Abstract
Oxidative stress has been implicated in the pathogenesis of bronchial asthma. Besides granulocytes, the airway epithelium can produce large amounts of reactive oxygen species and can contribute to asthma-related oxidative stress. Histamine is a major inflammatory mediator present in large quantities in asthmatic airways. Whether histamine triggers epithelium-derived oxidative stress is unknown. We therefore aimed at characterizing human airway epithelial H2O2 production stimulated by histamine. We found that air–liquid interface cultures of primary human bronchial epithelial cells (BECs) and an immortalized BEC model (Cdk4/hTERT HBEC) produce H2O2 in response to histamine. The main source of airway epithelial H2O2 is an NADPH dual oxidase, Duox1. Out of the four histamine receptors (H1R–H4R), H1R has the highest expression in BECs and mediates the H2O2–producing effects of histamine. IL-4 induces Duox1 gene and protein expression levels and enhances histamine-induced H2O2 production by epithelial cells. Using HEK-293 cells expressing Duox1 or Duox2 and endogenous H1R, histamine triggers an immediate intracellular calcium signal and H2O2 release. Overexpression of H1R further increases the oxidative output of Duox-expressing HEK-293 cells. Our observations show that BECs respond to histamine with Duox-mediated H2O2 production. These findings reveal a mechanism that could be an important contributor to oxidative stress characteristic of asthmatic airways, suggesting novel therapeutic targets for treating asthmatic airway disease.
Keywords: Duox, NADPH oxidase, asthma, airway epithelium, oxidative stress
Clinical Relevance
This study identifies a novel source of airway oxidative stress, which is characteristic of asthma disease pathogenesis, by showing that airway epithelial cells release hydrogen peroxide in response to histamine through H1 receptor–mediated activation of dual oxidase (Duox) enzymes. Furthermore, Th2 cytokines relevant to asthma promote these responses to histamine by augmenting Duox1 expression. These findings identify novel targets for therapeutic intervention for asthma.
Bronchial asthma is a chronic respiratory disorder characterized by airway obstruction, hyperresponsiveness, and chronic inflammation (1). Several lines of evidence suggest roles for oxidative stress in the pathogenesis of bronchial asthma. Oxidative damage results in airway hyperresponsiveness (2). Elevated H2O2 levels in the airways of patients with asthma positively correlate with disease severity and symptom scores and negatively correlate with their FEV1 values (3). Elevated H2O2 levels in exhaled breath condensates were proposed to be a disease marker for airway inflammation in asthma (4–6). Airway mucus hypersecretion is another characteristic hallmark of asthma (7). Oxidative stress triggered by a variety of agents (e.g., bacteria, silica, and growth factors) induces mucin synthesis and secretion in airway epithelial cells through activation of the epidermal growth factor receptor pathway (8–14).
Enhanced levels of reactive oxygen species (ROS) in asthmatic airways are thought to result from granulocytic infiltration (15, 16). Eosinophils and neutrophils are present in substantial numbers in asthmatic airways and produce large amounts of superoxide, H2O2, and downstream ROS (4, 9, 17). In addition to granulocytes, the human bronchial epithelium can generate significant quantities of H2O2, but its contribution as a source of ROS to asthma pathogenesis has not been investigated (13, 18–20).
Dual oxidase 1 (Duox1) and Dual oxidase 2 (Duox2) are NADPH oxidases expressed in bronchial epithelial cells (BECs) and are the main sources of epithelial ROS (11, 19, 21). Duox localizes to the apical plasma membrane of BECs, producing H2O2 into the airway lumen by consuming intracellular NADPH (20, 22). Both Duox enzymes are regulated by increases in intracellular calcium levels (12, 18, 20, 23). Several in vitro studies proposed roles of Duox in diverse epithelial functions (bacterial detection and killing, mucin secretion, wound healing, acid secretion, and inflammatory cytokine release) (13, 18, 19, 24–29). Recently, two studies suggested in vivo roles of Duox1 in airway epithelial wound repair and leukocyte recruitment in mouse models of airway injury and allergic asthma, respectively (30, 31).
Chronic inflammation in asthmatic airways is also characterized by high levels of the important pluripotent inflammatory mediator histamine (32). Histamine is a biogenic amine, is one of the most studied compounds in medical research, and has been implicated in diverse biological functions including hematopoiesis, wound healing, development, and inflammation (32). In bronchial asthma, histamine is released by mast cells or basophils upon stimulation of their Fcε receptors (33). Secreted histamine directly affects airway smooth muscle, endothelial, and different immune cells, but its impact on BECs has been vaguely studied (33). It has been shown recently in BECs that histamine stimulates mucin secretion, proinflammatory cytokine release, and airway remodeling through activation of epidermal growth factor receptor signaling (34–37). In other studies, Duox activation was shown to lead to the same effects in airway epithelial cells (24, 25, 27). Despite this, no studies have reported links between histamine and Duox activity in airway epithelial cells. We aimed at investigating whether histamine is capable of stimulating H2O2 production in BECs and the potential involvement of Duox in this mechanism.
Here we found that air–liquid interface (ALI) cultures of primary and immortalized human BECs (HBECs) release H2O2 in response to histamine. Gene expression data and experiments using specific agonists and inhibitors indicated that the histamine receptor H1R mediates histamine-stimulated Duox activation and ROS production. We characterized Duox expression and activation in an immortalized HBEC model and show that Duox1 is the major Duox isoform expressed. Furthermore, Th2 cytokine treatment induces Duox1 gene expression and amplifies the stimulatory effects of histamine on ROS production. Our report identifies histamine as a novel stimulus of airway epithelial Duox (H2O2 production) and proposes Duox as a potential source of excess ROS in asthmatic airways.
Materials and Methods
Cell Culture
Primary HBECs (Lonza, Basel, Switzerland) were cultured on ALI as described (18, 38).
Immortalized (nononcogenic) (CDk4/hTERT) HBECs were created by retroviral introduction of Cdk4 and hTERT in primary HBECs and were provided by Dr. John Minna (University of Texas Southwestern Medical Center, Dallas, TX) (39, 40). We used CDK4/hTERT HBECs seeded on collagen-coated, 24-well Costar transwell (6.5 mm) inserts without lung fibroblasts. These cells are referred to as “immortalized BECs” herein. For cytokine treatment, submerged cell cultures in 6-well plates (no ALI) were induced by 10 ng/ml human IL-4 or IL-13 (R&D Systems, Minneapolis, MN) for 2 days. Further details are provided in the online supplement.
The 16HBE140 HBEC line was obtained from Dr. D. Gruenert (Cystic Fibrosis Research Center, University of California, San Francisco, CA) and was cultured in Eagle’s minimum essential medium (MEM) containing l-glutamine, glucose, NaHCO3, 10% FBS, and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA) on surfaces coated with 1% BSA, 0.03 mg/ml bovine collagen, and 0.01 mg/ml human fibronectin (41).
The human mucoepidermoid BEC line NCI-H292 (ATCC, Manassas, VA) was cultured as described (12).
IB3–1 and C38 BECs (ATCC) were maintained in MEM medium containing l-glutamine, glucose, 10% FBS, and 1% penicillin-streptomycin (Invitrogen).
BEAS-2B cells (ATCC) were cultured on coated surfaces (BSA, bovine collagen, and bovine fibronectin) in LHC-8 medium containing l-glutamine, 10% FBS, and 1% penicillin-streptomycin (Invitrogen).
HEK-293 cells were cultured in MEM-α medium on collagen-coated plates. The creation of HEK-293 cell lines stably expressing human Duox1+DuoxA1α or Duox2+DuoxA2 (selected Flp-In-293 clones transfected with bicistronic DuoxA/Duox cassettes) is described in detail elsewhere (27).
Measurement of H2O2
H2O2 production in primary HBECs cultured on ALI was measured by Amplex Red/horseradish peroxidase (HRP) assay (Life Technologies, Grand Island, NY) (18). Histamine-triggered extracellular H2O2 production by HEK-293 cells or immortalized airway epithelial cells was measured using 0.25 mM homovanillic acid (Sigma, St. Louis, MO) (22). Extracellular H2O2 release stimulated by ionomycin or ATP in immortalized airway epithelial cells was assessed by luminol/HRP-based chemiluminescence assay (17). Histamine interferes with the luminol/HRP assay (data not shown). Details are provided in the online supplement.
Western Blotting
NCI-H292 or Cdk4/hTERT cell lysates were processed for Western blotting as described (13). Primary antibodies used were anti-Duox (rabbit, 1:2,000; a gift from Dr. Francois Miot [IRIBHM, Université Libre de Bruxelles]), anti–α-tubulin (mouse, 1:2,000; Santa Cruz Biotechnology, Santa Cruz, CA), anti-TLR4 (rabbit, 1:2,000; Novus Biologicals, Littleton, CO), anti-TLR5 (rabbit, 1:2,000; Santa Cruz Biotechnology), anti-goat Histamine H1 Receptor (A-20) antibody (Santa Cruz Biotechnology), rabbit polyclonal anti-GAPDH antibody (Trevigen, Gaithersburg, MD), and antiactin (rabbit, 1:4,000; Sigma). Secondary antibodies used were HRP-linked anti-rabbit IgG from donkey (1:1,000; GE Healthcare) and HRP-linked anti-mouse IgG from sheep (1:1,000; GE Healthcare).
The supplemental material contains details of RNA isolation, qualitative RT-PCR, quantitative real-time PCR, calcium measurements, and cloning of the H1R construct.
Statistical Analysis
Data are represented as mean ± SEM or mean ± SD. Significance was calculated with Student’s t test and was marked as *P < 0.05, **P < 0.01, and ***P < 0.001.
Results
Primary BECs Release H2O2 in Response to Histamine
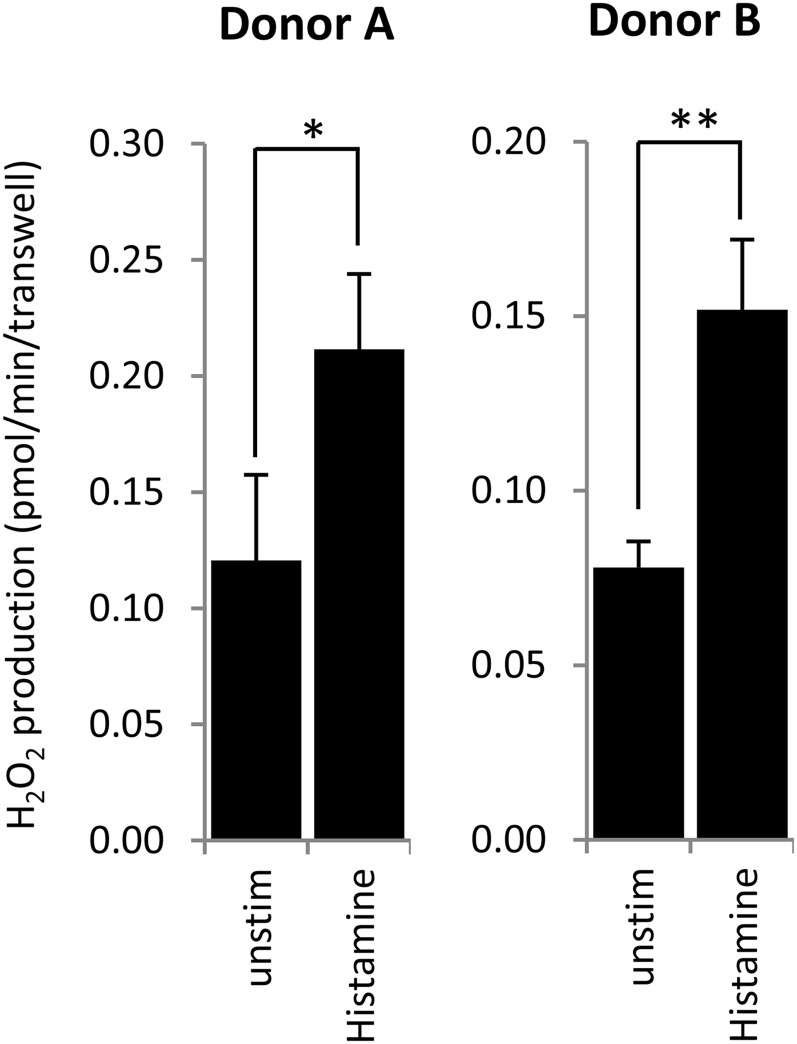
To determine if histamine induces ROS production in normal HBECs (NHBECs), NHBECs were differentiated for 3 to 4 weeks on ALI. These NHBEC cultures are polarized, develop transepithelial resistance, express large amounts of Duox, and are capable of producing H2O2 after stimulation by ionomycin or ATP (18, 28). Histamine added to the luminal surface of the airway cells increased apical H2O2 generation by 74.5% (donor A) or 94.7% (donor B) (Figure 1A).
Figure 1.
Histamine stimulates apical release of H2O2 from polarized cultures of primary human bronchial epithelial cells. Cells obtained from two different donors were differentiated on air–liquid interface (ALI) cultures (6.5-mm transwells, 24 wells) for 21 days. Apical H2O2 production was measured on adherent cells over 30 minutes by the Amplex Red/HRP assay in the presence or absence of 300 μM histamine (triplicates, mean ± SD). *P < 0.05 and **P < 0.01 (Student’s t test). Unstim, unstimulated.
ALI Cultures of Cdk4/hTERT Immortalized HBECs
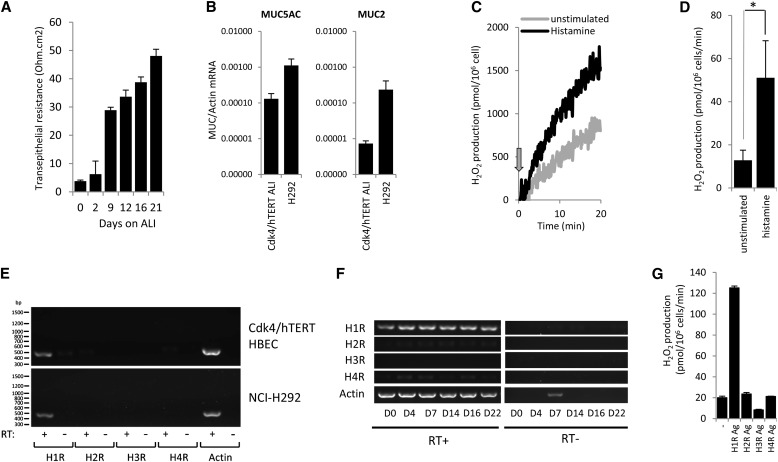
Primary airway cells provide the best in vitro model to study airway epithelial physiology, but they have limitations (e.g., donor-to-donor variations and limited expansion potential) preventing large-scale studies. Several cancer cell lines have been used as alternative models, but these cultures lack most of the features of differentiated BECs, including high Duox expression. Cancer lines acquire several novel features due to their oncogenic nature. To circumvent this, Minna and colleagues derived immortalized BEC lines from primary human cells by transfections with retroviral constructs coding cyclin-dependent kinase (Cdk) 4 and human telomerase reverse transcriptase (hTERT) (39). This resulted in immortalized cultures that exhibit an epithelial morphology and express mature epithelial markers (39). These cells were created without oncogenic transformation, provide an excellent model to study airway epithelial biology in vitro, and have been well characterized in a three-dimensional organotypic culture model (40). We cultured Cdk4/hTERT-immortalized (Cdk4/hTERT) HBECs on collagen-coated transwell supports exposed to ALI without using collagen plugs or a lung fibroblast feeder layer (40). Under these conditions, Cdk4/hTERT HBECs develop into monolayers with increased transepithelial resistance (Figure 2A), which are easy to maintain and can be expanded easily and show minimum variations between experiments. These cultures release MUC5AC (measured by ELISA; data not shown) and stain positively for mucins by Periodic acid-Schiff staining (data not shown). Real-time PCR data detected MUC5AC as the main MUC gene expressed (Figure 2B). MUC5AC gene expression detected by real-time PCR (MUC5AC/actin mRNA) in Cdk4/hTERT HBE ALI cultures (mean, 1.31 × 10−4; n = 3) is comparable to gene expression in the mucus-producing NCI-H292 human mucoepidermoid BEC line (mean, 1.12 × 10−3; n = 3) (12). Expression of another airway mucin gene, MUC2 (MUC2/actin mRNA), in CDK4/hTERT HBE ALI cultures was low (mean, 7.33 × 10−6; n = 3) compared with that of NCI-H292 cells (mean, 2.35 × 10−4; n = 3) (Figure 2B) (12). Ciliated cells were not observed in these ALI cultures. However, Cdk4/hTERT ALI cells expressed the anion channel pendrin (real-time PCR and Western blot; data not shown) also found in differentiated BECs (42). Because of these advantages, we used this BEC model, referred to as “immortalized BECs” or “Cdk4/hTERT HBECs,” throughout the study to describe histamine-stimulated Duox activity.
Figure 2.
Histamine H1 receptor mediates H2O2 production in immortalized human bronchial epithelial cells (HBECs) stimulated with histamine. (A) ALI cultures of immortalized Cdk4/hTERT HBECs develop transepithelial resistance over time. Cells were cultured for 3 weeks on collagen-coated, 24-well transwell (6.5-mm) inserts, and transepithelial resistance (Ohm/cm2) was measured at the indicated time points by voltohmeter. Data show mean ± SEM of four independent experiments. (B) Comparison of gene expression levels of two mucin genes, MUC2 and MUC5AC, between Cdk4/hTERT HBEs (ALI) and H292 cells. Total RNA was isolated from airway cells and reverse transcribed, and MUC gene expression was evaluated by real-time PCR (MUC/actin gene expression, mean ± SEM; n = 3) (C) Immortalized Cdk/hTERT cells were cultured on ALI for 2.5 weeks and trypsinized, and histamine-stimulated (100 μM) extracellular H2O2 release was measured in suspension by the homovanillic acid/HRP assay (one representative experiment; n = 3). Arrow indicates time of histamine stimulation. (D) Histamine (100 μM)-stimulated H2O2 production rates obtained in three independent experiments presented in B were analyzed, and mean ± SEM values are presented. (E) Gene expression of histamine receptors (H1R–H4R) was analyzed in two different bronchial epithelial cell lines (Cdk4/hTERT and NCI-H292) maintained in submerged cultures (one representative gel out of four independent experiments). (F) Gene expression changes of histamine receptors in ALI cultures of Cdk4/hTERT cells over time. At Days 0, 4, 7, 14, 16, and 22, cells were harvested, total RNA was prepared, and histamine receptor expression was determined using gene-specific primers (one representative gel; two other experiments gave similar data). Samples without reverse transcription (RT) were included as controls. (G) Extracellular H2O2 release of 2.5-week-old ALI cultures of Cdk4/hTERT cells stimulated by histamine receptor specific agonists (HR Ag) was quantified in suspension by the homovanillic acid/horseradish peroxidase assay. Another experiment gave similar data (mean ± SD). Student’s t test. *P < 0.05.
H1R Is the Dominant Histamine Receptor in HBECs
We confirmed that Cdk4/hTERT HBECs also respond to histamine with enhanced H2O2 production. Basal H2O2 production of immortalized BECs was significantly increased by histamine (Figures 2C and 2D). Next we asked which histamine receptors (HRs) are expressed in differentiated ALI cultures of Cdk4/hTERT HBECs. H1R was the main HR expressed (Figure 2E). H1R was found to be the dominant HR expressed in other airway epithelial cell lines (BEAS-2B, NCI-H292) as well (Figure 2E) (37, 43). H1R expression appears early and remains dominant during differentiation on ALI (Figure 2F). Testing HR-specific agonists, we found that only H1R agonist stimulated H2O2 production in differentiated Cdk4/hTERT HBECs (Figure 2G). Thus, we conclude that histamine H1R is the main HR expressed in immortalized BECs and mediates the H2O2–producing effect of histamine.
Characterization of Duox Function in Cdk4/hTERT HBECs Cultured on ALI
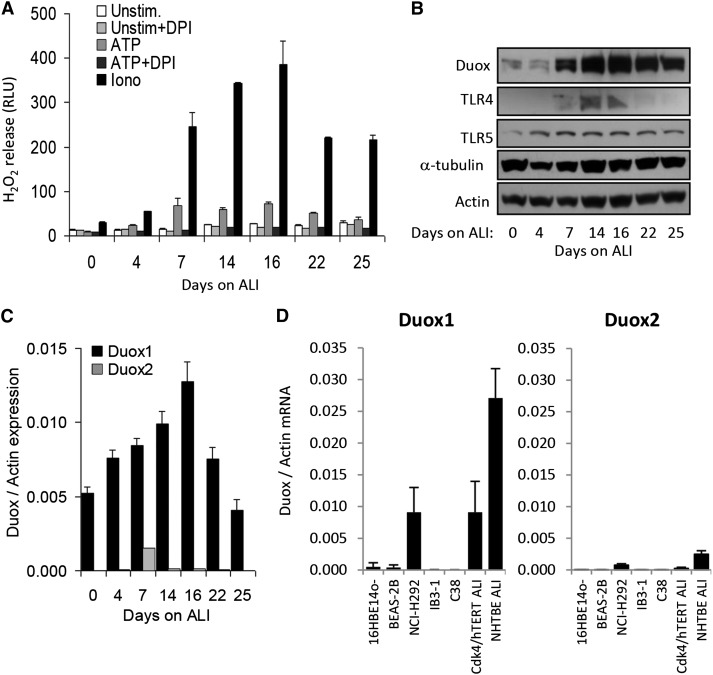
Duox expression and function has not been investigated before in Cdk4/hTERT HBECs (39, 40). Therefore, we cultured HBECs on ALI for different periods of time and measured H2O2 production in the presence of the well-known Duox activators ionomycin (1 μM) and ATP (300 μM). H2O2 production gradually developed and peaked at 16 days (Figure 3A). Later, H2O2 production decreased with time (Figure 3A). The flavoenzyme inhibitor diphenylene iodonium (10 μM), which also inhibits Duox, blocked ATP-stimulated H2O2 production (Figure 3A). In the same set of samples, we determined Duox protein expression by Western blotting. Duox expression followed functional H2O2 production data with time (Figures 3A and 3B), such that the most intense Duox protein band was observed also at Day 16 and the intensity of protein expression dropped afterward (Figure 3B). In addition to Duox, we detected expression of important innate immune receptors Toll-like receptor 2 and Toll-like receptor 5 (Figure 3B). These data confirm that Cdk4/hTERT HBECs represent an excellent in vitro model to study Duox function in airway epithelium.
Figure 3.
Dual oxidase (Duox)1 is the main source of H2O2 in immortalized HBECs. (A) Immortalized Cdk4/HTERT HBECs were cultured on ALI for different times. Cells in suspension were stimulated by 1 μM ionomycin or 300 μM ATP with or without 10 μM diphenylene iodonium (DPI), and H2O2 production was measured by the Luminol/HRP luminescence assay. Data are from one representative measurement (n = 3). (B) Cell lysates were prepared from Cdk4/hTERT cells cultured under the conditions mentioned in A, and expressions of the following proteins were measured by Western blotting: Duox, TLR4, TLR5, α-tubulin, and actin. Data are from one representative measurement (n = 3). (C) Human Duox1 and Duox2 gene expression levels in ALI cultures of Cdk4/hTERT cells were determined by real-time RT-PCR. Duox gene expression is given relative to actin. Data are from one representative experiment (n = 3). (D) Human Duox1 and Duox2 mRNA levels (relative to actin) in different bronchial epithelial cell lines were compared with primary HBEC ALI cultures. Values are mean ± SEM (n = 4). Iono, ionomycin; RLU, relative luminescence unit; TLR, Toll-like receptor; unstim, unstimulated.
Duox1 Is the Major Isoform Expressed in Immortalized HBECs
The H2O2 measurement and Western blot data do not determine which Duox isoform is dominant in Cdk4/hTERT HBECs. Therefore, we measured human Duox1 and Duox2 gene expression levels by real-time RT-PCR in the same set of cultures previously assessed for Western blotting and H2O2 release (Figures 3A and 3B). Duox1 mRNA levels were almost two orders of magnitude higher than Duox2 mRNA levels at most of the time points tested during ALI differentiation (Figure 3C). Changes in Duox1 mRNA levels followed the same pattern as Duox protein expression data and H2O2 release results (Figures 3A and 3B). These data clearly show that Duox1 is the main Duox isoform expressed in this cell culture model. We also detected gene expression of Duox activator 1 and Duox activator 2 in ALI cultures of immortalized HBECs (data not shown). We also compared Duox1 and Duox2 gene expression levels among numerous HBEC lines and primary cells (Figure 3D). All of the cell lines included in our survey are widely used in airway epithelial studies. However, several of these models lack significant Duox expression in comparison to primary ALI NHBECs expressing large amounts of Duox (Figure 3D) (18). In addition to primary ALI cells, the NCI-H292 cell model expresses Duox and provides an excellent in vitro model to study the airway epithelial functions of Duox (18, 24). Our data indicate that Duox1 expression in Cdk4/hTERT cells is similar to that of primary cells or NCI-H292 cells (Figure 3D). Duox2 expression in all cell models tested was significantly lower than Duox1 (Figure 3D). These results further establish Cdk4/hTERT HBECs as an excellent in vitro model to study Duox functions in the human airways.
Th2 Cytokines Induce Duox1 Expression in Cdk4/hTERT HBECs
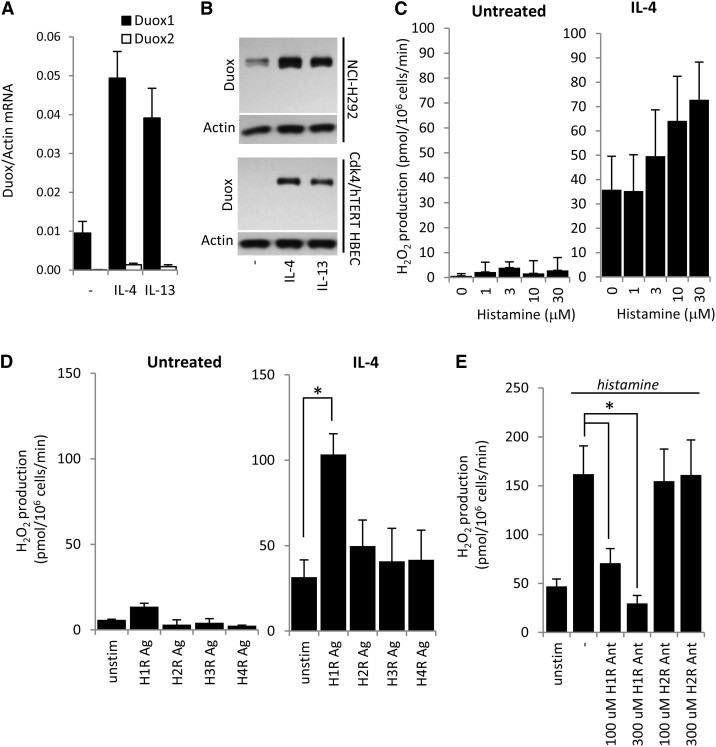
Th2 cytokines (IL-4 and IL-13) have been shown to up-regulate Duox1 in airway epithelial cells (18, 44). To confirm this observation in our cell model, we exposed Cdk4/hTERT HBECs (submerged cultures, non-ALI) to human IL-4 or IL-13 and measured Duox1 and Duox2 gene expression levels by real-time PCR. Duox1 expression was found to be dominant over Duox2 confirming previous data (Figures 3C and 3D). Both Th2 cytokines induced Duox1 expression, IL-4 by 5.1-fold and IL-13 by 4.1-fold (mean; n = 3) (Figure 4A). Untreated immortalized HBECs expressed relatively low amounts of Duox, but its expression was strongly boosted by IL-4 or IL-13 in Cdk4/hTERT cells, similar to NCI-H292 cells (Figure 4B).
Figure 4.
IL-4 amplifies histamine-triggered H2O2 production in bronchial epithelial cells. (A) Submerged cultures of Cdk4/hTERT HBECs were treated with human IL-4 or IL-13 (10 ng/ml) for 2 days, and Duox gene expression levels were assessed by real-time RT-PCR. Data are from one representative experiment (n = 3). (B) Duox protein expression levels were induced in NCI-H292 or Cdk4/hTERT HBECs by IL-4 or IL-13 (10 ng/ml, 2 d). Data are from one representative experiment (n = 3). (C) Histamine stimulates H2O2 production in a dose-dependent manner (0–30 μM) in IL-4–induced Cdk4/hTERT HBECs (IL-4 treatment: 10 ng/ml, 2 d). Uninduced cells do not produce H2O2. H2O2 production was measured by homovanillic acid/horseradish peroxidase assay. Values are mean ± SEM. IL-4 induction: n = 4; no induction: n = 3. (D) Histamine 1 receptor agonist (100 μM) stimulates H2O2 production in IL-4–induced Cdk4/hTERT HBECs. Histamine 2,3,4 receptor (H1R–H4R) agonists had no effect. Noninduced cells were unresponsive to the agonists tested. (E) H1R antagonist inhibits histamine-stimulated (100 μM) H2O2 production in IL-4–induced Cdk4/hTERT HBECs in a dose-dependent manner. H2R antagonist had no effect. H2O2 production was measured by homovanillic acid/horseradish peroxidase assay. Values are mean ± SEM (n = 3). Ag, agonist; Ant, antagonist; unstim, unstimulated.
H1R Mediates Histamine-Activated H2O2 Production in IL-4–Induced HBECs
Untreated Cdk4/hTERT HBECs did not produce detectable H2O2 in response to histamine, consistent with their low Duox expression (Figures 4B and 4C). IL-4 treatment, however, enhanced basal H2O2 production that was further increased by histamine in a dose-dependent manner (Figure 4C). Similar results were obtained with IL-13 treatments (data not shown). To identify the HR responsible for histamine-stimulated H2O2 production, we exposed IL-4–treated Cdk4/hTERT cells to HR-specific agonists. Only H1R agonist stimulation resulted in significantly increased H2O2 production (Figure 4D). We also pretreated IL-4–induced Cdk4/hTERT HBECs with H1R and H2R antagonists before histamine challenge. H1R antagonist inhibited histamine’s effect on H2O2 production in a dose-dependent manner, whereas H2R antagonism had no effect (Figure 4E). These data confirm that histamine stimulates ROS production in immortalized BECs through H1R.
Duox-Expressing HEK-293 Cells as a Model to Study Histamine-Stimulated Duox Activation
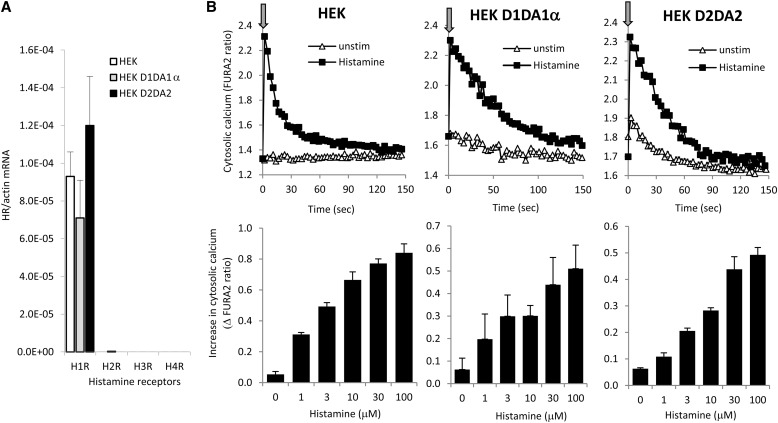
In addition to airway epithelial cells, we studied Duox activation by histamine in independent Duox1- or Duox2-reconstituted cell models for better characterization of the process. We used our established HEK-293 cell models stably expressing bicistronic pcDNA5.1/FRT constructs of human Duox1+DuoxA1α or human Duox2+DuoxA2 (22). These cells, referred to as Duox1/DuoxA1α or Duox2/DuoxA2 Flp-In-293 cells, express Duox1 or Duox2 and produce large amounts of H2O2 in response to calcium-mobilizing agonists (22). Histamine-stimulated responses in these cell lines have not been characterized before (22). By real-time quantitative PCR, we detected similar gene expression levels of H1R in HEK-293 cells and its Duox/DuoxA-expressing derivative lines (Figure 5A). No H2R, H3R, or H4R gene expression was detected (Figure 5A). To show that HEK cells respond to histamine, we followed changes in intracellular calcium in FURA2-loaded cells. All three cell lines showed immediate and robust calcium signals after the addition of histamine (Figure 5B, upper panels). The calcium kinetics showed a rapid peak within seconds after histamine addition, which dropped to basal levels in a few minutes (Figure 5B, upper panels). The amplitude of increases in intracellular calcium was enhanced by increasing doses of histamine (Figure 5B, lower panels). Thus, we conclude that HEK cells express H1R and respond to histamine with rapid increases in cytosolic calcium and that histamine signaling is not altered by stable expression of the Duox/DuoxA systems.
Figure 5.
Endogenous H1R expression in HEK-293 cells mediates histamine-stimulated increases in cytosolic calcium levels. (A) Gene expression levels (H1R–H4R) were measured in HEK-293 cells by real-time RT-PCR (mean ± SEM; n = 3). (B) Histamine (100 μM)-stimulated changes in cytosolic calcium concentrations were followed for 2.5 minutes in FURA2-loaded cells. Parental HEK-293 cells and HEK-293 cells stably transfected with the Duox1/DuoxA1α or the Duox2/DuoxA2 system were tested. Arrows indicate time of histamine stimulation. One representative experiment for each cell line is shown (n = 4). Lower graphs: histamine dose dependence of maximal calcium level increases in the three cell lines tested (mean ± SEM; n = 4).
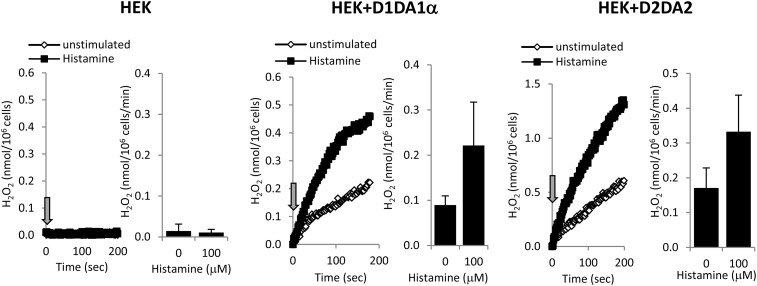
We next measured extracellular H2O2 production in all three cell lines stimulated by histamine. As expected, HEK-293 cells without transfected Duox did not produce H2O2 (Figure 6). HEK-293 cells expressing Duox1/DuoxA1α or Duox2/DuoxA2 release large amounts of H2O2 without stimulation, which was more than doubled after the addition of histamine (D1 system: 2.25 ± 0.49 fold; D2 system: 1.97 ± 0.21-fold [n = 3]) (Figure 6).
Figure 6.
Histamine stimulates Duox-mediated H2O2 production in HEK-293 cells stably expressing Duox1/DuoxA1α or Duox2/DuoxA2. HEK-293 cells stably expressing the Duox1/DuoxA1α (D1A1α) or the Duox2/DuoxA2 (D2A2) system were stimulated with 100 μM histamine, and H2O2 production was measured by the homovanillic acid/horseradish peroxidase assay. Representative kinetics and barographs with average ± SEM values are shown (n = 3).
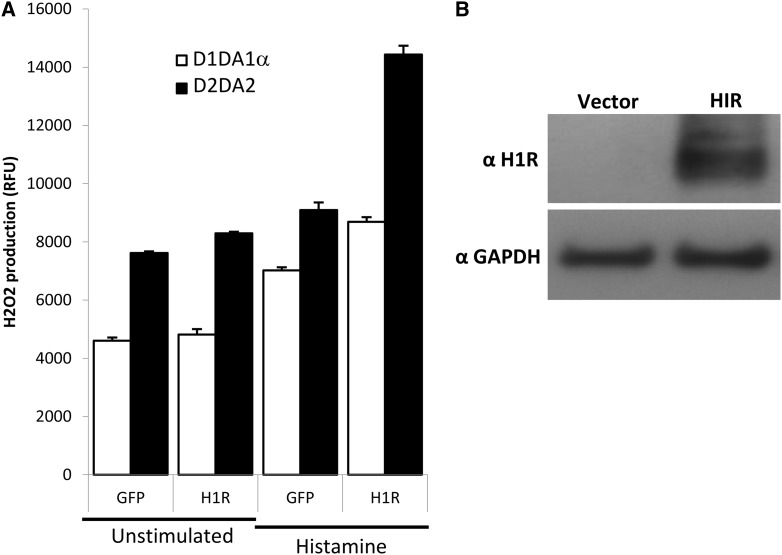
Overexpression of H1R in Duox-Expressing HEK-293 Cells Increases Extracellular H2O2 Production by Histamine
To confirm that H1R is responsible for histamine-mediated H2O2 production, we transiently transfected Duox1/DuoxA1α- or Duox2/DuoxA2-expressing HEK-293 cells with human H1R cDNA and measured H2O2 production 48 hours after transfection. Control cells were transfected with the same vector expressing green fluorescent protein (GFP). Histamine increased basal H2O2 production in Duox/DuoxA-expressing cells transfected with the GFP plasmid due to endogenous levels of H1R, which was further increased by the expression of H1R (Figure 7A). Duox2/DuoxA2-expressing HEK cells showed higher basal and histamine-stimulated H2O2 production than the cells expressing Duox1/DuoxA1α (Figure 7A). Western blot data of cell lysates of HEK-293 cells probed with an anti-H1R antibody confirm enhanced H1R protein levels in H1R-transfected cells compared with the GFP-transfected controls (Figure 7B).
Figure 7.
H1R overexpression enhances histamine-stimulated H2O2 production in Duox/DuoxA-expressing HEK-293 cells. (A) HEK-293 cells stably expressing the Duox1/DuoxA1α (D1A1α) or the Duox2/DuoxA2 (D2A2) system were transiently transfected with a plasmid coding the H1R or a with green fluorescent protein (GFP)-expressing plasmid (control). H2O2 production stimulated by histamine (100 μM) was measured 2 days after transfection by homovanillic acid/horseradish peroxidase assay (values are normalized on unstimulated GFP-expressing cells). Mean ± SEM of three independent experiments is shown. (B) H1R expression in cell lysates of nontransfected HEK-293 cells or cells transiently transfected with the H1R-coding plasmid was assessed by Western blotting using an anti-H1R antibody. One representative experiment is shown.
Discussion
The results presented in this study provide new mechanistic details on the action of histamine in airway epithelial cells. Our study is the first to show that histamine induces release of ROS (H2O2) by airway epithelial cells. Histamine release induced by ROS in mast cells and basophils has already been recognized, but histamine-stimulated ROS production by airway epithelial cells has not been documented before (45, 46).
We show that differentiated primary HBECs release H2O2 in response to histamine (Figure 1). The limitations of primary cell cultures prohibit large-scale experimentation; therefore, we modified and characterized a previously established airway model of immortalized BECs (Cdk4/hTERT HBECs) by culturing them in transwells on an ALI. We found the originally described organotypic cultures not suitable for large-scale studies and therefore simplified the culturing conditions. By omitting the fibroblast support and the collagen plug, we cultured the immortalized Cdk4/hTERT cells on collagen-coated transwell inserts similar to several other studies published on ALI cultures of primary human cells or cancer lines. This immortalized cell line offers several advantages over other in vitro airway epithelial cell models. Most cancer lines obtained from patients’ airways exhibit less differentiated airway features than immortalized (noncancer) lines and express low or undetectable levels of Duox (13, 18, 47). Several cancer lines do not become polarized once cultured on ALI. The biggest advantage of this immortalized line is its high Duox expression on ALI or when induced by cytokines. We have assayed several airway epithelial cell models for Duox expression and found that only the NCI-H292 cell line and our Cdk4/hTERT model showed high Duox expression (comparable to ALI cultures of primary cells). We and others have already used the NCI-H292 cell model to study Duox, but this line cannot be cultured on ALI, and it is a transformed line isolated from a cancer patient (18, 24). Similar to ALI cultures of primary cells, Duox1 is the major isoform found in ALI cultures of Cdk4/hTERT cells. H2O2 production, Duox protein production, and Duox1 mRNA levels change in parallel with time during cultivation on ALI (Figure 3).
Typically, primary NHBE ALI cells have a substantial baseline H2O2 production without treatment with any stimuli (18). Cdk4/hTERT ALI cultures have very low background signal of ROS species production that is mostly diphenylene iodonium resistant (Figure 2A), suggesting that, unlike primary cells, Duox is not constitutively active in this model. This feature of the Cdk4/hTERT ALI cultures makes them ideal for studies on Duox activation by external stimuli.
ALI Cdk4/hTERT immortalized airway epithelial cells also respond to histamine with H2O2 production like primary ALI cell cultures (Figures 1 and 2). We determined H1R receptor to have the highest expression among HRs in our cell model (Figure 2). This is consistent with findings also identifying H1R as the main HR expressed in other airway epithelial cell lines (34, 43). H1R signals through intracellular calcium, which is a known activator of Duox (18, 19, 34, 48). Furthermore, H1R has a crucial role in mediating allergic responses in the airways (49).
The data presented here show that histamine stimulates H2O2 production in airway epithelial cells by signaling through H1R and dual oxidases. The Th2 cytokines IL-4 and IL-13 were shown to induce Duox1 in primary airway epithelial cells (18, 44). We also observed enhanced Duox1 expression and histamine-stimulated H2O2 release in immortalized Cdk4/hTERT cells by IL-4 and IL-13 (Figure 4). Allergic and asthmatic airways are characterized by high levels of the Th2 cytokines IL-4 and IL-13 (50, 51). Elevation of Th2 cytokine levels and histamine suggests that induced higher levels of Duox1 and Duox-derived production of H2O2 in allergic/asthmatic airways could contribute to disease pathogenesis. Our work suggests Duox as a potential source of excess ROS that could be targeted in allergic/asthmatic airways (5, 52).
Acknowledgments
Acknowledgments
The authors thank Dr. John Minna (The University of Texas Southwestern Medical Center, Dallas, Texas) for providing the Cdk4/hTERT-transformed bronchial epithelial cell line and the culturing protocol; Drs. Krisztián Németh, Balázs Mayer, and Éva Mezey (Stem Cell Research Unit, NIDCR, NIH) for providing the histamine receptor agonists and antagonists and for help with optimizing the PCR reactions; and Dr. Francois Miot (IRIBHM, Université Libre de Bruxelles, Faculté de Médecine, Campus Erasme, BatC) for providing the Duox antibody.
Footnotes
Supported by funds from the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0254OC on August 20, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Leonardi S, Vitaliti G, Marseglia GL, Caimmi D, Lionetti E, Miraglia Del Giudice M, Salpietro C, Spicuzza L, Ciprandi G, La Rosa M. Function of the airway epithelium in asthma. J Biol Regul Homeost Agents. 2012;26:S41–S48. [PubMed] [Google Scholar]
- 2.Hulsmann AR, Raatgeep HR, den Hollander JC, Stijnen T, Saxena PR, Kerrebijn KF, de Jongste JC. Oxidative epithelial damage produces hyperresponsiveness of human peripheral airways. Am J Respir Crit Care Med. 1994;149:519–525. doi: 10.1164/ajrccm.149.2.8306055. [DOI] [PubMed] [Google Scholar]
- 3.Emelyanov A, Fedoseev G, Abulimity A, Rudinski K, Fedoulov A, Karabanov A, Barnes PJ. Elevated concentrations of exhaled hydrogen peroxide in asthmatic patients. Chest. 2001;120:1136–1139. doi: 10.1378/chest.120.4.1136. [DOI] [PubMed] [Google Scholar]
- 4.Loukides S, Bouros D, Papatheodorou G, Panagou P, Siafakas NM. The relationships among hydrogen peroxide in expired breath condensate, airway inflammation, and asthma severity. Chest. 2002;121:338–346. doi: 10.1378/chest.121.2.338. [DOI] [PubMed] [Google Scholar]
- 5.Jobsis Q, Raatgeep HC, Hermans PW, de Jongste JC. Hydrogen peroxide in exhaled air is increased in stable asthmatic children. Eur Respir J. 1997;10:519–521. [PubMed] [Google Scholar]
- 6.Horvath I, Donnelly LE, Kiss A, Kharitonov SA, Lim S, Chung KF, Barnes PJ. Combined use of exhaled hydrogen peroxide and nitric oxide in monitoring asthma. Am J Respir Crit Care Med. 1998;158:1042–1046. doi: 10.1164/ajrccm.158.4.9710091. [DOI] [PubMed] [Google Scholar]
- 7.Rogers DF. Airway mucus hypersecretion in asthma: an undervalued pathology? Curr Opin Pharmacol. 2004;4:241–250. doi: 10.1016/j.coph.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Nadel JA, Takeyama K. Mechanisms of hypersecretion in acute asthma, proposed cause of death, and novel therapy. Pediatr Pulmonol Suppl. 1999;18:54–55. [PubMed] [Google Scholar]
- 9.Takeyama K, Dabbagh K, Jeong Shim J, Dao-Pick T, Ueki IF, Nadel JA. Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor receptor: role of neutrophils. J Immunol. 2000;164:1546–1552. doi: 10.4049/jimmunol.164.3.1546. [DOI] [PubMed] [Google Scholar]
- 10.Deshpande A, Narayanan PK, Lehnert BE. Silica-induced generation of extracellular factor(s) increases reactive oxygen species in human bronchial epithelial cells. Toxicol Sci. 2002;67:275–283. doi: 10.1093/toxsci/67.2.275. [DOI] [PubMed] [Google Scholar]
- 11.De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem. 2000;275:23227–23233. doi: 10.1074/jbc.M000916200. [DOI] [PubMed] [Google Scholar]
- 12.Rada B, Gardina P, Myers TG, Leto TL. Reactive oxygen species mediate inflammatory cytokine release and EGFR-dependent mucin secretion in airway epithelial cells exposed to Pseudomonas pyocyanin. Mucosal Immunol. 2011;4:158–171. doi: 10.1038/mi.2010.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rada B, Leto TL. Characterization of hydrogen peroxide production by Duox in bronchial epithelial cells exposed to Pseudomonas aeruginosa. FEBS Lett. 2010;584:917–922. doi: 10.1016/j.febslet.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rada B, Leto TL. Pyocyanin effects on respiratory epithelium: relevance in Pseudomonas aeruginosa airway infections. Trends Microbiol. 2013;21:73–81. doi: 10.1016/j.tim.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Vliet A. Nox enzymes in allergic airway inflammation. Biochim Biophys Acta. 2011;1810:1035–1044. doi: 10.1016/j.bbagen.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoskins A, Reiss S, Wu P, Chen N, Han W, Do RH, Abdolrasulnia R, Dworski R. Asthmatic airway neutrophilia after allergen challenge is associated with the glutathione S-transferase M1 genotype. Am J Respir Crit Care Med. 2013;187:34–41. doi: 10.1164/rccm.201204-0786OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgel PR, Lazarus SC, Tam DC, Ueki IF, Atabai K, Birch M, Nadel JA. Human eosinophils induce mucin production in airway epithelial cells via epidermal growth factor receptor activation. J Immunol. 2001;167:5948–5954. doi: 10.4049/jimmunol.167.10.5948. [DOI] [PubMed] [Google Scholar]
- 18.Rada B, Lekstrom K, Damian S, Dupuy C, Leto TL. The Pseudomonas toxin pyocyanin inhibits the dual oxidase-based antimicrobial system as it imposes oxidative stress on airway epithelial cells. J Immunol. 2008;181:4883–4893. doi: 10.4049/jimmunol.181.7.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 2003;17:1502–1504. doi: 10.1096/fj.02-1104fje. [DOI] [PubMed] [Google Scholar]
- 20.Forteza R, Salathe M, Miot F, Forteza R, Conner GE. Regulated hydrogen peroxide production by Duox in human airway epithelial cells. Am J Respir Cell Mol Biol. 2005;32:462–469. doi: 10.1165/rcmb.2004-0302OC. [DOI] [PubMed] [Google Scholar]
- 21.Dupuy C, Ohayon R, Valent A, Noel-Hudson MS, Deme D, Virion A. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase: cloning of the porcine and human cdnas. J Biol Chem. 1999;274:37265–37269. doi: 10.1074/jbc.274.52.37265. [DOI] [PubMed] [Google Scholar]
- 22.Morand S, Ueyama T, Tsujibe S, Saito N, Korzeniowska A, Leto TL. Duox maturation factors form cell surface complexes with Duox affecting the specificity of reactive oxygen species generation. FASEB J. 2009;23:1205–1218. doi: 10.1096/fj.08-120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ameziane-El-Hassani R, Morand S, Boucher JL, Frapart YM, Apostolou D, Agnandji D, Gnidehou S, Ohayon R, Noel-Hudson MS, Francon J, et al. Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. J Biol Chem. 2005;280:30046–30054. doi: 10.1074/jbc.M500516200. [DOI] [PubMed] [Google Scholar]
- 24.Shao MX, Nadel JA. Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci USA. 2005;102:767–772. doi: 10.1073/pnas.0408932102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wesley UV, Bove PF, Hristova M, McCarthy S, van der Vliet A. Airway epithelial cell migration and wound repair by ATP-mediated activation of dual oxidase 1. J Biol Chem. 2007;282:3213–3220. doi: 10.1074/jbc.M606533200. [DOI] [PubMed] [Google Scholar]
- 26.Schwarzer C, Machen TE, Illek B, Fischer H. NADPH oxidase-dependent acid production in airway epithelial cells. J Biol Chem. 2004;279:36454–36461. doi: 10.1074/jbc.M404983200. [DOI] [PubMed] [Google Scholar]
- 27.Boots AW, Hristova M, Kasahara DI, Haenen GR, Bast A, van der Vliet A. ATP-mediated activation of the NADPH oxidase DUOX1 mediates airway epithelial responses to bacterial stimuli. J Biol Chem. 2009;284:17858–17867. doi: 10.1074/jbc.M809761200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moskwa P, Lorentzen D, Excoffon KJ, Zabner J, McCray PB, Jr, Nauseef WM, Dupuy C, Banfi B. A novel host defense system of airways is defective in cystic fibrosis. Am J Respir Crit Care Med. 2007;175:174–183. doi: 10.1164/rccm.200607-1029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerson C, Sabater J, Scuri M, Torbati A, Coffey R, Abraham JW, Lauredo I, Forteza R, Wanner A, Salathe M, et al. The lactoperoxidase system functions in bacterial clearance of airways. Am J Respir Cell Mol Biol. 2000;22:665–671. doi: 10.1165/ajrcmb.22.6.3980. [DOI] [PubMed] [Google Scholar]
- 30.Chang S, Linderholm A, Franzi L, Kenyon N, Grasberger H, Harper R. Dual oxidase regulates neutrophil recruitment in allergic airways. Free Radic Biol Med. 2013;65C:38–46. doi: 10.1016/j.freeradbiomed.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorissen SH, Hristova M, Habibovic A, Sipsey LM, Spiess PC, Janssen-Heininger YM, van der Vliet A. Dual oxidase-1 is required for airway epithelial cell migration and bronchiolar reepithelialization after injury. Am J Respir Cell Mol Biol. 2013;48:337–345. doi: 10.1165/rcmb.2012-0393OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jutel M, Akdis M, Akdis CA. Histamine, histamine receptors and their role in immune pathology. Clin Exp Allergy. 2009;39:1786–1800. doi: 10.1111/j.1365-2222.2009.03374.x. [DOI] [PubMed] [Google Scholar]
- 33.O'Mahony L, Akdis M, Akdis CA. Regulation of the immune response and inflammation by histamine and histamine receptors. J Allergy Clin Immunol. 2011;128:1153–1162. doi: 10.1016/j.jaci.2011.06.051. [DOI] [PubMed] [Google Scholar]
- 34.Matsubara M, Tamura T, Ohmori K, Hasegawa K. Histamine H1 receptor antagonist blocks histamine-induced proinflammatory cytokine production through inhibition of Ca2+-dependent protein kinase C, Raf/MEK/ERK and IKK/I kappa B/NF-kappa B signal cascades. Biochem Pharmacol. 2005;69:433–449. doi: 10.1016/j.bcp.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Hirota N, Risse PA, Novali M, McGovern T, Al-Alwan L, McCuaig S, Proud D, Hayden P, Hamid Q, Martin JG. Histamine may induce airway remodeling through release of epidermal growth factor receptor ligands from bronchial epithelial cells. FASEB J. 2012;26:1704–1716. doi: 10.1096/fj.11-197061. [DOI] [PubMed] [Google Scholar]
- 36.Kim YM, Won TB, Kim SW, Min YG, Lee CH, Rhee CS. Histamine induces MUC5AC expression via a hCLCA1 pathway. Pharmacology. 2007;80:219–226. doi: 10.1159/000104419. [DOI] [PubMed] [Google Scholar]
- 37.Kim HM, Lee CH, Rhee CS. Histamine regulates mucin expression through H1 receptor in airway epithelial cells. Acta Otolaryngol. 2012;132:S37–S43. doi: 10.3109/00016489.2012.661075. [DOI] [PubMed] [Google Scholar]
- 38.Gray TE, Guzman K, Davis CW, Abdullah LH, Nettesheim P. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol. 1996;14:104–112. doi: 10.1165/ajrcmb.14.1.8534481. [DOI] [PubMed] [Google Scholar]
- 39.Ramirez RD, Sheridan S, Girard L, Sato M, Kim Y, Pollack J, Peyton M, Zou Y, Kurie JM, Dimaio JM, et al. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res. 2004;64:9027–9034. doi: 10.1158/0008-5472.CAN-04-3703. [DOI] [PubMed] [Google Scholar]
- 40.Vaughan MB, Ramirez RD, Wright WE, Minna JD, Shay JW. A three-dimensional model of differentiation of immortalized human bronchial epithelial cells. Differentiation. 2006;74:141–148. doi: 10.1111/j.1432-0436.2006.00069.x. [DOI] [PubMed] [Google Scholar]
- 41.Gruenert DC, Basbaum CB, Welsh MJ, Li M, Finkbeiner WE, Nadel JA. Characterization of human tracheal epithelial cells transformed by an origin-defective simian virus 40. Proc Natl Acad Sci USA. 1988;85:5951–5955. doi: 10.1073/pnas.85.16.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedemonte N, Caci E, Sondo E, Caputo A, Rhoden K, Pfeffer U, Di Candia M, Bandettini R, Ravazzolo R, Zegarra-Moran O, et al. Thiocyanate transport in resting and IL-4-stimulated human bronchial epithelial cells: role of pendrin and anion channels. J Immunol. 2007;178:5144–5153. doi: 10.4049/jimmunol.178.8.5144. [DOI] [PubMed] [Google Scholar]
- 43.Muller T, Myrtek D, Bayer H, Sorichter S, Schneider K, Zissel G, Norgauer J, Idzko M. Functional characterization of histamine receptor subtypes in a human bronchial epithelial cell line. Int J Mol Med. 2006;18:925–931. [PubMed] [Google Scholar]
- 44.Harper RW, Xu C, Eiserich JP, Chen Y, Kao CY, Thai P, Setiadi H, Wu R. Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett. 2005;579:4911–4917. doi: 10.1016/j.febslet.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Ogasawara H, Fujitani T, Drzewiecki G, Middleton E., Jr The role of hydrogen peroxide in basophil histamine release and the effect of selected flavonoids. J Allergy Clin Immunol. 1986;78:321–328. doi: 10.1016/s0091-6749(86)80083-5. [DOI] [PubMed] [Google Scholar]
- 46.Boros M, Kaszaki J, Nagy S. Histamine release during intestinal ischemia-reperfusion: role of iron ions and hydrogen peroxide. Circ Shock. 1991;35:174–180. [PubMed] [Google Scholar]
- 47.Luxen S, Belinsky SA, Knaus UG. Silencing of DUOX NADPH oxidases by promoter hypermethylation in lung cancer. Cancer Res. 2008;68:1037–1045. doi: 10.1158/0008-5472.CAN-07-5782. [DOI] [PubMed] [Google Scholar]
- 48.Bakker RA, Schoonus SB, Smit MJ, Timmerman H, Leurs R. Histamine H(1)-receptor activation of nuclear factor-kappa B: roles for G beta gamma- and G alpha(q/11)-subunits in constitutive and agonist-mediated signaling. Mol Pharmacol. 2001;60:1133–1142. doi: 10.1124/mol.60.5.1133. [DOI] [PubMed] [Google Scholar]
- 49.Bryce PJ, Mathias CB, Harrison KL, Watanabe T, Geha RS, Oettgen HC. The H1 histamine receptor regulates allergic lung responses. J Clin Invest. 2006;116:1624–1632. doi: 10.1172/JCI26150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ingram JL, Kraft M.IL-13 in asthma and allergic disease: asthma phenotypes and targeted therapies J Allergy Clin Immunol 2012130829–842.quiz 843–824. [DOI] [PubMed] [Google Scholar]
- 51.Maes T, Joos GF, Brusselle GG. Targeting interleukin-4 in asthma: lost in translation? Am J Respir Cell Mol Biol. 2012;47:261–270. doi: 10.1165/rcmb.2012-0080TR. [DOI] [PubMed] [Google Scholar]
- 52.Loukides S, Kontogianni K, Hillas G, Horvath I. Exhaled breath condensate in asthma: from bench to bedside. Curr Med Chem. 2011;18:1432–1443. doi: 10.2174/092986711795328418. [DOI] [PubMed] [Google Scholar]