Abstract
The statins are now recognized to have pleiotropic properties, including augmentation of endothelial barrier function. To explore the mechanisms involved, we investigated the effect of simvastatin on endothelial cell (EC) tight junctions. Western blotting of human pulmonary artery ECs treated with simvastatin (5 μM) confirmed a significant time-dependent increase (16–48 h) in claudin-5 protein expression compared with controls, without detectable alterations in zonula occludens-1 or occludin. These effects were associated with membrane translocation of VE-cadherin, whereas translocation of vascular endothelial cadherin (VE-cadherin; silencing RNA) inhibited simvastatin-induced claudin-5 up-regulation. Moreover, simvastatin treatment of ECs induced increased phosphorylation of both FoxO1 and β-catenin, transcriptional regulators of claudin-5 expression mediated by VE-cadherin. Subsequently, we found no effect of claudin-5 silencing on EC barrier protection by simvastatin in response to thrombin stimulation, as measured by either transendothelial electrical resistance or by EC monolayer flux of FITC-dextran (2,000 kD). However, silencing of claudin-5 did significantly attenuate simvastatin-mediated EC barrier protection in response to thrombin, as measured by monolayer flux of sodium fluorescein (376 Da). Finally, employing a murine model of LPS-induced acute lung injury, there was no effect of claudin-5 silencing in vivo (intratracheal injection) on bronchoalveolar lavage fluid protein or cell counts, but LPS-induced lung tissue extravasation of the small molecular weight markers, sodium fluorescein and Hochst stain (562 Da), were significantly increased in claudin-5–silenced animals compared with simvastatin-treated control animals. These findings implicate a distinct mechanism underlying size-selective endothelial barrier–protective properties of statins, and may ultimately lead to new novel therapeutic targets for patients with acute lung injury.
Keywords: statins, claudins, endothelial cells, permeability
Clinical Relevance
Acute lung injury (ALI) is a devastating syndrome associated with significant morbidity and mortality. We previously reported that simvastatin confers protection in a mouse model of ALI. In the current study, we confirm a significant role for claudin-5, an endotheial cell tight junctional protein, in simvastatin-mediated ALI protection, thus implicating claudin-5 as a potential novel therapeutic target in patients with ALI.
Statins are now well recognized to have pleiotropic properties beyond their ability to lower serum cholesterol levels, including the augmentation of endothelial cell (EC) barrier function, which we have reported is associated with the protective effects of these drugs in murine acute lung injury (ALI) (1, 2). The direct effects of statins on EC signaling and activation are complex, and include actin cytoskeletal rearrangement via differential effects on the small GTPases, RhoA and Rac1 (2), inhibition of superoxide generation via reduced nicotinamide adenine dinucleotide phosphate oxidase (3), and the up-regulation of integrin-β4 (2), a molecule that attenuates EC inflammatory responses via effects on mitogen-activated protein kinase signaling, and mediates the lung-protective effects of simvastatin in murine ALI (4). To more fully characterize the statin protection of EC barrier function in vitro and murine ALI in vivo, we investigated the effects of simvastatin on lung EC tight junctional proteins, and have identified an important role for claudin-5 in this context.
EC tight junctions are composed of transmembrane proteins, including claudins, occludins, and junctional adhesion molecules that associate with cytoplasmic proteins, including zonula occludens (ZO). Notably, mice deficient in claudin-5, which is specifically expressed in ECs (5), demonstrate a selective increase in permeability of the blood–brain barrier to small molecules (6), whereas the up-regulation of claudin-5 has been reported in rat brain ECs treated with pitavastatin (7). Separately, expression levels of lung claudin-5 correlate inversely with ALI severity in a mouse model (8). However, the role of claudin-5 in statin-mediated ALI protection is unknown.
We identified a significant up-regulation of claudin-5 in human pulmonary artery ECs in response to simvastatin, and linked this finding to increased membrane translocation of vascular endothelial cadherin (VE-cadherin), known to regulate claudin-5 via the inhibition of the transcriptional regulators, FoxO1 and β-catenin, resulting in increased claudin-5 mRNA expression (9). These effects of simvastatin were associated with a reduction in agoinst-induced EC barrier permeability to small molecules both in vitro and in vivo in our murine ALI model. These data identify claudin-5 as an important mediator of ALI protection by simvastatin, and implicate claudin-5 as a potential novel therapeutic target in patients with ALI.
Materials and Methods
Cell Culture
Human pulmonary artery ECs were purchased from Clonetics (San Diego, CA), and were cultured in EGM-2 supplemented with 2% FBS, hydrocortisone, hFGF, VEGF, ascorbic acid, hEGF, GA-1000, heparin, and R3-IGF-1 (Clonetics). Cells were incubated in 75-cm2 flasks and cultured at 37°C in 5% CO2 and 95% air. All cells were used at passages 4–8.
Materials and Reagents
Claudin-5 antibody was purchased from Life Technologies (Grand Island, NY). FoxO1 antibody was purchased from Cell Signaling (Danvers, MA). ZO-1 antibody was purchased from BD Biosciences (San Jose, CA). All other antibodies and reagents were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Transfection with Silencing RNA
The target sequence for silencing RNA (siRNA) specific for claudin-5 was 5′-CCAACAUUGUCGUCCGCGAtt-3′ (Life Technologies). All other siRNAs were purchased from Dharmacon (Layfayette, CO). For β-catenin siRNA, the target sequence was 5′-GGAUGUUCACAACCGAAUUtt-3′. SMARTpool siRNA was used for FoxO1 and VE-cadherin (Dharmacon). The target sequence for nonspecific RNA (nsRNA) used as a control was 5′-UAGCGACUAAACACAUCAA-3′. ECs were plated (60–80% confluent) and were transfected with nsRNA or siRNA specific for claudin-5 (100 nM) using siPORT Amine (Ambion, Austin, TX). After incubating for 72 hours, knockdown of protein was verified by Western blotting.
Western Blotting
Samples were prepared according to standard protocols. Western blotting was performed using primary antibodies and horseradish peroxidase-conjugated secondary antibodies before visualization via chemiluminescence (Amersham Biosciences, Piscataway, NJ). Blot density was determined by Alpha Imager software (Alpha Innotech, San Leandro, CA).
Immunofluorescent Microscopy
Confluent ECs grown on coverslips were exposed to experimental conditions, fixed with 3.7% formaldehyde, and permeabilized with 0.25% Triton X-100. After blocking with 2% bovine serum albumin, cells were exposed to primary antibodies for 60 minutes. Fluorescently tagged secondary antibodies were applied for 60 minutes. Cells were imaged using a Nikon video imaging system (Nikon, Melville, NY).
Transendothelial Electrical Resistance Measurements
ECs were grown to confluence over evaporated gold microelectrodes connected to a phase-sensitive lock-in amplifier, as previously described (10). Transendothelial electrical resistance (TER) was measured in response to specific agonists using an electrical cell substrate impedance sensing system (ECIS; Applied BioPhysics Inc., Troy, NY).
Transwell Permeability Assay
A commercially available kit (Millipore, Billerica, CA) was used to measure EC monolayer permeability to high– and low–molecular weight proteins on the basis of the Transwell model that our laboratory previously described (11). In separate experiments, 100 μl FITC-dextran (2,000 kD) or 2 μg/ml sodium fluorescein (376 Da) was added to cells and incubated for 1 hour. The Transwell insert was then removed and 100 μl medium collected. Fluorescent density was analyzed on a Titertek Fluoroskan II Microplate Fluorometer (Diversified Equipment, Lorton, VA) at excitation and emission wavelengths of 485 and 530 nm, respectively.
Murine ALI Model
All experiments and animal care procedures were approved by the University of Illinois at Chicago Animal Care and Use Committee. C57/Bl6 mice (8–12 wk old; Jackson Laboratory, Bar Harbor, ME) were administered a single dose of siRNA via intratracheal injection (10 μg/g body weight), as we have previously described (12), 3 days before simvastatin (20 mg/kg) or vehicle via intraperitoneal injection. The mice were treated again 24 hours later with simvastatin or vehicle, followed by intratracheal LPS (1.25 mg/kg body weight, 4 d after siRNA delivery). The next day, 24 hours after LPS, the mice were killed and bronchoalveolar lavage (BAL) was performed as previously described (1). In select animals, sodium fluorescein was injected via internal jugular (IJ) vein 24 hours after LPS and BAL fluid collected 1 hour later. In a separate group of animals, Hoechst stain H33258 (562 Da) was injected (IJ) 24 hours after LPS and lungs then flushed with PBS 2 hours later and harvested for histological analysis.
Statistical Analysis
Student’s t test was used to compare the means of data from two experimental groups, whereas significant differences (P < 0.05) among multiple group comparisons were confirmed by one-way ANOVA followed by Tukey’s Studentized range test. Results are expressed as means (± SE).
Results
Effect of Simvastatin on EC Expression of Tight Junctional Proteins
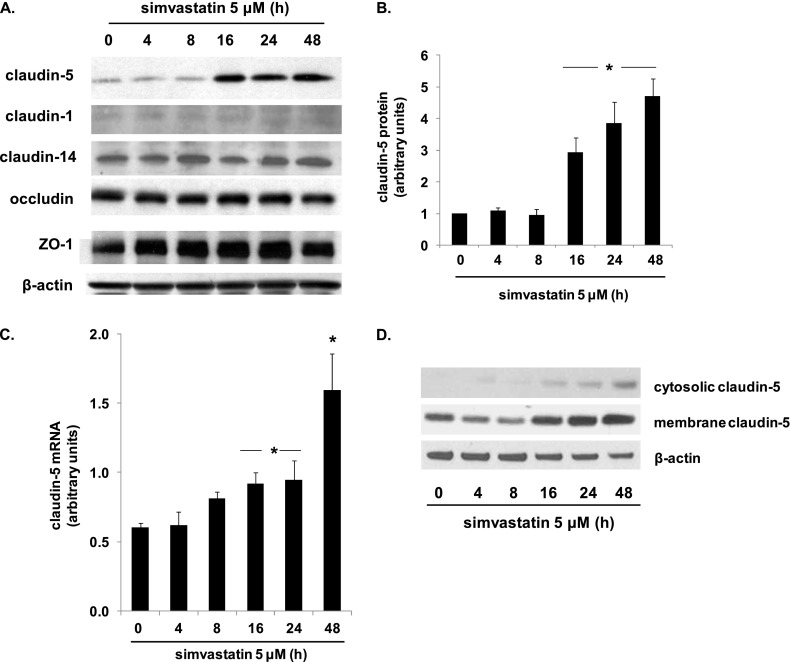
ECs were treated with simvastatin (5 μM, 4–48 h) and lysates subjected to Western blotting for the tight junctional proteins, claudin-5, ZO-1, and occludin (Figure 1). Although there was no change in ZO-1 or occludin expression levels at any time point, a significant increase in claudin-5 protein was evident 16 hours after simvastatin treatment, and was sustained at 48 hours. There was no change in claudin-5 expression at 4 or 8 hours after simvastatin treatment. This time course corresponds to the time course of simvastatin-mediated EC barrier protection as we have previously reported (2). Of note, two other claudins, claudin-1 and claudin-14, were also assessed, and no changes in their expression levels were evident at any time point (0–48 h) in response to simvastatin treatment. Claudin-5 mRNA levels were also evaluated, and a significant increase in response to simvastatin at 16 hours was evident, with a further increase at 48 hours. Subsequently, membrane fractionation, followed by Western blotting, confirmed increased claudin-5 expression induced by simvastatin was localized predominantly in the membrane.
Figure 1.
Simvastatin up-regulates endothelial cell (EC) claudin-5 expression. (A) ECs were treated with simvastatin (5 μM, 4–48 h) and lysates subjected to Western blotting for the tight junctional proteins, claudin-5, zonula occludens (ZO)-1, and occludin (representative blots shown). (B) Densitometry confirmed a significant increase in claudin-5 at 16 hours after simvastatin treatment that was sustained at 48 hours. (C) Similarly, claudin-5 mRNA levels were significantly increased at 16 hours, and were further increased at 48 hours after simvastatin (*P < 0.05, n = 3/condition). (D) Representative blots are shown from membrane fractionation experiments followed by Western blotting for claudin-5 at various time points after treatement with simvastatin (5 μM, 4–48 h). Results are expressed as means (± SE).
Role of VE-Cadherin in Claudin-5 Up-Regulation by Simvastatin
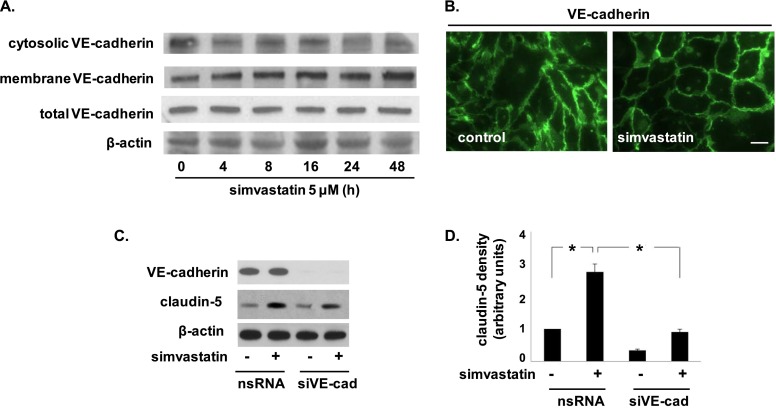
As VE-cadherin is a known mediator of claudin-5 expression (9), we next examined the effects of simvastatin on EC VE-cadherin expression and localization. There was no change in protein levels of VE-cadherin in response to simvastatin (5 μM, 16 h), however, there was increased translocation of VE-cadherin to the EC membrane evident by both Western blotting of membrane fractions and immunohistochemistry, which demonstrated more discrete localization of VE-cadherin at the cell periphery in response to simvastatin (Figures 2A and 2B). Notably, evidence of VE-cadherin translocation was apparent as early as 4 hours after simvastatin treatment (Figure 2A), before detectable changes in claudin-5 mRNA or protein levels. In addition, silencing of VE-cadherin significantly attenuated the increased expression of claudin-5 induced by simvastatin (Figures 2C and 2D). These data are consistent with the idea that the up-regulation of claudin-5 by simvastatin is mediated by the translocation of VE-cadherin to the cell membrane.
Figure 2.
Vascular endothelial cadherin (VE-cadherin) translocates in response to simvastatin and mediates claudin-5 up-regulation. (A) ECs were either untreated or treated with simvastatin (5 μM, 4–48 h) and then subjected to membrane fractionation before Western blotting of cytosolic and membrane fractions for VE-cadherin (representative blots shown). (B) Immunofluorescence imaging of control ECs and simvastatin-treated ECs demonstrated membrane translocation of VE-cadherin in response to simvastatin at 16 hours. Scale bar, 10 μm. (C) Subsequently, ECs were transfected with nonspecific RNA (nsRNA) or silencing RNA (siRNA) specific for VE-cadherin (siVE-cad) before treatment with simvastatin (5 μM, 16 h), and then immunblotted for claudin-5 (representative blots shown). (D) Densitometry confirmed significantly reduced claudin-5 expression in simvastatin-treated ECs transfected with siVE-cad compared with simvastatin-treated EC controls (nsRNA) (*P < 0.05, n = 3/condition). Results are expressed as means (± SE).
Role of FoxO1 and β-Catenin in Claudin-5 Up-Regulation by Simvastatin
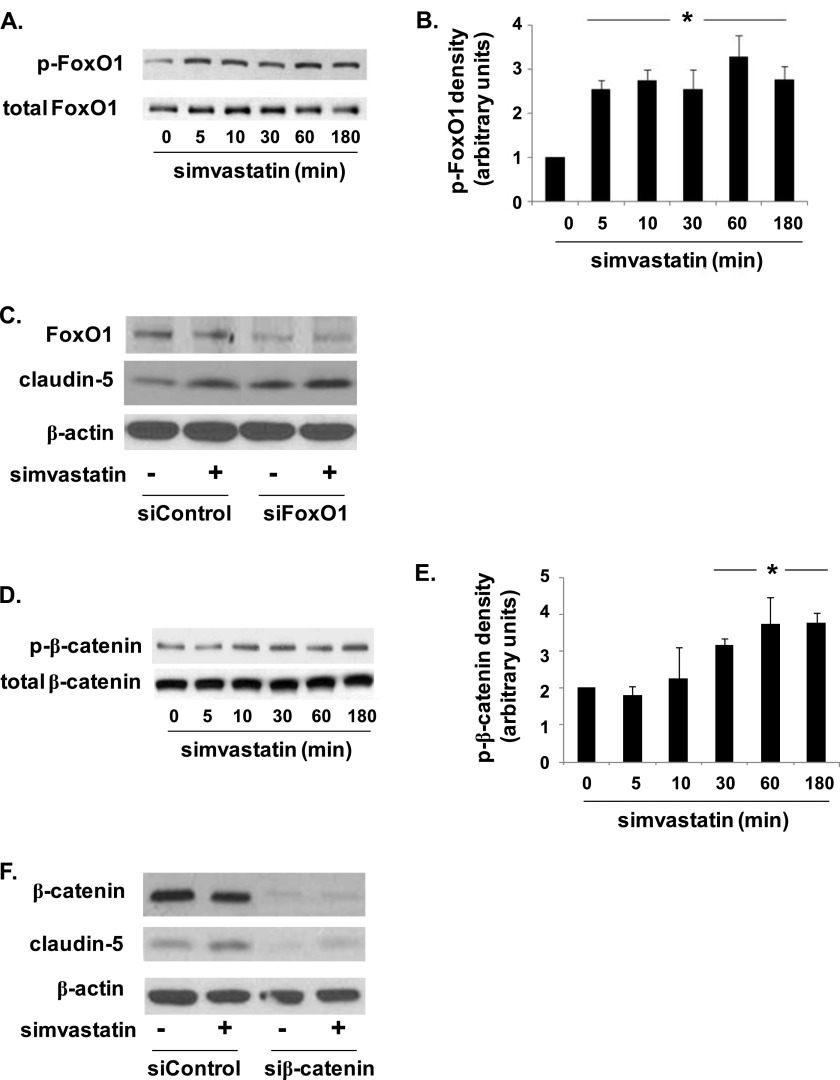
VE-cadherin mediates claudin-5 expression via the inhibition of the nuclear accumulation of the transcriptional repressors, FoxO1 and β-catenin (9). To further investigate the link between VE-cadherin and claudin-5 expression in response to simvastatin, we assessed the effects of simvastatin on FoxO1 and β-catenin phosphorylation. Simvastatin treatment (5 μM) of ECs was associated with a significant time-dependent increase in both FoxO1 and β-catenin phosphorylation that was evident at 5 and 30 minutes, respectively, and was sustained at 3 hours (Figure 3). Moreover, silencing of FoxO1 was associated with an increase in both basal and simvastatin-induced (5 μM, 16 h) claudin-5 expression, consistent with the known role of FoxO1 as a transcriptional repressor (that translocates from the nucleus on phosphorylation) (9). Conversely, silencing of β-catenin effected a decrease in both basal and simvastatin-induced (5 μM, 16 h) claudin-5 expression, suggesting that it acts as a potential enhancer of transcription in this context.
Figure 3.
Simvastatin induces time-dependent phosphorylation of FoxO1 and β-catenin. (A) ECs were treated with simvastatin (5 μM, 0–180 min), and lysates were subjected to Western blotting for phosphorylated and total FoxO1 (representative blots shown). (B) FoxO1 phosphorylation was significantly increased at 5 minutes, and was sustained at 180 minutes, as confirmed by densitometry analyses (*P < 0.05, n = 3/condition). (C) Western blots confirmed increased claudin-5 expression in simvastatin-treated (5 μM, 16 h) ECs transfected with either control siRNA or siRNA specific for FoxO1 (siFoxO1) compared with their respective, untreated controls (representative blots shown). (D) In separate experiments, ECs were treated with simvastatin (5 μM, 0–180 min) and immunoblotted for phophorylated and total β-catenin (representative blots shown). (E) Densitometry revealed a significant increase in phosphorylated β-catenin (p-β-catenin) at 30 minutes that was sustained at 180 minutes (*P < 0.05, n = 3/condition). (F) Western blots confirmed decreased claudin-5 expression in simvastatin-treated (5 μM, 16 h) ECs transfected with either control siRNA or siRNA specific for β-catenin (siβ-catenin) compared with their respective, untreated controls (representative blots shown). Results are expressed as means (± SE).
Role of Claudin-5 in EC Barrier Protection by Simvastatin
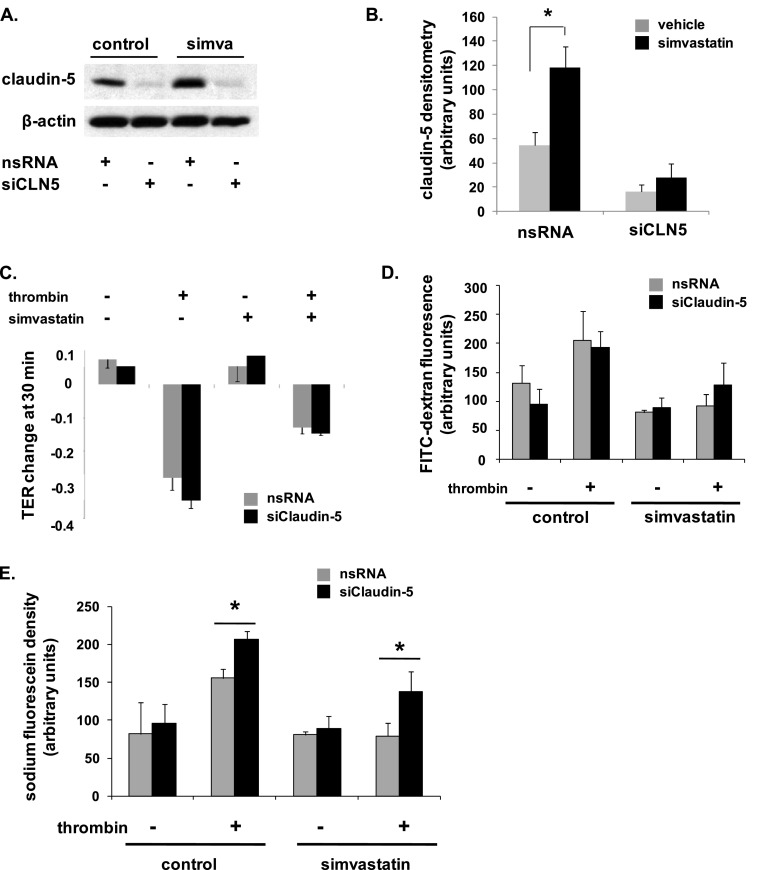
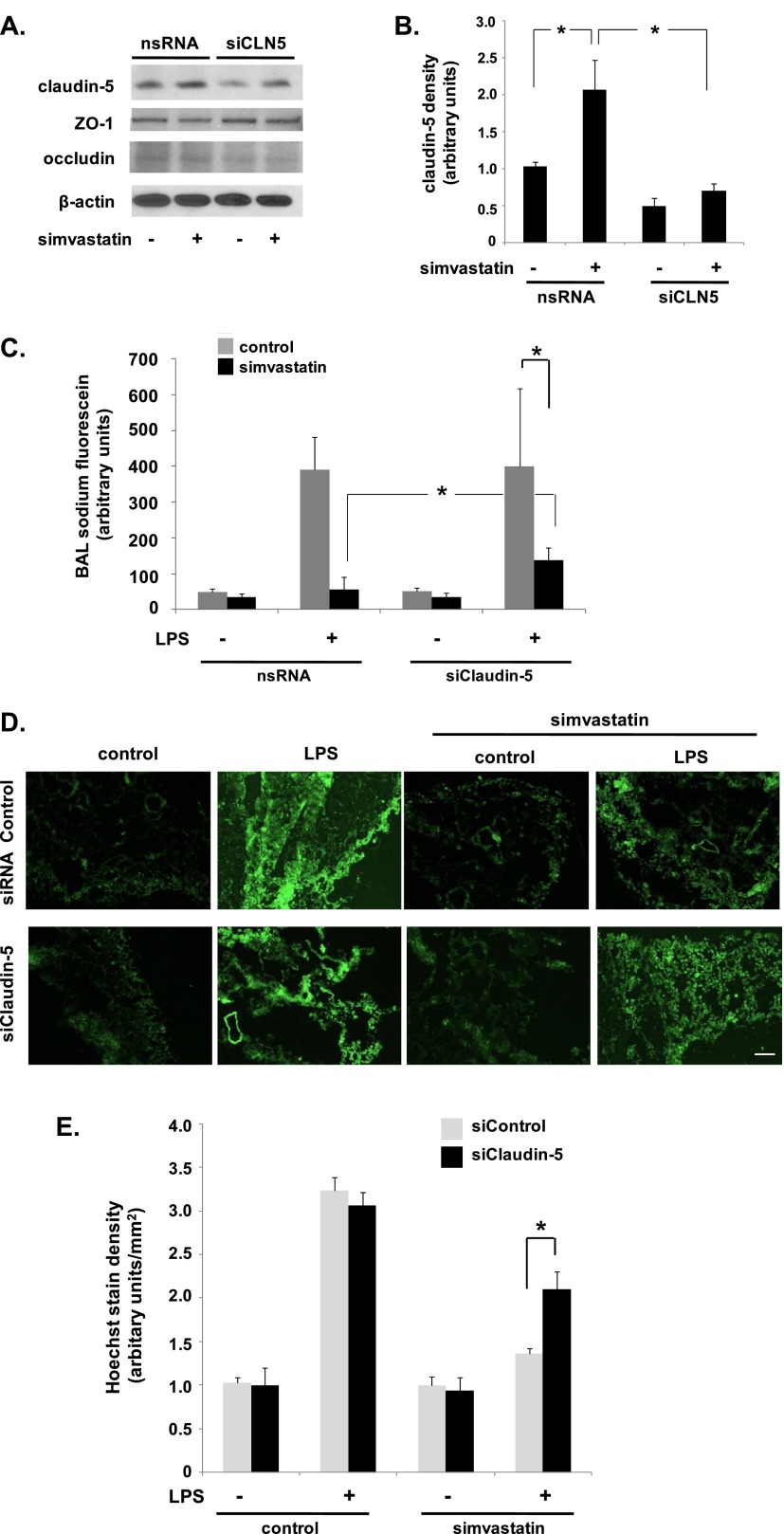
We have previously characterized potent EC barrier–protective properties of simvastatin both in vitro and in vivo (1–3). To determine the role of claudin-5 in simvastatin-mediated EC barrier protection, we initially measured TER in claudin-5–silenced ECs pretreated with simvastatin (5 μM, 16 h) before thrombin stimulation (1 U/ml). Maximal barrier disruption was achieved at 30 minutes after thrombin administration, and was significantly attenuated in simvastatin-treated ECs (Figure 4C). However, silencing of claudin-5 had no effect on either the degree of barrier disruption induced by thrombin or the degree of protection conferred by simvastatin. In separate experiments with identical experimental conditions, measurement of FITC–dextran across EC monolayers grown in transwell inserts also did not identify a significant effect of claudin-5 silencing on EC barrier function (Figure 4D).
Figure 4.
Differential effects of claudin-5 silencing on endothelial barrier function. (A) ECs were transfected with nsRNA or siRNA specific for claudin-5 (siCLN5) and then either remained untreated or were treated with simvastatin (5 μM, 16 h) with silencing subsequently confirmed by Western blotting (representative blots shown). (B) Densitometry confirmed a significant increase in claudin-5 expression in simvastatin-treated cells transfected with control siRNA compared with untreated controls and significantly decreased claudin-5 expression in siCLN5-transfected cells, with no effect in response to simvastatin. (C) Subsequently, transfected ECs were grown to confluence overlying gold-plated microelectrodes to measure transendothelial electrical resistance (TER), and were treated with simvastatin (5 μM, 16 h) before thrombin stimulation (1 U/ml). Fold TER change at 30 minutes is shown corresponding to the time to maximal barrier disruption after thrombin administration (n = 3/condition). (D) In separate experiments under the same conditions, transfected ECs were grown in transwell inserts and FITC–dextran (2,000 kD) monolayer flux measured 1 hour after thrombin (n = 3/group). (E) Finally, ECs were transfected with nsRNA or siCLN5 and grown on transwell inserts. Cells were treated with simvastatin (5 μM, 16 h) before measurements of sodium fluorescein (376 Da) monolayer flux 1 hour after thrombin stimulation (1 U/ml) (*P < 0.05, n = 3/group). Results are expressed as means (± SE).
As TER is an indirect measurement of barrier function, and the FITC–dextran used was a particularly large molecule (2,000 kD), we next studied the role of claudin-5 in EC barrier regulation assessed by transwell flux of the low–molecular weight marker, sodium fluorescein (376 Da). Although silencing of claudin-5 did not affect a significant change in permeability at baseline, it did result in both a significant increase in thrombin-induced permeability and a significant attenuation of simvastatin protection (Figure 4E).
Claudin-5 as a Mediator of Murine ALI Protection by Simvastatin
Having confirmed a size-selective effect of claudin-5 on EC barrier regulation in vitro, we sought to confirm a role for claudin-5 in simvastatin-mediated murine ALI protection. Mice were initially administered 10 mg/kg siRNA specific for claudin-5 (siCLN5) or nsRNA via intratracheal injection, and lungs were harvested from select animals for Western blotting of homogenates to confirm claudin-5 knockdown. After siRNA administration, animals were either untreated or pretreated with simvastatin (20 mg/kg via intraperitoneal injection, 16 h) and then received intratracheal LPS. Consistent with our prior report (1), these studies confirmed a significant protective effect of simvastatin on LPS-induced increases in BAL fluid cell counts and total protein levels at 24 hours, but there was no significant difference between mice treated with siCLN5 and nsRNA-treated control animals (data not shown). Notably, Western blotting of whole-lung homogenates demonstrated a significant decrease in claudin-5 protein levels associated with siCLN5 treatment relative to the respective untreated or simvastatin-treated controls that received nsRNA (Figures 5A and 5B).
Figure 5.
Silencing of claudin-5 attenuates simvastatin protection against LPS-induced lung vascular leak of low–molecular weight molecules in vivo. (A) Mice were administered siCLN5 (10 mg/kg, 3 d) or nsRNA via intratracheal injection before pretreatment with simvastatin (20 mg/kg via intraperitoneal injection, 16 h), followed by intratracheal LPS (1.25 mg/ml, 24 h). In select animals, whole-lung homogenates were subjected to Western blotting for claudin-5 to confirm silencing (representative blots shown). (B) Densitometry confirmed a significant increase in whole-lung claudin-5 expression in response to simvastatin and decreased claudin-5 expression in animals treated with siCLN5 that was not affected by simvastatin treatment (*P < 0.05, n = 3/group). (C) Mice were then administered sodium fluorescein (1 mg/ml) via the internal jugular (IJ) vein 24 hours after LPS, and bronchoalveolar lavage (BAL) was collected 1 hour later (*P < 0.05 compared with LPS control animals transfected with nsRNA and pretreated with simvastatin; n = 3/group). (D) In subsequent experiments under the same conditions, Hoechst stain H33258 (562 Da) was injected (IJ) 24 hours after LPS and lungs were then harvested after 2 hours. Fluorescent microscopy was used to assess extravasation of Hoechst into the lung (representative images shown). Scale bar, ×200. (E) Quantification (ImageJ; National Institutes of Health, Washington, DC) confirmed a significant increase in LPS-induced Hoechst stain extravasation in simvastatin-treated animals that were pretreated with siCLN5 compared with animals treated with control siRNA (*P < 0.05, n = 3/group). Results are expressed as means (± SE).
To assess a potential effect of claudin-5 on lung vascular permeability to small molecules in our ALI model, mice were administered sodium fluorescein (1 mg/ml) via the IJ vein 24 hours after LPS, and BAL was collected 1 hour later. These studies confirmed a significant attenuation of LPS-induced BAL fluid sodium fluorescein content by simvastatin that was attenuated in animals treated with siCLN5 (Figure 5C). Notably, siCLN5 had no effect on baseline or LPS-induced BAL sodium fluorescein levels in animals that did not receive simvastatin.
Finally, in subsequent experiments under the same conditions, Hoechst stain H33258 (562 Da) was injected (IJ) 24 hours after LPS, and lungs were then flushed and harvested after 2 hours. Fluorescent microscopy confirmed a significant extravasation of Hoechst in LPS-treated animals that was markedly attenuated by simvastatin pretreatment (Figures 5D and 5E). However, this effect of simvastatin was partially reversed in animals also treated with siCLN5.
Discussion
The potential role for statins in the treatment of ALI is an area of ongoing investigation, and is supported by abundant in vitro data (2, 3, 13), findings in animal models (1, 14–17), numerous observational reports involving relevant patient populations (18–21), as well as data from recent human studies (22, 23). These drugs are recognized to have pleiotropic properties, including numerous direct effects on EC signaling and activation. In this study, we further elucidate the remarkably complex effects of statins as they relate to their therapeutic potential in ALI, and characterize claudin-5 as a critical mediator of the protective properties of simvastatin in a murine model of ALI.
The endothelial barrier regulates permeability to fluid, proteins, and inflammatory cells via two distinct pathways: the paracellular pathway, which plays a prominent role in these functions, and the transcellular pathway (24). The paracellular pathway is regulated largely by tight junctional complexes comprised of two types of transmembrane proteins, occludins and claudins (25). The claudins represent a family of more than 20 proteins, 20–24 kD in size, characterized by four transmembrane domains, two extracellular loops, and two cytoplasmic tails. Their ability to regulate tight junctions is evidenced by the sensitive response to cell conductance and permeability to charged molecules affected by changes in claudin expression or by specific claudin mutations (26, 27). Claudins can also regulate cell permeability via size selectivity, as evidenced by increased permeability of small molecules (< 800 Da) across the blood–brain barrier of mice deficient in claudin-5 (6).
Despite the important role of claudins as regulators of tight junctional complexes and cell permeability, the role of claudin-5 in the increased vascular permeability associated with ALI remains poorly characterized. In a murine model of acroelin-induced ALI, decreased lung claudin-5 expression was found to be associated with increased susceptibility to injury (8). In a separate study, human lung microvascular ECs transfected with a replication-deficient human influenza virus were found to have increased permeability in association with increased degradation of claudin-5 (28). The functional role of claudin-5 in these studies, however, was not investigated.
We previously reported the protective effects of simvastatin in a murine model of ALI (1). These effects are associated with EC barrier protection conferred by simvastatin and mediated by differential Rho GTPase activation (2), inhibition of superoxide generation via reduced nicotinamide adenine dinucleotide phosphate oxidase (3), and the up-regulation of integrin-β4 (2). However, circumstantial evidence of a role for claudin-5 in ALI protection by statins is suggested by increased expression of EC claudin-5 in response to statin treatment (7, 29). Moreover, a functional link between statins and claudin-5 is suggested by statin regulation of integrin-β1 (30, 31), which has been identified as a mediator of increased vascular permeability via effects on claudin-5 (32).
This study demonstrates, for the first time, a role for claudin-5 on size-selective lung vascular permeability in ALI, and strongly suggests that the attenuation of agonist-induced EC permeability to small molecules by simvastatin, both in vitro and in vivo, is mediated by claudin-5. Moreover, evidence of membrane translocation of VE-cadherin in response to simvastatin and increased phosphorylation of FoxO1 is consistent with the observed increase in claudin-5 expression as the down-regulation of FoxO1, a transcriptional repressor of claudin-5, is mediated by its phosphorylation via PI3K-Akt in response to VE-cadherin clustering at the cell membrane (9). Notably, although it has been reported that β-catenin serves to augment FoxO1 binding to the claudin-5 promoter, and thus acts as a transcriptional repressor in this context (9), our results indicate that silencing of β-catenin is associated with a marked decrease in claudin-5 protein expression, suggesting that phosphorylated β-catenin is, in fact, a transcriptional activator of claudin-5. This is consistent with the known role of β-catenin in the transcriptional activation of other genes (33, 34). Accordingly, additional studies aimed at further characterizing the regulation of claudin-5 by both FoxO1 and β-catenin, as well as their functional interactions with each other, are needed.
A potential limitation of our study is the use of siRNA delivery in vivo via intratracheal administration, which raises questions regarding cell specificity. Although this approach is not specific for targeting of lung ECs, we were able to confirm both significant knockdown of whole-lung claudin-5 expression as well as significant effects on simvastatin-mediated ALI protection, thus strongly implicating a novel role for claudin-5 in this context. Moreover, as we have previously reported, there is evidence to suggest that the naked delivery of siRNA via intratracheal injection does, in fact, target lung ECs without evidence of marked nonspecific effects otherwise (12).
Although our findings are consistent with increased lung vascular permeability to small molecules associated with claudin-5 knockdown in our mouse model, we did not observe a significant effect on lung vascular permeability to inflammatory cells or large proteins, which raises the question as to the clinical significance of these observations. This is an important area of future investigation, although we speculate that these effects are relevant to the complex pathobiology of ALI, perhaps, for example, via increased paracellular flux of microparticles that contribute to injury (35). Accordingly, it is certainly possible that strategies aimed at augmenting either claudin-5 expression directly or upstream signaling pathways may lead to novel and effective therapies for patients with ALI. Our results also further bolster the justification for clinical trials, some of which are currently underway, to evaluate the efficacy of statins as a treatment in these patients.
Footnotes
This work was supported by National Institutes of Health grant HL096887 (J.R.J.).
Originally Published in Press as DOI: 10.1165/rcmb.2013-0058OC on September 12, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Jacobson JR, Barnard JW, Grigoryev DN, Ma SF, Tuder RM, Garcia JG. Simvastatin attenuates vascular leak and inflammation in murine inflammatory lung injury. Am J Physiol. 2005;288:L1026–L1032. doi: 10.1152/ajplung.00354.2004. [DOI] [PubMed] [Google Scholar]
- 2.Jacobson JR, Dudek SM, Birukov KG, Ye SQ, Grigoryev DN, Girgis RE, Garcia JG. Cytoskeletal activation and altered gene expression in endothelial barrier regulation by simvastatin. Am J Respir Cell Mol Biol. 2004;30:662–670. doi: 10.1165/rcmb.2003-0267OC. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Pendyala S, Natarajan V, Garcia JG, Jacobson JR. Endothelial cell barrier protection by simvastatin: GTPase regulation and NADPH oxidase inhibition. Am J Physiol. 2008;295:L575–L583. doi: 10.1152/ajplung.00428.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen W, Sammani S, Mitra S, Ma SF, Garcia JG, Jacobson JR. Critical role for integrin-β4 in the attenuation of murine acute lung injury by simvastatin. Am J Physiol. 2012;303:L279–L285. doi: 10.1152/ajplung.00361.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999;147:185–194. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood–brain barrier in claudin-5–deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morofuji Y, Nakagawa S, So G, Hiu T, Horai S, Hayashi K, Tanaka K, Suyama K, Deli MA, Nagata I, et al. Pitavastatin strengthens the barrier integrity in primary cultures of rat brain endothelial cells. Cell Mol Neurobiol. 2010;30:727–735. doi: 10.1007/s10571-010-9497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jang AS, Concel VJ, Bein K, Brant KA, Liu S, Pope-Varsalona H, Dopico RA, Jr, Di YP, Knoell DL, Barchowsky A, et al. Endothelial dysfunction and claudin 5 regulation during acrolein-induced lung injury. Am J Respir Cell Mol Biol. 2011;44:483–490. doi: 10.1165/rcmb.2009-0391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S, Dejana E. Endothelial adherens junctions control tight junctions by VE-cadherin–mediated upregulation of claudin-5. Nat Cell Biol. 2008;10:923–934. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]
- 10.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia JG, Siflinger-Birnboim A, Bizios R, Del Vecchio PJ, Fenton JW, II, Malik AB. Thrombin-induced increase in albumin permeability across the endothelium. J Cell Physiol. 1986;128:96–104. doi: 10.1002/jcp.1041280115. [DOI] [PubMed] [Google Scholar]
- 12.Mirzapoiazova T, Moitra J, Moreno-Vinasco L, Sammani S, Turner JR, Chiang ET, Evenoski C, Wang T, Singleton PA, Huang Y, et al. Non-muscle myosin light chain kinase isoform is a viable molecular target in acute inflammatory lung injury. Am J Respir Cell Mol Biol. 2011;44:40–52. doi: 10.1165/rcmb.2009-0197OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 14.Belli S, Basaran O, Ozdemir BH, Türkoğlu S, Karabay G, Kut A, Karakayali H, Haberal M. Protective role of simvastatin on lung damage caused by burn and cotton smoke inhalation in rats. J Surg Res. 2011;167:e283–e290. doi: 10.1016/j.jss.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 15.Kim JW, Rhee CK, Kim TJ, Kim YH, Lee SH, Yoon HK, Kim SC, Lee SY, Kwon SS, Kim KH, et al. Effect of pravastatin on bleomycin-induced acute lung injury and pulmonary fibrosis. Clin Exp Pharmacol Physiol. 2010;37:1055–1063. doi: 10.1111/j.1440-1681.2010.05431.x. [DOI] [PubMed] [Google Scholar]
- 16.Merx MW, Liehn EA, Janssens U, Lutticken R, Schrader J, Hanrath P, Weber C. HMG-CoA reductase inhibitor simvastatin profoundly improves survival in a murine model of sepsis. Circulation. 2004;109:2560–2565. doi: 10.1161/01.CIR.0000129774.09737.5B. [DOI] [PubMed] [Google Scholar]
- 17.Muller HC, Hellwig K, Rosseau S, Tschernig T, Schmiedl A, Gutbier B, Schmeck B, Hippenstiel S, Peters H, Morawietz L, et al. Simvastatin attenuates ventilator-induced lung injury in mice. Crit Care. 2010;14:R143. doi: 10.1186/cc9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almog Y, Shefer A, Novack V, Maimon N, Barski L, Eizinger M, Friger M, Zeller L, Danon A. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation. 2004;110:880–885. doi: 10.1161/01.CIR.0000138932.17956.F1. [DOI] [PubMed] [Google Scholar]
- 19.Kruger P, Fitzsimmons K, Cook D, Jones M, Nimmo G. Statin therapy is associated with fewer deaths in patients with bacteraemia. Intensive Care Med. 2006;32:75–79. doi: 10.1007/s00134-005-2859-y. [DOI] [PubMed] [Google Scholar]
- 20.O’Neal HR, Jr, Koyama T, Koehler EA, Siew E, Curtis BR, Fremont RD, May AK, Bernard GR, Ware LB. Prehospital statin and aspirin use and the prevalence of severe sepsis and acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2011;39:1343–1350. doi: 10.1097/CCM.0b013e3182120992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt H, Hennen R, Keller A, Russ M, Muller-Werdan U, Werdan K, Buerke M. Association of statin therapy and increased survival in patients with multiple organ dysfunction syndrome. Intensive Care Med. 2006;32:1248–1251. doi: 10.1007/s00134-006-0246-y. [DOI] [PubMed] [Google Scholar]
- 22.Craig TR, Duffy MJ, Shyamsundar M, McDowell C, O’Kane CM, Elborn JS, McAuley DF. A randomized clinical trial of hydroxymethylglutaryl-coenzyme A reductase inhibition for acute lung injury (the HARP study) Am J Respir Crit Care Med. 2011;183:620–626. doi: 10.1164/rccm.201003-0423OC. [DOI] [PubMed] [Google Scholar]
- 23.Liu PY, Liu YW, Lin LJ, Chen JH, Liao JK. Evidence for statin pleiotropy in humans: differential effects of statins and ezetimibe on Rho-associated coiled-coil containing protein kinase activity, endothelial function, and inflammation. Circulation. 2009;119:131–138. doi: 10.1161/CIRCULATIONAHA.108.813311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- 25.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol. 2002;283:C142–C147. doi: 10.1152/ajpcell.00038.2002. [DOI] [PubMed] [Google Scholar]
- 27.Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest. 2001;107:1319–1327. doi: 10.1172/JCI12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armstrong SM, Wang C, Tigdi J, Si X, Dumpit C, Charles S, Gamage A, Moraes TJ, Lee WL. Influenza infects lung microvascular endothelium leading to microvascular leak: role of apoptosis and claudin-5. PLoS One. 2012;7:e47323. doi: 10.1371/journal.pone.0047323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Béziaud T, Ru Chen X, El Shafey N, Fréchou M, Teng F, Palmier B, Beray-Berthat V, Soustrat M, Margaill I, Plotkine M, et al. Simvastatin in traumatic brain injury: effect on brain edema mechanisms. Crit Care Med. 2011;39:2300–2307. doi: 10.1097/CCM.0b013e3182227e4a. [DOI] [PubMed] [Google Scholar]
- 30.Takeda I, Maruya S, Shirasaki T, Mizukami H, Takahata T, Myers JN, Kakehata S, Yagihashi S, Shinkawa H. Simvastatin inactivates beta1-integrin and extracellular signal–related kinase signaling and inhibits cell proliferation in head and neck squamous cell carcinoma cells. Cancer Sci. 2007;98:890–899. doi: 10.1111/j.1349-7006.2007.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, Nishimura H, Losordo DW, Asahara T, Isner JM. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow–derived endothelial progenitor cells. Circulation. 2002;105:3017–3024. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 32.Huang RL, Teo Z, Chong HC, Zhu P, Tan MJ, Tan CK, Lam CR, Sng MK, Leong DT, Tan SM, et al. ANGPTL4 modulates vascular junction integrity by integrin signaling and disruption of intercellular VE-cadherin and claudin-5 clusters. Blood. 2011;118:3990–4002. doi: 10.1182/blood-2011-01-328716. [DOI] [PubMed] [Google Scholar]
- 33.Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W. Essential role of BCL9–2 in the switch between beta-catenin’s adhesive and transcriptional functions. Genes Dev. 2004;18:2225–2230. doi: 10.1101/gad.317604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282:11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McVey M, Tabuchi A, Kuebler WM. Microparticles and acute lung injury. Am J Physiol. 2012;303:L364–L381. doi: 10.1152/ajplung.00354.2011. [DOI] [PubMed] [Google Scholar]