Abstract
Pulmonary hypertension (PH) occurs in 25 to 35% of premature infants with significant bronchopulmonary dysplasia (BPD). Neonatal mice exposed to 14 days of hyperoxia develop BPD-like lung injury and PH. To determinne the impact of hyperoxia on pulmonary artery (PA) cyclic guanosine monophosphate (cGMP) signaling in a murine model of lung injury and PH, neonatal C57BL/6 mice were placed in room air, 75% O2 for 14 days (chronic hyperoxia [CH]) or 75% O2 for 24 hours, followed by 13 days of room air (acute hyperoxia with recovery [AHR]) with or without sildenafil. At 14 days, mean alveolar area, PA medial wall thickness (MWT), right ventricular hypertrophy (RVH), and vessel density were assessed. PA protein was analyzed for cGMP, soluble guanylate cyclase, and PDE5 activity. CH and AHR mice had RVH, but only CH mice had increased alveolar area and MWT and decreased vessel density. In CH and AHR PAs, soluble guanylate cyclase activity was decreased, and PDE5 activity was increased. In CH mice, sildenafil attenuated MWT and RVH but did not improve mean alveolar area or vessel density. In CH and AHR PAs, sildenafil decreased PDE5 activity and increased cGMP. Our results indicate that prolonged hyperoxia leads to lung injury, PH, RVH, and disrupted PA cGMP signaling. Furthermore, 24 hours of hyperoxia causes RVH and disrupted PA cGMP signaling that persists for 13 days. Sildenafil reduced RVH and restored vascular cGMP signaling but did not attenuate lung injury. Thus, hyperoxia can rapidly disrupt PA cGMP signaling in vivo with sustained effects, and concurrent sildenafil therapy can be protective.
Keywords: bronchopulmonary dysplasia, phosphodiesterases, soluble guanylate cyclase, right ventricular hypertrophy
Clinical Relevance
Using a murine model of hyperoxia-induced lung injury and pulmonary hypertension, we demonstrate that 24 hours of exposure to 75% O2 causes disrupted cyclic guanosine monophosphate (cGMP) signaling in the small pulmonary arteries of mice and right ventricular hypertrophy that persists long after exposure. In addition, treatment with low doses of sildenafil prevents hyperoxia-induced pulmonary hypertension and restores cGMP signaling in the small pulmonary arteries.
Bronchopulmonary dysplasia (BPD) is a well-described and common complication of prematurity. It has been recognized that 25 to 35% of infants with moderate to severe BPD develop pulmonary hypertension (PH) and right ventricular hypertrophy (RVH) (1–3). Infants with BPD have simplified alveolarization and stunted vascularization. Although there has been mixed success in the prevention of BPD using inhaled nitric oxide, vitamin A, and caffeine, there is no definitive treatment to prevent BPD (4–7). Moreover, the underlying pathophysiology involving BPD-associated PH is poorly understood, and there are no proven therapeutic options.
In this study, we used a previously described murine model to approximate BPD (8). Rodents are well suited for the study of BPD because their lungs at birth are structurally similar to human neonates born at 24 to 28 weeks of gestation (9). The murine model for hyperoxia-induced lung injury is well established: neonatal rodents exposed to hyperoxia for 14 days develop alveolar simplification, thickened alveolar septa, decreased vessel density, vascular remodeling, and RVH, similar to what is seen in the histology of infants with BPD-associated PH (10, 11). Although the lung signaling pathways involved in BPD pathogenesis have been extensively studied in this model, numerous questions remain regarding the pathogenesis and signaling alterations of BPD-associated PH.
The NO–cyclic guanosine monophosphate (cGMP) signaling pathway is a key regulator of perinatal vascular tone, but it has not been well studied in BPD-associated PH. This pathway offers multiple attractive potential targets for treatment, including the cGMP-specific phosphodiesterases (PDEs), which are a group of enzymes that regulate the downstream signaling of cyclic nucleotides, including cGMP. PDE5, the most prevalent isoform in the lung and pulmonary arteries (PAs), degrades cGMP into inactive GMP, thus limiting NO-mediated vasorelaxation. PDE5 has been shown to be important in the vascular transition that occurs after birth at term, and it is developmentally regulated after birth (12–14).
Growing evidence suggests that even brief exposure to high levels of O2 leads to damage that persists well beyond the initial exposure. For example, mice exposed to 72 hours of hyperoxia after birth had a significantly shortened life span (13). Pertinent to pulmonary vascular function, we recently demonstrated that in the PAs of adult mice, PDE5 activity significantly increased after just 45 minutes of hyperoxia, and in near-term sheep, 24 hours of ventilation with 100% O2 increased PA PDE5 activity (15, 16). Thus, the hyperoxia exposure that predisposes premature infants to BPD may also predispose them to increased PA PDE5 activity and decreased PA cGMP levels. Sildenafil is a selective PDE5 inhibitor approved to treat adult pulmonary arterial hypertension. Previous studies in a neonatal rat hyperoxia-induced lung injury model showed that high doses of sildenafil (50–150 mg/kg/d) improved alveolar growth, decreased RVH, and increased lung cGMP levels (17–19).
Despite these advances, significant questions remain. The specific signaling changes in the small PAs of mice with PH associated with hyperoxia-induced lung injury are not well understood. Furthermore, 14 days of hyperoxia is an exposure that extends long past the stage of lung development where preterm infants have typically weaned off O2 (9, 20). To address these gaps in knowledge, in the present study we developed a new methodology to isolate small PAs from 14-day–exposed mice and detected significant disruptions of cGMP signaling in small PAs from mice exposed to the “classic” hyperoxia-induced lung injury model. We found that significant changes in PA cGMP signaling and RVH were triggered after just 24 hours of hyperoxia exposure and were detectable even after 13 days despite recovery in room air. Finally, we demonstrate that sildenafil administration before and during hyperoxia exposure normalizes PA cGMP signaling and ameliorates RVH in 24-h–exposed mice and in 14-day–exposed mice.
Materials and Methods
Animal Protocols
This study was approved by the Institutional Animal Care and Use Committee at Northwestern University. Aged-matched C57Bl/6 litters (Charles River, Wilmington, MA) were placed in room air (control) or 75% O2 (chronic hyperoxia [CH]) in a Plexiglass chamber (Biospherix, Lacona, NY) within 24 hours of birth (10). Dams were rotated every 24 hours. After 14 days of exposure, pups were killed. Other aged-matched litters were exposed to room air (control) or 75% O2 (acute hyperoxia and recovery [AHR]) for 24 hours and were allowed to recover in room air for 13 days. Pulmonary artery protein was isolated using an iron particle infusion (15) and is described in more detail in the online supplement.
Sildenafil Treatment
Litters of control and CH pups received sildenafil (0.8 mg/ml sildenafil in 5% dextrose) (Revatio; Pfizer, New York, NY) or vehicle (5% dextrose) injections (3 mg/kg/dose, subcutaneous) every other day starting from P0, for a total of seven injections (21). AHR litters received one injection of sildenafil/vehicle before O2 exposure. Control mice for AHR received one injection of sildenafil/vehicle after birth and were left in room air for 14 days.
Measurement of RVH
Mouse hearts were dissected, and right ventricle (RV) and left ventricle plus septum (LV+S) were weighed. Fulton’s Index (RV weight divided by LV+S weight) was used to assess RVH (10).
Measurement of Medial Wall Thickness
PH was assessed by medial wall thickness (MWT) of small PAs and is further described in the online supplement. MWT was measured as the area of the small PA wall divided by the total PA area (10, 17).
Measurement of Alveolar Area
Lung morphometry was assessed as described in the online supplement. Mean alveolar area was measured using Scion software (Scion Corporation, Frederick, MD).
PDE5 Activity Assay
PA protein was assayed for cGMP-hydrolytic activity using a commercially available kit (Enzo, Farmingdale, NY) as described in the online supplement. Results are shown as PDE5-specific pmol cGMP hydrolyzed/min/mg total protein.
Cyclic GMP Enzyme Immunoassay
cGMP levels in the small PAs were measured by enzyme immunoassay using a commercially available kit (Cayman Chemical, Ann Arbor, MI) as described in the online supplement (16). Results are shown as pmol cGMP/ml/mg total protein.
Soluble Guanylate Cyclase Activity Assay
Soluble guanylate cyclase (sGC) activity assay was performed on PA protein as described in the online supplement (16). sGC activity results are shown as pmol cGMP/min/mg total protein.
Immunohistochemistry
von Willebrand factor and nitrotyrosine (3NT) staining was performed as described in the online supplement.
Western Blot Analysis
PA protein expression was assessed via Western blot for PDE5, sGCα, and sGCβ and is described in the online supplement (16). Data are shown as fold ± SEM relative to control mice.
Statistical Analysis
All data are expressed as means ± SEM, with “n” representing the number of animals in each group and significance at P < 0.05. Results were analyzed by ANOVA with post hoc Bonferroni’s analysis using Prism software (Graphpad Software Inc., San Diego, CA).
Results
Neonatal Mice Develop RVH and Increased Alveolar Area after 14 Days of Chronic O2 Exposure but Develop only RVH after 24 Hours of O2 Exposure
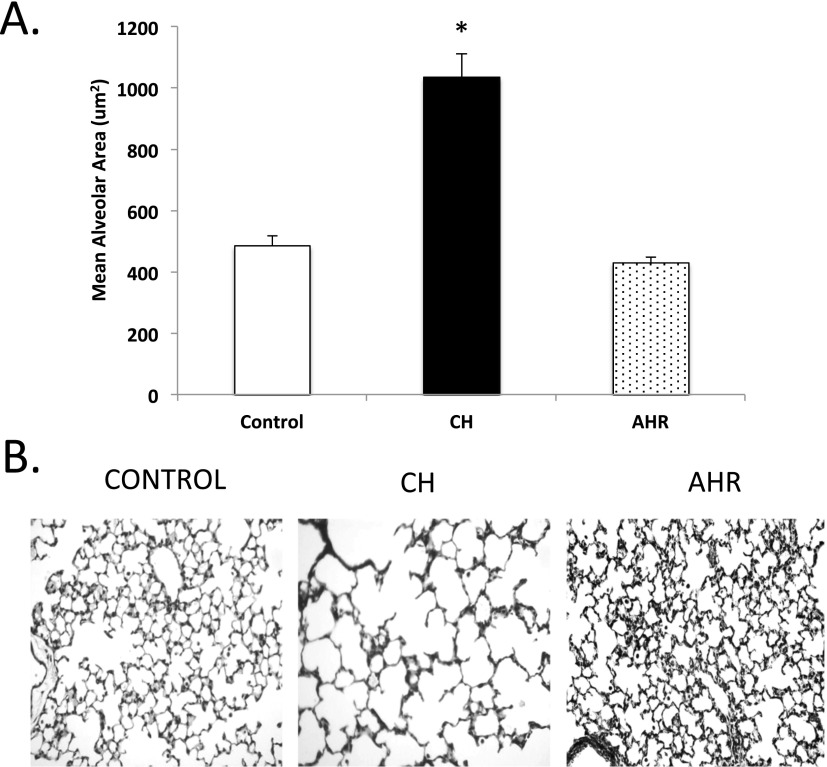
CH animals had a 2.1 ± 0.2-fold increase in mean alveolar area (Figure 1A). Representative hematoxylin images from the lungs are shown in Figure 1B. Chronic 14-day O2 exposure also led to vessel remodeling in the lungs, with increased MWT (Figure 2A) and RVH measured by Fulton’s index (Figure 2B). Representative vessel images are shown in Figure 2C.
Figure 1.
Mice develop alveolar simplification after 14 days of hyperoxia but not after 24 hours of O2 exposure. Mice exposed to hyperoxia (24 h [acute hyperoxia with recovery, AHR] or 14 d [chronic hyperoxia, CH]) or room air (controls) were killed, and lungs were inflation fixed. Hematoxylin-stained sections were imaged (original magnification: ×20), and mean alveolar area was measured using Scion software. (A) CH mice have increased mean alveolar area when compared with age-matched control mice, whereas AHR mice did not (n = 5–7 mice per group). (B) Representative hematoxylin-stained lung sections (original magnification: ×20) are shown for control, CH, and AHR mice. Data are shown as mean ± SEM. *P < 0.05 versus control mice.
Figure 2.
Right ventricular hypertrophy (RVH) and oxidative stress are present in CH and AHR mice, but only CH mice have remodeled vessels. Mice exposed to hyperoxia (24 h [AHR] or 14 d [CH]) or room air (controls) were killed, and lungs were inflation fixed and hearts were dissected. Lung sections were stained with hematoxylin and eosin (H&E) or nitrotyrosine antibody and were imaged (original magnification: ×40). Medial wall thickness (MWT) was measured using ImageJ. Fulton’s index was measured as the weight of the right ventricle (RV) divided by the weight of the left ventricle plus septum (LV+S). (A) CH mice (n = 43 vessels from six animals), but not AHR mice (n = 53 vessels from six animals), had increased MWT compared with control mice (n = 42 vessels from six mice). (B) Fulton’s index was significantly increased in CH (n = 15) and AHR mice (n = 14) compared with age-matched control mice (n = 19). (C) Representative H&E-stained lung sections (original magnification: ×40) for control, CH, and AHR mice. (D) CH and AHR mice have qualitative evidence of peroxynitrite formation in the pulmonary artery (PA), as evaluated by nitrotyrosine staining when compared with control mice. Representative fluorescent and phase images (original magnification: ×40) are shown for control, CH, and AHR mice. Data are shown as mean ± SEM. *P < 0.05 versus control mice. AW = airway.
The effects of acute O2 exposure followed by a 13-day recovery period were also investigated. Although the alveolar area and the MWT in the AHR mice remained unchanged from the control mice (Figures 1A and 2A), a small but significant and reproducible increase in RVH was measured by the Fulton’s index when compared with control mice (Figure 2B).
CH and AHR Mice Have Higher Oxidative Stress and Disrupted cGMP Signaling in the Small PAs
To evaluate if oxidative stress is increased in the PAs, lung sections of control, CH, and AHR mice were stained for 3NT, a marker of peroxynitrite formation. Qualitatively, these fluorescence images indicate that the PAs of CH and AHR have increased levels of 3NT staining. Representative images are shown in Figure 2D.
We also isolated small pulmonary arteries in 14-day mice exposed to O2 and analyzed the PA protein for sGC activity, PDE5 activity, cGMP content, and PDE5, sGCα, and sGCβ expression (Figure 3). In their small PAs, CH mice had significantly decreased sGC activity (Figure 3A), increased PDE5 activity (Figure 3B), and lower levels of cGMP (Figure 3C). Although protein expression for PDE5 and sGCα remained unchanged, sGCβ expression was decreased by about half when compared with control mice (Figure 3D).
Figure 3.
Cyclic guanosine monophosphate (cGMP) signaling is disrupted in the small PAs after exposure to hyperoxia. Mice exposed to hyperoxia (24 h [AHR] or 14 d [CH]) or room air (controls) were killed, and PA protein was harvested. (A) sGC activity is decreased in the small PAs of CH (n = 6) and AHR mice (n = 13) compared with aged-matched control mice (n = 7). (B) PDE5 activity is increased in the small PAs of CH (n = 11) and AHR mice (n = 17) compared with aged-matched control mice (n = 9). (C) cGMP levels are decreased in the small PAs of CH mice (n = 12) compared with aged-matched control mice (n = 14) but are not significantly lower in the small PAs of AHR mice (n = 12). (D) Expression was measured in PA protein by Western blot in control (n = 6), CH (n = 6), and AHR mice (n = 6 or 7). sGCβ expression is decreased in small PAs of CH mice, but PDE5 and sGCα expression is unchanged for all other groups. (E) Representative Western blots of PDE5, sGCα, and sGCβ expression that were normalized to β-actin. Data are shown as fold ± SEM. *P < 0.05 versus control mice.
AHR mice also exhibited significantly lower sGC activity (Figure 3A) and higher PDE5 activity (Figure 3B) in the small PAs. Trends toward lower cGMP levels were observed in AHR mice but were not significantly different from the control mice (P = 0.1) (Figure 3C). There were no significant changes in PDE5, sGCα, or sGCβ expression in the AHR mice (Figure 3D).
Sildenafil Does Not Improve Lung Morphometry or Vessel Density
We established in our CH mouse model that 14 days of hyperoxia significantly increased the mean alveolar area. Treatment of CH mice with low-dose sildenafil (3 mg/kg per dose every other day) did not improve the mean alveolar area in these mice when compared with vehicle-treated mice (Figure 4A). Representative hematoxylin-stained lung sections of the treated mice are shown in Figure 4B.
Figure 4.
Sildenafil does not improve lung morphometry in CH mice. Vehicle- or sildenafil-treated mice exposed to hyperoxia (24 h [AHR] or 14 d [CH]) or room air (controls) were killed, and lungs were inflation fixed. Hematoxylin-stained sections were imaged (original magnification: ×20), and mean alveolar area was measured using Scion software. (A) Mean alveolar area is unchanged in sildenafil-treated CH mice when compared with vehicle-treated mice (n = 5–7 animals per group). (B) Representative hematoxylin-stained lung sections of vehicle- or sildenafil-treated control and CH mice (original magnification: ×20). Data are shown as mean ± SEM. *P < 0.05 versus control vehicle. #P < 0.05 versus control sildenafil.
We also investigated the effects of neonatal hyperoxia on the vessel density in these mice by immunostaining the small nonmuscularized vessels with von Willebrand factor. As previously reported (11), we observed decreased vascular growth in CH+vehicle mice compared with control+vehicle mice (P < 0.05) (Figure 5A). Sildenafil treatment did not improve vascular growth in CH+sildenafil compared with CH+vehicle mice (Figure 5A). Representative phase and fluorescent images from the lung sections are shown in Figure 5C. Vessel density is also unaffected in the AHR mice when compared with control mice, and sildenafil had no further effect on vascular growth (Figures 5B and 5D).
Figure 5.
Vessel density is decreased in CH mice but not in AHR mice, and sildenafil does not improve vascularization. Vehicle- or sildenafil-treated mice exposed to hyperoxia (24 h [AHR] or 14 d [CH]) or room air (controls) were killed, and lungs were inflation fixed. Vessels were labeled by von Willebrand factor staining and imaged (original magnification: ×10) using a Nikon Eclipse TE-300 fluorescent microscope. (A) Vessel density is decreased in CH mice when compared with control+vehicle mice and remains lower after treatment with sildenafil (n = 8–10 images per high powered field, four animals per group). (B) Vessel density is unchanged in AHR and control mice (n = 8–10 images per high powered field, four animals per group). (C and D) Representative phase and fluorescent images (original magnification: ×10) are shown for vehicle- and sildenafil-treated CH, AHR, and control mice. Data are shown as mean ± SEM. *P < 0.05 versus control+vehicle. #P < 0.05 versus control+sildenafil.
Sildenafil Prevents Vascular Remodeling in CH Mice
Although sildenafil treatment does not repair mean alveolar area or vascular density in CH mice, it significantly decreases vascular remodeling by reducing MWT in small PAs compared with CH+vehicle mice (Figure 6A). Representative hematoxylin and eosin–stained lung sections from these mice are shown in Figure 6B.
Figure 6.
Sildenafil protects hyperoxia-induced vessel remodeling and RVH in CH and AHR mice. Vehicle- or sildenafil-treated mice exposed to hyperoxia (24 h [AHR] or 14 d [CH]) or room air (controls) were killed, and lungs were inflation fixed and hearts were dissected. H&E-stained sections were imaged (original magnification: ×40), and MWT was measured using ImageJ. Fulton’s index was measured as the weight of the RV divided by LV+S. (A) MWT was decreased in CH+sildenafil mice (n = 50 vessels from seven mice) when compared with CH+vehicle (n = 48 vessels from five mice). MWT for CH+sildenafil was also statistically similar to control mice (n = 40 vessels from five mice for control+vehicle; n = 56 vessels from six animals for control+sildenafil). (B) Representative H&E-stained lung sections from vehicle- and sildenafil-treated control and CH mice. (C) RVH is decreased in CH+sildenafil mice (n = 8) when compared with CH+vehicle (n = 6). Fulton’s index for CH+sildenafil was similar to control mice (n = 5 for control vehicle; n = 6 for control sildenafil). (D) RVH is decreased in AHR+sildenafil mice (n = 12) when compared with AHR+vehicle animals (n = 11). Sildenafil-treated AHR animals were also similar to control mice (n = 5 for control vehicle; n = 6 for control sildenafil). Data are shown as mean ± SEM. *P < 0.05 versus control+vehicle. &P < 0.05 versus CH+vehicle.
Sildenafil Reduces RVH in CH and AHR Mice
Sildenafil-treated mice also demonstrated attenuated RVH. CH+sildenafil mice had significantly lower Fulton’s index compared with CH+vehicle mice and were statistically similar to the control+sildenafil mice (Figure 6C).
We also observed that a single dose of sildenafil prevented the effects of acute hyperoxia in the AHR mice. Figure 6D shows that the Fulton’s index is reduced in the AHR+sildenafil mice when compared with the AHR+vehicle mice.
Sildenafil Repairs the Hyperoxia-Induced Disrupted Components of the cGMP Signaling Pathway
Sildenafil attenuated hyperoxia-induced changes in PDE5 activity and cGMP levels in the small PAs of CH mice. PDE5 activity was significantly decreased in CH+sildenafil versus CH+vehicle mice (Figure 7A). We detected measurable PA PDE5 activity in CH+sildenafil animals, which confirms that the lower drug dose selected for this study does not completely abolish PDE5 activity. Figure 7C shows that sildenafil increases vascular cGMP levels when compared with CH+vehicle mice.
Figure 7.
Sildenafil restores PDE5 activity in the small PA of CH and AHR mice and in PA cGMP levels in CH mice. Vehicle- or sildenafil-treated mice exposed to hyperoxia (24 h [AHR] or 14 d [CH]) or room air (controls) were killed, and PA protein was harvested. (A) PDE5 activity is decreased in the small PAs of CH+sildenafil mice (n = 12) when compared with CH+vehicle (n = 11). (B) PDE5 activity is decreased in the small PAs of AHR+sildenafil mice (n = 13) when compared with AHR+vehicle (n = 12). (C) Sildenafil restores cGMP levels in the small PAs of CH+sildenafil mice (n = 11) when compared with CH+vehicle (n = 12). (D) Sildenafil increases cGMP levels in the small PAs of AHR mice (n = 11) when compared with AHR+vehicle (n = 8). Data are shown as mean ± SEM. *P < 0.05 versus control mice. &P < 0.05 versus CH+vehicle or AHR+vehicle.
In the small PAs of AHR mice, treatment with sildenafil significantly decreased PDE5 activity (Figure 7B). cGMP levels were also significantly increased in the AHR+sildenafil mice as compared with AHR+vehicle mice (Figure 7D).
Discussion
This study provides the first evidence that cGMP signaling is disrupted in the small PAs of mice with hyperoxia-associated PH. We show that neonatal CH mice have simplified alveoli, remodeled vessels, and RVH. The CH mice also have qualitatively higher levels of 3NT, a marker of oxidative stress, in the small PAs. Futhermore, cGMP signaling is disrupted in the small PAs of CH mice. We also show for the first time that lasting cardiovascular injury occurs rapidly after brief exposure to hyperoxia. An acute exposure to 24 hours of high levels of O2 was sufficient to cause disrupted vascular cGMP signaling and RVH that persists after 13 days of room air recovery. The small PAs also retain qualitatively higher levels of 3NT despite the short hyperoxia exposure period when compared with control mice. Finally, we demonstrate that low-dose sildenafil successfully prevented hyperoxia-induced RVH and pulmonary vascular remodeling and restored cGMP signaling in the small PAs in the acute and 14-day–exposed mice.
It has been previously shown in term lambs that cGMP signaling is disrupted in persistent PH of the newborn (PPHN) (16, 22). However, all information regarding pulmonary vascular signaling in murine BPD models has been derived from analysis of whole lung (11, 13, 18, 19, 23), which includes multiple other cell types that may have similar or different responses as compared with vascular endothelial and smooth muscle cells. In the present study, we focused on hyperoxia-induced disruptions of the cGMP signaling pathway by using a novel technique to isolate small PAs from 14-day-old mice. Similar to our previous observations in neonatal lambs with PPHN (16), hyperoxia exposure decreases sGC activity and cGMP and dramatically increases PDE5 activity. We saw no changes in PDE5 expression in the small PAs, indicating that there was likely posttranslational modification of PDE5 in the small PAs. Our study represents the first report of vascular cGMP signaling disruption in mice with BPD-associated PH and provides important new information to support future investigations of potential clinical therapies, such as sGC activators and PDE5 inhibitors.
By 14 days, the lung development of the mouse has structurally matured to a stage equivalent to a 2-year-old human (9, 20). Thus, a more prolonged exposure to hyperoxia may not fully mimic the effects of O2 exposure in human infants at risk for BPD. We have previously published that even a brief 30-minute exposure to hyperoxia induces significant oxidant stress and disrupted cGMP signaling in ovine fetal pulmonary artery smooth muscle cells (15). We further demonstrated that hyperoxic ventilation of adult mice for just 45 minutes triggers changes in pulmonary vascular PDE5 activity and cGMP levels (15). Here, we demonstrate for the first time that newborn mice pups exposed to just 24 hours of 75% O2 followed by 13 days of room air recovery still have significant RVH as well as decreased sGC activity and increased PDE5 activity in the small PAs. These results suggest that cGMP signaling in the PA is affected rapidly after exposure to hyperoxia and that these changes persist long after the initial exposure. Increased PDE5 activity persists despite unchanged protein expression. The mechanism of persistent PDE5 activity is unknown but may be due to hyperoxia-induced epigenetic changes in the enzymes regulating PDE5 activity, such as cGMP-dependent protein kinase. Our results may help explain why some premature infants with BPD and PH develop persistent abnormalities in pulmonary vascular tone long after their baseline clinical status has improved. These data may also partially explain why these infants remain at higher risk for clinical decompensation and intensive care unit readmission with later insults (24).
Finally, we examined the role of sildenafil as a possible preventative therapy for BPD-associated PH. Sildenafil, a PDE5 inhibitor, is FDA approved to treat adult pulmonary arterial hypertension. Recent clinical trials have explored the use of sildenafil for the treatment of PH in term infants. A small placebo-controlled study by Baquero and colleagues showed improved oxygenation and survival in term infants with PPHN who received sildenafil versus placebo (85% vs. 17%) (25). A recent clinical trial of intravenous sildenafil in 36 term infants with PPHN also showed improved oxygenation (26). However, results in preterm infants with BPD-associated PH have not been as positive. Most recently, Nyp and colleagues demonstrated that in infants with established BPD and PH, late administration of sildenafil improved PA pressures but had no effect on oxygenation or need for ventilatory support (27–29).
Our study focused on the use of low-dose sildenafil for relative brief periods of time. This is an important and timely consideration because of the August 2012 warning released by the FDA against the use of sildenafil for pediatric patients (ages 1–17 yr) with pulmonary arterial hypertension (30). The FDA warning stemmed from an analysis of the long-term follow-up data associated with an extension phase of the STARTS trial, which reported that children chronically taking a high dose of Revatio had a higher risk of death than children taking a low dose (31). As noted in reviews of this study (32, 33), there was no increase in mortality for low-dose patients who uptitrated to a higher dose of sildenafil, and there were issues related to standardization of care because this study was conducted in several countries with significant variability in rates and availability of follow-up care.
Given this complicated clinical landscape, it is important to note that we used subcutaneous injections of sildenafil at a dosage of 3 mg/kg every other day, which is significantly lower than the 50 to 150 mg/kg/d used in other sildenafil studies in the rat hyperoxia-induced lung injury model (17–19) and lower than the high-dose group reported in the STARTS-2 trial (34). Based on the original cross-species pharmacokinetic analysis, we believe the low dose used in the present study more closely mimics the doses given to term infants in clinical trials (26, 35). Even at this low dose, we observed an improvement in RVH and MWT in our 14-day hyperoxia–exposed mice, together with restoration of normal cGMP signaling in the small PA. Previously, it has been reported that sildenafil (at 100 mg/kg) improves alveolar and vascular growth in rats (17, 19). Low-dose sildenafil failed to improve the alveolarization or vascularization within the lungs in our 14-day hyperoxia–exposed mice. Thus, it appears that at this low dose, sildenafil improves PH and RVH via normalization of PA cGMP signaling and pulmonary vascular tone but does not prevent the underlying lung injury. Another possible reason for this discrepancy could be the fact that the mice were only injected every other day, but we found that daily injections caused the neonatal mice and the dams to be stressed and thus scaled back the dosage to every other day. We speculate that although sildenafil may represent an important option for the prevention and/or treatment of PH in preterm infants, it is unlikely to improve lung function or ventilatory parameters at any clinically relevant dose.
In conclusion, we have developed a novel technique to isolate small PAs in 14-day mice, which allows for more specific study of vascular signaling pathways in the developing mouse lung. We show that 14 days of hyperoxia causes RVH, vascular remodeling, and decreased alveolarization and vessel formation, consistent with previous literature (10, 36), and demonstrate for the first time disrupted cGMP signaling in the small PAs of these mice. We also show for the first time that relatively brief exposures of hyperoxia in newborn mice caused RVH and disrupted PA cGMP signaling that persisted for 13 days after the initial exposure. Finally, we demonstrate that low doses of sildenafil prevent RVH and vessel remodeling and restore normal patterns of cGMP signaling in the small PAs. Our findings suggest the need for future studies investigating sildenafil as a rescue therapy for the cardiovascular complications of BPD-associated PH because systemic sildenafil therapy may restore normal cGMP signaling in resistance PA.
Acknowledgments
Acknowledgments
The authors thank Dr. Gregory Waypa for assistance with the development of the iron-particle infusion technique to isolate small PA, Dr. Molly Ball for instruction in the techniques for vessel wall thickness measurement and heart dissection, and Jennifer Whitesides for assistance in mouse surgeries.
Footnotes
This study was supported by National Institutes of Health Grants HD052902 (S.B.), HL54705 (R.H.S.), HD007446 (K.M.O.), and HL109478 (K.F.) and by the Society of Pediatric Research Summer Research Program (K.M.O.).
Author Contributions: K.J.L., S.K.B., G.A.K., J.M.T., K.M.O., and K.N.F. planned, designed, and performed the experiments. K.J.L., S.K.B., and K.N.F. analyzed data. K.J.L., S.K.B., R.H.S., and K.N.F. contributed to the writing of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents online at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0118OC on September 13, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.An HS, Bae EJ, Kim GB, Kwon BS, Beak JS, Kim EK, Kim HS, Choi JH, Noh CI, Yun YS. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ J. 2010;40:131–136. doi: 10.4070/kcj.2010.40.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, Mullen MP. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics. 2007;120:1260–1269. doi: 10.1542/peds.2007-0971. [DOI] [PubMed] [Google Scholar]
- 3.Check J, Gotteiner N, Liu X, Su E, Porta N, Steinhorn R, Mestan KK. Fetal growth restriction and pulmonary hypertension in premature infants with bronchopulmonary dysplasia. J Perinatol. 2013;33:553–557. doi: 10.1038/jp.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PL, Merrill JD, Walsh MC, Durand DJ, Mayock DE, Eichenwald EC, et al. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med. 2006;355:343–353. doi: 10.1056/NEJMoa061088. [DOI] [PubMed] [Google Scholar]
- 5.Kinsella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart C, Sekar KC, Auten RL, Bhutani VK, Gerdes JS, et al. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. N Engl J Med. 2006;355:354–364. doi: 10.1056/NEJMoa060442. [DOI] [PubMed] [Google Scholar]
- 6.Tyson JE, Wright LL, Oh W, Kennedy KA, Mele L, Ehrenkranz RA, Stoll BJ, Lemons JA, Stevenson DK, Bauer CR, et al. Vitamin a supplementation for extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med. 1999;340:1962–1968. doi: 10.1056/NEJM199906243402505. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, Solimano A, Tin W. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354:2112–2121. doi: 10.1056/NEJMoa054065. [DOI] [PubMed] [Google Scholar]
- 8.Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol. 1998;275:L110–L117. doi: 10.1152/ajplung.1998.275.1.L110. [DOI] [PubMed] [Google Scholar]
- 9.Amy RW, Bowes D, Burri PH, Haines J, Thurlbeck WM. Postnatal growth of the mouse lung. J Anat. 1977;124:131–151. [PMC free article] [PubMed] [Google Scholar]
- 10.Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA, Kourembanas S. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. 2009;180:1122–1130. doi: 10.1164/rccm.200902-0242OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balasubramaniam V, Mervis CF, Maxey AM, Markham NE, Abman SH. Hyperoxia reduces bone marrow, circulating, and lung endothelial progenitor cells in the developing lung: implications for the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1073–L1084. doi: 10.1152/ajplung.00347.2006. [DOI] [PubMed] [Google Scholar]
- 12.Maclean MR, Johnston ED, McCulloch KM, Pooley L, Houslay MD, Sweeney G. Phosphodiesterase isoforms in the pulmonary arterial circulation of the rat: changes in pulmonary hypertension. J Pharmacol Exp Ther. 1997;283:619–624. [PubMed] [Google Scholar]
- 13.Yee M, White RJ, Awad HA, Bates WA, McGrath-Morrow SA, O'Reilly MA. Neonatal hyperoxia causes pulmonary vascular disease and shortens life span in aging mice. Am J Pathol. 2011;178:2601–2610. doi: 10.1016/j.ajpath.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farrow KN, Steinhorn RH. Phosphodiesterases: emerging therapeutic targets for neonatal pulmonary hypertension. Handb Exp Pharmacol. 2011;204:251–277. doi: 10.1007/978-3-642-17969-3_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farrow KN, Lee KJ, Perez M, Schriewer JM, Wedgwood S, Lakshminrusimha S, Smith CL, Steinhorn RH, Schumacker PT. Brief hyperoxia increases mitochondrial oxidation and increases phosphodiesterase 5 activity in fetal pulmonary artery smooth muscle cells. Antioxid Redox Signal. 2012;17:460–470. doi: 10.1089/ars.2011.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrow KN, Lakshminrusimha S, Czech L, Groh BS, Gugino SF, Davis JM, Russell JA, Steinhorn RH. Superoxide dismutase and inhaled nitric oxide normalize phosphodiesterase 5 expression and activity in neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2010;299:L109–L116. doi: 10.1152/ajplung.00309.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ladha F, Bonnet S, Eaton F, Hashimoto K, Korbutt G, Thebaud B. Sildenafil improves alveolar growth and pulmonary hypertension in hyperoxia-induced lung injury. Am J Respir Crit Care Med. 2005;172:750–756. doi: 10.1164/rccm.200503-510OC. [DOI] [PubMed] [Google Scholar]
- 18.de Visser YP, Walther FJ, Laghmani el H, Boersma H, van der Laarse A, Wagenaar GT. Sildenafil attenuates pulmonary inflammation and fibrin deposition, mortality and right ventricular hypertrophy in neonatal hyperoxic lung injury. Respir Res. 2009;10:30. doi: 10.1186/1465-9921-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park HS, Park JW, Kim HJ, Choi CW, Lee HJ, Kim BI, Chun YS. Sildenafil alleviates bronchopulmonary dysplasia in neonatal rats by activating the hypoxia-inducible factor signaling pathway. Am J Respir Cell Mol Biol. 2013;48:105–113. doi: 10.1165/rcmb.2012-0043OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thurlbeck WM. Postnatal human lung growth. Thorax. 1982;37:564–571. doi: 10.1136/thx.37.8.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Chopp M, Szalad A, Liu Z, Bolz M, Alvarez FM, Lu M, Zhang L, Cui Y, Zhang RL, et al. Phosphodiesterase-5 is a therapeutic target for peripheral neuropathy in diabetic mice. Neuroscience. 2011;193:399–410. doi: 10.1016/j.neuroscience.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deruelle P, Grover TR, Abman SH. Pulmonary vascular effects of nitric oxide-cGMP augmentation in a model of chronic pulmonary hypertension in fetal and neonatal sheep. Am J Physiol Lung Cell Mol Physiol. 2005;289:L798–L806. doi: 10.1152/ajplung.00119.2005. [DOI] [PubMed] [Google Scholar]
- 23.Thebaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation. 2005;112:2477–2486. doi: 10.1161/CIRCULATIONAHA.105.541524. [DOI] [PubMed] [Google Scholar]
- 24.Ambalavanan N, Carlo WA, McDonald SA, Yao Q, Das A, Higgins RD. Identification of extremely premature infants at high risk of rehospitalization. Pediatrics. 2011;128:e1216–e1225. doi: 10.1542/peds.2011-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baquero H, Soliz A, Neira F, Venegas ME, Sola A. Oral sildenafil in infants with persistent pulmonary hypertension of the newborn: a pilot randomized blinded study. Pediatrics. 2006;117:1077–1083. doi: 10.1542/peds.2005-0523. [DOI] [PubMed] [Google Scholar]
- 26.Steinhorn RH, Kinsella JP, Pierce C, Butrous G, Dilleen M, Oakes M, Wessel DL.Intravenous sildenafil in the treatment of neonates with persistent pulmonary hypertension J Pediatr 2009155841–847.e841. [DOI] [PubMed] [Google Scholar]
- 27.Nyp M, Sandritter T, Poppinga N, Simon C, Truog WE. Sildenafil citrate, bronchopulmonary dysplasia and disordered pulmonary gas exchange: any benefits? J Perinatol. 2012;32:64–69. doi: 10.1038/jp.2011.131. [DOI] [PubMed] [Google Scholar]
- 28.Farrow KN, Steinhorn RH. Sildenafil therapy for bronchopulmonary dysplasia: not quite yet. J Perinatol. 2012;32:1–3. doi: 10.1038/jp.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mourani PM, Sontag MK, Ivy DD, Abman SH. Effects of long-term sildenafil treatment for pulmonary hypertension in infants with chronic lung disease. J Pediatr. 2009;154:379–384. doi: 10.1016/j.jpeds.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.U.S. Food and Drug Administration. Revatio (sildenafil): drug safety communication--recommendation against use in children [updated 2012 Aug 30; accessed 2013 Feb 20]. Available from: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm317743.htm
- 31.Barst RJ, Ivy DD, Gaitan G, Szatmari A, Rudzinski A, Garcia AE, Sastry BK, Pulido T, Layton GR, Serdarevic-Pehar M, et al. A randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naive children with pulmonary arterial hypertension. Circulation. 2012;125:324–334. doi: 10.1161/CIRCULATIONAHA.110.016667. [DOI] [PubMed] [Google Scholar]
- 32.Abman SH, Kinsella JP, Rosenzweig EB, Krishnan U, Kulik T, Mullen M, Wessel DL, Steinhorn R, Adatia I, Hanna B, et al. Implications of the FDA warning against the use of sildenafil for the treatment of pediatric pulmonary hypertension. Am J Respir Crit Care Med. 2013;187:572–575. doi: 10.1164/rccm.201210-1928PP. [DOI] [PubMed] [Google Scholar]
- 33.Steinhorn RH, Kinsella JP, Abman SH. Beyond pulmonary hypertension: sildenafil for chronic lung disease of prematurity. Am J Respir Cell Mol Biol. 2013;48:iii–v. doi: 10.1165/rcmb.2012-0441ED. [DOI] [PubMed] [Google Scholar]
- 34.Barst RJ, Layton GR, Konourina I, Richardson H, Beghetti M, Ivy DD. STARTS-2: long-term survival with oral sildenafil monotherapy in treatment-naive patients with pediatric pulmonary arterial hypertension. Eur Heart J. 2012;33:979. doi: 10.1161/CIRCULATIONAHA.113.005698. [DOI] [PubMed] [Google Scholar]
- 35.Walker DK, Ackland MJ, James GC, Muirhead GJ, Rance DJ, Wastall P, Wright PA. Pharmacokinetics and metabolism of sildenafil in mouse, rat, rabbit, dog and man. Xenobiotica. 1999;29:297–310. doi: 10.1080/004982599238687. [DOI] [PubMed] [Google Scholar]
- 36.Balasubramaniam V, Ryan SL, Seedorf GJ, Roth EV, Heumann TR, Yoder MC, Ingram DA, Hogan CJ, Markham NE, Abman SH. Bone marrow-derived angiogenic cells restore lung alveolar and vascular structure after neonatal hyperoxia in infant mice. Am J Physiol Lung Cell Mol Physiol. 2010;298:L315–L323. doi: 10.1152/ajplung.00089.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]