Abstract
The emergence of functional neuronal connectivity in the developing cerebral cortex depends on neuronal migration. This process enables appropriate positioning of neurons and the emergence of neuronal identity so that the correct patterns of functional synaptic connectivity between the right types and numbers of neurons can emerge. Delineating the complexities of neuronal migration is critical to our understanding of normal cerebral cortical formation and neurodevelopmental disorders resulting from neuronal migration defects. For the most part, the integrated cell biological basis of the complex behavior of oriented neuronal migration within the developing mammalian cerebral cortex remains an enigma. This review aims to analyze the integrative mechanisms that enable neurons to sense environmental guidance cues and translate them into oriented patterns of migration toward defined areas of the cerebral cortex. We discuss how signals emanating from different domains of neurons get integrated to control distinct aspects of migratory behavior and how different types of cortical neurons coordinate their migratory activities within the developing cerebral cortex to produce functionally critical laminar organization.
Keywords: cerebral cortex, laminar organization, projection neurons, interneurons, radial migration, tangential migration
INTRODUCTION
Neuronal migration and the resultant placement of neurons enable the establishment of functional neuronal connectivity in the developing brain. The two major types of cortical neurons, glutamatergic excitatory projection (pyramidal) neurons and GABAergic inhibitory interneurons, arise from distinct sets of progenitor cells within the telencephalon and migrate over remarkably long distances to their unique areal and laminar locations in the developing neocortex. Correct positioning of newborn neurons within the six-layered neocortex ensures the emergence of proper identities and, thus, appropriate patterns of functional connectivity between the right types and numbers of neurons. Disruptions in neuronal migration result in a plethora of neurodevelopmental disorders, ranging from gross brain malformations such as lissencephaly to neurobehavioral disorders such as autism, underscoring the importance of this process in proper cortical development and function. Postnatally, neuronal migration continues to play an important role in the ongoing maintenance of neuronal circuitry in selective niches of the adult brain. Unraveling the complexities of neuronal migration is thus essential to our understanding of both normal cortical development and neurodevelopmental disorders.
Significant progress has been made toward defining many mechanisms underlying neuronal migration. However, we still do not fully understand how neurons integrate a multitude of signals to produce oriented and coordinated patterns of migration evident in the developing cerebral cortex. In this review, we analyze how neurons navigate from their sites of birth to their target positions within the cerebral cortex, and we focus on mechanisms that enable neurons to sense and integrate the diverse cellular signals necessary to produce distinct patterns of oriented migration toward defined cortical areas. We discuss how signals emanating from different cellular domains (e.g., cell soma, primary cilia, and growth cones) are integrated and how they control distinct aspects of neuronal migratory behavior. Furthermore, we address how different types of neurons (projection neurons and interneurons) coordinate their oriented migratory activities within the developing cerebral cortex to ultimately produce functional laminar organization.
MIGRATION OF PROJECTION NEURONS: RADIAL MIGRATION
Cortical projection neurons arise from undifferentiated neuroepithelial progenitor cells in the ventricular zone (VZ) and subventricular zone (SVZ) of the telencephalon (Ayala et al. 2007, Bystron et al. 2008, Götz & Huttner 2005, Higginbotham et al. 2011, Kriegstein et al. 2006). At the start of corticogenesis, radial glial progenitors (RGPs) in the VZ expand their population by dividing symmetrically to produce two daughter RGPs. As neurogenesis begins, the majority of RGPs in the VZ divide asymmetrically (Higginbotham et al. 2011; Noctor et al. 2001, 2004, 2008). Several modes of asymmetric cell division are recognized within the VZ: (a) neurogenic division (produces a self-renewing RGP and a daughter neuron), (b) asymmetric progenitor division [results in a self-renewing RGP and an intermediate progenitor (IP) cell that migrates into the SVZ], and (c) final gliogenic division (results in a neuron and a daughter cell that translocates away from the VZ to differentiate into astroglia) (Kriegstein et al. 2006; Noctor et al. 2004, 2008). In the SVZ, all of the IPs divide symmetrically to produce either two neurons (the majority of divisions) or two daughter IPs (Kriegstein et al. 2006; Martínez-Cerdeño et al. 2006; Noctor et al. 2004, 2007, 2008). In addition, a novel class of asymmetrically dividing radial glia–like progenitor cells, termed outer radial glial cells, has been identified recently in the outer SVZ region (Hansen et al. 2010, LaMonica et al. 2012, X. Wang et al. 2011). Neurogenesis subsides when the progenitor pool is depleted owing to both terminal IP divisions into neurons and terminal RGP divisions into glia (Ayala et al. 2007, Higginbotham et al. 2011, Noctor et al. 2004).
Newly born projection neurons, which constitute the majority of cortical neurons, reach their target locations within the developing cortex via radial migration (reviewed in Ayala et al. 2007, Marín & Rubenstein 2003, Marín et al. 2010) (Figure 1). The earliest-arriving neurons form the transient preplate (PP) and are followed by the neurons that form the cortical plate (CP). The CP neurons split the PP into the superficial marginal zone (MZ, or layer 1) and the subplate (SP), which is located below the newly forming cortical layer 6 (Ayala et al. 2007, Ghashghaei et al. 2007, Kwan et al. 2012, Marín & Rubenstein 2003, Marín et al. 2010). Successive waves of migrating neurons arrive to occupy progressively more superficial cortical layers in an inside-out fashion; in other words, neurons belonging to the deepest cortical layers are generated first and arrive at their destinations first, followed by neurons that will reside in the upper layers. However, recent data also suggest that at least a subset of RGPs is specified to generate only upper-layer neurons irrespective of niche and birthdate (Franco et al. 2012).
Figure 1.
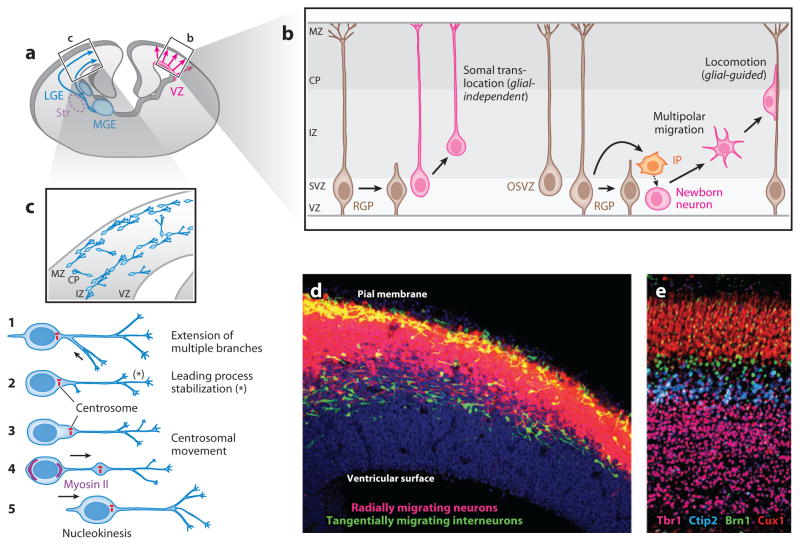
Neuronal migration in the developing cerebral cortex. (a) Projection neurons ( pink) and interneurons (blue) originate from distinct proliferative domains and migrate into the developing cerebral wall. (b) Projection neurons, generated from radial glial progenitors (RGPs, brown) or intermediate precursors (IPs, orange), migrate using either radial-glial-independent somal translocation or glial-guided locomotion. Newborn neurons undergo a multipolar transition phase prior to glial-guided radial migration. (c) Interneurons migrate in multiple streams into the pallium. They extend multiple leading branches ➀, followed by branch stabilization ➁, centrosomal movement ➂, ➃, and forward nucleokinesis ➄. (d ) Radially migrating neurons (magenta) and tangentially migrating interneurons ( green) in the E14.5 mouse cerebral wall. (e) Coordinated migration of these neurons during development leads to the laminar organization of neurons in the cerebral cortex. Neurons in different layers of postnatal day 0 cortex are labeled with antibodies to Ctip2 (blue, layer V), Cux1 (red, layers II–IV), Brn1 ( green, layers II–V), and Tbr1 (magenta, layer VI). Adapted from Higginbotham et al. (2012). Abbreviations: CP, cortical plate; IZ, intermediate zone; LGE, lateral ganglionic eminence; MGE, medial ganglionic eminence; MZ, marginal zone; OSVZ, outer subventricular zone progenitor; Str, striatum; SVZ, subventricular zone; VZ, ventricular zone.
Radial migration occurs in two different modes: somal translocation and locomotion (Kriegstein & Noctor 2004, Marín & Rubenstein 2003, Nadarajah & Parnavelas 2002, Nadarajah et al. 2001) (Figure 1). The earliest neurons that form the PP use somal translocation, whereas most cortical neurons forming the CP employ locomotion.
Somal Translocation Versus Locomotion
Early-born cortical neurons possess a long, branched leading process attached to the pial surface (Miyata et al. 2001, Nadarajah et al. 2001). The leading process gets progressively shorter as the cell soma translocates upward (Nadarajah et al. 2001) (Figure 1). These somally translocating neurons move continuously, without significant pausing (Nadarajah et al. 2001). Importantly, this mode of migration does not depend on radial glial guides, but attachment of the leading process to the intact pial basement membrane is likely required (Hawthorne et al. 2010, Marín & Rubenstein 2003).
Neurons that migrate via locomotion are morphologically distinct from the translocating neurons. They possess short, unbranched leading and trailing processes that extend and retract rapidly, resulting in forward movements of the entire cell, interrupted by stationary phases (Ayala et al. 2007, Marín & Rubenstein 2003, Nadarajah et al. 2001). A fundamental feature of migration via locomotion is the involvement of radial glial cells, highly polarized cortical progenitor cells that not only serve as precursors to the majority of cortical neurons but also provide an instructive scaffold for neuronal migration (Marín & Rubenstein 2003, Marín et al. 2010, Noctor et al. 2001, Rakic 1972) (Figure 1). Polarized glial cells have a characteristic pear-shaped soma positioned in the VZ, a short apical process anchored at the ventricular surface, and a long basal process that extends through the cerebral wall toward the pial surface and is attached to the pial basal membrane (Ayala et al. 2007) (Figure 1). Radial glial processes function as guides that direct migrating neurons from their birthplace in the VZ to their final destination in the cerebral cortex. Radial glial scaffold is by no means static, because adjacent radial glial cells constantly probe each other and the neurons in contact with them (Yokota et al. 2010). This constant remodeling of the dynamic radial glial scaffold may restrict the dispersion and patterns of radial migration of isogenic cohorts of neurons and, thus, may affect columnar and laminar organization of the neocortex.
Neurons migrating by locomotion switch to somal translocation during the final stages of their migration, right after their leading process makes contact with the MZ (Nadarajah et al. 2001), which implies that these two modes of radial migration are not entirely neuronal-type specific. However, considering differences in the morphologies of locomoting and translocating cells, as well as differences in how they move (continuous translocation versus saltatory movement of locomoting neurons), the two modes of radial migration are likely mediated by different mechanisms (Franco et al. 2011, Marín & Rubenstein 2003, Nadarajah et al. 2001). Different effects of gene mutations that disrupt cortical development support this possibility. For example, mutations that affect glial-independent translocation result in unsplit PP, whereas mutations that affect glial-dependent locomotion result in the formation of normal PP but a failure to position late-born neurons properly (Marín & Rubenstein 2003, Nadarajah et al. 2001). Further, the two modes of radial migration may have evolved independently (Marín & Rubenstein 2003, Nadarajah et al. 2001) and reflect the differences in cortical organization complexity: Somal translocation is used when the cerebral wall is still thin and distances that neurons need to migrate are relatively short, whereas radial glia–dependent locomotion is required to guide migrating neurons along more convoluted (and longer) paths in complex cortices (Nadarajah & Parnavelas 2002).
Multipolar Phase of Radial Migration
Radial glia–guided neurons assume a characteristic bipolar morphology, with a long leading process oriented toward the pial surface and a shorter trailing process in the direction of the VZ. However, several studies have identified a distinct population of migrating cells within the lower intermediate zone (IZ) and SVZ: multipolar neurons that possess multiple thin processes that extend and retract in a dynamic but apparently random fashion (LoTurco & Bai 2006, Tabata & Nakajima 2003, Tabata et al. 2009). These multipolar neurons do not seem to require radial glia and move at a slower speed in the direction of the pial surface, occasionally reverting back to the ventricular surface and then reassuming pia-oriented migration (Tabata & Nakajima 2003, Tabata et al. 2009). Importantly, the multipolar stage is transient and is followed by a switch back to the bipolar morphology and locomotion-based mechanism of migration (Noctor et al. 2004, Tabata et al. 2009). Several recent studies have suggested that this transient stage is critical for the progressive emergence of different neuronal layer identities and proper cortical lamination ( Jossin & Cooper 2011, Miyoshi & Fishell 2012, Ohshima et al. 2007, Pacary et al. 2011).
MIGRATION OF INTERNEURONS: TANGENTIAL MIGRATION
The inhibitory interneurons of the cerebral cortex arise mainly from the medial and caudal ganglionic eminences (MGE and CGE) and the preoptic area (POA) within the ventral telencephalon, and they migrate tangentially (orthogonal to the radial glial scaffold) into the developing neocortex (Anderson et al. 1997, 2001; Batista-Brito & Fishell 2009; Faux et al. 2012; Gelman & Marín 2010; Gelman et al. 2009; Ghashghaei et al. 2007; Kriegstein & Noctor 2004; Lavdas et al. 1999; Marín & Rubenstein 2001; Miyoshi et al. 2010; Nery et al. 2002; Tan et al. 1998; Wichterle et al. 1999, 2001; Wonders & Anderson 2006; Yozu et al. 2005) (Figure 1). Similarly to projection neurons, interneurons contribute to the inside-out pattern of the neocortex lamination (Ang et al. 2003, Rakić et al. 2009, Valcanis & Tan 2003). However, correct laminar positioning of interneurons also depends on their place of origin; for example, CGE-derived interneurons preferentially migrate to the more superficial cortical layers, irrespective of their time of birth (Faux et al. 2012, Miyoshi & Fishell 2011, Miyoshi et al. 2010, Rymar & Sadikot 2007, Yozu et al. 2004).
Unlike projection neurons, cortical interneurons comprise a highly heterogeneous group of neurons with different molecular, morphological, and electrophysiological characteristics (Corbin & Butt 2011, DeFelipe et al. 2013, Faux et al. 2012, Gelman & Marín 2010, Rudy et al. 2011). Generation of interneuron diversity is controlled by a spatially and temporally regulated transcription factor matrix within the GEs (Butt et al. 2005, 2008; Faux et al. 2012; Kwan et al. 2012; Marín & Rubenstein 2003; Miyoshi et al. 2007; Wonders et al. 2008; Xu et al. 2004). Distinct interneuron subtypes exhibit different migration patterns and migratory dynamics (Marín & Rubenstein 2003, Yokota et al. 2007).
Migration of cortical interneurons involves oriented exit from the GE toward the cortex as well as migration within the cortex toward specific areal and laminar positions. Interneurons exiting from the GE avoid entering the striatum and migrate into the cortex through the MZ or SP or stream along the lower IZ/SVZ (Ang et al. 2003; Faux et al. 2012; Kriegstein & Noctor 2004; Lavdas et al. 1999; Marín & Rubenstein 2001, 2003; Marín et al. 2001; Métin et al. 2006) (Figure 1). Once in the cortex, several modes of migration are used by the interneurons. First, a significant number of interneurons undergo multidirectional tangential migration in multiple zones of the cortex (Ang et al. 2003; Tanaka et al. 2003, 2006; Yokota et al. 2007), during which interneurons migrate over long distances and in different directions. This mode of migration may help disperse interneurons across the neocortex to achieve proper laminar organization (Tanaka et al. 2006). Second, a subpopulation of interneurons exhibit ventricle-oriented migration (Nadarajah et al. 2002), during which interneurons within the IZ migrate first toward the ventricle before migrating radially to their position within the CP, possibly to obtain layer information for correct cortical positioning or to modulate proliferation of ventricular progenitors. Lastly, tangentially migrating streams of interneurons switch to radial migration as they move toward specific laminar locations within the CP (Faux et al. 2012, Nadarajah et al. 2002, Yokota et al. 2007).
Although it has been suggested that interneurons do not depend on radial glia for their migration (Marín & Rubenstein 2003) and may rely more on developing axons as substrates, recent evidence suggests that dynamic interactions between specific subtypes of interneurons and radial glial guides may influence the final trajectories of interneuron migration and, thus, their positioning in the developing cortex (Polleux et al. 2002, Poluch & Juliano 2007, Yokota et al. 2007). Once within the developing cortical layers, interactions with the excitatory projection neurons enable the final positioning of the interneurons (Lodato et al. 2011). In summary, the ventral germinal zone of origin of interneurons, interactions with developing axonal fibers and radial glial scaffold, and local interactions with projection neurons in the emerging CP influence the final areal and laminar positioning of interneurons.
CELLULAR AND MOLECULAR MECHANISMS OF ORIENTED NEURONAL MIGRATION
Migrating cortical neurons in general follow three major steps as they navigate toward their target: (a) formation and extension of a leading process, (b) nucleokinesis, and (c) retraction of the trailing process (Marín et al. 2010) (Figure 1). Distinct types of neurons possess morphologically distinct leading processes (i.e., the single unbranched leading process of the radially migrating projection neurons, the multiple thin leading processes of multipolar neurons, and the constantly branching leading process of the tangentially migrating interneurons), but in all cases the leading process serves as a compass that directs oriented migration by responding to various chemotactic, chemoattractant, or chemorepellent gradients (Faux et al. 2012, Marín et al. 2010, Trivedi & Solecki 2011). The cell soma remains largely static while the leading process explores the environment. The dynamism of the leading process and its influence on directional migration is particularly prominent in the interneurons. In these neurons, the leading process branches continuously until a single branch is selected and oriented toward the direction of movement, and the rest of the branches retract (Faux et al. 2012, Marín et al. 2010, Trivedi & Solecki 2011). Signaling from the primary cilium in these neurons may help them sense orienting cues (Baudoin et al. 2012, Higginbotham et al. 2012) (see below). Stabilization of the selected leading process is followed by translocation of the centrosome and the Golgi complex toward the selected branch, followed by nuclear translocation and trailing-process retraction (Marín et al. 2010, Trivedi & Solecki 2011) (Figure 1). This process is repeated to facilitate directional movement of interneurons into the cerebral cortex. Trailing-process retraction is thought to push the nucleus forward by squeezing it at the rear, thus enabling nuclear translocation. The leading-process extension, nucleokinesis, and trailing-process retraction of the migrating neurons depend on integration of extrinsic cues and the resultant dynamic rearrangements of the cytoskeletal functions.
A coordinated network of signaling pathways emanating from different domains of the neurons (e.g., cell soma, growth cone, and primary cilium) controls cytoskeletal rearrangements that ultimately lead to oriented neuronal movement. However, the integration of the components of these networks in controlling oriented neuronal migration remains elusive. Nevertheless, glimpses into the common mechanisms that are used by these networks to regulate both radial and tangential migration, and into how different signaling pathways converge on the cytoskeleton to drive oriented neuronal migration, are beginning to emerge.
Leading-Process Activities, Secreted or Cell-Surface Guidance Cues, and Patterns of Cortical Neuronal Migration
Regulation of both the actin and microtubule cytoskeleton in the leading process of migrating neurons is important for their dynamics. Many aspects of the guidance cue–mediated leading-process extension are shared between neuronal migration and axon guidance. Growth cones, which are dilated ends of both axons and dendrites, are highly dynamic structures that constantly extend and retract membrane protrusions to probe the environment (Dent et al. 2011). Both the actin and microtubule cytoskeleton are involved in changing growth cone morphology in response to guidance cues, many of which have also been shown to regulate leading-process dynamics in migrating neurons (Dent et al. 2011, Marín et al. 2010).
Secreted guidance cues and patterns of neuronal migration
A large number of secreted molecules and their corresponding receptors regulate oriented neuronal migration (Table 1) (Figure 2). Semaphorins provide an illustrative model of how secreted cues can coordinately regulate distinct patterns of neuronal migration in the neocortex. Class 3 semaphorins, a class of chemorepellents originally identified as regulators of axon guidance, bind to their corresponding coreceptors, neuropilins and plexins (Ayala et al. 2007, Kolodkin & Tessier-Lavigne 2011, Manns et al. 2012, Raper 2000, Tamagnone & Comoglio 2004), and regulate cytoskeletal dynamics, mainly via Rho GTPases and their associated proteins. In axon guidance, semaphorin-mediated signaling leads to growth cone collapse via several mechanisms. The GTPase-activating domain of plexins promotes activation of the Rho GTPase Rac1, which in turn activates LIMK1, a kinase that phosphorylates and inactivates cofilin, an important regulator of actin dynamics. Semaphorin 3A/LIMK1-mediated inhibition of cofilin results in decreased actin turnover and decreased motility, leading to growth cone collapse (Aizawa et al. 2001, Endo et al. 2003, Liu & Strittmatter 2001, Pasterkamp 2012, Västrik et al. 1999, Zhou et al. 2008). Alternatively, semaphorin 4D/plexin-B1 signaling mediates growth cone collapse via activation of a GTPase RhoA in the growth cone, which results in increased actin contractility (Swiercz et al. 2002). Lastly, semaphorin-mediated signaling has also been shown to stimulate RapGAP (GTPase-activating protein) activity of full-length plexin (Wang et al. 2012), thus contributing to growth cone collapse. Additionally, semaphorin-mediated signaling regulates microtubule dynamics during growth come collapse via GSK3β-mediated phosphorylation of CRMP2, leading to microtubule destabilization (Gu & Ihara 2000, Uchida et al. 2005, Zhou et al. 2008).
Table 1.
Genes regulating cortical neuronal migration
| Secreted and substrate-bound guidance cues | ||||||
|---|---|---|---|---|---|---|
| Gene | Protein | Role | Tangential migration | Radial migration | Both | References |
| Bdnf | BDNF (brain-derived neurotrophic factor) | Stimulates tangential migration, transition from tangential to radial migration of medial ganglionic eminence (MGE)-derived interneurons | ✓ | Alcántara et al. 2006, Polleux et al. 2002 | ||
| Cxcl12 | CXCL12 (C-X-C motif ligand 12)/SDF-1 (stromal cell-derived factor-1) | Ligand of CXCR4 and CXCR7 receptors, attractive guidance cue for interneurons | ✓ | Stumm et al. 2003 | ||
| Efna5 | Ephrin A5 | Ephrin ligand, repulsive guidance cue | ✓ | Nomura et al. 2006, Villar-Cerviño et al. 2013 | ||
| Egf | EGF (epidermal growth factor) | EGFR ligand, attractive guidance cue | ✓ | Caric et al. 2001, Puehringer et al. 2013 | ||
| GABA (gamma-aminobutyric acid) | Inhibitory neurotransmitter that binds to GABA receptors, regulates tangential and radial migration | ✓ | ✓ | ✓ | Behar et al. 1996, Behar et al. 1998, Bolteus & Bordey 2004, Bortone & Polleux 2009, Cuzon et al. 2006, López-Bendito et al. 2003 | |
| Gdnf | GDNF (glial cell line–derived neurotrophic factor) | GFRα-1 ligand, attractive guidance cue for interneurons | ✓ | Pozas & Ibáñez, 2005 | ||
| Glutamate | Excitatory neurotransmitter that binds to AMPA and NMDA receptors and regulates tangential and radial migration | ✓ | ✓ | ✓ | Behar et al. 1999, Bortone & Polleux 2009, Hirai et al. 1999, Jansson et al. 2013, Métin et al. 2000, Soria & Valdeolmillos 2002 | |
| Hgf | HGF/SF (hepatocyte growth factor/scatter factor) | Motogenic activity for interneuron migration | ✓ | Powell et al. 2001 | ||
| NRG1 | Neuregulin 1 | ErbB4 ligand, substrate-bound and secreted guidance cue for interneuron migration | ✓ | ✓ | ✓ | Anton et al. 1997, Flames et al. 2004, Li et al. 2012 |
| NTF4 | NTF4 (neurotrophin-4) | Stimulation of tangential migration of MGE-derived cells | ✓ | Polleux et al. 2002 | ||
| Ntn1 | Netrin 1 | Secreted guidance cue Orientation of the migration of cortical neurons and promotion of the transition from multipolar to bipolar morphology |
✓ | ✓ | ✓ | Ju et al. 2013, Stanco et al. 2009 |
| Reln | Reelin | Extracellular matrix (ECM) protein released by Cajal-Retzius cells, VLDLR/ApoER2 ligand, regulates terminal phase of neuronal migration and placement | ✓ | Franco et al. 2011, Jossin & Cooper 2011, Ogawa et al. 1995 | ||
| Sema3A, Sema3F | Semaphorin 3A/3F | Neuropilin ligand, repulsive guidance cue | ✓ | ✓ | ✓ | Chen et al. 2008, Marín et al. 2001 |
| Sema4D | Semaphorin 4D | Plexin B1 ligand, promotes tangential and radial migration | ✓ | ✓ | ✓ | Hirschberg et al. 2010 |
| Serotonin | Neurotransmitter, decreases interneuron migration | ✓ | Riccio et al. 2009 | |||
| Shh | Sonic hedgehog | Binds to Patched 1 receptor, releasing inhibition of Smoothened receptor Transition from tangential to radial migration of MGE-derived interneurons |
✓ | Baudoin et al. 2012 | ||
| Slit | Slit | Robo ligand, repulsive guidance cue | ✓ | ✓ | ✓ | Andrews et al. 2008, Zheng et al. 2012 |
| Sparcl1 | SPARCL1 (secreted protein, acidic and rich in cysteines-like 1) or SC1 | Antiadhesive ECM protein, terminates radial glia–based migration through its antiadhesive properties | ✓ | Gongidi et al. 2004 | ||
| Receptors and membrane-bound proteins | ||||||
| Cacna | Voltage-dependent L-type calcium channel or VSCC (voltage-sensitive calcium channel) | Frequency of spontaneous intracellular calcium transients initiated by L-type voltage-sensitive calcium channel (VSCC) activation is decreased upon GABAA receptor activation during interneuron migration | ✓ | Bortone & Polleux 2009, Cross-Disord. Group Psychiatr. Genomics Consort. 2013, Komuro & Kumada 2009, Zheng & Poo 2007 | ||
| Cxcr4, Cxcr7 | CXCR4, CXCR7 (CXC chemokine receptor) | Receptor of CXCL12 or SDF-1, expressed by tangentially migrating interneurons | ✓ | Sánchez-Alcañiz et al. 2011, Stumm et al. 2003, Y. Wang et al. 2011 | ||
| Dcc | DCC (Deleted in Colorectal Cancer) | Corrects the orientation of radially migrating projection neurons | ✓ | Ju et al. 2013, Yee et al. 1999 | ||
| Drd1 | Dopamine D1 receptor | Promotes interneuron migration | ✓ | Crandall et al. 2007 | ||
| Drd2 | Dopamine D2 receptor | Inhibits interneuron migration | ✓ | Crandall et al. 2007 | ||
| Efnb1 | Ephrin B1 | Ephrin ligand | ✓ | Stuckmann et al. 2001, Villar-Cerviño et al. 2013 | ||
| Egfr | EGFR (epidermal growth factor receptor) | Receptor for EGF, promotes radial migration | ✓ | Caric et al. 2001 | ||
| ErbB4 | ErbB-4 (v-erb-a erythroblastic leukemia viral oncogene homolog 4) | NRG1 receptor | ✓ | Anton et al. 2004, Flames et al. 2004 | ||
| Gabra1, Gabbr1, Gabrr1 | GABAA, GABAB, and GABAC receptors | Receptors for GABA, regulate tangential and radial migration | ✓ | ✓ | ✓ | Behar et al. 1996, Behar et al. 1998, Bolteus & Bordey 2004, Bortone & Polleux 2009, Cuzon et al. 2006, López-Bendito et al. 2003 |
| Gfra1 | GFRα-1 (GDNF family coreceptor α1) | GPI-anchored receptor: GDNF receptor | ✓ | Pozas & Ibanez 2005 | ||
| Gpr56 | GPR56 | Adhesion G-protein-coupled adhesion receptors, regulation of basal lamina and glial endfeet–pial membrane interactions | ✓ | Li et al. 2008 | ||
| Gria1, 2, 3, 4 | AMPA (α-amino-3- hydroxy-5-methyl-4- isoxazolepropionic acid) receptor | Receptor for glutamate, regulates radial and tangential migration | ✓ | ✓ | ✓ | Bortone & Polleux 2009, Jansson et al. 2013, Métin et al. 2000 |
| Grin1, Grin2a,b,d | NMDA (N-methyl D-aspartate) receptor | Receptor for glutamate, regulates radial and tangential migrations | ✓ | ✓ | ✓ | Behar et al. 1999, Bortone & Polleux 2009, Hirai et al. 1999, Soria & Valdeolmillos 2002 |
| Itga3, Itga5, Itgb1, Itgav | Integrin receptors | Modulates glial-guided locomotion and interneuron migration | ✓ | ✓ | ✓ | Anton et al. 1999, Belvindrah et al. 2007, Graus-Porta et al. 2001, McCarty et al. 2005, Schmid et al. 2004, Sekine et al. 2012, Stanco et al. 2009 |
| Lrp8 (ApoER2) | LRP8, low-density lipoprotein receptor–related protein 8 (ApoER2, apolipoprotein E receptor 2) | Reelin receptor | ✓ | ✓ | ✓ | D’Arcangelo et al. 1999, Hiesberger et al. 1999, Howell et al. 1999, Trommsdorff et al. 1999 |
| Met | MET | Receptor for HGF/SF | ✓ | Powell et al. 2001 | ||
| Notch1 | Neurogenic locus notch homolog protein 1 | Role in Reln regulation of neuronal migration | ✓ | Hashimoto-Torii et al. 2008 | ||
| Nrp1, Nrp2 | Neuropilin 1/2 | Semaphorin receptor | ✓ | ✓ | ✓ | Chen et al. 2008, Marín et al. 2001 |
| Ntrk2 | TrkB | Receptor of BDNF and NT4, promotes tangential migration | ✓ | Polleux et al. 2002 | ||
| Plxnb1 | Plexin | Semaphorin receptor | ✓ | ✓ | ✓ | Hirschberg et al. 2010 |
| Ptch1 | Patched 1 | Shh receptor, releases inhibition of Smoothened receptor upon Shh binding | ✓ | Baudoin et al. 2012, Ruat et al. 2012 | ||
| Robo | Robo | Slit receptor | ✓ | ✓ | ✓ | Andrews et al. 2006, Marín et al. 2003, Zheng et al. 2012 |
| Rtn4 | Reticulon-4 (Nogo-A) | Myelin-associated protein, required for tangential migration | ✓ | Mingorance-Le Meur et al. 2007 | ||
| Slc6a4 | 5-HTR6 | Serotonin transporter, decreases interneuron migration | ✓ | Riccio et al. 2009 | ||
| Smo | Smo (Smoothened) | G-protein-coupled receptor, translocates to primary cilia, mediates Shh signaling | ✓ | Baudoin et al. 2012 | ||
| Vldlr | VLDLR (very low density lipoprotein receptor) | Reelin receptor | ✓ | ✓ | ✓ | D’Arcangelo et al. 1999, Hiesberger et al. 1999, Howell et al. 1999, Trommsdorff et al. 1999 |
| Adhesion molecules | ||||||
| Astn1, Astn2 | ASTN1 and ASTN2, Astrotactin | Adhesion glycoprotein, ASTN2 complexes with ASTN1 to regulate ASTN1 membrane targeting, allowing glial-guided locomotion | ✓ | Wilson et al. 2010, Zheng et al. 1996 | ||
| Cdh2 | N-Cadherin | Cell-adhesion molecule, membrane levels decreased by Reelin/Rap pathway during migration of multipolar neurons | ✓ | Imai et al. 2006, Jossin & Cooper 2011, Shikanai et al. 2011 | ||
| Cntn2 (or TAG-1) | Contactin-2 | Neural cell–adhesion molecule expressed by cortical axons, supports migration of early-born MGE interneurons | ✓ | McManus et al. 2004 | ||
| Cntnap2 | Contactin associated protein–like 2 | Cell-adhesion molecule, member of the neurexin family, role in neuron-glia interactions | ✓ | Peñagarikano et al. 2011 | ||
| Dscam | DSCAM (Down syndrome cell adhesion molecule homolog) | Cell-adhesion molecule from Ig superfamily, axon guidance and branching Expression repressed by the transcription factor Dlx1/2 |
✓ | Chédotal & Rijli 2009, Cobos et al. 2007 | ||
| Gja1, Gjb2 | Connexins 43, 26 | Mediates dynamic adhesive interactions between migrating neurons and radial glia | ✓ | Elias et al. 2007, 2010; Fushiki et al. 2003; Liu et al. 2012 | ||
| Mdga1 | MDGA1 (MAM domain–containing glycosylphosphatidylinositol anchor 1) | IgCAM anchored to the extracellular surface of the cell membrane by a GPI-linkage, controls migration of superficial layer cortical neurons | ✓ | Takeuchi & O’Leary 2006 | ||
| Ncam1 | NCAM (neural cell-adhesion molecule 1) | Neural cell-adhesion molecule from Ig superfamily | ✓ | Battista & Rutishauser 2010 | ||
| Transcription factors | ||||||
| Arx | Aristaless-related homeobox | Upregulated by Dlx1/2, required for tangential migration of GABAergic neurons, targets the expression of guidance cues (Semaphorin 6, Slit2) | ✓ | Colasante et al. 2008, Friocourt & Parnavelas 2011, Marcorelles et al. 2010 | ||
| Ascl1 (Mash1) | Mammalian achaete-scute homolog 1 | Induction of the GABAergic phenotype, activates Dlx1/2 expression | ✓ | Fode et al. 2000, Letinic et al. 2002, Parras et al. 2002, Poitras et al. 2007 | ||
| Dlx1, Dlx2 | Distal-less homeobox | Necessary for interneuron migration from basal forebrain to neocortex | ✓ | Anderson et al. 1997 | ||
| Ebf3 | Early B-cell factor 3 | Downregulated by Arx, enriched in interneuron transcriptome, potential candidate for the regulation of interneuron migration | ✓ | Friocourt & Parnavelas 2011, Fulp et al. 2008 | ||
| Foxg1 | FoxG1, Forkhead box protein G1 | Required for tangential migration of multipolar cells (pyramidal precursors) and their migration to the cortical plate | ✓ | Miyoshi & Fishell 2012 | ||
| Foxp2 | Forkhead box P2 | Repression of Foxp2 by miRNAs promotes radial neuronal migration | ✓ | Clovis et al. 2012 | ||
| Gbx1 | Gastrulation brain homeobox 1 | Upregulated by Arx, required for cortical interneuron migration | ✓ | Colombo et al. 2007 | ||
| Gsh2 | Gsh2 | Involved in the determination of pallium/subpallium border | ✓ | Corbin et al. 2003 | ||
| Klf4 | Krüppel-like factor 4 | Regulation of multipolar-to-bipolar transition of radially migrating neurons | ✓ | Qin & Zhang 2012 | ||
| Lhx6 | LIM/homeobox protein 6 | Upregulated by Nk2x-1, required for the normal pattern of tangential migration of GABAergic interneurons and for their correct distribution in the cortical layers | ✓ | Liodis et al. 2007 | ||
| Lmo1 | LIM domain only 1 | Downregulated by Arx, enriched in interneuron transcriptome, potential candidate for the regulation of interneuron migration | ✓ | Friocourt & Parnavelas 2011, Fulp et al. 2008 | ||
| Neurog2 | Neurogenin 2 | Controls radial migration through the regulation of Rho GTPase Rnd2 | ✓ | Heng et al. 2008, Pacary et al. 2011 | ||
| Nkx2-1 | NK2 homeobox 1 | Represses the expression of neuropilin 2 (semaphorin 3A/3F receptor) | ✓ | Nobrega-Pereira et al. 2008 | ||
| Nr2f1 | Nuclear receptor subfamily 2, group F, member 1 (COUP transcription factor 1) | Regulates the expression of the cortical migration regulator Rho GTPase 2 Rnd2, modulating late-born neuron migration | ✓ | Alfano et al. 2011 | ||
| Pou3f2 (BRN-2), Pou3f3 (BRN-1) | POU domain, class 3, transcription factor 2 (or 3) | Regulation of the expression of the p35 and p39 regulatory subunits of Cdk5 in migrating cortical neurons, control of the initiation of radial migration | ✓ | McEvilly et al. 2002, Sugitani et al. 2002 | ||
| Rest | Repressor element 1–silencing transcription factor | Transcriptional repressor, regulates radial migration via its downstream target Dcx | ✓ | Mandel et al. 2011 | ||
| Satb2 | Special AT-rich sequence binding protein 2 | Required for layer-dependent control of migration | ✓ | Alcamo et al. 2008, Britanova et al. 2008, J. Zhang et al. 2012 | ||
| Scrt1, 2 | Scratch 1 and 2 | Neural-specific zinc-finger transcription factor, regulates radial migration during (a) the onset of migration by downregulating E-cadherin expression and (b) the multipolar phase by modulating transcriptional activation of Neurog2 | ✓ | Itoh et al. 2013, Paul et al. 2012 | ||
| Shox2 | Short stature homeobox 2 | Downregulated by Arx, potential candidate for the regulation of interneuron migration | ✓ | Fulp et al. 2008 | ||
| Sox5 | Sex determining region Y-box 5 | Regulates migration of deep-layer neurons | ✓ | Kwan et al. 2008, Lai et al. 2008 | ||
| Srf | SRF (serum response factor) | Controls expression of actin-regulating protein gelsolin | ✓ | Alberti et al. 2005 | ||
| Tbr1 | T-box brain factor 1 | Required for cortical neuronal positioning | ✓ | Han et al. 2011, Hevner et al. 2001, McKenna et al. 2011 | ||
| Zeb2 | Zinc finger E-box binding homeobox 2 (or Sip1) | Essential for cortical interneuron guidance by decreasing levels of the repulsive receptor Unc5b | ✓ | van den Berghe et al. 2013 | ||
| miRNAs and miRNA machinery | ||||||
| Dicer1 | DICER | RNase III ribonuclease that cleaves double-stranded miRNA precursors to produce mature miRNAs | ✓ | ✓ | ✓ | McLoughlin et al. 2012 |
| Mir9-1 | miR-9 | miRNA, regulates expression of FoxG1, Foxp2, and stathmin | ✓ | Clovis et al. 2012, Delaloy et al. 2010, Shibata et al. 2008 | ||
| Mir124a-1 | miR-124 | miRNA, regulates expression of neuropilin-1 in the growth cone | ✓ | ✓ | ✓ | Baudet et al. 2012 |
| Mir132 | miR-132 | miRNA, regulates expression of Foxp2 | ✓ | Clovis et al. 2012 | ||
| Intracellular signal transduction machinery | ||||||
| Acta1, Actb | Actin α1, Actin β | Cytoskeletal component | ✓ | ✓ | ✓ | Govek et al. 2011 |
| Akt | Akt | Mediates Reelin signaling, acts downstream of Dab-1 | ✓ | Jossin & Goffinet 2007 | ||
| Apc | APC | Regulates radial glia cell polarity and thus glial-guided migration | ✓ | Yokota et al. 2009 | ||
| Arfgef2 | BIG2 | Interacts with Filamin A, promotes radial migration | ✓ | J. Zhang et al. 2012 | ||
| Arl13b | Arl13b | Promotes tangential migration, regulates cilia dynamics during migration and ciliary localization of signaling receptors | ✓ | Higginbotham et al. 2012 | ||
| Cdc42 | Cdc42 | Rho GTPase necessary for growth cone extension | ✓ | Cappello et al. 2006, Etienne-Manneville & Hall 2001 | ||
| Cdk5 | Cdk5, Cyclin-dependent kinase 5 | Neuron-specific serine/threonine kinase, regulates actin and microtubule dynamics | ✓ | ✓ | ✓ | Chae et al. 1997, Gilmore et al. 1998, Ko et al. 2001, Kwon & Tsai 1998, Ohshima et al. 1996, Rakić et al. 2009 |
| Cdk5r1 | p35 | Regulatory subunit 1 of Cdk5 | ✓ | ✓ | ✓ | Gupta et al. 2003; Hammond 2004, Ko et al. 2001, Kwon & Tsai 1998, Rakić et al. 2009 |
| Cdk5r2 | p39 | Regulatory subunit 2 of Cdk5 | ✓ | Humbert et al. 2000, Ko et al. 2001 | ||
| Cdkn1b | p27(Kip1) | Microtubule-associated protein, promotes tangential migration | ✓ | Godin et al. 2012 | ||
| Clip1 | CLIP170 | Microtubule plus tip protein, candidate role in linking microtubules to actin during cell motility | ✓ | Kholmanskikh et al. 2006 | ||
| Crk | Crk (CT10 Regulator of Kinase) | Adaptor protein, part of Reelin-signaling pathway | ✓ | Assadi et al. 2003, Ballif et al. 2003, Chen et al. 2004, Huang et al. 2004 | ||
| Ctnnb1 | β-Catenin | Protein associated with cadherins and involved in Wnt signaling pathway | ✓ | Fu et al. 2006, Valenta et al. 2012 | ||
| Ctnnd1 | p120 Catenin | Transport of N-cadherin containing vesicles to the membrane surface | ✓ | Chauvet et al. 2003 | ||
| Cul5 | Cullin-5 | E3 ubiquitin ligase component, forms complex with SOCS leading to degradation of Dab-1 | ✓ | Feng et al. 2007 | ||
| Dab1 | Dab-1 (Disabled-1) | Adaptor protein mediating Reelin signaling | ✓ | Franco et al. 2011 | ||
| Dclk | Doublecortin-like kinase | Microtubule-associated protein related to doublecortin, stabilization of microtubules and necessary for nucleokinesis | ✓ | Deuel et al. 2006, Friocourt et al. 2007, Koizumi et al. 2006, Marín et al. 2010, Shu et al. 2006 | ||
| Dcx | Doublecortin | Microtubule-associated protein, stabilizes microtubules and regulates nucleokinesis | ✓ | Deuel et al. 2006, Friocourt et al. 2007, Gleeson et al. 1999, Koizumi et al. 2006, Marín et al. 2010, Shu et al. 2006, Tanaka et al. 2004 | ||
| Diap1 | mDia1, diaphanous homolog 1 (Drosophila) | Formin family, actin-polymerization factor | ✓ | Shinohara et al. 2012 | ||
| Disc1 | Disrupted in schizophrenia 1 | Localized at the centrosome Necessary for microtubule dynamics |
✓ | Ishizuka et al. 2011; Kamiya et al. 2006, 2008 | ||
| Dmd | Dystrophin | Protein linking the dystrophin-glycoprotein complex to the actin cytoskeleton Promotes radial migration |
✓ | Pawlisz & Feng 2011 | ||
| Dpysl2 | CRMP2 (Collapsin response mediator protein 2) | Microtubule-associated protein promoting microtubule polymerization | ✓ | Sun et al. 2010 | ||
| Dync1h1, Dync1i1, Dynll1 | Dynein | Microtubule molecular motor, couples nucleus–centrosome | ✓ | Niethammer et al. 2000, Sasaki et al. 2000 | ||
| Dyx1c1 | Dyslexia susceptibility 1 candidate 1 | Regulation of primary cilium morphology and signaling, required for radial migration | ✓ | Hoh et al. 2012, Wang et al. 2006 | ||
| Efhc1 | EF-hand domain (C terminal) containing 1 | Microtubule-associated protein, localizes to the centrosome and mitotic spindle, required for tangential and radial migration | ✓ | ✓ | ✓ | De Nijs et al. 2012 |
| Ena/Vasp | Ena/VASP | Actin-nucleation factor | ✓ | Goh et al. 2002 | ||
| Ezh2 | Ezh2, Histone-lysine N-methyltransferase | Repression of Netrin1 by counteraction of Shh pathway, promoting tangential migration of mouse precerebellar neurons | ✓ | Di Meglio et al. 2013 | ||
| Filip1 | FILIP1, Filamin A interacting protein | Interacts with Filamin A, promotes radial migration | ✓ | Nagano et al. 2004, Sato & Nagano 2005 | ||
| Flna | FLNA, Filamin A | Actin-binding protein determining cell morphology during migration | ✓ | Nagano et al. 2004, Sato & Nagano 2005, J. Zhang et al. 2012 | ||
| Fyn | Fyn | Tyrosine kinase Activation of Dab-1 | ✓ | Arnaud et al. 2003, Bock & Herz 2003 | ||
| Gsn | Gelsolin | Actin-severing protein | ✓ | Alberti et al. 2005 | ||
| Ift88 | Intraflagellar transport protein homolog 88 | Member of the tetratrico peptide repeat family, part of the intraflagellar transport (IFT) machinery Necessary for primary cilia–dependent tangential migration |
✓ | Baudoin et al. 2012 | ||
| Iqgap1 | IQGAP1 | Calcium-sensitive GTPase scaffolding protein, links CLIP170 to cortical actin cytoskeleton through Lis1 regulation | ✓ | Kholmanskikh et al. 2006 | ||
| Katna1 | Katanin p60 | Microtubule-severing protein recruited by Ndel1, necessary for radial migration | ✓ | Toyo-Oka et al. 2005 | ||
| Kif11 | Kinesin family member 11, kinesin-5 | Microtubule molecular motor regulating transport of short microtubules in neuronal processes | ✓ | Falnikar et al. 2011 | ||
| Kif3A | Kinesin family protein 3A | Microtubule molecular motor, necessary for primary cilia–dependent tangential migration | ✓ | Baudoin et al. 2012 | ||
| Map2 | MAP2 | Microtubule-associated protein regulating microtubule stabilization, expression of Map2 is repressed by Dlx1/2 | ✓ | Cobos et al. 2007 | ||
| Mapt | Tau | Microtubule-associated protein regulating microtubule stabilization, important for the maintenance of the neuronal process | ✓ | Cobos et al. 2007, Colasante et al. 2008 | ||
| Mark2 | MARK2 (Mammalian Par-1 ortholog) | Necessary for multipolar-to-bipolar transition of migrating projection neurons | ✓ | Sapir et al. 2008 | ||
| Myh2 | Myosin II | Generates actin contractility, necessary for growth cone migration, nucleokinesis, and trailing-process retraction | ✓ | ✓ | ✓ | Bellion et al. 2005; Solecki et al. 2004, 2009 |
| Nap1 | NCK-associated protein 1 (NCKAP1 or NAP1) | Part of the WAVE complex that regulates lamellipodia formation necessary for neurite extension | ✓ | Yokota et al. 2007 | ||
| Ndel1 | NUDEL | Interacting partner of Lis1, required for nucleokinesis | ✓ | Niethammer et al. 2000, Sasaki et al. 2000 | ||
| Nova2 | Neuro-oncological ventral antigen 2 | RNA-binding protein Modulates neuronal migration by regulating the alternative splicing of Dab1 |
✓ | Yano et al. 2010 | ||
| Pafah1b1/Lis1 | Platelet-activating factor acetylhydrolase 1b, regulatory subunit 1, LIS1 | Noncatalytic alpha subunit of the intracellular Ib isoform of platelet-activating factor acetylhydrolase Interacts with microtubules, dynein, doublecortin, Ndel1 Necessary for nucleokinesis and subsequent neuronal migration |
✓ | ✓ | ✓ | des Portes et al. 1998, Pilz et al. 1999, Sapir et al. 1997 |
| Pak3 | PAK3 | Repressed by Dlx1/2, promotes neurite extension, potential role in interneuron migration termination | ✓ | Cobos et al. 2007 | ||
| Pard6a | Par-6 | Member of cell polarity pathway Role in establishing and/or maintaining the direction of neuronal locomotion |
✓ | Solecki et al. 2004 | ||
| Pik3r3 | Pi3K (phosphatidylinositol 3-kinase) | Mediation of Reelin signaling downstream of Dab-1 | ✓ | Jossin & Goffinet 2007 | ||
| Pomt1, Pomt2 | POMT1, POMT2 |
O-Mannosyltransferase, mediates biosynthesis of O-mannosylglycans Deletion leads to overmigration of neocortical neurons |
✓ | Hu et al. 2011 | ||
| Ptk2 | FAK (Focal adhesion kinase) | Tyrosine kinase, substrate of Cdk5, involved in microtubule organization and nucleokinesis of radial migrating neurons | ✓ | Xie et al. 2003 | ||
| Rab5, Rab7, Rab11 | Rab5, Rab7, Rab11 | Rab GTPases, regulate N-cadherin endocytic pathway | ✓ | Kawauchi et al. 2010 | ||
| Rac1 | Rac1 | Rho GTPase, promotes radial and tangential migration | ✓ | ✓ | ✓ | Minobe et al. 2009 |
| Rap1a | Rap1 | Small GTPase involved in the Reelin signaling pathway, regulates multipolar-to-bipolar transition and radial glia–independent somal translocation | ✓ | Franco et al. 2011, Gao & Godbout 2013, Jossin & Cooper 2011 | ||
| Rhoa | RhoA | Rho GTPase necessary for actin remodeling | ✓ | Govek et al. 2011 | ||
| Rnd2,3 | Rho GTPase 2,3 | Atypical Rho GTPase inhibiting RhoA activity | ✓ | Heng et al. 2008, Pacary et al. 2011 | ||
| Slc12a5 | KCC2 | Potassium-chloride cotransporter Promotes termination of interneuron migration |
✓ | Bortone & Polleux 2009 | ||
| Socs1 | SOCS (Suppressors of Cytokine Signaling) | Complexes with Cul5 to facilitate Dab-1 degradation | ✓ | Feng et al. 2007 | ||
| Src | Src | Tyrosine kinase activating Dab-1 | ✓ | Arnaud et al. 2003, Bock & Herz 2003 | ||
| Stk11 | LKB1 (Liver Kinase B1) | Serine-threonine kinase, regulates multipolar-to-bipolar transition | ✓ | Asada et al. 2007, Bony et al. 2013 | ||
| Stmn2 | Stathmin-2 (SCG10) | Microtubule-polymerization regulator | ✓ | Westerlund et al. 2011 | ||
| Tuba1a, Tubb2b, Tuba8 | Tubulin α1 A, Tubulin β2B, Tubulin β8 | Tubulin isotypes, necessary for microtubule formation | ✓ | Abdolahhi et al. 2009, Poirier et al. 2007, Jaglin et al. 2009 | ||
| Wasf1 | WAVE (Wiskott–Aldrich syndrome protein family, verprolin-homologous protein) | Actin-nucleation protein | ✓ | ✓ | ✓ | Tahirovic et al. 2010 |
Figure 2.
Molecular control of neuronal migration in the developing cerebral cortex. Radially and tangentially migrating neurons are shown in pink and blue, respectively. Molecules known to regulate radial, tangential, or both modes of neuronal migration are indicated in pink, blue, and purple, respectively. Molecules regulating the switch from tangential to radial mode of migration of interneurons are indicated in orange. Regulators of multipolar-to-radial transition of projection neurons are indicated in green. Abbreviations: AM, adhesion molecules; C, connexins; CP, cortical plate; IZ, intermediate zone; MZ, marginal zone; SVZ, subventricular zone; VZ, ventricular zone.
Similar signaling mechanisms may operate during semaphorin-regulated cytoskeletal rearrangements in leading processes of neurons during neuronal migration. Class 3 semaphorins expressed by the striatal cells repel tangentially migrating interneurons away from the striatum and channel them into distinct paths leading to the neocortex (Marín et al. 2001). Semaphorins expressed by the CP prevent some of the incoming interneurons from entering the CP and guide them into the migrating streams in the lower IZ (Ito et al. 2008, Tamamaki et al. 2003). However, semaphorins can also serve as chemoattractants for both migrating neurons and developing axons (Chauvet et al. 2007, Raper 2000). For example, a semaphorin 3A gradient produced by the cortical layers serves as a chemoattractant for the neuropilin-expressing layer-II/III projection neurons and promotes their radial migration (Chen et al. 2008). Thus, semaphorins appear to serve as either repellents or attractants to coordinate the migration of projection neurons and interneurons into the developing cerebral wall.
Slit proteins are another class of chemorepulsive guidance molecules secreted from the VZ and SVZ of the GE (Andrews et al. 2007, Marillat et al. 2002, Yuan et al. 1999). Binding of Slit proteins to their corresponding receptors of the Robo family, expressed by interneurons, repels interneurons from the GE, thus initiating their migration toward the neocortex (Hu 1999, Wu et al. 1999). However, the role of Slit in mediating this process was brought into question when tangential migration was found to be unaffected in Slit1−/−/Slit2−/− double-mutant mice (Marín et al. 2003), which suggests that different ligands may mediate Robo signaling. Later studies discovered an increase in interneuron proliferation in Robo−/− mice as well as an increase in the length of the leading process and branching in a Slit-dependent manner (Andrews et al. 2008), which suggests that Slit/Robo signaling is indeed important for establishing correct morphology of the interneuron population. Recent data also suggest that a new member of the Robo family, Robo4, regulates radial migration, partly by suppressing the repulsive activity of Slit (Zheng et al. 2012).
Slit/Robo signaling affects the cytoskeleton in several different ways, most of which converge on the actin cytoskeleton. Slit binding to Robo promotes interaction between Robo and a GAP protein, srGAP (Wong et al. 2001). srGAP inactivates Rho GTPase Cdc42, preventing it from activating an actin-nucleating factor, N-WASP, and thus abolishing actin polymerization associated with Arp2/3 protein activity (Wong et al. 2001). Alternatively, Slit binding may regulate actin polymerization by decreasing WASP and Arp2/3 expression (Ning et al. 2011). In addition, Robo has been shown to form a complex with Abl tyrosine kinase, β-catenin, and N-cadherin. Abl-mediated phosphorylation of β-catenin leads to its dissociation from the complex and its translocation into the nucleus, resulting in disrupted cell adhesion (Rhee et al. 2007). This Slit/Robo-mediated down-regulation of the N-cadherin-dependent adhesion may regulate extension/retraction of neuronal processes during migration (Wong et al. 2012).
Another, well-studied example of a guidance cue necessary for oriented neuronal migration is neuregulin 1 (Nrg1) growth factor, which binds to the ErbB family of receptor tyrosine kinases (Falls 2003, Rico & Marín 2011). One of the ErbB receptors, ErbB4, is expressed mainly by interneurons in the developing brain (Flames et al. 2004, Yau et al. 2003). Nrg1/ErbB4 signaling controls the initial exit of the MGE-derived interneurons through the permissive lateral ganglionic eminence (LGE) corridor and toward the dorsal cortex (Flames et al. 2004). Importantly, this corridor is formed by cells expressing high levels of the membrane-bound Nrg1 (an Nrg1-CRD isoform that possesses an extracellular, cysteine-rich domain) throughout the LGE, together with inhibitory striatal cells that secrete semaphorins (Flames et al. 2004). Secreted forms of Nrg1 (Nrg1-Ig type-I and -II isoforms that possess an immunoglobulin-like domain) expressed by the neocortex then act as long-range chemoattractants to promote interneuron movement into the dorsal cortex.
ErbB tyrosine kinase receptors can influence cell migration by modulating cell-adhesive properties via integrins, receptors of the extracellular matrix molecules. Nrg1 modulation of integrin-dependent adhesion has been shown to trigger the PI3K/AKT-signaling pathway (Kanakry et al. 2007). Integrins can also regulate Rho GTPase activity, thus linking Nrg1 signaling to the actin cytoskeleton in migrating neurons (Sparrow et al. 2012).
Another guidance cue that regulates both radial and tangential neuronal migration is netrin1. Netrin1 can bind to three different receptors, DCC, DSCAM, and Unc5 (Lai Wing Sun et al. 2011), and can serve as either a repellent or an attractant, depending on the receptor binding (Marín & Rubenstein 2003). Recent evidence suggests that DCC is involved in establishing correct orientation of the radially migrating projection neurons ( Ju et al. 2013), which points to a role of netrin1-mediated signaling in radial migration. Netrin1 has also been shown to localize along the migratory routes of interneurons, where it interacts with the α3β1 integrin expressed by the migrating interneurons to promote tangential migration (Stanco et al. 2009). At the mechanistic level, netrin1-mediated signaling likely affects both the actin and microtubule cytoskeleton, at least in part via CDK5- and GSK3β-dependent phosphorylation of MAP1B (Ayala et al. 2007).
Lastly, several lines of evidence suggest that classic neurotransmitters glutamate and GABA serve as extracellular cues to regulate both radial and tangential neuronal migration (Behar et al. 1996, 1998, 1999; Bolteus & Bordey 2004; Bony et al. 2013; Cuzon et al. 2006; Hirai et al. 1999; Jansson et al. 2013; López-Bendito et al. 2003). For example, GABA- or glutamate-mediated de-polarization of tangentially migrating interneurons induces intracellular calcium transients that stimulate interneuron motility (Bortone & Polleux 2009). Conversely, interneuron hyperpolarization mediated by upregulation of a potassium/chloride exchanger, KCC2, negatively regulates the frequency of intracellular calcium transients and stops interneuron migration (Bortone & Polleux 2009). Further, glutamate NMDA receptor/N-type calcium channel–mediated calcium dynamics have been shown to regulate saltatory patterns of radial migration (Komuro & Rakic 1993, 1996, 1998). These data suggest that regulation of calcium dynamics by neurotransmitter receptors is a common mechanism for different patterns of neuronal migration (Behar et al. 1996, Bortone & Polleux 2009, Komuro & Kumada 2005, Kumada & Komuro 2004, Zheng & Poo 2007).
Extracellular matrix and cell-surface guidance cue–activated pathways as models for integration of molecular networks underlying neuronal migration
Reelin, a large extra-cellular glycoprotein secreted by Cajal-Retzius (CR) cells in the MZ, affects two aspects of radial neuronal migration: the ability of migrating neurons to split the PP and the ability of radial glia–guided neurons to migrate past older neurons to generate the inside-out pattern of cortical layering (D’Arcangelo et al. 1995, Leemhuis & Bock 2011, Marín et al. 2010, Ogawa et al. 1995, Stranahan et al. 2013). Reelin binds to two lipoprotein receptors expressed on the migrating neurons and radial glia: very low density lipoprotein receptor (VLDR) and apolipoprotein E receptor 2 (ApoER2) (D’Arcangelo et al. 1999). Reelin binding induces phosphorylation of the adaptor protein disabled-1 (Dab1) by protein kinases Src and Fyn (Bock & Herz 2003; Hiesberger et al. 1999; Howell et al. 1999, 2000; Kuo et al. 2005).
Phosphorylated Dab1 acts as a hub for binding of various interacting proteins that activate several signaling networks that control neuronal migration and positioning (Gao & Godbout 2013). Reelin may affect radial neuronal migration through the Dab1-Crk/CrkL-C3G-Rap1-N-cadherin signaling pathway in at least three different ways. Binding of Reelin to its receptors promotes Dab1-Crk interaction and C3G phosphorylation, resulting in activation of a Ras-related GTPase, Rap1 (Gao & Godbout 2013). Inhibition of Rap1 in migrating multipolar neurons results in loss of neuronal ability to migrate in a CP-oriented manner in response to Reelin ( Jossin & Cooper 2011). This effect is due to an increase in the level of cell-surface N-cadherin by active Rap1 ( Jossin & Cooper 2011). Additionally, cell-surface levels of N-cadherin are regulated by Rab GTPases—members of the endocytosis machinery—thus linking endocytosis to Reelin-mediated neuronal migration (Kawauchi et al. 2010). Additionally, the effect of Rap1/N-cadherin was abolished when migrating neurons assumed bipolar morphology, which suggests that the Reelin-Rap1 pathway does not regulate radial glia–dependent locomotion (Franco et al. 2011, Jossin & Cooper 2011).
Another line of evidence established a role for the Reelin/Dab1/Rap1 pathway in radial glia–independent somal translocation of both early- and late-born neurons (Franco et al. 2011). Dab1-deficient and Rap1-inactivated neurons failed to orient and attach their leading processes to the MZ, resulting in neuronal migration defects. These defects were rescued by overexpression of cadherins, which suggests that the Reelin/Dab1/Rap1/cadherin pathway stabilizes leading processes of migrating neurons and regulates their attachment to the MZ (Franco et al. 2011). In this context, Sekine et al. (2012) recently showed that the Reelin-Crk/CrkL-Dab1-Rap1 signaling pathway regulates terminal translocation by activating integrin α5β1 in the leading processes of migrating neurons. This activation promotes neuronal adhesion to fibronectin present in the MZ, which signals an end to translocation in the migrating neurons.
At the cytoskeletal level, Reelin signaling modulates both microtubule and actin dynamics. Dab1 phosphorylation is coupled to the PI3K/Akt/GSK3β pathway (Gao & Godbout 2013, Marín et al. 2010): Reelin-induced serine phosphorylation of GSK3β results in GSK3β inactivation and, as a result, hypophosphorylation of a microtubule-stabilizing protein, tau. However, Reelin-mediated phosphorylation of GSK3β on specific tyrosine residues results in GSK3β activation and phosphorylation of another microtubule-associated protein, MAP1B, which also regulates microtubule stability (Marín et al. 2010). The Reelin-PI3K-Akt pathway can also regulate the actin cytoskeleton by activating LIMK1, a Ser/Thr kinase that phosphorylates an actin-depolymerizing protein, n-cofilin, thus blocking its function (Chai et al. 2009). Inactivation of n-cofilin in the leading processes of migrating neurons may affect the stability of the processes as well as their attachment to the MZ (Chai et al. 2009, Gao & Godbout 2013). Together, these data suggest that integration of Reelin signaling regulates a diverse set of molecular networks, ultimately leading to appropriate migration and placement of neurons in the cerebral cortex.
Ephrins, a class of membrane-anchored (A-type) and transmembrane (B-type) guidance molecules, bind to Eph receptor tyrosine kinases (Rodger et al. 2012). Both Ephs and ephrins can serve as receptors or ligands (Davy & Soriano 2005, Pasquale 2008), leading to different signaling outcomes, including repulsion, attraction, or changes in motility (Himanen et al. 2007, Pitulescu & Adams 2010, Poliakov et al. 2004, Rodger et al. 2012). In the developing brain, Eph-ephrin signaling has been shown to regulate different aspects of both radial and tangential neuronal migration. Ephrin-Eph interactions mediate contact repulsion and cortical distribution of the CR cells, which regulate migration of projection neurons via release of Reelin (Villar-Cerviño et al. 2013). Further, transmembrane ephrin Bs associate with the Reelin receptors, VLDR and ApoER2, and bind Reelin. Clustering of ephrin Bs on the membrane recruits Dab1 and promotes its phosphorylation (Sentürk et al. 2011), and Reelin was recently shown to induce EphB activation (Bouché et al. 2013), suggesting that cross talk between the Eph-ephrin and Reelin signaling pathways may play a role in controlling radial neuronal migration. Additionally, Torii et al. (2009) showed that ephrin A–mediated forward signaling regulates lateral dispersion of radially migrating neurons during the multipolar stage of their migration, thus ensuring proper intermixing of neuronal types within the developing cortical columns.
Ephrins and Ephs regulate tangential migration mostly via their class-A proteins. For example, both early- and late-born interneurons express EphA4 receptor. Ephrin A3 is expressed in the striatum and repulses the early-born EphA4-expressing interneurons, restricting them from entering the striatum (Rudolph et al. 2010). Ephrin A5 is expressed in the VZ of the GE and repels EphA4-expressing, late-born interneurons from entering the VZ on their way to the cortex (Rodger et al. 2012, Zimmer et al. 2008). Together, ephrin A3– and A5–mediated signaling pathways create ventral (along the MZ and SP) and dorsal (along the lower IZ/SVZ) corridors for interneuronal migration, respectively (Rodger et al. 2012). Additionally, bidirectional signaling by ephrin B3-EphA4 segregates migrating interneurons into two migratory streams: Ephrin B3–expressing POA interneurons repel MGE-derived interneurons from the stream that migrates through the MZ via forward signaling, whereas EphA4-expressing MGE interneurons repel POA interneurons from the stream that migrates through the SVZ via reverse signaling (Zimmer et al. 2011). Two of the semaphorin receptors, neuropilin 1 and neuropilin 2, are also differentially expressed between these two migratory streams; neuropilin 1 is expressed on the MGE-derived interneurons, and neuropilin 2 is expressed on the POA interneurons (Zimmer et al. 2010, 2011). Together, these data suggest that integration between ephrin and semaphorin signaling pathways may create exclusion zones for migrating interneurons and help form corridors for streams of interneurons migrating into the cerebral cortex.
During this process, ephrin signaling may activate Rho GTPases directly (Noren & Pasquale 2004) or via Src-family kinases (Kullander & Klein 2002, Zimmer et al. 2007), resulting in rearrangements of the actin cytoskeleton (Carter et al. 2002). Ephrins have also been implicated in the regulation of cell-cell adhesion via their physical or functional interaction with integrins (Davy & Soriano 2005) and the MAPK pathway (Poliakov et al. 2004). In summary, guidance cues for oriented neuronal migration represent a diverse class of secreted or substrate-bound molecules that act as either chemotactic, chemoattractant, or chemorepellent guides. Common signaling pathways triggered by the guidance molecules result in coordinated cytoskeletal rearrangements that enable oriented neuronal migration and, thus, proper lamination of the cerebral cortex.
Mechanisms of Nucleokinesis and Patterns of Neuronal Migration
Nucleokinesis relies on extensive and rapid cytoskeletal rearrangements involving both micro-tubules and actin. During nucleokinesis, the centrosome and its accompanying organelles move into the swelling formed by the base of the leading process, followed by translocation of the nucleus and cell body (Trivedi & Solecki 2011). The centrosome is linked to a cage-like micro-tubule structure surrounding the nucleus, which is essential for forward nuclear movement (Tsai & Gleeson 2005, Xie et al. 2003).
Nuclear translocation is powered by dynein and dynein regulatory factors, such as Lissencephaly 1 (Lis1). Lis1 protein localizes to the centrosome and interacts with dynein via Ndel1; Ndel1 is also required for anchoring of microtubules at the centrosome (Guo et al. 2006, Li et al. 2005, Smith et al. 2000). Thus, the Lis1-Ndel1-dynein complex regulates nucleokinesis by promoting centrosome-nucleus coupling. Furthermore, a microtubule-associated protein, doublecortin (DCX), and its related protein, DCLK, may play a similar role in nucleokinesis (Deuel et al. 2006, Gleeson et al. 1999, Koizumi et al. 2006, Marín et al. 2010, Shu et al. 2006, Tanaka et al. 2004). In addition, a cyclin-dependent kinase (CDK5) is known to phosphorylate both Ndel1 and DCX, suggesting that it plays a crucial role in regulating the microtubule cytoskeleton during nucleokinesis (Marín et al. 2010).
Several lines of evidence suggest that mechanisms of nucleokinesis may be different between radially and tangentially migrating neurons (Trivedi & Solecki 2011). In interneurons, the nucleus is tethered to the centrosome by the microtubules, and movement of the centrosome, along with the Golgi complex, to the swelling at the base of the leading process creates a pulling force for the nuclear movement (Bellion et al. 2005). This nuclear movement is also supported by the actomyosin contractions at the rear of the cell that push the nucleus forward (Bellion et al. 2005). However, nuclear movement in subsets of radially migrating, glial-guided neurons, such as cerebellar granule neurons, does not depend on centrosome positioning and is powered by a specific subset of acetylated microtubules that are not associated with the centrosome (Umeshima et al. 2007). In these neurons, the nucleus moves ahead of the centrosome in a dynein-powered process driven by the leading-process actomyosin contraction that pulls the nucleus forward (Solecki et al. 2009). These differences in the mechanisms of nucleokinesis employed by radially and tangentially migrating neurons may in part reflect the interactions with different adhesion molecules and subsequent selective cytoskeletal rearrangements during radial and tangential migration (Trivedi & Solecki 2011). Further, adhesion mechanisms have been shown to regulate both nuclear translocation and leading-process dynamics during radial migration (Elias et al. 2007). Connexins 26 and 43, both of which are gap junction subunits, promote adhesions between radial glial fibers and radially migrating neurons (Elias et al. 2007); connexin 26–mediated adhesion promotes nuclear translocation, whereas connexin 43–mediated adhesion regulates the stability of the leading-process branching (Elias et al. 2007). Considering that both connexins and other adhesion molecules interact with different components of the cytoskeleton, differences in the adhesion mechanisms between radially and tangentially migrating neurons may explain different mechanisms of nuclear translocation and leading-process dynamics evident in these neurons.
Trailing-Process Dynamics During Neuronal Migration
Formation and retraction of the trailing process are characteristic features of both interneuron and projection neuron migration. During neuronal migration, radially migrating neurons trail an extensive proto-axon from the cell, whereas interneurons in general display only a minor trailing process (Trivedi & Solecki 2011). These differences in trailing-process morphology may underlie the differences in translocation mechanisms used by these two groups of neurons. In tangentially migrating neurons, retraction of the trailing process is thought to depend on myosin II–mediated contraction at the neuron’s rear (Bellion et al. 2005, Schaar & McConnell 2005, Trivedi & Solecki 2011). This myosin-based retraction of the trailing process is thought to impel the interneuron forward. However, in radially migrating neurons, the concentration of myosin II motor is highest in the leading process, where it promotes centrosome translocation (He et al. 2010, Solecki et al. 2009, Trivedi & Solecki 2011). These differences in actomyosin localization and function, as related to trailing-process retraction, further highlight the distinguishing cellular mechanisms between the radial and tangential modes of migration.
PRIMARY CILIUM AS A SIGNALING CENTER DURING NEURONAL MIGRATION
The primary cilium, a microtubule-based sensory organelle, is a critical regulator of several signaling pathways (Han & Alvarez-Buylla 2010, Lee & Gleeson 2011, Louvi & Grove 2011). Primary cilia, found on cortical progenitor cells and developing neurons, are important for cortical morphogenesis (Arellano et al. 2012, Besse et al. 2011, Breunig et al. 2008, Willaredt et al. 2008, Wilson et al. 2012). Disrupted primary cilia functions may be responsible for cognitive defects and brain malformations in human ciliopathies (Han & Alvarez-Buylla 2010, Hildebrandt et al. 2011).
Recent studies have shown that primary cilia are essential regulators of interneuronal migration (Baudoin et al. 2012, Higginbotham et al. 2012). Interneuron-specific deletion of the cilia-specific Arl13b, a small GTPase of the Arf/Arl family, results in disrupted tangential migration (Higginbotham et al. 2012). Arl13b mutant interneurons are defective in their ability to respond to gradients of guidance cues from the dorsal cortex and have altered cAMP and Erk signaling (Higginbotham et al. 2012). The finding that Arl13b-related cilia defects do not affect radial migration suggests that cilium-mediated signaling may play a vital role in neuronal migration that depends on gradients of extrinsic guidance cues, as opposed to the substrate-guided mechanisms used by radially migrating neurons. High concentrations of signaling receptors in the primary cilium may enable it to act as a sensor of shallow gradients during oriented interneuronal migration. Further, a study by Baudoin and colleagues suggested that cilia-mediated Sonic hedgehog (Shh) signaling promotes tangential-to-radial transition of migrating interneurons, thus facilitating interneuronal colonization of the CP (Baudoin et al. 2012, Corbit et al. 2005). Together, these studies suggest that primary cilium–mediated signaling is instrumental in interneuronal navigation.
Although the mechanism via which ciliary proteins such as Arl13b may affect the neuronal cytoskeleton during migration is still unclear, the Arl proteins have been described as emerging regulators of microtubule dynamics (Kahn et al. 2005, Zhou et al. 2006). In Caenorhabditis elegans arl-13 mutants, effects of cilia disruption could be rescued by changing the acetylation state of the microtubules (Li et al. 2010, Loktev et al. 2008). It is tempting to speculate that similar mechanisms operate in interneuron cilia upon Shh or other guidance cue binding and promote oriented interneuronal migration by influencing microtubule dynamics. Consistently, Shh signaling–regulated tangential migration is accompanied by changes in microtubule organization and Golgi complex morphology (the latter relies on the microtubule cytoskeleton) (Baudoin et al. 2012).
Both primary cilia and growth cones sense and respond to extrinsic guidance cues and transduce signals that result in cytoskeletal rearrangements and oriented neuronal migration. Although several signaling receptors are shared between cilia and growth cones (Higginbotham et al. 2012), whether signaling emanating from these receptors in primary cilia and growth cones regulates distinct aspects of neuronal migratory behavior is currently unknown. Primary cilium’s proximity to the centrosome and nucleus may make it ideally positioned to promote nucleokinesis and neuron movement in response to appropriate guidance cues.
TRANSCRIPTIONAL, POSTTRANSCRIPTIONAL, AND EPIGENETIC REGULATION OF NEURONAL MIGRATION
Emerging data suggest that neuronal migration, positioning, and acquisition of correct neuronal identity are regulated by cell type– and layer-specific transcriptional programs in the developing neocortex (Kwan et al. 2012). Distinct transcriptional factors control migration of early- and late-born neurons, but several common principles govern this regulation. For example, transcription factors that are expressed by both early- and late-born projection neurons regulate neuronal migration in a neuronal type–specific manner. Sox5, specifically expressed in projection neurons destined for the SP and neocortical layers 5 and 6 (Kwan et al. 2008, Lai et al. 2008), is responsible for the correct migration of the deep-layer neurons past earlier-born neurons, but it does not affect migration of the late-born neurons. POU3F2 and -3, related transcription factors expressed by the late-born neurons, exhibit cell type–specific regulation of projection neurons destined to form layers 2–5 of the developing neocortex (Kwan et al. 2012). However, early neuron transcription factor TBR1 and late neuron transcription factor Satb2 regulate neuronal migration in a layer-specific manner (Alcamo et al. 2008, Bedogni et al. 2010, Britanova et al. 2008, Kwan et al. 2012). The downstream mechanisms of this transcriptional regulation have not yet been explored fully, but it is intriguing that POU3 transcription factors exhibit their regulatory effect via Cdk5 and Dab1, suggesting that migration-dependent transcriptional programs modulate intracellular signaling pathways known to be essential for oriented neuronal migration.
Importantly, distinct transcription factors have been shown to regulate radial and tangential migration. For example, transcription factors Dlx1/2 regulate interneuronal migration and tangential-to-radial migratory switches by affecting leading-process dynamics: Leading processes of the Dlx1/2−/− interneurons are longer and exhibit decreased branching (Anderson et al. 1997, 1999; Chédotal & Rijli 2009; Cobos et al. 2007). Another interneuron-specific transcription factor, Arx, which acts downstream of Dlx (Cobos et al. 2005, Colasante et al. 2008), controls tangential migration through a similar mechanism (Colombo et al. 2007). Importantly, expression of guidance cues and their receptors appears to be regulated by Arx as well as by other interneuron-specific transcription factors (Chédotal & Rijli 2009, Friocourt & Parnavelas 2011). For example, semaphorin 6 and Slit2 have been identified as potential Arx targets (Friocourt & Parnavelas 2011), whereas expression of the semaphorin 3A/3F receptor neuropilin 2 is controlled by transcription factors Nkx2-1 (Nóbrega-Pereira et al. 2008) and Dlx (Le et al. 2007). Interneurons are also enriched in the transcription factor Sip1, which, as van den Berghe et al. (2013) recently described, controls acquisition of proper interneuron identity as well as tangential migration.
During radial migration, transcription factor KLF4 (Krüppel-like factor 4) regulates multipolar-to-bipolar transition by affecting both leading and trailing processes (Qin & Zhang 2012). Paul et al. (2012) showed that transcription factor Scratch2 regulates radial migration during the multipolar phase as well. Recently, Miyoshi & Fishell (2012) showed that temporally regulated expression of transcription factor FoxG1 is required for the exit of projection neurons from the multipolar stage, as well as their migration to the CP. Additionally, Mandel et al. (2011) showed that the transcriptional repressor REST regulates radial migration, at least in part via its downstream target, Dcx. Together, these data suggest that combinations of projection neuron– and interneuron-specific transcription factors and repressors trigger signaling programs to facilitate appropriate patterns of neuronal migration.
Although the current evidence for posttranscriptional regulation of neuronal migration is limited, several recent studies highlight the importance of this process in neuronal migration. miRNAs, a class of small, noncoding RNAs that exhibit imperfect complementarity to their target mRNAs, regulate a multitude of transcripts by either promoting mRNA degradation or affecting mRNA translation (Bartel 2009). Temporal and spatial patterns of miRNA expression are important for brain development (Gao 2010, Krichevsky et al. 2003, Miska et al. 2004). The miRNA biogenesis pathway involves the activity of Dicer, an RNase III ribonuclease that cleaves miRNA precursors to produce mature miRNA duplexes. McLoughlin et al. (2012) showed that depleting Dicer from the neuronal progenitor cells disrupts progenitor localization in the VZ and SVZ and results in defective radial migration and an increase in the CR cells, suggesting that miRNAs control neuronal migration, and production of the CR cells, specifically. Furthermore, miR-9, one of the brain-specific miRNAs, negatively regulates expression of FoxG1, a transcription factor that regulates production and differentiation of the CR cells (Shibata et al. 2008). Delaloy et al. (2010) showed that miR-9 also regulates migration of the human neuronal progenitor cells in vitro and in vivo, suggesting that miR-9 may be an important regulator of neuronal migration. Importantly, stathmin, a known regulator of microtubule dynamics and radial migration (Westerlund et al. 2011), was identified as a miR-9 target (Delaloy et al. 2010), suggesting that miRNAs may partly control cytoskeletal rearrangements in neurons during neuronal migration.
Several miRNAs are known to regulate guidance cue–mediated signaling (e.g., ephrin and semaphorin signaling) (Baudet et al. 2012, Gao 2010). miRNAs may therefore regulate neuronal migration indirectly by regulating signaling pathways that drive migration. For example, miR-124, the most abundant miRNA in the mouse brain (Lagos-Quintana et al. 2002), regulates semaphorin 3A–mediated signaling by controlling the expression of neuropilin 1 in the growth cone (Baudet et al. 2012). Moreover, reciprocal regulation of miRNAs by transcription factors and repressors, and vice versa, constitutes yet another layer of regulation that may control neuronal migration. For example, repression of transcription factor Foxp2 by miR-9 and miR-132 has been shown to promote radial neuronal migration (Clovis et al. 2012), and transcriptional repressor REST, which has been implicated in radial migration, suppresses expression of miR-124 (Conaco et al. 2006). Thus, characteristic cell type–specific expression of many miRNAs, as well as their ability to target multiple transcripts, makes them attractive candidates as regulators of neuronal migration.
Alternative splicing is another major regulator of transcript diversity, and it has been estimated that up to 95% of all genes are alternatively spliced (Wang et al. 2008). The role of alternative splicing in neuronal migration is largely unexplored; however, splicing of Dab1, a crucial adaptor molecule in Reelin signaling, is one of the few splicing events that has been addressed in the context of neuronal migration. Alternative splicing of Dab1 results in several isoforms that differ in the number and type of tyrosine phosphorylation motifs. Different phosphorylated isoforms of Dab1 bind and activate different intracellular targets (Gao & Godbout 2013). Activation of different subsets of Dab1 targets may then lead to different outputs of Reelin-mediated signaling and differential effects on neuronal migration (Gao & Godbout 2013). Neuron-specific RNA-binding protein Nova2 regulates alternative splicing of Dab1 by suppressing inclusion of Dab1 exons 7b/c in radially migrating neurons (Yano et al. 2010). This suppression establishes a balance between Dab1 and Dab1.7bc isoforms that favors radial migration (Yano et al. 2010). Alternative splicing and alternative promoter usage also regulate expression of Nrg1, resulting in the production of membrane-bound and soluble isoforms (Falls 2003, Flames et al. 2004).
Lastly, epigenetic mechanisms have emerged recently as important regulators of both radial and tangential neuronal migration. S-Nitrosylation of histone deacetylase 2 has been shown to regulate radial neuronal migration via a signaling pathway that controls expression of a subunit of an ATP-dependent chromatin-remodeling complex (Nott et al. 2013). Further, histone methyltransferase Ezh2 regulates several transcription factors to promote tangential migration of mouse precerebellar neurons (Di Meglio et al. 2013). Diverse transcriptional, posttranscriptional, and epigenetic regulatory mechanisms likely control different aspects of neuronal migration. Experiments to unravel mechanistic aspects of this regulation, as well as direct neuronal and cytoskeletal targets of these processes, will further our understanding of integrative mechanisms that regulate neuronal migration.
NEURONAL MIGRATION AND THE WIRING OF THE CEREBRAL CORTEX
Both neuronal migration and axon guidance are essential for establishing functional brain circuitry. Although the two processes are usually examined independently, recent studies indicate that neuronal migration regulates axonal guidance in the cerebral cortex. Guidepost neurons, cortical neurons that are positioned along axonal migration routes, have been shown to guide axonal pathways. For example, two transient populations of glutamatergic and GABAergic neurons arrive at the corpus callosum just before the arrival of callosal axons and act together with glial cells to provide attractive cues for axonal navigation (Shu et al. 2003). Glutamatergic neurons secrete semaphorin 3C, which attracts neuropilin 1–expressing callosal axons, whereas MGE- and CGE-derived GABAergic neurons use semaphorin 3A– and ephrin-signaling pathways, as well as cell-adhesion mechanisms, to control callosal axon navigation (Niquille et al. 2009, 2013). Interestingly, known chemorepellents semaphorin and ephrin act as attractants in this case (Niquille et al. 2013).
Development of the thalamocortical projection depends on the tangential migration of a population of the LGE-derived interneurons, which create a permissive corridor for thalamocortical axon navigation in an otherwise inhibitory MGE environment (López-Bendito et al. 2006, Molnar et al. 2012). Nrg1/ErbB4 signaling is largely responsible for creating this permissive corridor (López-Bendito et al. 2006). Importantly, the Slit/Robo signaling pathway is instrumental in repelling corridor neurons from the ventral MGE and POA, thus controlling oriented migration of these cells and their correct positioning within the corridor (Bielle et al. 2011a). Within the corridor itself, opposing gradients of Slit1 and netrin1 act to establish topography of different types of thalamocortical axons: Slit1 repels intermediate thalamocortical axons while at the same time enabling netrin1 to serve as an attractant for the rostral axons (Bielle et al. 2011b). Recent data also suggest that thalamocortical axons traveling through this permissive corridor guide pathfinding of the pioneer corticothalamic axons and that temporally regulated semaphorin 3E/plexin-D1 signaling in pioneer cortical neurons is required for this regulation (Deck et al. 2013).
Finally, lateral olfactory tract (LOT) cells, which are early-generated excitatory projection neurons (Hirata et al. 2012, Sato et al. 1998), migrate ventrally and tangentially toward the area between the neocortex and the GE (the LOT area), where they abruptly change their orientation and disperse in a rostrocaudal direction (Kawasaki et al. 2006, Marín et al. 2010, Tomioka et al. 2000). A network of LOT guidepost cells then serves as a scaffold for the olfactory bulb axons. Several signaling pathways serve to create this network of guidepost cells. Ephrin A5 acts as a repellent that prevents LOT cells from crossing over into the ventral telencephalon (Nomura et al. 2006). Semaphorin 3F excludes LOT cells from the GE (Ito et al. 2008). Netrin1 locally attracts LOT cells into the ventral LOT area during later stages of their migration (Kawasaki et al. 2006). Together, these examples suggest that neuronal migration can influence multiple aspects of axon guidance in the developing brain, which is essential for establishing functional brain circuitry.
ANALYSIS OF PATHWAYS UNDERLYING CORTICAL NEURONAL MIGRATION
To evaluate the signaling pathways most influential in radial or tangential migration, we ran DAVID functional analysis (Dennis et al. 2003) on the gene lists (Table 1) for radial and tangential migration independently. We restricted the results to pathways listed in the Biological Biochemical Image Database (BBID) (Becker et al. 2000), BioCarta (Nishimura 2001), and the Kyoto Encyclopedia of Genes and Genomes (KEGG) (Kanehisa & Goto 2000). For each analysis, we considered pathways to be significant if the DAVID p-value was less than 0.05 and the number of input genes in the pathway was greater than 2. Under these criteria, we found 13 pathways significant for both tangential and radial migration, 3 for tangential only, and 24 for radial only. The top five pathways for each are shown in Table 2. Although this analysis is limited by the input list of genes currently known to modulate cortical neuronal migration (Table 1), it provides insight into emergent pathways vital for establishing specific patterns of cortical neuronal migration. Additionally, STRING (Search Tool for the Retrieval of Interacting Genes/Proteins) analysis ( Jensen et al. 2009) of proteins regulating migration indicates the strength of interactions between groups of proteins implicated in different patterns of cortical neuronal migration (see Supplemental Figure 1 at the Supplemental Material link in the online version of this article or at http://www.annualreviews.org/). As additional genes are identified to be regulators of specific aspects of neuronal migration (e.g., migratory stage or neuronal-subtype specific), such analysis may help identify nodal pathways critical for different aspects of neuronal migration and explain how disease-causing gene mutations may impinge on these pathways during brain development. Further, analysis of gene expression changes in mouse models of human neuronal migration defects [e.g., Lis1 neuronal migration complex (Lis1, Dcx, 14-3-3ε, Ndel1)] has led to the identification of commonly dysregulated signaling pathways and genes that are novel candidates of human neurodevelopmental disorders (Pramparo et al. 2011).
Table 2.
Analysis of signaling pathways regulating cortical neuronal migration
| Radial and tangential migration | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of genes | p-Value | Genes | Number of over lapping genes | Link | |||||
| Database | Pathway | Tangential | Radial | Tangential | Radial | Tangential | Radial | ||
| Kyoto Encyclopedia of Genes and Genomes (KEGG) | Axon guidance | 14 | 12 | 1.38e-12 | 3.18e-10 | NRP1, MET, CXCL12, SLIT1, ITGB1, NTN1, CDK5, PAK3, CXCR4, SEMA3F, RAC1, EFNA5, SEMA3A, SEMA4D | CDC42, PTK2, NRP1, FYN, SEMA3F, EFNB1, RAC1, RHOA, SEMA3A, SEMA4D, CDK5, ITGB1 | 5 | http://david.abcc.ncifcrf.gov/kegg.jsp?path=hsa04360$Axon%20guidance&termId=470038851&source=kegg |
| KEGG | Focal adhesion | 8 | 13 | 0.0004 | 2.68e-9 | ACTB, PAK3, MET, RAC1, RELN, ITGA3, HGF, ITGB1 | ACTB, ITGA3, ITGB1, FLNA, SRC, CDC42, PTK2, FYN, RAC1, RHOA, RELN, PIK3R3, CRK | 5 | http://david.abcc.ncifcrf.gov/kegg.jsp?path=hsa04510$Focal%20adhesion&termId=470038853&source=kegg |
| BIOCARTA | Lissencephaly gene (LIS1) in neuronal migration and development | 3 | 7 | 0.0111 | 3.81e-9 | CDK5R1, DCX, CDK5 | CDK5R1, NDEL1, DYNLL1, CLIP1, LRP8, DCX, CDK5 | 3 | http://david.abcc.ncifcrf.gov/biocarta.jsp?path=h_Lis1Pathway$Lissencephaly%20gene%20(LIS1)%20in%20neuronal%20migration%20and%20development&termId=30000173&source=biocarta |
| KEGG | Regulation of actin cytoskeleton | 7 | 11 | 0.0036 | 7.97e-7 | ACTB, PAK3, GSN, WASF1, RAC1, ITGA3, ITGB1 | ACTB, CDC42, PTK2, WASF1, RAC1, RHOA, ITGA3, PIK3R3, CRK, ITGB1, IQGAP1 | 5 | http://david.abcc.ncifcrf.gov/kegg.jsp?path=hsa04810$Regulation%20of%20actin%20cytoskeleton&termId=470038881&source=kegg |
| KEGG | Adherens junction | 4 | 8 | 0.0167 | 5.37e-7 | ACTB, WASF1, MET, RAC1 | ACTB, CDC42, FYN, WASF1, RAC1, RHOA, IQGAP1, SRC | 3 | http://david.abcc.ncifcrf.gov/kegg.jsp?path=hsa04520$Adherens%20junction&termId=470038856&source=kegg |
See related pathways in the database link to identify closely linked pathways relevant to neuronal migration.
DISORDERS CAUSED BY DISRUPTION IN NEURONAL MIGRATION
The importance of neuronal migration for cerebral cortical formation and function is evident in neurodevelopmental disorders resulting from disrupted neuronal migration. Major neuronal migration defects lead to severe brain malformations, as summarized in Table 3. These mutations in cytoskeletal regulators, extracellular matrix molecules, or posttranslational modifiers tend to affect both radial and tangential migration (Valiente & Marín 2010).
Table 3.
Neuronal migration disorders
| Syndrome | Gene | Defect | References |
|---|---|---|---|
| Periventricular heterotopia | FLNA | Decreased Filamin A affinity for actin cytoskeleton leading to cell-adhesion defect, disrupted onset of neuronal migration | Fox et al. 1998, Sheen et al. 2004, J. Zhang et al. 2012 |
| ARFGEF2 | Impaired vesicular trafficking of adhesion molecules and promotion of Filamin A phosphorylation (leading to a decreased affinity for actin), disrupted neuronal migration | Sheen et al. 2004, J. Zhang et al. 2012 | |
| Subcortical band heterotopia | DCX | Disrupted microtubule bundling Defect in nucleokinesis, disrupted maintenance of neuronal migration |
Corbo et al. 2002, des Portes et al. 1998, Gleeson et al. 1998, Kappeler et al. 2006, Pilz et al. 1999, Tanaka et al. 2004 |
| RhoA | Destabilization of actin in radial glial cells and disrupted radial glia scaffold, leading to radial migration defect Disruption of multipolar-to-bipolar transition, neurite extension, and locomotion of radially migrating neurons |
Cappello et al. 2012, Kouchi et al. 2011, Pacary et al. 2011 | |
| Type I lissencephaly | TUBA1A | Defects in the tubulin folding and heterodimer assembly pathway Disrupted radial migration, abnormal lamination of hippocampus and cortex |
Keays et al. 2007, Poirier et al. 2007 |
| PAFAH1B1 | Destabilization of microtubules and defective centrosome functions Defect in nucleokinesis and disrupted maintenance of radial and tangential migrations of neurons |
des Portes et al. 1998, Pilz et al. 1999, Sapir et al. 1997 |
|
| ARX | Direct target of Dlx2 transcription factor Disrupted tangential migration of cortical interneurons |
Cobos et al. 2005, Colasante et al. 2008, Marcorelles et al. 2010 | |
| RELN | Disruption of multipolar neuron polarization, terminal phase of neuronal migration, and inside-out layer formation | Chang et al. 2007, Hong et al. 2000, Jossin & Cooper 2011 | |
| Type II lissencephaly (cobblestone lissencephaly) | Pomt1 |
O-Mannosyltransferase mediating biosynthesis of O-mannosyl glycans Mutation leads to hypoglycosylation of α-dystroglycan, defective basal lamina and glial endfeet-pial membrane interactions, overmigration of cortical neurons |
Currier et al. 2005, Hu et al. 2011 |
| Dmd | Member of the dystrophin-glycoprotein complex (DGC), linking DGC to actin Disruption of radial glia scaffold and basal lamina leading to overmigration of cortical neurons |
Moore et al. 2002, Pawlisz & Feng 2011 | |
| Polymicrogyria | TUBB2B | Tubulin heterodimer assembly defect Disrupted radial migration: accumulation of late-born neurons in deep cortical layers (SVZ/IZ) |
Jaglin et al. 2009 |
| TUBA8 | Variant of α-tubulin with unknown function Mutated in the autosomal recessive syndrome characterized by generalized polymicrogyria and associated optic nerve hypoplasia |
Abdollahi et al. 2009 | |
| GPR56 | Defective basal lamina and glial endfeet-pial membrane interactions, malpositioning of neurons in the developing cortical plate | Li et al. 2008, Piao et al. 2005 | |
| Ciliopathies | Arl13b ( Joubert syndrome) | Disrupted cilia formation and ciliary localization of signaling receptors Aberrant patterns of tangential migration of interneurons |
Cantagrel et al. 2008, Caspi et al. 2000, Higginbotham et al. 2012 |
| DYX1C1 (Dyslexia) | Disruption of cilia morphology and signaling, defect in radial migration | Hoh et al. 2012, Massinen et al. 2011, Wang et al. 2006 | |
| Schizophrenia | NRG1/ | Decreased PI3K recruitment and activation | Anton et al. 1997, 2004; |
| ERBB4 | Disrupted tangential migration of cortical interneurons | Flames et al. 2004; Gambarotta et al. 2004; Li et al. 2012 | |
| DISC1 | Abnormal dynein function Disrupted Disc1–NDEL1, Disc1-ErbB interactions Accumulation of late-born neurons in the SVZ and IZ Neuronal migration defects in the hippocampus |
Duan et al. 2007, Seshadri et al. 2010 | |
| POU3F2 | Regulation of the expression of the p35 and p39 regulatory subunits of Cdk5 in migrating cortical neurons Neurons are unable to express Dab1, defective migration of cortical neurons |
McEvilly et al. 2002, Potkin et al. 2009, Sugitani et al. 2002 | |
| 22q1.2 deletion syndrome | 1.5- to 3-megabase hemizygous deletion on human chromosome 22 Leads to multiple CNS defects, including interneuron migration deficits |
Karayiorgou et al. 2010; Meechan et al. 2009, 2012 | |
| Autism | CXCR4 | Altered SVZ interneuron migratory stream More interneurons in the VZ, with frequent radially oriented leading processes |
Fine et al. 2005; Meechan et al. 2009, 2012; Niklasson et al. 2009 |
| CNTNAP2 | Neuronal transmembrane protein member (neurexin superfamily) involved in neuron-glia interactions Disrupted CNTNAP2 activity leads to ectopic neurons in corpus callosum, lamination defects, and decreased number of interneurons |
Alarcón et al. 2008, Penagarikaño et al. 2011 |
|
| SOX5 | Transcription factor Inactivation leads to disrupted preplate partition and laminar positioning defects of subplate and deep-layer neurons |
Kwan et al. 2008, Rosenfeld et al. 2010 | |
| Juvenile myoclonic epilepsy ( JME) | EFHC1 | Microtubule-associated protein JME mutations disrupt the morphology of radial and tangential migrating neurons and disrupt their migration (accumulation of radial migrating neurons in the IZ, reduced number of interneurons reaching the cortex) |
de Nijs et al. 2012, Suzuki et al. 2004 |
| Depression related to polymorphism 5-HTTLPR | Slc6a4 | Serotonin transporter Disruption of 5-HTR6 leads to aberrant interneuron migration and placement in the cerebral wall |
Pezawas et al. 2005, Riccio et al. 2009 |
| Mental retardation | PAK3 | Rho GTPase effector, expression decreased in migrating interneurons, increased when they reach the cortex Deletion disrupts tangential migration of cortical interneurons Overexpression of PAK3 leads to arrest of interneuron migration |
Allen et al. 1998, Cobos et al. 2007 |
| Autism spectrum disorder, attention deficit, hyperactivity disorder, bipolar disorder, major depressive disorder, and schizophrenia | L-type voltage-gated calcium channel subunits: CACNA1C and CACNB2 |
Risk loci Potential changes in terminal phase of interneuron migration |
Bortone & Polleux 2009, Cross-Disord. Group Psychiatr. Genomics Consort. 2013 |
However, minor changes in temporal or spatial patterns of neuronal migration may also affect the formation and activity of cortical circuits. Genes associated with autism spectrum disorders and psychotic and bipolar spectrum disorders can modulate neuronal migration. Subtle changes in neuronal positioning resulting from minor disruptions in these gene functions during development may contribute to the formation of aberrant neuronal circuitry underlying these disorders (Table 3).
CONCLUSIONS AND OPEN QUESTIONS
The emergence of functional neuronal connectivity in the developing cerebral cortex depends on appropriate neuronal placement. Placement of the right numbers and types of cortical neurons in the right areas depends on neuronal migration. Significant progress has been made in identifying individual molecules and mechanisms that regulate neuronal migration, specifically which guidance cues and cell-cell adhesion, transcriptional, and posttranscriptional mechanisms modulate migration. However, the modes of coordination between these mechanisms and how they converge on the cytoskeleton to drive neuronal navigation from their site of birth to their target locations in the cerebral cortex remain to be fully elucidated.
Several open questions remain. For example, how are various guidance cue–mediated changes in the leading processes of neurons coupled to nuclear translocation that drives neuronal migration, and how do the mechanics of this integration differ between radial and tangential modes of neuronal migration? The question of how different signaling pathways are integrated or modified during different modes of migration (e.g., somal translocation, multipolar migration, and radial glia–guided locomotion in projection neurons or tangential migration, ventricle-oriented migration, and tangential-to-radial transition in interneurons) within the same neuron remains unclear. Further, the emergence of the primary cilium as a regulator of neuronal migration raises the question of how cross talk between signals emanating from different domains of the migrating neurons (e.g., cilium, growth cone) may coordinate and control distinct aspects of oriented neuronal migration. The mechanisms of secondary, local migration of neurons that are necessary for the final allocation and placement of neurons during early postnatal stages (Marìn et al. 2010) also remain to be explored fully.
Importantly, both projection neurons and interneurons often migrate next to each other in the developing pallium. Yet they respond differently to cues in the same environment (for example, multipolar-to-radial transition of migrating projection neurons and ventricle-directed or tangential migration of interneurons occurring at the same time in the SVZ). How the different subtypes of developing projection neurons and interneurons achieve the molecular context to undergo different migratory programs that enable them to reach specific locations within the cerebral cortex, as well as the cell-intrinsic components of this process, is yet to be deciphered. Lastly, further evidence on subtle changes in migration patterns resulting from mutant susceptibility genes associated with circuit dysfunction in complex neurobehavioral disorders will help to delineate the role of migration in the organization and formation of functional neuronal circuitry. Moreover, it will be important to examine how networks of signaling pathways employed by neurons during development are adopted by newly generated neurons in the neurogenic niches of the postnatal brain to maintain and modify neuronal circuitry.
Supplementary Material
Acknowledgments
This research was supported by National Institutes of Health grant MH060929 to E.A. We thank M. Deshmukh and W. Snider for helpful comments, A. Byrnes for help with pathway analysis, and G. Wilkins for help with Figure 1d. We apologize to all colleagues whose important work could not be cited here due to the large scope of this review and space limitations.
Glossary
- GABA
gamma-aminobutyric acid
- VZ
ventricular zone
- SVZ
subventricular zone
- RGP
radial glial progenitor
- IP
intermediate progenitor
- PP
preplate
- CP
cortical plate
- MZ
marginal zone
- SP
subplate
- IZ
intermediate zone
- MGE
medial ganglionic eminence
- CGE
caudal ganglionic eminence
- POA
preoptic area
- LGE
lateral ganglionic eminence
- CR
Cajal-Retzius
- LOT
lateral olfactory tract
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Abdollahi MR, Morrison E, Sirey T, Molnar Z, Hayward BE, et al. Mutation of the variant α-tubulin TUBA8 results in polymicrogyria with optic nerve hypoplasia. Am J Hum Genet. 2009;85:737–44. doi: 10.1016/j.ajhg.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa H, Wakatsuki S, Ishii A, Moriyama K, Sasaki Y, et al. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat Neurosci. 2001;4(4):367–73. doi: 10.1038/86011. [DOI] [PubMed] [Google Scholar]
- Alarcón M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, et al. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008;82:150–59. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti S, Krause SM, Kretz O, Philippar U, Lemberger T, et al. Neuronal migration in the murine rostral migratory stream requires serum response factor. Proc Natl Acad Sci USA. 2005;102:6148–53. doi: 10.1073/pnas.0501191102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcamo EA, Chirivella L, Dautzenbuerg M, Dobreva G, Fariñas I, et al. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;557:364–77. doi: 10.1016/j.neuron.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Alcántara S, Pozas E, Ibañez CF, Soriano E. BDNF-modulated spatial organization of Cajal-Retzius and GABAergic neurons in the marginal zone plays a role in the development of cortical organization. Cereb Cortex. 2006;16:487–99. doi: 10.1093/cercor/bhi128. [DOI] [PubMed] [Google Scholar]
- Alfano C, Viola L, Heng JIT, Pirozzi M, Clarkson M, et al. COUP-TFI promotes radial migration and proper morphology of callosal projection neurons by repressing Rnd2 expression. Development. 2011;138:4685– 97. doi: 10.1242/dev.068031. [DOI] [PubMed] [Google Scholar]
- Allen KM, Gleeson JG, Bagrodia S, Partington MW, MacMillan JC, et al. PAK3 mutation in nonsyndromic X-linked mental retardation. Nat Genet. 1998;20:25–30. doi: 10.1038/1675. [DOI] [PubMed] [Google Scholar]
- Anderson S, Mione M, Yun K, Rubenstein JLR. Differential origins of neocortical projection and local circuit neurons: role of Dlx genes in neocortical interneuronogenesis. Cereb Cortex. 1999;9(6):646–54. doi: 10.1093/cercor/9.6.646. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JLR. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–76. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Marín O, Horn C, Jennings K, Rubenstein JLR. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–63. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- Andrews W, Barber M, Hernandez-Miranda LR, Xian J, Rakic S, et al. The role of Slit-Robo signaling in the generation, migration and morphological differentiation of cortical interneurons. Dev Biol. 2008;313(2):648–58. doi: 10.1016/j.ydbio.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Andrews WD, Barber M, Parnavelas JG. Slit-Robo interactions during cortical development. J Anat. 2007;211(2):188–98. doi: 10.1111/j.1469-7580.2007.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews W, Liapi A, Plachez C, Camurri L, Zhang J, et al. Robo1 regulates the development of major axon tracts and interneuron migration in the forebrain. Development. 2006;133:2243–52. doi: 10.1242/dev.02379. [DOI] [PubMed] [Google Scholar]
- Ang ES, Jr, Haydar TF, Gluncic V, Rakic P. Four-dimensional migratory coordinates of GABAergic interneurons in the developing mouse cortex. J Neurosci. 2003;23:5805–15. doi: 10.1523/JNEUROSCI.23-13-05805.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton ES, Ghashghaei HT, Weber JL, McCann C, Fischer TM, et al. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci. 2004;7(12):1319–28. doi: 10.1038/nn1345. [DOI] [PubMed] [Google Scholar]
- Anton ES, Kreidberg JA, Rakic P. Distinct functions of α3 and αv integrin receptors in neuronal migration and laminar organization of the cerebral cortex. Neuron. 1999;22(2):277–89. doi: 10.1016/s0896-6273(00)81089-2. [DOI] [PubMed] [Google Scholar]
- Anton ES, Marchionni MA, Lee KF, Rakic P. Role of GGF/neuregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development. 1997;124:3501–10. doi: 10.1242/dev.124.18.3501. [DOI] [PubMed] [Google Scholar]
- Arellano JI, Guadiana SM, Breunig JJ, Rakic P, Sarkisian MR. Development and distribution of neuronal cilia in mouse neocortex. J Comp Neurol. 2012;520:848–73. doi: 10.1002/cne.22793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud L, Ballif BA, Förster E, Cooper JA. Fyn tyrosine kinase is a critical regulator of disabled-1 during brain development. Curr Biol. 2003;13:9–17. doi: 10.1016/s0960-9822(02)01397-0. [DOI] [PubMed] [Google Scholar]
- Asada N, Sanada K, Fukada Y. LKB1 regulates neuronal migration and neuronal differentiation in the developing neocortex through centrosomal positioning. J Neurosci. 2007;27(43):11769–75. doi: 10.1523/JNEUROSCI.1938-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assadi AH, Zhang G, Beffert U, McNeil RS, Renfro AL, et al. Interaction of reelin signaling and Lis1 in brain development. Nat Genet. 2003;35:270–76. doi: 10.1038/ng1257. [DOI] [PubMed] [Google Scholar]
- Ayala R, Shu T, Tsai L-H. Trekking across the brain: the journey of neuronal migration. Cell. 2007;128:29–43. doi: 10.1016/j.cell.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Ballif BA, Arnaud L, Cooper JA. Tyrosine phosphorylation of Disabled-1 is essential for Reelin-stimulated activation of Akt and Src family kinases. Mol Brain Res. 2003;117:152–59. doi: 10.1016/s0169-328x(03)00295-x. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista-Brito R, Fishell G. The developmental integration of cortical interneurons into a functional network. Curr Top Dev Biol. 2009;87:81–118. doi: 10.1016/S0070-2153(09)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista D, Rutishauser U. Removal of polysialic acid triggers dispersion of subventricularly derived neuroblasts into surrounding CNS tissues. J Neurosci. 2010;30:3995–4003. doi: 10.1523/JNEUROSCI.4382-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudet ML, Bellon A, Holt CE. Role of microRNAs in Semaphorin function and neural circuit formation. Semin Cell Dev Biol. 2012;24(3):146–55. doi: 10.1016/j.semcdb.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Baudoin JP, Viou L, Launay PS, Luccardini C, Espeso Gil S, et al. Tangentially migrating neurons assemble a primary cilium that promotes their reorientation to the cortical plate. Neuron. 2012;76:1108–22. doi: 10.1016/j.neuron.2012.10.027. [DOI] [PubMed] [Google Scholar]
- Becker KG, Whilte SL, Muller J, Engel J. BBID: the biological biochemical image database. Bioinformatics. 2000;16(8):745–46. doi: 10.1093/bioinformatics/16.8.745. [DOI] [PubMed] [Google Scholar]
- Bedogni F, Hodge RD, Elsen GE, Nelson BR, Daza RAM, et al. Tbr1 regulates regional and laminar identity of postmitotic neurons in the developing neocortex. Proc Natl Acad Sci USA. 2010;107:13129–34. doi: 10.1073/pnas.1002285107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar TN, Li YX, Tran HT, Ma W, Dunlap V, et al. GABA stimulates chemotaxis and chemokinesis of embryonic cortical neurons via calcium-dependent mechanisms. J Neurosci. 1996;16(5):1808–18. doi: 10.1523/JNEUROSCI.16-05-01808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar TN, Schaffner AE, Scott CA, O’Connell C, Barker JL. Differential response of cortical plate and ventricular zone cells to GABA as a migration stimulus. J Neurosci. 1998;18(16):6378–87. doi: 10.1523/JNEUROSCI.18-16-06378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar TN, Scott CA, Greene CL, Wen X, Smith SV, et al. Glutamate acting at NMDA receptors stimulates embryonic cortical neuronal migration. J Neurosci. 1999;19:4449–61. doi: 10.1523/JNEUROSCI.19-11-04449.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellion A, Baudoin JP, Alvarez C, Bornens M, Métin C. Nucleokinesis in tangentially migrating neurons comprises two alternating phases: forward migration of the Golgi/centrosome associated with centrosome splitting and myosin contraction at the rear. J Neurosci. 2005;25(24):5691–99. doi: 10.1523/JNEUROSCI.1030-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvindrah R, Graus-Porta D, Goebbels S, Nave KA, Müller U. β1 integrins in radial glia but not in migrating neurons are essential for the formation of cell layers in the cerebral cortex. J Neurosci. 2007;27:13854–65. doi: 10.1523/JNEUROSCI.4494-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besse L, Neti M, Anselme I, Gerhardt C, Rüther U, et al. Primary cilia control telencephalic patterning and morphogenesis via Gli3 proteolytic processing. Development. 2011;138(10):2079–88. doi: 10.1242/dev.059808. [DOI] [PubMed] [Google Scholar]
- Bielle F, Marcos-Mondéjar P, Keita M, Mailhes C, Verney C, et al. Slit2 activity in the migration of guidepost neurons shapes thalamic projections during development and evolution. Neuron. 2011a;69(6):1085–98. doi: 10.1016/j.neuron.2011.02.026. [DOI] [PubMed] [Google Scholar]
- Bielle F, Marcos-Mondéjar P, Levva-Díaz E, Lokmane L, Mire E, et al. Emergent growth cone responded to combinations of Slit and Netrin 1 in thalamocortical axon topography. Curr Biol. 2011b;21(20):1748– 55. doi: 10.1016/j.cub.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Bock HH, Herz J. Reelin activates SRC family tyrosine kinases in neurons. Curr Biol. 2003;13:18–26. doi: 10.1016/s0960-9822(02)01403-3. [DOI] [PubMed] [Google Scholar]
- Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci. 2004;24(35):7623–31. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bony G, Szczurkowska J, Tamagno I, Shelly M, Contestabile A, Cancedda L. Non-hyperpolarizing GABAB receptor activation regulates neuronal migration and neurite growth and specification by cAMP/LKB1. Nat Commun. 2013;4:1800. doi: 10.1038/ncomms2820. [DOI] [PubMed] [Google Scholar]
- Bortone D, Polleux F. KCC2 expression promotes the termination of cortical interneuron migration in a voltage-sensitive calcium-dependent manner. Neuron. 2009;62:53–71. doi: 10.1016/j.neuron.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouché E, Romero-Ortega MI, Henkemeyer M, Catchpole T, Leemhuis J, et al. Reelin induces EphB activation. Cell Res. 2013;23:473–90. doi: 10.1038/cr.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breunig JJ, Sarkisian MR, Arellano JI, Morozov YM, Ayoub AE, et al. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc Natl Acad Sci USA. 2008;105(35):13127–32. doi: 10.1073/pnas.0804558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britanova O, de Juan Romero C, Cheung A, Kwan KY, Schwark M, et al. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron. 2008;57:378–92. doi: 10.1016/j.neuron.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Butt SJB, Fuccillo M, Nery S, Noctor S, Kriegstein A, et al. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Butt SJB, Sousa VH, Fuccillo MV, Hjerling-Leffler J, Miyoshi G, et al. The requirement of Nkx2-1 in the temporal specification of cortical interneuron subtypes. Neuron. 2008;59(5):722–32. doi: 10.1016/j.neuron.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9:110–22. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- Cantagrel V, Silhavy JL, Bielas SL, Swistun D, Marsh SE, et al. Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am J Hum Genet. 2008;83:170–79. doi: 10.1016/j.ajhg.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappello S, Attardo A, Wu X, Iwasato T, Itohara S, et al. The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat Neurosci. 2006;9:1099–107. doi: 10.1038/nn1744. [DOI] [PubMed] [Google Scholar]
- Cappello S, Böhringer CR, Bergami M, Conzelmann KK, Ghanem A, et al. A radial glia-specific role of RhoA in double cortex formation. Neuron. 2012;73:911–24. doi: 10.1016/j.neuron.2011.12.030. [DOI] [PubMed] [Google Scholar]
- Caric D, Raphael H, Viti J, Feathers A, Wancio D, Lillien L. EGFRs mediate chemotactic migration in the developing telencephalon. Development. 2001;128:4203–16. doi: 10.1242/dev.128.21.4203. [DOI] [PubMed] [Google Scholar]
- Carter N, Nakamoto T, Hirai H, Hunter T. EphrinA1-induced cytoskeletal re-organization requires FAK and p130cas. Nat Cell Biol. 2002;4(8):565–73. doi: 10.1038/ncb823. [DOI] [PubMed] [Google Scholar]
- Caspi M, Atlas R, Kantor A, Sapir T, Reiner O. Interaction between LIS1 and doublecortin, two lissencephaly gene products. Hum Mol Genet. 2000;9:2205–13. doi: 10.1093/oxfordjournals.hmg.a018911. [DOI] [PubMed] [Google Scholar]
- Chae T, Kwon YT, Bronson R, Dikkes P, Li E, Tsai LH. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron. 1997;18:29–42. doi: 10.1016/s0896-6273(01)80044-1. [DOI] [PubMed] [Google Scholar]
- Chai X, Förster E, Zhao S, Bock HH, Frotscher M. Reelin stabilizes the actin cytoskeleton of neuronal processes by inducing n-cofilin phosphorylation at serine 3. J Neurosci. 2009;29(1):288–99. doi: 10.1523/JNEUROSCI.2934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BS, Duzcan F, Kim S, Cinbis M, Aggarwai A, et al. Role of RELN in lissencephaly and neuropsychiatric disease. Med Genet B Neuropsychiatr Genet. 2007;144B(1):58–63. doi: 10.1002/ajmg.b.30392. [DOI] [PubMed] [Google Scholar]
- Chauvet S, Cohen S, Yoshida Y, Fekrane L, Livet J, et al. Gating of Sema3E/Plexin D1 signaling by neuropilin-1 switches axonal repulsion to attraction during brain development. Neuron. 2007;56(5):807–22. doi: 10.1016/j.neuron.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet N, Prieto M, Fabre C, Noren NK, Privat A. Distribution of p120 catenin during rat brain development: potential role in regulation of cadherin-mediated adhesion and actin cytoskeleton organization. Mol Cell Neurosci. 2003;22(4):467–86. doi: 10.1016/s1044-7431(03)00030-7. [DOI] [PubMed] [Google Scholar]
- Chédotal A, Rijli FM. Transcriptional regulation of tangential neuronal migration in the developing forebrain. Curr Opin Neurobiol. 2009;19(2):139–45. doi: 10.1016/j.conb.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Chen G, Sima J, Jin M, Wang KY, Xue XJ, et al. Semaphorin-3A guides radial migration of cortical neurons during development. Nat Neurosci. 2008;11:36–44. doi: 10.1038/nn2018. [DOI] [PubMed] [Google Scholar]
- Chen K, Ochalski PG, Tran TS, Sahir N, Schubert M, et al. Interaction between Dab1 and CrkII is promoted by Reelin signaling. J Cell Sci. 2004;117:4527–36. doi: 10.1242/jcs.01320. [DOI] [PubMed] [Google Scholar]
- Clovis YM, Enard W, Marinaro F, Huttner WB, De Pietri Tonelli D. Convergent repression of Foxp2 3′UTR by miR-9 and miR-132 in embryonic mouse neocortex: implications for radial migration of neurons. Development. 2012;139(18):3332–42. doi: 10.1242/dev.078063. [DOI] [PubMed] [Google Scholar]
- Cobos I, Borello U, Rubenstein JLR. Dlx transcription factors promote migration through repression of axon and dendrite growth. Neuron. 2007;54(6):873–88. doi: 10.1016/j.neuron.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos I, Broccoli V, Rubenstein JL. The vertebrate ortholog of Aristaless is regulated by Dlx genes in the developing forebrain. J Comp Neurol. 2005;483(3):292–303. doi: 10.1002/cne.20405. [DOI] [PubMed] [Google Scholar]
- Colasante G, Collombat P, Raimondi V, Bonanomi D, Ferrai C, et al. Arx is a direct target of Dlx2 and thereby contributes to the tangential migration of GABAergic interneurons. J Neurosci. 2008;28(42):10674–86. doi: 10.1523/JNEUROSCI.1283-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo E, Collombat P, Colasante G, Bianchi M, Long J, et al. Inactivation of Arx, the murine ortholog of the X-linked lissencephaly with ambiguous genitalia gene, leads to severe disorganization of the ventral telencephalon with impaired neuronal migration and differentiation. J Neurosci. 2007;27(17):4786–98. doi: 10.1523/JNEUROSCI.0417-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaco C, Otto S, Han JJ, Mandel G. Reciprocal actions of REST and a microRNA promote neuronal identity. Proc Natl Acad Sci USA. 2006;103(7):2422–27. doi: 10.1073/pnas.0511041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin JG, Butt SJB. Developmental mechanisms for the generation of telencephalic interneurons. Dev Neurobiol. 2011;71:710–32. doi: 10.1002/dneu.20890. [DOI] [PubMed] [Google Scholar]
- Corbin JG, Rutlin M, Gaiano N, Fishell G. Combinatorial function of the homeodomain proteins Nkx2.1 and Gsh2 in ventral telencephalic patterning. Development. 2003;130:4895–906. doi: 10.1242/dev.00717. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–21. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- Corbo JC, Deuel TA, Long JM, LaPorte P, Tsai E, et al. Doublecortin is required in mice for lamination of the hippocampus but not the neocortex. J Neurosci. 2002;22:7548–57. doi: 10.1523/JNEUROSCI.22-17-07548.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall JE, McCarthy DM, Araki KY, Sims JR, Ren JQ, Bhide PG. Dopamine receptor activation modulates GABA neuron migration from the basal forebrain to the cerebral cortex. J Neurosci. 2007;27:3813–22. doi: 10.1523/JNEUROSCI.5124-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross-Disord. Group Psychiatr. Genomics Consort. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–79. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier SC, Lee CK, Chang BS, Bodell AL, Pai GS, et al. Mutations in POMT1 are found in a minority of patients with Walker-Warburg syndrome. Am J Med Genet. 2005;133A:53–57. doi: 10.1002/ajmg.a.30487. [DOI] [PubMed] [Google Scholar]
- Cuzon VC, Yeh PW, Cheng Q, Yeh HH. Ambient GABA promotes cortical entry of tangentially migrating cells derived from the medial ganglionic eminence. Cereb Cortex. 2006;16(10):1377–88. doi: 10.1093/cercor/bhj084. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–23. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Homayouni R, Keshvara L, Rice DS, Sheldon M, Curran T. Reelin is a ligand for lipoprotein receptors. Neuron. 1999;24:471–79. doi: 10.1016/s0896-6273(00)80860-0. [DOI] [PubMed] [Google Scholar]
- Davy A, Soriano P. Ephrin signaling in vivo: Look both ways. Dev Dyn. 2005;232:1–10. doi: 10.1002/dvdy.20200. [DOI] [PubMed] [Google Scholar]
- Deck M, Lokmane L, Chauvet S, Mailhes C, Keita M, et al. Pathfinding of corticothalamic axons relies on a rendezvous with thalamic projections. Neuron. 2013;77(3):472–84. doi: 10.1016/j.neuron.2012.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, López-Cruz PL, Benavides-Piccione R, Bielza C, Larrañaga P, et al. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci. 2013;14(3):202–16. doi: 10.1038/nrn3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaloy C, Liu L, Lee JA, Su H, Shen F, et al. MicroRNA-9 coordinates proliferation and migration of human embryonic stem cell-derived neural progenitors. Cell Stem Cell. 2010;6(4):323–35. doi: 10.1016/j.stem.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nijs L, Wolkoff N, Coumans B, Delgado-Escueta AV, Grisar T, Lakaye B. Mutations of EFHC1, linked to juvenile myoclonic epilepsy, disrupt radial and tangential migrations during brain development. Hum Mol Genet. 2012;21:5106–17. doi: 10.1093/hmg/dds356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4(5):P3. [PubMed] [Google Scholar]
- Dent EW, Gupton SL, Gertler FB. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb Perspect Biol. 2011;3:a001800. doi: 10.1101/cshperspect.a001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- des Portes V, Pinard JM, Billuart P, Vinet MC, Koulakoff A, et al. A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell. 1998;92:51–61. doi: 10.1016/s0092-8674(00)80898-3. [DOI] [PubMed] [Google Scholar]
- Deuel TAS, Liu JS, Corbo JC, Yoo SY, Rorke-Adams LB, Walsh CA. Genetic interactions between doublecortin and doublecortin-like kinase in neuronal migration and axon outgrowth. Neuron. 2006;49(1):41–53. doi: 10.1016/j.neuron.2005.10.038. [DOI] [PubMed] [Google Scholar]
- Di Meglio T, Kratochwil CF, Vilain N, Loche A, Vitobello A, et al. Ezh2 orchestrates topographic migration and connectivity of mouse precerebellar neurons. Science. 2013;339(6116):204–7. doi: 10.1126/science.1229326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–58. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias LA, Turmaine M, Parnavelas JG, Kriegstein AR. Connexin 43 mediates the tangential to radial migratory switch in ventrally derived cortical interneurons. J Neurosci. 2010;30:7072–77. doi: 10.1523/JNEUROSCI.5728-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias LA, Wang DD, Kriegstein AR. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448(7156):901–7. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- Endo M, Ohashi K, Sasaki Y, Goshima Y, Niwa R, et al. Control of growth cone motility and morphology by LIM kinase and Slingshot via phosphorylation and dephosphorylation of cofilin. J Neurosci. 2003;23(7):2527–37. doi: 10.1523/JNEUROSCI.23-07-02527.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCζ. Cell. 2001;106:489–98. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003;284(1):14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- Falnikar A, Tole S, Baas PW. Kinesin-5, a mitotic microtubule-associated motor protein, modulates neuronal migration. Mol Biol Cell. 2011;22:1561–74. doi: 10.1091/mbc.E10-11-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faux C, Rakic S, Andrews W, Britto JM. Neurons on the move: migration and lamination of cortical interneurons. Neurosignals. 2012;20:164–85. doi: 10.1159/000334489. [DOI] [PubMed] [Google Scholar]
- Feng L, Allen NS, Simo S, Cooper JA. Cullin 5 regulates Dab1 protein levels and neuron positioning during cortical development. Genes Dev. 2007;21:2717–30. doi: 10.1101/gad.1604207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine SE, Weissman A, Gerdes M, Pinto-Martin J, Zackai EH, et al. Autism spectrum disorders and symptoms in children with molecularly confirmed 22q11.2 deletion syndrome. J Autism Dev Disord. 2005;35:461–70. doi: 10.1007/s10803-005-5036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flames N, Long JE, Garratt AN, Fischer TM, Gassmann M, et al. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron. 2004;44(2):251–61. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Fode C, Ma Q, Casarosa S, Ang SL, Anderson DJ, Guillemot F. A role for neural determination genes in specifying the dorsoventral identity of telencephalic neurons. Genes Dev. 2000;14:67–80. [PMC free article] [PubMed] [Google Scholar]
- Fox JW, Lamperti ED, Eksioglu YZ, Hong SE, Feng Y, et al. Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron. 1998;21:1315–25. doi: 10.1016/s0896-6273(00)80651-0. [DOI] [PubMed] [Google Scholar]
- Franco SJ, Gil-Sanz C, Martinez-Garay I, Espinosa A, Harkins-Perry SR, et al. Fate-restricted neural progenitors in the mammalian cerebral cortex. Science. 2012;337(6095):746–49. doi: 10.1126/science.1223616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco SJ, Martinez-Garay I, Gil-Sanz C, Harkins-Perry SR, Müller U. Reelin regulates cadherin function via Dab1/Rap1 to control neuronal migration and lamination in the neocortex. Neuron. 2011;69(3):482– 97. doi: 10.1016/j.neuron.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friocourt G, Liu JS, Antypa M, Rakic S, Walsh CA, Parnavelas JG. Both doublecortin and doublecortin-like kinase play a role in cortical interneuron migration. J Neurosci. 2007;27(14):3875–83. doi: 10.1523/JNEUROSCI.4530-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friocourt G, Parnavelas JG. Identification of Arx targets unveils new candidates for controlling cortical interneuron migration and differentiation. Front Cell Neurosci. 2011;5:28. doi: 10.3389/fnbeh.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Sun H, Klein WH, Mu X. β-Catenin is essential for lamination but not neurogenesis in mouse retinal development. Dev Biol. 2006;299:424–37. doi: 10.1016/j.ydbio.2006.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulp CT, Cho G, Marsh ED, Nasrallah IM, Labosky PA, Golden JA. Identification of Arx transcriptional targets in the developing basal forebrain. Hum Mol Genet. 2008;17:3740–60. doi: 10.1093/hmg/ddn271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fushiki S, Perez Velazquez JL, Zhang L, Bechberger JF, Carlen PL, Naus CC. Changes in neuronal migration in neocortex of connexin43 null mutant mice. J Neuropathol Exp Neurol. 2003;62:304–14. doi: 10.1093/jnen/62.3.304. [DOI] [PubMed] [Google Scholar]
- Gambarotta G, Garzotto D, Destro E, Mautino B, Giampietro C, et al. ErbB4 expression in neural progenitor cells (ST14A) is necessary to mediate neuregulin-1β1-induced migration. J Biol Chem. 2004;279:48808–16. doi: 10.1074/jbc.M408374200. [DOI] [PubMed] [Google Scholar]
- Gao FB. Context-dependent function of specific microRNAs in neuronal development. Neural Dev. 2010;5:25. doi: 10.1186/1749-8104-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Godbout R. Reelin-Disabled-1 signaling in neuronal migration: Splicing takes the stage. Cell Mol Life Sci. 2013;70(13):2319–29. doi: 10.1007/s00018-012-1171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman DM, Marín O. Generation of interneuron diversity in the mouse cerebral cortex. Eur J Neurosci. 2010;31:2136–41. doi: 10.1111/j.1460-9568.2010.07267.x. [DOI] [PubMed] [Google Scholar]
- Gelman DM, Martini FJ, Nóbrega-Pereira S, Pierani A, Kessaris N, Marín O. The embryonic preoptic area is a novel source of cortical GABAergic interneurons. J Neurosci. 2009;29:9380–89. doi: 10.1523/JNEUROSCI.0604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Lai C, Anton ES. Neuronal migration in the adult brain: are we there yet? Nat Rev Neurosci. 2007;8:141–51. doi: 10.1038/nrn2074. [DOI] [PubMed] [Google Scholar]
- Gilmore EC, Ohshima T, Goffinet AM, Kulkarni AB, Herrup K. Cyclin-dependent kinase 5-deficient mice demonstrate novel developmental arrest in cerebral cortex. J Neurosci. 1998;18:6370–77. doi: 10.1523/JNEUROSCI.18-16-06370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson JG, Allen KM, Fox JW, Lamperti ED, Berkovic S, et al. doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92:63–72. doi: 10.1016/s0092-8674(00)80899-5. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23(2):257–71. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- Godin JD, Thomas N, Laguesse S, Malinouskaya L, Close P, et al. p27Kip1 is a microtubule-associated protein that promotes microtubule polymerization during neuron migration. Dev Cell. 2012;23:729–44. doi: 10.1016/j.devcel.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Goh KL, Cai L, Cepko CL, Gertler FB. Ena/VASP proteins regulate cortical neuronal positioning. Curr Biol. 2002;12:565–69. doi: 10.1016/s0960-9822(02)00725-x. [DOI] [PubMed] [Google Scholar]
- Gongidi V, Ring C, Moody M, Brekken R, Sage EH, et al. SPARC-like 1 regulates the terminal phase of radial glia-guided migration in the cerebral cortex. Neuron. 2004;41:57–69. doi: 10.1016/s0896-6273(03)00818-3. [DOI] [PubMed] [Google Scholar]
- Götz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–88. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Govek EE, Hatten ME, Van Aelst L. The role of Rho GTPase proteins in CNS neuronal migration. Dev Neurobiol. 2011;71:528–53. doi: 10.1002/dneu.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus-Porta D, Blaess S, Senften M, Littlewood-Evans A, Damsky C, et al. β1-Class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron. 2001;31(3):367–79. doi: 10.1016/s0896-6273(01)00374-9. [DOI] [PubMed] [Google Scholar]
- Gu Y, Ihara Y. Evidence that collapsin response mediator protein-2 is involved in the dynamics of microtubules. J Biol Chem. 2000;275(24):17917–20. doi: 10.1074/jbc.C000179200. [DOI] [PubMed] [Google Scholar]
- Guo J, Yang Z, Song W, Chen Q, Wang F, et al. Nudel contributes to microtubule anchoring at the mother centriole and is involved in both dynein-dependent and -independent centrosomal protein assembly. Mol Biol Cell. 2006;17(2):680–89. doi: 10.1091/mbc.E05-04-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Sanada K, Miyamoto DT, Rovelstad S, Nadarajah B, et al. Layering defect in p35 deficiency is linked to improper neuronal-glial interaction in radial migration. Nat Neurosci. 2003;6:1284–91. doi: 10.1038/nn1151. [DOI] [PubMed] [Google Scholar]
- Hammond V, Tsai LH, Tan SS. Control of cortical neuron migration and layering: cell and non cell-autonomous effects of p35. J Neurosci. 2004;24:576–87. doi: 10.1523/JNEUROSCI.4529-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YG, Alvarez-Buylla A. Role of primary cilia in brain development and cancer. Curr Opin Neurobiol. 2010;20:58–67. doi: 10.1016/j.conb.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Kwan KY, Shim S, Lam MM, Shin Y, et al. TBR1 directly represses Fezf2 to control the laminar origin and development of the corticospinal tract. Proc Natl Acad Sci USA. 2011;108(7):3041–46. doi: 10.1073/pnas.1016723108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Parker PRL, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–61. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- Hashimoto-Torii K, Torii M, Sarkisian MR, Bartley CM, Shen J, et al. Interaction between Reelin and Notch signaling regulates neuronal migration in the cerebral cortex. Neuron. 2008;60:273–84. doi: 10.1016/j.neuron.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorne AL, Wylie CJ, Landmesser LT, Deneris ES, Silver J. Serotonergic neurons migrate radially through the neuroepithelium by dynamin-mediated somal translocation. J Neurosci. 2010;30(2):420–30. doi: 10.1523/JNEUROSCI.2333-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Zhang ZH, Guan CB, Xia D, Yuan XB. Leading tip drives soma translocation via forward F-actin flow during neuronal migration. J Neurosci. 2010;30(32):10885–98. doi: 10.1523/JNEUROSCI.0240-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng JI, Nguyen L, Castro DS, Zimmer C, Wildner H, et al. Neurogenin 2 controls cortical neuron migration through regulation of Rnd2. Nature. 2008;455:114–18. doi: 10.1038/nature07198. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Shi L, Justice N, Hsueh Y, Sheng M, et al. Tbr1 regulates differentiation of the preplate and layer 6. Neuron. 2001;29(2):353–66. doi: 10.1016/s0896-6273(01)00211-2. [DOI] [PubMed] [Google Scholar]
- Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, et al. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 1999;24:481–89. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- Higginbotham H, Eom T-Y, Mariano LE, Bachleda A, Hirt J, et al. Arl13B in primary cilia regulates the migration and placement of interneurons in the developing cerebral cortex. Dev Cell. 2012;23:925–38. doi: 10.1016/j.devcel.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginbotham H, Yokota Y, Anton ES. Strategies for analyzing neuronal progenitor development and neuronal migration in the developing cerebral cortex. Cereb Cortex. 2011;21:1465–74. doi: 10.1093/cercor/bhq197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med. 2011;364:1533–43. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanen JP, Saha N, Nikolov DB. Cell-cell signaling via Eph receptors and ephrins. Curr Opin Cell Biol. 2007;19(5):534–42. doi: 10.1016/j.ceb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K, Yoshioka H, Kihara M, Hasegawa K, Sakamoto T, et al. Inhibiting neuronal migration by blocking NMDA receptors in the embryonic rat cerebral cortex: a tissue culture study. Brain Res Dev Brain Res. 1999;114(1):63–67. doi: 10.1016/s0165-3806(99)00019-x. [DOI] [PubMed] [Google Scholar]
- Hirata T, Kumada T, Kawasaki T, Furukawa T, Aiba A, et al. Guidepost neurons for the lateral olfactory tract: expression of metabotropic glutamate receptor 1 and innervation by glutamatergic olfactory bulb axons. Dev Neurobiol. 2012;72:1559–76. doi: 10.1002/dneu.22030. [DOI] [PubMed] [Google Scholar]
- Hirschberg A, Deng S, Korostylev A, Paldy E, Costa MR, et al. Gene deletion mutants reveal a role for semaphorin receptors of the plexin-B family in mechanisms underlying corticogenesis. Mol Cell Biol. 2010;30:764–80. doi: 10.1128/MCB.01458-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoh RA, Stowe TR, Turk E, Stearns T. Transcriptional program of ciliated epithelial cells reveals new cilium and centrosome components and links to human disease. PLoS ONE. 2012;7:e52166. doi: 10.1371/journal.pone.0052166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SE, Shugart YY, Huang DT, Shawan SA, Grant PE, et al. Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat Genet. 2000;26(1):93–96. doi: 10.1038/79246. [DOI] [PubMed] [Google Scholar]
- Howell BW, Herrick TM, Cooper JA. Reelin-induced tyrosine phosphorylation of disabled 1 during neuronal positioning. Genes Dev. 1999;13:643–48. doi: 10.1101/gad.13.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BW, Herrick TM, Hildebrand JD, Zhang Y, Cooper JA. Dab1 tyrosine phosphorylation sites relay positional signals during mouse brain development. Curr Biol. 2000;10:877–85. doi: 10.1016/s0960-9822(00)00608-4. [DOI] [PubMed] [Google Scholar]
- Hu H. Chemorepulsion of neuronal migration by Slit2 in the developing mammalian forebrain. Neuron. 1999;23(4):703–11. doi: 10.1016/s0896-6273(01)80029-5. [DOI] [PubMed] [Google Scholar]
- Hu H, Li J, Gagen CS, Gray NW, Zhang Z, et al. Conditional knockout of protein O-mannosyltransferase 2 reveals tissue-specific roles of O-mannosyl glycosylation in brain development. J Comp Neurol. 2011;519:1320–37. doi: 10.1002/cne.22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Magdaleno S, Hopkins R, Slaughter C, Curran T, Keshvara L. Tyrosine phosphorylated Disabled 1 recruits Crk family adapter proteins. Biochem Biophys Res Commun. 2004;318:204–12. doi: 10.1016/j.bbrc.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Humbert S, Dhavan R, Tsai L. p39 activates cdk5 in neurons, and is associated with the actin cytoskeleton. J Cell Sci. 2000;113(Pt. 6):975–83. doi: 10.1242/jcs.113.6.975. [DOI] [PubMed] [Google Scholar]
- Imai F, Hirai S, Akimoto K, Koyama H, Miyata T, et al. Inactivation of aPKCλ results in the loss of adherens junctions in neuroepithelial cells without affecting neurogenesis in mouse neocortex. Development. 2006;133(9):1735–44. doi: 10.1242/dev.02330. [DOI] [PubMed] [Google Scholar]
- Ishizuka K, Kamiya A, Oh EC, Kanki H, Seshadri S, et al. DISC1-dependent switch from progenitor proliferation to migration in the developing cortex. Nature. 2011;473:92–96. doi: 10.1038/nature09859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Kawasaki T, Takashima S, Matsuda I, Aiba A, Hirata T. Semaphorin 3F confines ventral tangential migration of lateral olfactory tract neurons onto the telencephalon surface. J Neurosci. 2008;28:4414–22. doi: 10.1523/JNEUROSCI.0372-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Moriyama Y, Hasegawa T, Endo TA, Toyoda T, Gotoh Y. Scratch regulates neuronal migration onset via and epithelial-mesenchymal transition-like mechanism. Nat Neurosci. 2013;16(4):416–25. doi: 10.1038/nn.3336. [DOI] [PubMed] [Google Scholar]
- Jaglin XH, Poirier K, Saillour Y, Buhler E, Tian G, et al. Mutations in the β-tubulin gene TUBB2B result in asymmetrical polymicrogyria. Nat Genet. 2009;41:746–52. doi: 10.1038/ng.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson LC, Louhivuori L, Wigren HK, Nordström T, Louhivuori V, et al. Effect of glutamate receptor antagonists on migrating neural progenitor cells. Eur J Neurosci. 2013;37(9):1369–82. doi: 10.1111/ejn.12152. [DOI] [PubMed] [Google Scholar]
- Jensen LJ, Kuhn M, Stark M, Chaffron S, Creevey C, et al. STRING 8—a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res. 2009;37:D412–16. doi: 10.1093/nar/gkn760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jossin Y, Cooper JA. Reelin, Rap1 and N-cadherin orient the migration of multipolar neurons in the developing neocortex. Nat Neurosci. 2011;14:697–703. doi: 10.1038/nn.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jossin Y, Goffinet AM. Reelin signals through phosphatidylinositol 3-kinase and Akt to control cortical development and through mTor to regulate dendritic growth. Mol Cell Biol. 2007;27:7113–24. doi: 10.1128/MCB.00928-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju XD, Guo Y, Wang NN, Huang Y, Lai MM, et al. Both myosin-10 isoforms are required for radial neuronal migration in the developing cerebral cortex. Cereb Cortex. 2013 doi: 10.1093/cercor/bhs407. In press. [DOI] [PubMed] [Google Scholar]
- Kahn RA, Volpicelli-Daley L, Bowzard B, Shrivastava-Ranjan P, Li Y, et al. Arf family GTPases: roles in membrane traffic and microtubule dynamics. Biochem Soc Trans. 2005;33:1269–72. doi: 10.1042/BST0331269. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Tan PL, Kubo K, Engelhard C, Ishizuka K, et al. Recruitment of PCM1 to the centrosome by the cooperative action of DISC1 and BBS4: a candidate for psychiatric illnesses. Arch Gen Psychiatr. 2008;65:996–1006. doi: 10.1001/archpsyc.65.9.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya A, Tomoda T, Chang J, Takaki M, Zhan C, et al. DISC1-NDEL1/NUDEL protein interaction, an essential component for neurite outgrowth, is modulated by genetic variations of DISC1. Hum Mol Genet. 2006;15:3313–23. doi: 10.1093/hmg/ddl407. [DOI] [PubMed] [Google Scholar]
- Kanakry CG, Li Z, Nakai Y, Sei Y, Weinberger DR. Neuregulin-1 regulates cell adhesion via an ErbB2/phosphoinositide-3 kinase/Akt-dependent pathway: potential implications for schizophrenia and cancer. PLoS ONE. 2007;2(12):e1369. doi: 10.1371/journal.pone.0001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000;28(1):27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappeler C, Saillour Y, Baudoin JP, Tuy FPD, Alvarez C, et al. Branching and nucleokinesis defects in migrating interneurons derived from doublecortin knockout mice. Hum Mol Genet. 2006;15:1387–400. doi: 10.1093/hmg/ddl062. [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, Simon TJ, Gogos JA. 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci. 2010;11(6):402–16. doi: 10.1038/nrn2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T, Ito K, Hirata T. Netrin 1 regulates ventral tangential migration of guidepost neurons in the lateral olfactory tract. Development. 2006;133:845–53. doi: 10.1242/dev.02257. [DOI] [PubMed] [Google Scholar]
- Kawauchi T, Sekine K, Shikanai M, Chihama K, Tomita K, et al. Rab GTPases-dependent endocytic pathways regulate neuronal migration and maturation through N-cadherin trafficking. Neuron. 2010;67:588– 602. doi: 10.1016/j.neuron.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Keays DA, Tian G, Poirier K, Huang GJ, Siebold C, et al. Mutations in α-tubulin cause abnormal neuronal migration in mice and lissencephaly in humans. Cell. 2007;128:45–57. doi: 10.1016/j.cell.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholmanskikh SS, Koeller HB, Wynshaw-Boris A, Gomez T, Letourneau PC, Ross ME. Calcium-dependent interaction of Lis1 with IQGAP1 and Cdc42 promotes neuronal motility. Nat Neurosci. 2006;9:50–57. doi: 10.1038/nn1619. [DOI] [PubMed] [Google Scholar]
- Ko J, Humbert S, Bronson RT, Takahashi S, Kulkarni AB, et al. p35 and p39 are essential for cyclin-dependent kinase 5 function during neurodevelopment. J Neurosci. 2001;21:6758–71. doi: 10.1523/JNEUROSCI.21-17-06758.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi H, Tanaka T, Gleeson JG. doublecortin-like kinase functions with doublecortin to mediate fiber tract decussation and neuronal migration. Neuron. 2006;49(1):55–66. doi: 10.1016/j.neuron.2005.10.040. [DOI] [PubMed] [Google Scholar]
- Kolodkin AL, Tessier-Lavigne M. Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb Perspect Biol. 2011;3:895–910. doi: 10.1101/cshperspect.a001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro H, Kumada T. Ca2+ transients control CNS neuronal migration. Cell Calcium. 2005;37(5):387–93. doi: 10.1016/j.ceca.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Modulation of neuronal migration by NMDA receptors. Science. 1993;260(5104):95–97. doi: 10.1126/science.8096653. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Intracellular Ca2+ fluctuations modulate the rate of neuronal migration. Neuron. 1996;17(2):275–85. doi: 10.1016/s0896-6273(00)80159-2. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Orchestration of neuronal migration by activity of ion channels, neurotransmitter receptors, and intracellular Ca2+ fluctuations. J Neurobiol. 1998;37(1):110–30. [PubMed] [Google Scholar]
- Kouchi Z, Igarashi T, Shibayama N, Inanobe S, Sakurai K, et al. Phospholipase Cδ3 regulates RhoA/Rho kinase signaling and neurite outgrowth. J Biol Chem. 2011;286:8459–71. doi: 10.1074/jbc.M110.171223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS. A microRNA array reveals extensive regulation of microRNAs during brain development. RNA. 2003;9(10):1274–81. doi: 10.1261/rna.5980303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein AR, Noctor SC. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci. 2004;27:392–99. doi: 10.1016/j.tins.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Kriegstein AR, Noctor S, Martínez-Cerdeño V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat Rev Neurosci. 2006;7:883–90. doi: 10.1038/nrn2008. [DOI] [PubMed] [Google Scholar]
- Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3(7):475–86. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- Kumada T, Komuro H. Completion of neuronal migration regulated by loss of Ca2+ transients. Proc Natl Acad Sci USA. 2004;101(22):8479–84. doi: 10.1073/pnas.0401000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo G, Arnaud L, Kronstad-O’Brien P, Cooper JA. Absence of Fyn and Src causes a reeler-like phenotype. J Neurosci. 2005;25:8578–86. doi: 10.1523/JNEUROSCI.1656-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Lam MMS, Krsnik Ž, Kawasawa YI, Lefebvre V, Šestan N. SOX5 postmitotically regulates migration, postmigratory differentiation, and projections of subplate and deep-layer neocortical neurons. Proc Natl Acad Sci USA. 2008;105:16021–26. doi: 10.1073/pnas.0806791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Šestan N, Anton ES. Transcriptional co-regulation of neuronal migration and laminar identity in the neocortex. Development. 2012;139:1535–46. doi: 10.1242/dev.069963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YT, Tsai LH. A novel disruption of cortical development in p35−/− mice distinct from reeler. J Comp Neurol. 1998;395:510–22. doi: 10.1002/(sici)1096-9861(19980615)395:4<510::aid-cne7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12(9):735–39. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- Lai T, Jabaudon D, Molyneaux BJ, Azim E, Arlotta P, et al. SOX5 controls the sequential generation of distinct cortifugal neuron subtypes. Neuron. 2008;57:232–47. doi: 10.1016/j.neuron.2007.12.023. [DOI] [PubMed] [Google Scholar]
- Lai Wing Sun K, Correia JP, Kennedy TE. Netrins: versatile extracellular cues with diverse functions. Development. 2011;138(11):2153–69. doi: 10.1242/dev.044529. [DOI] [PubMed] [Google Scholar]
- LaMonica B, Lui JH, Wang X, Kriegstein AR. OSVZ progenitors in the human cortex: an updated perspective on neurodevelopmental disease. Curr Opin Neurobiol. 2012;22:747–53. doi: 10.1016/j.conb.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavdas AA, Grigoriou M, Pachnis V, Parnavelas JG. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J Neurosci. 1999;19:7881–88. doi: 10.1523/JNEUROSCI.19-18-07881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TN, Du G, Fonseca M, Zhou QP, Wigle JT, Eisenstat DD. Dlx homeobox genes promote cortical interneuron migration from the basal forebrain by direct repression of the semaphorin receptor neuropilin-2. J Biol Chem. 2007;282(26):19071–81. doi: 10.1074/jbc.M607486200. [DOI] [PubMed] [Google Scholar]
- Lee JE, Gleeson JG. Cilia in the nervous system: linking cilia function and neurodevelopmental disorders. Curr Opin Neurobiol. 2011;24:98–105. doi: 10.1097/WCO.0b013e3283444d05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemhuis J, Bock HH. Reelin modulates cytoskeletal organization by regulating Rho GTPases. Commun Integr Biol. 2011;4(3):254–57. doi: 10.4161/cib.4.3.14890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex. Nature. 2002;417:645–49. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- Li H, Chou SJ, Hamasaki T, Perez-Garcia CG, O’Leary DD. Neuregulin repellent signaling via ErbB4 restricts GABAergic interneurons to migratory paths from ganglionic eminence to cortical destinations. Neural Dev. 2012;7:10. doi: 10.1186/1749-8104-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lee WL, Cooper JA. NudEL targets dynein to microtubule ends through LIS1. Nat Cell Biol. 2005;7(7):686–90. doi: 10.1038/ncb1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Jin Z, Koirala S, Bu L, Xu L, et al. GPR56 regulates pial basement membrane integrity and cortical lamination. J Neurosci. 2008;28:5817–26. doi: 10.1523/JNEUROSCI.0853-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wei Q, Zhang Y, Ling K, Hu J. The small GTPases ARL-13 and ARL-3 coordinate intraflagellar transport and ciliogenesis. J Cell Biol. 2010;189(6):1039–51. doi: 10.1083/jcb.200912001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liodis P, Denaxa M, Grigoriou M, Akufo-Addo C, Yanagawa Y, Pachnis V. Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J Neurosci. 2007;27(12):3078–89. doi: 10.1523/JNEUROSCI.3055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BP, Strittmatter SM. Semaphorin-mediated axonal guidance via Rho-related G proteins. Curr Opin Cell Biol. 2001;13(5):619–26. doi: 10.1016/s0955-0674(00)00260-x. [DOI] [PubMed] [Google Scholar]
- Liu X, Sun L, Torii M, Rakic P. Connexin 43 controls the multipolar phase of neuronal migration to the cerebral cortex. Proc Natl Acad Sci USA. 2012;109(21):8280–85. doi: 10.1073/pnas.1205880109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodato S, Rouaux C, Quast KB, Jantrachotechatchawan C, Studer M, et al. Excitatory projection neuron subtypes control the distribution of local inhibitory interneurons in the cerebral cortex. Neuron. 2011;69:763–79. doi: 10.1016/j.neuron.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loktev AV, Zhang Q, Beck JS, Searby CC, Scheetz TE, et al. A BBSome subunit links ciliogenesis, microtubule stability, and acetylation. Dev Cell. 2008;15(6):854–65. doi: 10.1016/j.devcel.2008.11.001. [DOI] [PubMed] [Google Scholar]
- López-Bendito G, Cautinat A, Sánchez JA, Bielle F, Flames N, et al. Tangential neuronal migration controls axon guidance: a role for neuregulin-1 in thalamocortical axon navigation. Cell. 2006;125:127–42. doi: 10.1016/j.cell.2006.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bendito G, Luján R, Shigemoto R, Ganter P, Paulsen O, Molnár Z. Blockade of GABAB receptors alters the tangential migration of cortical neurons. Cereb Cortex. 2003;13(9):932–42. doi: 10.1093/cercor/13.9.932. [DOI] [PubMed] [Google Scholar]
- LoTurco JJ, Bai J. The multipolar stage and disruptions in neuronal migration. Trends Neurosci. 2006;29(7):407–13. doi: 10.1016/j.tins.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Louvi A, Grove EA. Cilia in the CNS: a quiet organelle claims center stage. Neuron. 2011;69:1046–60. doi: 10.1016/j.neuron.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel G, Fiondella CG, Covey MV, Lu DD, LoTurco JJ, Ballas N. Repressor element 1 silencing transcription factor (REST) controls radial migration and temporal neuronal specification during neocortical development. Proc Natl Acad Sci USA. 2011;108(40):16789–94. doi: 10.1073/pnas.1113486108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns RP, Cook GM, Holt CE, Keynes RJ. Differing semaphorin 3A concentrations trigger distinct signaling mechanisms in growth cone collapse. J Neurosci. 2012;32(25):8554–59. doi: 10.1523/JNEUROSCI.5964-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcorelles P, Laquerrière A, Adde-Michel C, Marret S, Saugier-Veber P, et al. Evidence for tangential migration disturbances in human lissencephaly resulting from a defect in LIS1, DCX and ARX genes. Acta Neuropathol. 2010;120:503–15. doi: 10.1007/s00401-010-0692-z. [DOI] [PubMed] [Google Scholar]
- Marillat V, Cases O, Nguyen-Ba-Charvet KT, Tessier-Lavigne M, Sotelo C, Chédotal A. Spatiotemporal expression patterns of slit and robo genes in the rat brain. J Comp Neurol. 2002;442(2):130–55. doi: 10.1002/cne.10068. [DOI] [PubMed] [Google Scholar]
- Marín O, Plump AS, Flames N, Sánchez-Camacho C, Tessier-Lavigne M, Rubenstein JLR. Directional guidance of interneuron migration to the cerebral cortex relies on subcortical Slit1/2-independent repulsion and cortical attraction. Development. 2003;130(9):1889–901. doi: 10.1242/dev.00417. [DOI] [PubMed] [Google Scholar]
- Marín O, Rubenstein JLR. A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci. 2001;2:780–90. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- Marín O, Rubenstein JLR. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–83. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- Marín O, Valiente M, Ge X, Tsai L-H. Guiding neuronal cell migrations. Cold Spring Harb Perspect Biol. 2010;2:a001834. doi: 10.1101/cshperspect.a001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín O, Yaron A, Bagri A, Tessier-Lavigne M, Rubenstein JLR. Sorting of striatal and cortical interneurons regulated by semaphorin-neuropilin interactions. Science. 2001;293:872–75. doi: 10.1126/science.1061891. [DOI] [PubMed] [Google Scholar]
- Martínez-Cerdeño V, Noctor SC, Kriegstein AR. The role of intermediate progenitor cells in the evolutionary expansion of the cerebral cortex. Cereb Cortex. 2006;16(Suppl 1):i152–61. doi: 10.1093/cercor/bhk017. [DOI] [PubMed] [Google Scholar]
- Massinen S, Hokkanen ME, Matsson H, Tammimies K, Tapia-Páez I, et al. Increased expression of the dyslexia candidate gene DCDC2 affects length and signaling of primary cilia in neurons. PLoS ONE. 2011;6:e20580. doi: 10.1371/journal.pone.0020580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty JH, Lacy-Hulbert A, Charest A, Bronson RT, Crowley D, et al. Selective ablation of αV integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2005;132(1):165–76. doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- McEvilly RJ, Ortiz de Diaz M, Schonemann MD, Hooshmand F, Rosenfeld MG. Transcriptional regulation of cortical neuron migration by POU domain factors. Science. 2002;295:1528–32. doi: 10.1126/science.1067132. [DOI] [PubMed] [Google Scholar]
- McKenna WL, Betancourt J, Larkin KA, Abrams B, Guo C, et al. Tbr1 and Fezf2 regulate alternate corticofugal neuronal identities during neocortical development. J Neurosci. 2011;31:549–64. doi: 10.1523/JNEUROSCI.4131-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin HS, Fineberg SK, Ghosh LL, Tecedor L, Davidson BL. Dicer is required for proliferation, viability, migration and differentiation in corticoneurogenesis. Neuroscience. 2012;223:285–95. doi: 10.1016/j.neuroscience.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus MF, Nasrallah IM, Gopal PP, Baek WS, Golden JA. Axon mediated interneuron migration. J Neuropathol Exp Neurol. 2004;63:932–41. doi: 10.1093/jnen/63.9.932. [DOI] [PubMed] [Google Scholar]
- Meechan DW, Tucker ES, Maynard TM, LaMantia AS. Diminished dosage of 22q11 genes disrupts neurogenesis and cortical development in a mouse model of 22q11 deletion/DiGeorge syndrome. Proc Natl Acad Sci USA. 2009;106(38):16434–45. doi: 10.1073/pnas.0905696106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meechan DW, Tucker ES, Maynard TM, LaMantia AS. Cxcr4 regulation of interneuron migration is disrupted in 22q11.2 deletion syndrome. Proc Natl Acad Sci USA. 2012;109:18601–6. doi: 10.1073/pnas.1211507109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métin C, Baudoin JP, Rakić S, Parnavelas JG. Cell and molecular mechanisms involved in the migration of cortical interneurons. Eur J Neurosci. 2006;23:894–900. doi: 10.1111/j.1460-9568.2006.04630.x. [DOI] [PubMed] [Google Scholar]
- Métin C, Denizot JP, Ropert N. Intermediate zone cells express calcium-permeable AMPA receptors and establish close contact with growing axons. J Neurosci. 2000;20(2):696–708. doi: 10.1523/JNEUROSCI.20-02-00696.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingorance-Le Meur A, Zheng B, Soriano E, del Río JA. Involvement of the myelin-associated inhibitor Nogo-A in early cortical development and neuronal maturation. Cereb Cortex. 2007;17:2375–86. doi: 10.1093/cercor/bhl146. [DOI] [PubMed] [Google Scholar]
- Minobe S, Sakakibara A, Ohdachi T, Kanda R, Kimura M, et al. Rac is involved in the interkinetic nuclear migration of cortical progenitor cells. Neurosci Res. 2009;63:294–301. doi: 10.1016/j.neures.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Miska EA, Alvarez-Saavedra E, Townsend M, Yoshii A, Šestan N, et al. Microarray analysis of microRNA expression in the developing mammalian brain. Genome Biol. 2004;5(9):R68. doi: 10.1186/gb-2004-5-9-r68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–74. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- Miyoshi G, Butt SJ, Takebayashi H, Fishell G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J Neurosci. 2007;27:7786–98. doi: 10.1523/JNEUROSCI.1807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Fishell G. GABAergic interneuron lineages selectively sort into specific cortical layers during early postnatal development. Cereb Cortex. 2011;21:845–52. doi: 10.1093/cercor/bhq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Fishell G. Dynamic FoxG1 expression coordinates the integration of multipolar pyramidal neuron precursors into the cortical plate. Neuron. 2012;74(6):1045–58. doi: 10.1016/j.neuron.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa VH, Butt SJ, et al. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci. 2010;30:1582–94. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar Z, Garel S, López-Bendito G, Maness P, Price DJ. Mechanisms controlling the guidance of thalamocortical axons through the embryonic forebrain. Eur J Neurosci. 2012;35:1573–85. doi: 10.1111/j.1460-9568.2012.08119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SA, Saito F, Chen J, Michele DE, Henry MD, et al. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature. 2002;418:422–25. doi: 10.1038/nature00838. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Alifragis P, Wong RO, Parnavelas JG. Ventricle-directed migration in the developing cerebral cortex. Nat Neurosci. 2002;5:218–24. doi: 10.1038/nn813. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Brunstrom JE, Grutzendler J, Wong ROL, Pearlman AL. Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci. 2001;4:143–50. doi: 10.1038/83967. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Parnavelas JG. Modes of neuronal migration in the developing cerebral cortex. Nat Rev Neurosci. 2002;3:423–32. doi: 10.1038/nrn845. [DOI] [PubMed] [Google Scholar]
- Nagano T, Morikubo S, Sato M. Filamin A and FILIP (Filamin A-Interacting Protein) regulate cell polarity and motility in neocortical subventricular and intermediate zones during radial migration. J Neurosci. 2004;24:9648–57. doi: 10.1523/JNEUROSCI.2363-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci. 2002;5:1279–87. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- Niethammer M, Smith DS, Ayala R, Peng J, Ko J, et al. NUDEL is a novel Cdk5 substrate that associates with LIS1 and cytoplasmic dynein. Neuron. 2000;28:697–711. doi: 10.1016/s0896-6273(00)00147-1. [DOI] [PubMed] [Google Scholar]
- Niklasson L, Rasmussen P, Óskarsdóttir S, Gillberg C. Autism, ADHD, mental retardation and behavior problems in 100 individuals with 22q11 deletion syndrome. Res Dev Disabil. 2009;30:763–73. doi: 10.1016/j.ridd.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Ning Y, Sun Q, Dong Y, Zu W, Zhang W, et al. Slit2-N inhibits PDGF-induced migration in rat airway smooth muscle cells: WASP and Arp2/3 involved. Toxicology. 2011;283(1):32–40. doi: 10.1016/j.tox.2011.01.026. [DOI] [PubMed] [Google Scholar]
- Niquille M, Garel S, Mann F, Hornung JP, Otsmane B, et al. Transient neuronal populations are required to guide callosal axons: a role for semaphorin 3C. PLoS Biol. 2009;7:e1000230. doi: 10.1371/journal.pbio.1000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niquille M, Minocha S, Hornung JP, Rufer N, Valloton D, et al. Two specific populations of GABAergic neurons originating from the medial and the caudal ganglionic eminences aid in proper navigation of callosal axons. Dev Neurobiol. 2013;73:647–72. doi: 10.1002/dneu.22075. [DOI] [PubMed] [Google Scholar]
- Nishimura D. A view from the web: BioCarta. Biotechnol Softw Internet Rep. 2001;2(3):117–21. [Google Scholar]
- Nóbrega-Pereira S, Kessaris N, Du T, Kimura S, Anderson SA, Marín O. Postmitotic Nkx2-1 controls the migration of telencephalic interneurons by direct repression of guidance receptors. Neuron. 2008;59(5):733– 45. doi: 10.1016/j.neuron.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman BS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–20. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martínez-Cerdeño V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–44. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martínez-Cerdeño V, Kriegstein AR. Contribution of intermediate progenitor cells to cortical histogenesis. Arch Neurol. 2007;64:639–42. doi: 10.1001/archneur.64.5.639. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martínez-Cerdeño V, Kriegstein AR. Distinct behaviors of neural stem and progenitor cells underlie cortical neurogenesis. J Comp Neurol. 2008;508:28–44. doi: 10.1002/cne.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Holmberg J, Frisen J, Osumi N. Pax6-dependent boundary defines alignment of migrating olfactory cortex neurons via the repulsive activity of ephrin A5. Development. 2006;133:1335–45. doi: 10.1242/dev.02290. [DOI] [PubMed] [Google Scholar]
- Noren NK, Pasquale EB. Eph receptor-ephrin bidirectional signals that target Ras and Rho proteins. Cell Signal. 2004;16(6):655–66. doi: 10.1016/j.cellsig.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Nott A, Nitarska J, Veenvliet JV, Schacke S, Derijck AA, et al. S-nitrosylation of HDAC2 regulates the expression of the chromatin-remodeling factor Brm during radial neuron migration. Proc Natl Acad Sci USA. 2013;110(8):3113–18. doi: 10.1073/pnas.1218126110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Miyata T, Nakajuma K, Yagyu K, Seike M, et al. The reeler gene-associated antigen on Cajal- Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron. 1995;14:899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- Ohshima T, Hirasawa M, Tabata H, Mutoh T, Adachi T, et al. Cdk5 is required for multipolar-to-bipolar transition during radial neuronal migration and proper dendrite development of pyramidal neurons in the cerebral cortex. Development. 2007;134(12):2273–82. doi: 10.1242/dev.02854. [DOI] [PubMed] [Google Scholar]
- Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna, et al. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci USA. 1996;93:11173–78. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacary E, Heng J, Azzarelli R, Riou P, Castro D, et al. Proneural transcription factors regulate different steps of cortical neuron migration through Rnd-mediated inhibition of RhoA signaling. Neuron. 2011;69(6):1069–84. doi: 10.1016/j.neuron.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parras CM, Schuurmans C, Scardigli R, Kim J, Anderson DJ, Guillemot F. Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity. Genes Dev. 2002;16:324–38. doi: 10.1101/gad.940902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ. Getting neural circuits into shape with semaphorins. Nat Rev Neurosci. 2012;13(9):605–18. doi: 10.1038/nrn3302. [DOI] [PubMed] [Google Scholar]
- Paul V, Tonchev AB, Henningfeld KA, Pavlakis E, Rust B, et al. Scratch2 modulates neurogenesis and cell migration through antagonism of bHLH proteins in the developing neocortex. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs356. In press. [DOI] [PubMed] [Google Scholar]
- Pawlisz AS, Feng Y. Three-dimensional regulation of radial glial functions by Lis1-Nde1 and dystrophin glycoprotein complexes. PLoS Biol. 2011;9:e1001172. doi: 10.1371/journal.pbio.1001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–46. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–34. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Piao X, Chang BS, Bodell A, Woods K, Benzeev B, et al. Genotype-phenotype analysis of human frontoparietal polymicrogyria syndromes. Ann Neurol. 2005;58:680–87. doi: 10.1002/ana.20616. [DOI] [PubMed] [Google Scholar]
- Pilz DT, Kuc J, Matsumoto N, Bodurtha J, Bernadi B, et al. Subcortical band heterotopia in rare affected males can be caused by missense mutations in DCX (XLIS) or LIS1. Hum Mol Genet. 1999;8:1757–60. doi: 10.1093/hmg/8.9.1757. [DOI] [PubMed] [Google Scholar]
- Pitulescu ME, Adams RH. Eph/ephrin molecules—a hub for signaling and endocytosis. Genes Dev. 2010;24(22):2480–92. doi: 10.1101/gad.1973910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier K, Keays DA, Francis F, Saillour Y, Bahi N, et al. Large spectrum of lissencephaly and pachygyria phenotypes resulting from de novo missense mutations in tubulin alpha 1A (TUBA1A) Hum Mutat. 2007;28:1055–64. doi: 10.1002/humu.20572. [DOI] [PubMed] [Google Scholar]
- Poitras L, Ghanem N, Hatch G, Ekker M. The proneural determinant MASH1 regulates forebrain Dlx1/2 expression through the I12b intergenic enhancer. Development. 2007;134:1755–65. doi: 10.1242/dev.02845. [DOI] [PubMed] [Google Scholar]
- Poliakov A, Cotrina M, Wilkinson DG. Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev Cell. 2004;7(4):465–80. doi: 10.1016/j.devcel.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Polleux F, Whitford KL, Dijkhuizen PA, Vitalis T, Ghosh A. Control of cortical interneuron migration by neurotrophins and PI3-kinase signaling. Development. 2002;129:3147–60. doi: 10.1242/dev.129.13.3147. [DOI] [PubMed] [Google Scholar]
- Poluch S, Juliano SL. A normal radial glial scaffold is necessary for migration of interneurons during neocortical development. Glia. 2007;55:822–30. doi: 10.1002/glia.20488. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Turner JA, Guffanti G, Lakatos A, Fallon JH, et al. A genome-wide association study of schizophrenia using brain activation as a quantitative phenotype. Schizophr Bull. 2009;35:96–108. doi: 10.1093/schbul/sbn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell EM, Mars WM, Levitt P. Hepatocyte growth factor/scatter factor is a motogen for interneurons migrating from the ventral to dorsal telencephalon. Neuron. 2001;30:79–89. doi: 10.1016/s0896-6273(01)00264-1. [DOI] [PubMed] [Google Scholar]
- Pozas E, Ibáñez CF. GDNF and GFRα1 promote differentiation and tangential migration of cortical GABAergic neurons. Neuron. 2005;45:701–13. doi: 10.1016/j.neuron.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Pramparo T, Libiger O, Jain S, Li H, Youn YH, et al. Global developmental gene expression and pathway analysis of normal brain development and mouse models of human neuronal migration defects. PLoS Genet. 2011;7(3):e1001331. doi: 10.1371/journal.pgen.1001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puehringer D, Orel N, Lüningschrör P, Subramanian N, Herrmann T, et al. EGF transactivation of Trk receptors regulates the migration of newborn cortical neurons. Nat Neurosci. 2013;16:407–15. doi: 10.1038/nn.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Zhang CL. Role of Krüppel-like factor 4 in neurogenesis and radial neuronal migration in the developing cerebral cortex. Mol Cell Biol. 2012;32(21):4297–305. doi: 10.1128/MCB.00838-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61– 83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Rakić S, Yanagawa Y, Obata K, Faux C, Parnavelas JG, Nikolić M. Cortical interneurons require p35/Cdk5 for their migration and laminar organization. Cereb Cortex. 2009;19:1857–69. doi: 10.1093/cercor/bhn213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper JA. Semaphorins and their receptors in vertebrates and invertebrates. Curr Opin Neurobiol. 2000;10(1):88–94. doi: 10.1016/s0959-4388(99)00057-4. [DOI] [PubMed] [Google Scholar]
- Rhee J, Buchan T, Zukerberg L, Lilien J, Balsamo J. Cables links Robo-bound Abl kinase to N-cadherin-bound β-catenin to mediate Slit-induced modulation of adhesion and transcription. Nat Cell Biol. 2007;9(8):883–89. doi: 10.1038/ncb1614. [DOI] [PubMed] [Google Scholar]
- Riccio O, Potter G, Walzer C, Vallet P, Szabó G, et al. Excess of serotonin affects embryonic interneuron migration through activation of the serotonin receptor 6. Mol Psychiatr. 2009;14:280–90. doi: 10.1038/mp.2008.89. [DOI] [PubMed] [Google Scholar]
- Rico B, Marín O. Neuregulin signaling, cortical circuitry development and schizophrenia. Curr Opin Genet Dev. 2011;21(3):262–70. doi: 10.1016/j.gde.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Rodger J, Salvatore L, Migani P. Should I stay or should I go? Ephs and ephrins in neuronal migration. Neurosignals. 2012;20(3):190–201. doi: 10.1159/000333784. [DOI] [PubMed] [Google Scholar]
- Rosenfeld JA, Ballif BC, Torchia BS, Sahoo T, Ravnan JB, et al. Copy number variations associated with autism spectrum disorders contribute to a spectrum of neurodevelopmental disorders. Genet Med. 2010;12:694–702. doi: 10.1097/GIM.0b013e3181f0c5f3. [DOI] [PubMed] [Google Scholar]
- Ruat M, Roudaut H, Ferent J, Traiffort E. Hedgehog trafficking, cilia and brain functions. Differentiation. 2012;83:S97–104. doi: 10.1016/j.diff.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Rudolph J, Zimmer G, Steinecke A, Barchmann S, Bolz J. Ephrins guide migrating cortical interneurons in the basal telencephalon. Cell Adhes Migr. 2010;4:400–8. doi: 10.4161/cam.4.3.11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol. 2011;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymar VV, Sadikot AF. Laminar fate of cortical GABAergic interneurons is dependent on both birthdate and phenotype. J Comp Neurol. 2007;501:369–80. doi: 10.1002/cne.21250. [DOI] [PubMed] [Google Scholar]
- Sánchez-Alcañiz JA, Haege S, Mueller W, Pla R, Mackay F, et al. Cxcr7 controls neuronal migration by regulating chemokine responsiveness. Neuron. 2011;69:77–90. doi: 10.1016/j.neuron.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Sapir T, Elbaum M, Reiner O. Reduction of microtubule catastrophe events by LIS1, platelet-activating factor acetylhydrolase subunit. EMBO J. 1997;16:6977–84. doi: 10.1093/emboj/16.23.6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir T, Sapoznik S, Levy T, Finkelshtein D, Shmueli A, et al. Accurate balance of the polarity kinase MARK2/Par-1 is required for proper cortical neuronal migration. J Neurosci. 2008;28:5710–20. doi: 10.1523/JNEUROSCI.0911-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S, Shionoya A, Ishida M, Gambello MJ, Yingling J, et al. A LIS1/NUDEL/cytoplasmic dynein heavy chain complex in the developing and adult nervous system. Neuron. 2000;28:681–96. doi: 10.1016/s0896-6273(00)00146-x. [DOI] [PubMed] [Google Scholar]
- Sato Y, Hirata T, Ogawa M, Fujisawa H. Requirement for early-generated neurons recognized by monoclonal antibody Lot1 in the formation of lateral olfactory tract. J Neurosci. 1998;18:7800–10. doi: 10.1523/JNEUROSCI.18-19-07800.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Nagano T. Involvement of filamin A and filamin A-interacting protein (FILIP) in controlling the start and cell shape of radially migrating cortical neurons. Anat Sci Int. 2005;80:19–29. doi: 10.1111/j.1447-073x.2005.00101.x. [DOI] [PubMed] [Google Scholar]
- Schaar BT, McConnell SK. Cytoskeletal coordination during neuronal migration. Proc Natl Acad Sci USA. 2005;102(38):13652–57. doi: 10.1073/pnas.0506008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid RS, Shelton S, Stanco A, Yokota Y, Kreidberg JA, Anton ES. α3β1 integrin modulates neuronal migration and placement during early stages of cerebral cortical development. Development. 2004;131(24):6023– 31. doi: 10.1242/dev.01532. [DOI] [PubMed] [Google Scholar]
- Sekine K, Kawauchi T, Kubo K, Honda T, Herz J, et al. Reelin controls neuronal positioning by promoting cell-matrix adhesion via inside-out activation of intergrin α5β1. Neuron. 2012;76(2):353–69. doi: 10.1016/j.neuron.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentürk A, Pfennig S, Weiss A, Burk K, Acker-Palmer A. Ephrin Bs are essential components of the Reelin pathway to regulate neuronal migration. Nature. 2011;472:356–60. doi: 10.1038/nature09874. [DOI] [PubMed] [Google Scholar]
- Seshadri S, Kamiya A, Yokota Y, Prikulis I, Kano S, et al. Disrupted-in-Schizophrenia-1 expression is regulated by β-site amyloid precursor protein cleaving enzyme-1-neuregulin cascade. Proc Natl Acad Sci USA. 2010;107:5622–27. doi: 10.1073/pnas.0909284107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen VL, Ganesh VS, Topcu M, Sebire G, Bodell A, et al. Mutations in ARFGEF2 implicate vesicle trafficking in neural progenitor proliferation and migration in the human cerebral cortex. Nat Genet. 2004;36:69–76. doi: 10.1038/ng1276. [DOI] [PubMed] [Google Scholar]
- Shibata M, Kurokawa D, Nakao H, Ohmura T, Aizawa S. MicroRNA-9 modulates Cajal-Retzius cell differentiation by suppressing Foxg1 expression in mouse medial pallium. J Neurosci. 2008;28(41):10415–21. doi: 10.1523/JNEUROSCI.3219-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanai M, Nakajima K, Kawauchi T. N-cadherin regulates radial glial fiber-dependent migration of cortical locomoting neurons. Commun Integr Biol. 2011;4:326–30. doi: 10.4161/cib.4.3.14886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara R, Thumkeo D, Kamijo H, Kaneko N, Sawamoto K, et al. A role for mDia, a Rho-regulated actin nucleator, in tangential migration of interneuron precursors. Nat Neurosci. 2012;15:373–80. S1–2. doi: 10.1038/nn.3020. [DOI] [PubMed] [Google Scholar]
- Shu T, Li Y, Keller A, Richards LJ. The glial sling is a migratory population of developing neurons. Development. 2003;130:2929–37. doi: 10.1242/dev.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu T, Tseng HC, Sapir T, Stern P, Zhou Y, et al. Doublecortin-like kinase controls neurogenesis by regulating mitotic spindles and M phase progression. Neuron. 2006;1:25–39. doi: 10.1016/j.neuron.2005.10.039. [DOI] [PubMed] [Google Scholar]
- Smith DS, Niethammer M, Ayala R, Zhou Y, Ganbello MJ, et al. Regulation of cytoplasmic dynein behavior and microtubule organization by mammalian Lis1. Nat Cell Biol. 2000;2(11):767–75. doi: 10.1038/35041000. [DOI] [PubMed] [Google Scholar]
- Solecki DJ, Model L, Gaetz J, Kapoor TM, Hatten ME. Par6αsignaling controls glial-guided neuronal migration. Nat Neurosci. 2004;7:1195–203. doi: 10.1038/nn1332. [DOI] [PubMed] [Google Scholar]
- Solecki DJ, Trivedi N, Govek EE, Kerekes RA, Gleason SS, Hatten ME. Myosin II motors and F-actin dynamics drive the coordinated movement of the centrosome and some during CBS glial-guided neuronal migration. Neuron. 2009;63(1):63–80. doi: 10.1016/j.neuron.2009.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria JM, Valdeomillos M. Receptor-activated calcium signals in tangentially migrating cortical cells. Cereb Cortex. 2002;12(8):831–39. doi: 10.1093/cercor/12.8.831. [DOI] [PubMed] [Google Scholar]
- Sparrow N, Manetti ME, Bott M, Fabianac T, Petrilli A, et al. The actin-severing protein cofilin is downstream of neuregulin signaling and is essential for Schwann cell myelination. J Neurosci. 2012;32(15):5284–97. doi: 10.1523/JNEUROSCI.6207-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanco A, Szekeres C, Patel N, Rao S, Campbell K, et al. Netrin-1-α3β1 integrin interactions regulate the migration of interneuron through the cortical marginal zone. Proc Natl Acad Sci USA. 2009;106(18):7595– 600. doi: 10.1073/pnas.0811343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Erion JR, Wosiski-Kuhn M. Reelin signaling in development, maintenance, and plasticity of neural networks. Ageing Res Rev. 2013;12:815–22. doi: 10.1016/j.arr.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuckmann I, Weigmann A, Shevchenko A, Mann M, Huttner WB. Ephrin B1 is expressed on neuroepithelial cells in correlation with neocortical neurogenesis. J Neurosci. 2001;21:2726–37. doi: 10.1523/JNEUROSCI.21-08-02726.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm RK, Zhou C, Ara T, Lazarini F, Dubois-Dalcq M, et al. CXCR4 regulates interneuron migration in the developing neocortex. J Neurosci. 2003;23:5123–30. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugitani Y, Nakai S, Minowa O, Nishi M, Jishage K, et al. Brn-1 and Brn-2 share crucial roles in the production and positioning of mouse neocortical neurons. Genes Dev. 2002;16:1760–65. doi: 10.1101/gad.978002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Fei T, Yang T, Zhang F, Chen YG, et al. The suppression of CRMP2 expression by bone morphogenetic protein (BMP)-SMAD gradient signaling controls multiple stages of neuronal development. J Biol Chem. 2010;285:39039–50. doi: 10.1074/jbc.M110.168351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Delgado-Escueta AV, Aguan K, Alonso ME, Shi J, et al. Mutations in EFHC1 cause juvenile myoclonic epilepsy. Nat Genet. 2004;36:842–49. doi: 10.1038/ng1393. [DOI] [PubMed] [Google Scholar]
- Swiercz JM, Kuner R, Behrens J, Offermanns S. Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron. 2002;35(1):51–63. doi: 10.1016/s0896-6273(02)00750-x. [DOI] [PubMed] [Google Scholar]
- Tabata H, Kanatani S, Nakajima K. Differences of migratory behavior between direct progeny of apical progenitors and basal progenitors in the developing cerebral cortex. Cereb Cortex. 2009;19:2092–105. doi: 10.1093/cercor/bhn227. [DOI] [PubMed] [Google Scholar]
- Tabata H, Nakajima K. Multipolar migration: the third mode of radial neuronal migration in the developing cerebral cortex. J Neurosci. 2003;23:9996–10001. doi: 10.1523/JNEUROSCI.23-31-09996.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahirovic S, Hellal F, Neukirchen D, Hindges R, Garvalov BK, et al. Rac1 regulates neuronal polarization through the WAVE complex. J Neurosci. 2010;30:6930–43. doi: 10.1523/JNEUROSCI.5395-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A, O’Leary DD. Radial migration of superficial layer cortical neurons controlled by novel Ig cell adhesion molecule MDGA1. J Neurosci. 2006;26:4460–64. doi: 10.1523/JNEUROSCI.4935-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagnone L, Comoglio PM. To move or not to move? Semaphorin signalling in cell migration. EMBO Rep. 2004;5:356–61. doi: 10.1038/sj.embor.7400114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Fujimori K, Nojyo Y, Kaneko T, Takauji R. Evidence that Sema3a and Sema3f regulate the migration of GABAergic neurons in the developing neocortex. J Comp Neurol. 2003;455:238–48. doi: 10.1002/cne.10476. [DOI] [PubMed] [Google Scholar]
- Tan SS, Kalloniatis M, Sturm K, Tam PP, Reese BE, Faulkner-Jones B. Separate progenitors for radial and tangential cell dispersion during development of the cerebral neocortex. Neuron. 1998;21:295–304. doi: 10.1016/s0896-6273(00)80539-5. [DOI] [PubMed] [Google Scholar]
- Tanaka D, Nakaya Y, Yanagawa Y, Obata K, Murakami F. Multimodal tangential migration of neocortical GABAergic neurons independent of GPI-anchored proteins. Development. 2003;130:5803–13. doi: 10.1242/dev.00825. [DOI] [PubMed] [Google Scholar]
- Tanaka DH, Maekawa K, Yanagawa Y, Obata K, Murakami F. Multidirectional and multizonal tangential migration of GABAergic interneurons in the developing cerebral cortex. Development. 2006;133:2167–76. doi: 10.1242/dev.02382. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Serneo FF, Higgins C, Gambello MJ, Wynshaw-Boris A, Gleeson JG. Lis1 and doublecortin function with dynein to mediate coupling of the nucleus to the centrosome in neuronal migration. J Cell Biol. 2004;165(5):709–21. doi: 10.1083/jcb.200309025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka N, Osumi N, Sato Y, Inoue T, Nakamura S, et al. Neocortical origin and tangential migration of guidepost neurons in the lateral olfactory tract. J Neurosci. 2000;20:5802–12. doi: 10.1523/JNEUROSCI.20-15-05802.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii M, Hashimoto-Torii K, Levitt P, Rakic P. Integration of neuronal clones in the radial cortical columns by EphA and ephrin-A signalling. Nature. 2009;461:524–28. doi: 10.1038/nature08362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyo-Oka K, Sasaki S, Yano Y, Mori D, Kobayashi T, et al. Recruitment of katanin p60 by phosphorylated NDEL1, an LIS1 interacting protein, is essential for mitotic cell division and neuronal migration. Hum Mol Genet. 2005;14:3113–28. doi: 10.1093/hmg/ddi339. [DOI] [PubMed] [Google Scholar]
- Trivedi N, Solecki DJ. Neuronal migration illuminated. A look under the hood of the living neuron. Cell Adhes Migr. 2011;5:42–47. doi: 10.4161/cam.5.1.13609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommsdorf M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, et al. Reller/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Tsai LH, Gleeson JG. Nucleokinesis in neuronal migration. Neuron. 2005;46(3):383–88. doi: 10.1016/j.neuron.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Ohshima T, Sasaki Y, Suzuki H, Yanai S, et al. Semaphorin3A signaling is mediated via sequential Cdk5 and GSK3βphosphorylation of CRMP2: implication of common phosphorylating mechanism underlying axon guidance and Alzheimer’s disease. Genes Cells. 2005;10(2):165–79. doi: 10.1111/j.1365-2443.2005.00827.x. [DOI] [PubMed] [Google Scholar]
- Umeshima H, Hirano T, Kengaku M. Microtubule-based nuclear movement occurs independently of centrosome positioning in migrating neurons. Proc Natl Acad Sci USA. 2007;104(41):16182–87. doi: 10.1073/pnas.0708047104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcanis H, Tan SS. Layer specification of transplanted interneurons in developing mouse neocortex. J Neurosci. 2003;23:5113–22. doi: 10.1523/JNEUROSCI.23-12-05113.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta T, Hausmann G, Basler K. The many faces and functions of β-catenin. EMBO J. 2012;31:2714–36. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente M, Marín O. Neuronal migration mechanisms in development and disease. Curr Opin Neurobiol. 2010;20(1):68–78. doi: 10.1016/j.conb.2009.12.003. [DOI] [PubMed] [Google Scholar]
- van den Berghe V, Stappers E, Vandesande B, Dimidschstein J, Kroes R, et al. Directed migration of cortical interneurons depends on the cell-autonomous action of Sip1. Neuron. 2013;77(1):70–82. doi: 10.1016/j.neuron.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Västrik I, Eickholt BJ, Walsh FS, Ridley A, Doherty P. Sema3A-induced growth-cone collapse is mediated by Rac1 amino acids 17–32. Curr Biol. 1999;9(18):991–98. doi: 10.1016/s0960-9822(99)80447-3. [DOI] [PubMed] [Google Scholar]
- Villar-Cerviño V, Molano-Mazón M, Catchpole T, Valdeolmillos M, Henkemeyer M, et al. Contact repulsion controls the dispersion and final distribution of Cajal-Retzius cells. Neuron. 2013;77(3):457–71. doi: 10.1016/j.neuron.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang I, et al. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456(7221):470–76. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Tsai J-W, LaMonica B, Kriegstein AR. A new subtype of progenitor cell in the mouse embryonic neocortex. Nat Neurosci. 2011;14:555–561. doi: 10.1038/nn.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, He H, Srivastava N, Vikarunnessa S, Chen YB, et al. Plexins are GTPase-activating proteins for Rap and are activated by induced dimerization. Sci Signal. 2012;5(207):ra6. doi: 10.1126/scisignal.2002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li G, Stanco A, Long JE, Crawford D, et al. CXCR4 and CXCR7 have distinct functions in regulating interneuron migration. Neuron. 2011;69:61–76. doi: 10.1016/j.neuron.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Paramasivam M, Thomas A, Bai J, Kaminen-Ahola N, et al. DYX1C1 functions in neuronal migration in developing neocortex. Neuroscience. 2006;143:515–22. doi: 10.1016/j.neuroscience.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Westerlund N, Zdrojewska J, Padzik A, Komulainen E, Björkblom B, et al. Phosphorylation of SCG10/stathmin-2 determines multipolar stage exit and neuronal migration rate. Nat Neurosci. 2011;14(3):305–13. doi: 10.1038/nn.2755. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Garcia-Verdugo JM, Herrera DG, Alvarez-Buylla A. Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nat Neurosci. 1999;2:461–66. doi: 10.1038/8131. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–71. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- Willaredt MA, Hasenpusch-Theil K, Gardner HA, Kitanovic I, Hirschfeld-Warneken VC, et al. A crucial role for primary cilia in cortical morphogenesis. J Neurosci. 2008;28:12887–900. doi: 10.1523/JNEUROSCI.2084-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PM, Fryer RH, Fang Y, Hatten ME. Astn2, a novel member of the astrotactin gene family, regulates the trafficking of ASTN1 during glial-guided neuronal migration. J Neurosci. 2010;30:8529–40. doi: 10.1523/JNEUROSCI.0032-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SL, Wilson JP, Wang C, Wang B, McConnell SK. Primary cilia and Gli3 activity regulate cerebral cortical size. Dev Neurobiol. 2012;72:1196–212. doi: 10.1002/dneu.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;14:305–13. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Taylor L, Welagen J, Mbata IC, Xiang JZ, Anderson SA. A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Dev Biol. 2008;314:127–36. doi: 10.1016/j.ydbio.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong GK, Baudet ML, Norden C, Leung L, Harris WA. Slit1b-Robo3 signaling and N-cadherin regulate apical process retraction in developing retinal ganglion cells. J Neurosci. 2012;32(1):223–28. doi: 10.1523/JNEUROSCI.2596-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K, Ren XR, Huang YZ, Xie Y, Liu G, et al. Signal transduction in neuronal migration: roles of GTPase activating proteins and the small GTPase Cdc42 in the Slit-Robo pathway. Cell. 2001;107(2):209–21. doi: 10.1016/s0092-8674(01)00530-x. [DOI] [PubMed] [Google Scholar]
- Wu W, Wong K, Chen J, Jiang Z, Dupuis S, et al. Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature. 1999;400:331–36. doi: 10.1038/22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Sanada K, Samuels BA, Shih H, Tsai LH. Serine 732 phosphorylation of FAK by Cdk5 is important for microtubule organization, nuclear movement, and neuronal migration. Cell. 2003;114(4):469– 82. doi: 10.1016/s0092-8674(03)00605-6. [DOI] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–22. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M, Hayakawa-Yano Y, Mele A, Darnell RB. Nova2 regulates neuronal migration through an RNA switch in disabled-1 signaling. Neuron. 2010;66(6):848–58. doi: 10.1016/j.neuron.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau HJ, Wang HF, Lai C, Liu FC. Neural development of the neuregulin receptor ErbB4 in the cerebral cortex and the hippocampus: preferential expression by interneurons tangentially migrating from the ganglionic eminences. Cereb Cortex. 2003;13(3):252–64. doi: 10.1093/cercor/13.3.252. [DOI] [PubMed] [Google Scholar]
- Yee KT, Simon HH, Tessier-Lavigne M, O’Leary DM. Extension of long leading processes and neuronal migration in the mammalian brain directed by the chemoattractant netrin-1. Neuron. 1999;24(3):607–22. doi: 10.1016/s0896-6273(00)81116-2. [DOI] [PubMed] [Google Scholar]
- Yokota Y, Eom TY, Stanco A, Kim WY, Rao S, et al. Cdc42 and Gsk3 modulate the dynamics of radial glial growth, inter-radial glial interactions and polarity in the developing cerebral cortex. Development. 2010;137:4101–10. doi: 10.1242/dev.048637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y, Kim WY, Chen Y, Wang X, Stanco A, et al. The adenomatous polyposis coli protein is an essential regulator of radial glial polarity and construction of the cerebral cortex. Neuron. 2009;61(1):42–56. doi: 10.1016/j.neuron.2008.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y, Gashghaei HT, Han C, Watson H, Campbell KJ, Anton ES. Radial glial dependent and independent dynamics of interneuronal migration in the developing cerebral cortex. PLoS ONE. 2007;2:e794. doi: 10.1371/journal.pone.0000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yozu M, Tabata H, Nakajima K. Birth-date dependent alignment of GABAergic neurons occurs in a different pattern from that of non-GABAergic neurons in the developing mouse visual cortex. Neurosci Res. 2004;49:395–403. doi: 10.1016/j.neures.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Yozu M, Tabata H, Nakajima K. The caudal migratory stream: a novel migratory stream of interneurons derived from the caudal ganglionic eminence in the developing mouse forebrain. J Neurosci. 2005;25:7268–77. doi: 10.1523/JNEUROSCI.2072-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Zhou L, Chen JH, Wu JY, Rao Y, Ornitz DM. The mouse SLIT family: secreted ligands for ROBO expressed in patterns that suggest a role in morphogenesis and axon guidance. Dev Biol. 1999;12(2):290–306. doi: 10.1006/dbio.1999.9371. [DOI] [PubMed] [Google Scholar]
- Zhang J, Neal J, Lian G, Shi B, Ferland RJ, Sheen V. Brefeldin A-inhibited guanine exchange factor 2 regulates filamin A phosphorylation and neuronal migration. J Neurosci. 2012;32:12619–29. doi: 10.1523/JNEUROSCI.1063-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Song NN, Chen JY, Huang Y, Li H, Ding YQ. Satb2 is required for dendritic arborization and soma spacing in mouse cerebral cortex. Cereb Cortex. 2012;22(7):1510–19. doi: 10.1093/cercor/bhr215. [DOI] [PubMed] [Google Scholar]
- Zheng C, Heintz N, Hatten ME. CNS gene encoding astrotactin, which supports neuronal migration along glial fibers. Science. 1996;272:417–19. doi: 10.1126/science.272.5260.417. [DOI] [PubMed] [Google Scholar]
- Zheng W, Geng AQ, Li PF, Wang Y, Yuan XB. Robo4 regulates the radial migration of newborn neurons in developing neocortex. Cereb Cortex. 2012;22:2587–601. doi: 10.1093/cercor/bhr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JQ, Poo MM. Calcium signaling in neuronal motility. Annu Rev Cell Dev Biol. 2007;23:375–404. doi: 10.1146/annurev.cellbio.23.090506.123221. [DOI] [PubMed] [Google Scholar]
- Zhou C, Cunningham L, Marcus AI, Li Y, Kahn RA. Arl2 and Arl3 regulate different microtubule-dependent processes. Mol Biol Cell. 2006;17(5):2476–87. doi: 10.1091/mbc.E05-10-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Gunput RA, Pasterkamp RJ. Semaphorin signaling: progress made and promises ahead. Trends Biochem Sci. 2008;33(4):161–70. doi: 10.1016/j.tibs.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Zimmer G, Garcez P, Rudolph J, Niehage R, Weth F, et al. Ephrin-A5 acts as a repulsive cue for migrating cortical interneurons. Eur J Neurosci. 2008;28:62–73. doi: 10.1111/j.1460-9568.2008.06320.x. [DOI] [PubMed] [Google Scholar]
- Zimmer G, Kästner B, Weth F, Bolz J. Multiple effects of ephrin-A5 on cortical neurons are mediated by SRC family kinases. J Neurosci. 2007;27(21):5643–53. doi: 10.1523/JNEUROSCI.0954-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer G, Rudolph J, Landmann J, Gerstmann K, Steinecke A, et al. Bidirectional EphrinB3/EphA4 signaling mediates the segregation of medial ganglionic eminence- and preoptic area-derived interneurons in the deep and superficial migratory stream. J Neurosci. 2011;31:18364–80. doi: 10.1523/JNEUROSCI.4690-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer G, Schanuel SM, Bürger S, Weth F, Steinecke A, et al. Chondroitin sulfate acts in concert with semaphorin 3A to guide tangential migration of cortical interneurons in the ventral telencephalon. Cereb Cortex. 2010;20(10):2411–22. doi: 10.1093/cercor/bhp309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.