010;12:229–36. doi: 10.1007/s11926-010-0105-2. [DOI] [PubMed] [Google Scholar]
- 34.Crimmings M, Lerstrom K, Govoni M, et al. Impact of lupus on patients' employment, family relationships and overall well-being. 2010. Presented at: ACR 2010:Poster PO1 F.9. [Google Scholar]
- 35.Lerstrom K, Crimmings M, Govoni M, et al. Impact of systemic lupus erythematosus on patients’ employment, family relationships and overall well-being. Ann Rheum Dis. 2010;69(Suppl. 3):753. [Google Scholar]
- 36.McElhone K, Abbott J, Gray J, et al. Patient perspective of systemic lupus erythematosus in relation to health-related quality of life concepts: a qualitative study. Lupus. 2010;19:1640–7. doi: 10.1177/0961203310378668. [DOI] [PubMed] [Google Scholar]
- 37.Jolly M, Utset TO. Can disease specific measures for systemic lupus erythematosus predict patients health related quality of life? Lupus. 2004;13:924–6. doi: 10.1191/0961203304lu2034oa. [DOI] [PubMed] [Google Scholar]
- 38.Bernatsky S, Peschken C, Fortin PR, et al. Medication use in systemic lupus erythematosus. J Rheumatol. 2011;38:271–4. doi: 10.3899/jrheum.100414. [DOI] [PubMed] [Google Scholar]
- 39.Cresswell L, Yee CS, Farewell V, et al. Numerical scoring for the classic BILAG index. Rheumatology. 2009;48:1548–52. doi: 10.1093/rheumatology/kep183. [DOI] [PMC free article] [PubMed] [Google Scholar]