Abstract
G protein–coupled receptors (GPCRs), also known as seven-transmembrane receptors (7TMRs), transduce various sensory and nonsensory signals. It is now widely accepted that these receptors associate with each other as homomeric or heteromeric dimers or as higher-order oligomers. This realization raises a number of questions regarding the quaternary structure of GPCRs and the function of GPCR oligomers: How does ligand binding in one protomer affect an associated protomer? What is the functional unit that activates downstream signaling molecules? What parts of the receptor form the interfaces between protomers? Where along the pathway from synthesis to degradation do oligomers form? Do they ever dissociate? Until recently, this last question has attracted little attention, and GPCR dimers and oligomers have generally been considered to be stable structures. However, biophysical studies have now begun to address this question, and the answer that is emerging will require a reassessment of the stable dimer model.
Many transmembrane (TM) proteins laterally associate with themselves or with other TM proteins to function as dimers or higher-order oligomers. The stability of TM quaternary structures varies widely. Ion channels provide a good example of stable TM oligomers. In one well-studied case, bacterial KcsA channels remain homotetramers even in detergents such as SDS and do not revert to monomers over the course of 2 weeks (1). At the other extreme, receptor tyrosine kinases (RTKs) are thought to exist in the plasma membrane in a monomer-dimer equilibrium, and robust dimerization requires ligand binding (2). Preformed, unliganded RTK dimers are difficult to observe and as a species probably represent a small fraction of unstimulated RTKs.
Where between these two extremes do G protein–coupled receptors (GPCRs) fall? A relatively small subclass of these receptors, the class C GPCRs, are stable obligate dimers whose mechanisms of association are well understood (3). In contrast, much less is known about the mechanisms and stability of class A GPCR dimerization, a larger subclass of receptors that is epitomized by the photoreceptor rhodopsin. Various methods have shown the propensity of these receptors to self-associate in lipid bilayers and cell membranes (4). However, few of these methods have the capability to determine the stability of receptor dimers or oligomers (5). A recent single-molecule imaging study by Hern et al. has provided the best evidence yet that some GPCR dimers live a short life (6).
Hern et al. first synthesized slowly dissociating fluorescent ligands for M1 muscarinic acetylcholine receptors, which belong to the rhodopsin-like class A subfamily of GPCRs. Total internal reflectance fluorescence microscopy (TIRFM) then allowed imaging of single M1 receptors bound to fluorescent ligands on the surface of cells. When the fluorescent ligands photobleached, the fluorescence of most individual spots decreased to background amounts in a single step, suggesting that individual receptors possessed only a single ligand-binding site (and thus were likely to be monomers). A few fluorescent spots were approximately twice as bright as the majority and bleached in two steps, suggesting that a fraction of M1 receptors (estimated at 30%) existed as dimers. Individual ligand-bound M1 receptors could also be tracked over time as they diffused laterally in the plasma membrane (Fig. 1). This allowed the authors to directly observe the association and dissociation of M1 receptors and estimate the mean lifetime of M1 receptor dimers, which was calculated to be 0.5 s (6).
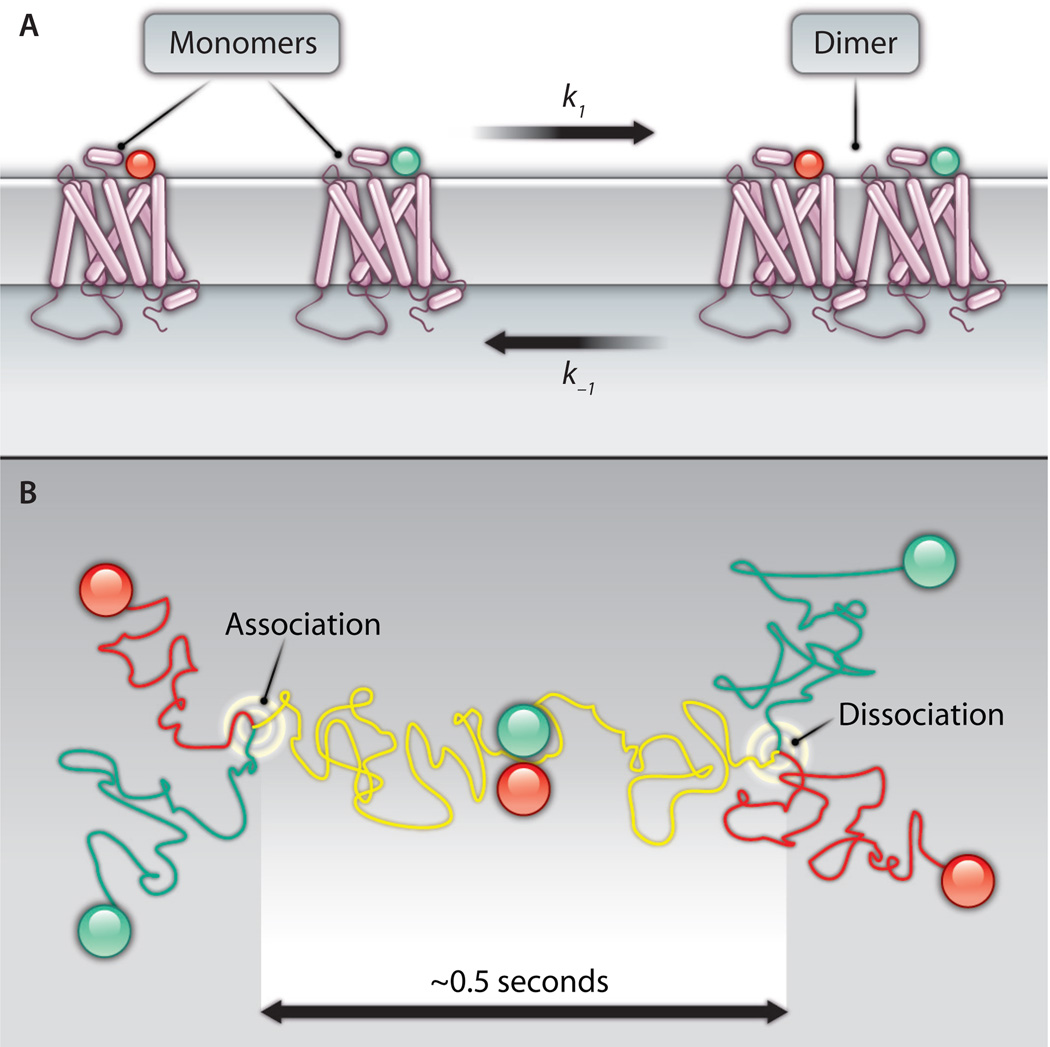
Fig. 1.
(A) Some class A GPCRs exist at the plasma membrane in a monomer-dimer equilibrium. The traditional view of GPCRs as freely diffusing monomers (left) and the newer view of GPCRs as dimers (right) are both accommodated by a dynamic equilibrium. The rate of dimer association (k1) will depend on the local concentration of monomers and time, whereas the rate of dimer dissociation (k−1) will depend only on time. Both rates might be altered by cellular factors such as lipid composition, the presence of ligands, and other factors. (B) A top-down view of GPCRs diffusing on the cell surface, such as in the experiments of Hern et al. (6). Two receptors labeled with differently colored ligands take separate random walks in the plasma membrane until they encounter each other (left) and (in some cases) associate. The two receptors diffuse together as a dimer (yellow track) until they dissociate (right) and go their separate ways. In the experiments of Hern et al., dimers existed for ~0.5 s on average, and ~30% of the receptors were dimers at any given instant.
The results of this study fit well with previous efforts to solve this problem by imaging populations of fluorescently labeled class A GPCRs. In these studies, a fraction of the receptors on the cell surface was immobilized by crosslinking with an antibody, and the mobility of the non-crosslinked remainder was measured by using fluorescence recovery after photobleaching (FRAP). Immobilization of protomers that are part of stable dimers or oligomers should also immobilize associated protomers that are not directly crosslinked. Instead, in one such study the mobility of β1 adrenoreceptors was changed only slightly when a fraction was immobilized by crosslinking (7). Increasing the fraction of crosslinked β1 adrenoreceptors decreased the mobility of the remaining protomers further, but not to the point of immobility. This suggested that, similar to M1 receptors, β1 adrenoreceptors transiently associate with each other. Similarly, antibody-crosslinked D2 dopamine receptors failed to impede the diffusion of other D2 receptors unless the interaction between them was made permanent by oxidative crosslinking (8). Taken together, these three studies suggest that at least some class A GPCRs remain associated for seconds or less.
What do these findings say about the probable structure of transient GPCR dimers? The crystal structures of several class A GPCRs have been solved, yet none of these receptors crystallized as dimers with a structure that has also been shown to exist in vivo (such as is the case for ion channels) (9). The quaternary structure of class A GPCRs has thus remained a problem to be solved. Crosslinking and fluorescence resonance energy transfer (FRET) studies have identified residues that are in or adjacent to interfaces between protomers, suggesting that GPCR oligomers adopt preferred interprotomer orientations (10–13). However, mutagenesis studies have not identified individual residues or motifs that are necessary for receptor association. Exchange of entire TM helices can alter the propensity of GPCRs to associate (14), but discrete structural elements responsible for oligomerization have not been reported.
The observation that GPCR dimers associate and dissociate on the time scale of seconds suggests that the interface between protomers may be smaller than previously imagined and less dependent on specific conserved structural motifs. Protein-protein association is a multistep process that involves formation of transient encounter complexes under the influence of long-range attractive forces, followed by desolvation, rearrangement of side-chains, and finally the formation of specific chemical bonds (15). This process can be described as falling into a funnel-shaped free-energy well as the number of bonds formed increases. Not all associations progress to the formation of multiple specific bonds, and the free-energy wells for such complexes will be correspondingly shallow. The depth of the free-energy well for a protein complex will determine how long, on average, the proteins remain associated.
The transient nature of GPCR dimers revealed by recent studies suggests that the free-energy wells for these particular dimers are shallow. Weak attractive forces are sufficient to promote the formation of transient dimers in bilayers because the entropic cost of protein-protein association is low in this two-dimensional environment (16, 17). For example, hydrogen bonds between single polar residues buried in the nonpolar bilayer core can promote the substantial association of model TM peptides (18). Similarly, minimization of mismatch between the hydrophobic length of TM helices and the hydrophobic bilayer thickness has been proposed to be a sufficient mechanism to drive oligomerization of TM proteins (19), including GPCRs (20). These or other “nonspecific” mechanisms are thus more likely to be responsible for transient GPCR association than a tight stereochemical fit between extensive regions of TM helices. Such nonspecific mechanisms would still impose preferred orientations on protomers and would thus be consistent with experimental data to that effect, but would not require well-defined and conserved structural elements.
This picture of a transient dimer interface is also consistent with the known promiscuity of GPCR interactions. Many individual GPCRs have been reported to form both homomeric and heteromeric complexes, in some cases with several other GPCRs (21). This lack of specificity is more easily accommodated by a model that involves relatively weak and chemically nonspecific mechanisms of association than by a model that involves large specific protein-protein interfaces.
In cases in which the physical links that connect GPCRs are fragile, the functional links between protomers may also be tenuous. Monomeric GPCRs can activate heterotrimeric G proteins (22), and GPCRs and arrestins interact in a 1:1 stoichiometry (23); thus, dimerization is not necessary for the defining functions of these receptors. However, more subtle functional effects have been attributed to direct intramolecular communication within GPCR dimers. These include changes in ligand-binding properties, trafficking, and downstream signaling (21, 24). Nonetheless, reports of GPCR physical association as a phenomenon still greatly outnumber reports describing the functional consequences of such associations. This is particularly true for ligand binding (the notable exception being opioid receptors) (25), suggesting that GPCR oligomerization rarely produces radical changes in the ligand-binding sites of the individual protomers. The relative dearth of described dimeror oligomer-specific functional properties may be partly due to the difficult process of unambiguously demonstrating such changes. However, it is also possible that this reflects the weak nature of some of these physical associations.
The recognition that class A GPCRs sometimes transiently associate with each other raises several questions. For example, how representative is the subsecond dimer life time determined for M1 receptors for class A GPCRs in general? Given the diversity of these receptors, it is likely that the stability of dimers and oligomers will vary widely. Indeed, the study that reported transient association of β1 adrenoreceptors also reported stable association of β2 adrenoreceptors (7). Similarly, reports of concentration-independent resonance energy transfer are difficult to reconcile with unstable self-association (10, 26–28). Transient association also suggests that physiologically meaningful regulation of dimer or oligomer abundance may occur in the short term. Several reports have indicated that ligand binding may regulate the self-association of GPCRs (10), suggesting that ligand binding and quaternary structure may regulate each other in a bidirectional manner. As Hern et al. point out, the proportion of transient dimers at the surface of a given cell will depend on the local concentration of protomers, and thus on rates of protomer delivery to and removal from the plasma membrane, as well as mechanisms that might locally confine protomers. Given the potential involvement of the lipid environment in driving or facilitating oligomerization, it is also possible that the monomer-dimer equilibrium will shift as GPCRs transit the various cellular compartments they encounter from synthesis to degradation.
The fact that GPCRs exist in a monomer-dimer equilibrium may help to explain some of the conflicting observations that have been made concerning GPCR dimerization. Random collision and permanent oligomerization are sometimes the only alternatives considered when this issue is discussed. Experiments that are designed to determine whether a given GPCR oligomerizes might yield results that are consistent with either interpretation, depending on which side of the equilibrium is favored by the conditions of the experiment. This is particularly true for experiments that involve populations of receptors. For this reason, it is likely that single-molecule methods, such as that used by Hern et al., will be useful in determining how often GPCR dimers come together and fall apart.
Acknowledgments
Funding: Work in N.L.’s laboratory is supported by NIH grant GM078319.
References and Notes
- 1.Heginbotham L, Odessey E, Miller C. Tetrameric stoichiometry of a prokaryotic K+ channel. Biochemistry. 1997;36:10335–10342. doi: 10.1021/bi970988i. [DOI] [PubMed] [Google Scholar]
- 2.Hubbard SR, Miller WT. Receptor tyrosine kinases: Mechanisms of activation and signaling. Curr. Opin. Cell Biol. 2007;19:117–123. doi: 10.1016/j.ceb.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pin JP, Galvez T, Prézeau L. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol. Ther. 2003;98:325–354. doi: 10.1016/s0163-7258(03)00038-x. [DOI] [PubMed] [Google Scholar]
- 4.Milligan G, Bouvier M. Methods to monitor the quaternary structure of G protein-coupled receptors. FEBS J. 2005;272:2914–2925. doi: 10.1111/j.1742-4658.2005.04731.x. [DOI] [PubMed] [Google Scholar]
- 5.Gurevich VV, Gurevich EV. How and why do GPCRs dimerize? Trends Pharmacol. Sci. 2008;29:234–240. doi: 10.1016/j.tips.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hern JA, Baig AH, Mashanov GI, Birdsall B, Corrie JET, Lazareno S, Molloy JE, Birdsall NJ. Formation and dissociation of M1 muscarinic receptor dimers seen by total internal reflection fluorescence imaging of single molecules. Proc. Natl. Acad. Sci. U.S.A. 2010;107:2693–2698. doi: 10.1073/pnas.0907915107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorsch S, Klotz KN, Engelhardt S, Lohse MJ, Bünemann M. Analysis of receptor oligomerization by FRAP microscopy. Nat. Methods. 2009;6:225–230. doi: 10.1038/nmeth.1304. [DOI] [PubMed] [Google Scholar]
- 8.Fonseca JM, Lambert NA. Instability of a class a G protein-coupled receptor oligomer interface. Mol. Pharmacol. 2009;75:1296–1299. doi: 10.1124/mol.108.053876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-proteincoupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fung JJ, Deupi X, Pardo L, Yao XJ, Velez-Ruiz GA, Devree BT, Sunahara RK, Kobilka BK. Ligand-regulated oligomerization of beta(2)-adrenoceptors in a model lipid bilayer. EMBO J. 2009;28:3315–3328. doi: 10.1038/emboj.2009.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo W, Shi L, Javitch JA. The fourth transmembrane segment forms the interface of the dopamine D2 receptor homodimer. J. Biol. Chem. 2003;278:4385–4388. doi: 10.1074/jbc.C200679200. [DOI] [PubMed] [Google Scholar]
- 12.Suda K, Filipek S, Palczewski K, Engel A, Fotiadis D. The supramolecular structure of the GPCR rhodopsin in solution and native disc membranes. Mol. Membr. Biol. 2004;21:435–446. doi: 10.1080/09687860400020291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kota P, Reeves PJ, Rajbhandary UL, Khorana HG. Opsin is present as dimers in COS1 cells: Identification of amino acids at the dimeric interface. Proc. Natl. Acad. Sci. U.S.A. 2006;103:3054–3059. doi: 10.1073/pnas.0510982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.González-Maeso J, Ang RL, Yuen T, Chan P, V.Weisstaub N, López-Giménez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schreiber G, Haran G, Zhou HX. Fundamental aspects of protein-protein association kinetics. Chem. Rev. 2009;109:839–860. doi: 10.1021/cr800373w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grasberger B, Minton AP, DeLisi C, Metzger H. Interaction between proteins localized in membranes. Proc. Natl. Acad. Sci. U.S.A. 1986;83:6258–6262. doi: 10.1073/pnas.83.17.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacKenzie KR, Fleming KG. Association energetics of membrane spanning alpha-helices. Curr. Opin. Struct. Biol. 2008;18:412–419. doi: 10.1016/j.sbi.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choma C, Gratkowski H, Lear JD, DeGrado WF. Asparagine-mediated self-association of a model transmembrane helix. Nat. Struct. Biol. 2000;7:161–166. doi: 10.1038/72440. [DOI] [PubMed] [Google Scholar]
- 19.Sparr E, Ash WL, Nazarov PV, Rijkers DT, Hemminga MA, Tieleman DP, Killian JA. Self-association of transmembrane alpha-helices in model membranes: Importance of helix orientation and role of hydrophobic mismatch. J. Biol. Chem. 2005;280:39324–39331. doi: 10.1074/jbc.M502810200. [DOI] [PubMed] [Google Scholar]
- 20.Botelho AV, Huber T, Sakmar TP, Brown MF. Curvature and hydrophobic forces drive oligomerization and modulate activity of rhodopsin in membranes. Biophys. J. 2006;91:4464–4477. doi: 10.1529/biophysj.106.082776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milligan G. G protein-coupled receptor heterodimerization: Contribution to pharmacology and function. Br. J. Pharmacol. 2009;158:5–14. doi: 10.1111/j.1476-5381.2009.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whorton MR, Bokoch MP, Rasmussen SG, Huang B, Zare RN, Kobilka B, Sunahara RK. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc. Natl. Acad. Sci. U.S.A. 2007;104:7682–7687. doi: 10.1073/pnas.0611448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanson SM, Gurevich EV, Vishnivetskiy SA, Ahmed MR, Song X, Gurevich VV. Each rhodopsin molecule binds its own arrestin. Proc. Natl. Acad. Sci. U.S.A. 2007;104:3125–3128. doi: 10.1073/pnas.0610886104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milligan G. G protein-coupled receptor dimerisation: Molecular basis and relevance to function. Biochim. Biophys. Acta. 2007;1768:825–835. doi: 10.1016/j.bbamem.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 25.van Rijn RM, Whistler JL, Waldhoer M. Opioidreceptor-heteromer-specific trafficking and pharmacology. Curr. Opin. Pharmacol. 2010;10:73–79. doi: 10.1016/j.coph.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salahpour A, Masri B. Experimental challenge to a ‘rigorous’ BRET analysis of GPCR oligomerization. Nat. Methods. 2007;4:599–600. doi: 10.1038/nmeth0807-599. , author reply 601. [DOI] [PubMed] [Google Scholar]
- 27.Mercier JF, Salahpour A, Angers S, Breit A, Bouvier M. Quantitative assessment of beta 1- and beta 2-adrenergic receptor homo- and heterodimerization by bioluminescence resonance energy transfer. J. Biol. Chem. 2002;277:44925–44931. doi: 10.1074/jbc.M205767200. [DOI] [PubMed] [Google Scholar]
- 28.Mansoor SE, Palczewski K, Farrens DL. Rhodopsin self-associates in asolectin liposomes. Proc. Natl. Acad. Sci. U.S.A. 2006;103:3060–3065. doi: 10.1073/pnas.0511010103. [DOI] [PMC free article] [PubMed] [Google Scholar]