Abstract
We describe what appears to be the first case of spondylodiscitis due to Candida dubliniensis. Our case adds to the current literature of the importance of C. dubliniensis as a cause of fungemia and subsequent deep seated infections. It highlights the importance of taking fungal as well as bacterial culture from biopsy specimens in patients with spondylodiscitis. We also review the literature covering the reported cases of Candida spondylodiscitis, which amount to about 100 over the last 5 decades.
Keywords: Spondylodiscitis, Vertebral osteomyelitis, Candida dubliniensis, Candida spp., Invasive fungal disease
1. Introduction
Spondylodiscitis is usually due to pyogenic bacteria (e.g. Staphylococcus aureus), but Mycobacterium tuberculosis and fungi are also occasionally involved [1,2]. Early recognition and timely intervention are important for successful management of osteomyelitis and spondylodiscitis. Blood cultures yield the etiological agent of pyogenic spondylodiscitis in more than a half of the cases. However, the yield may be significantly lower in non-pyogenic spinal infections such as fungal spondylodiscitis where fungemia may have been present only several months previously [3,4]. Surgical or needle biopsy specimens from the affected vertebral body or adjacent tissues are the cornerstone on etiological diagnosis. The importance of isolation of the etiological agent is underscored by the fact that treatment regimens for pyogenic bacteria, mycobacteria and fungi are completely different. Also, narrow-spectrum treatment options should be preferred to ameliorate the resistance problems related to the long treatments required.
No more than 5% of the cases of spondylodiscitis are caused by fungi; of these, Candida species are the most frequent agents [2]. Candida spondylodiscitis usually affects immunocompromised patients after hematogenous dissemination [5]. We report what appears to be the first case of spondylodiscitis caused by Candida dubliniensis in an immunocompetent patient.
2. Case
A 37-year old male who was an intravenous drug addict with chronic hepatitis C infection presented in May 2011 with severe lumbosacral pain. The pain radiated intermittently to both lower limbs but was not associated with any consistent changes in physical examination. Radiography of the lumbosacral spine in June 2011 was normal. As the pain escalated and became daily, new radiographs were obtained 1 month later, and now narrowing of the presacral space and a blurry edge adjacent to the fifth lumbar vertebral body were observed. This finding raised a suspicion of an infectious process and the patient was referred to our clinic. On admission (day 0) the erythrocyte sedimentation rate (ESR) was 45 mm/h, the C-reactive protein (CRP) 13 mg/l, the leukocytes 6.5×109/l and the hemoglobin 135 g/l. Magnetic resonance imaging (MRI) of the lumbosacral region on day 1 showed spondylodiscitis in the presacral space with bone edema, blurry edges of the end plates of the presacral discus, which was narrowed, and a presacral phlegmon 1 cm by depth (Fig. 1).
Fig. 1.
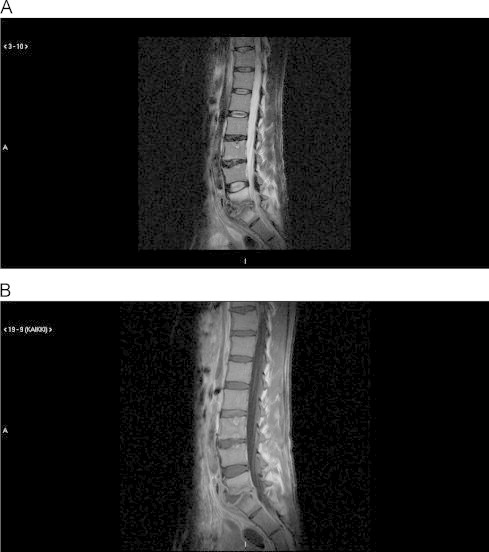
Magnetic resonance imaging (MRI) of the lumbosacral region on July 12th showed LV-SI spondylodiscitis and presacral phlegmon. (A) T2 image and (B) T1 image with gadolinium contrast.
On day 3 the patient was prepared for a needle biopsy in general anesthesia. After induction of anesthesia with midazolam, fentanyl, propofol and rocuronium bromide the patient sustained an anaphylactic reaction and the procedure was discontinued. After epinephrine and norepinephrine and corticosteroid injections he was admitted to intensive care unit. The procedure was postponed until after he had been tested for allergies and he was referred home for 3 weeks. Now the ESR was 9 mm/h, the CRP 6 mg/l and the liver function tests within reference ranges.
On day 32 the patient was readmitted to the clinic for a new needle biopsy. Two days before admission his ESR was 26 mm/h and the CRP was 9 mg/l. Blood cultures were drawn on nine different occasions between 11th July and 13th August (Bactec 9240 system, Becton Dickinson, Sparks USA), and were negative for bacteria and fungi. Specific fungal vials (Bactec Mycosis) were not used. Interferon gamma testing for mycobacteria was negative.
After needle biopsy samples for bacterial, fungal and mycobacterial cultures had been drawn an intravenous treatment regimen (cloxacillin 2 g every 4 h and oral levofloxacin 500 mg once daily) was instituted. Bacterial cultures of the needle biopsy specimen turned out negative as well as PCR testing for bacterial 16S ribosomal RNA. Importantly, a fungal culture of the needle biopsy specimen grew Candida species. Isolate identification by the Vitek 2 Yeast identification system (ID-YST, bioMérieux, Marcy-l´Etoile) showed C. dubliniensis (excellent confidence level, bionumber 6102546061125771). The isolate was sensitive to amphotericin B, fluconazole, flucytosine and voriconazole. Antibacterial treatment was stopped and intravenous liposomal amphotericin B at a dose of 3 mg/kg was started on day 35. After 4 weeks the liposomal amphotericin B was discontinued and the patient was put on oral fluconazole 400 mg once daily. After 2 months of the start of antifungal therapy, on day 92, a lumbosacral MRI showed decreased edema in the LV vertebra, presacral disc space and prespinal soft tissues. After 5 months of antifungal therapy, on day 190, MRI showed only minimal residual edema and the dose of fluconazole was decreased to 200 mg once daily. Fluconazole was stopped after a total duration of 32 weeks of antifungal treatment. Three months later, the patient was symptomless and ESR was 2 mm/h and CRP 4 mg/l.
3. Discussion
Invasive fungal infections including nosocomial bloodstream infections due to Candida species have increased significantly over the last years. Candida infections are common among immunocompromised patients and intravenous drug users, but spondylodiscitis caused by Candida species is infrequent. Over the past 47 years 94 cases of Candida spondylodiscitis (the vast majority of them caused by Candida albicans) have been reported. Miller and coworkers (1966–2000) and Moon and coworkers (2006) found together 82 cases [3,6]. We searched the published literature through the PubMed for Candida spondylodiscitis from 2007 through 2012 and found 12 additional cases.
Candida spondylodiscitis is a uniquely rare condition and a high level of clinical suspicion is needed to identify it among patients presenting with low back pain. Important clues are the presence of risk factors for fungal infections and a history of preceding candidemia [3,4]. Well identified risk factors include a history of central venous catheter, immune suppression, parenteral nutrition, hemodialysis, surgery, massive burns, neutropenia, diabetes or prolonged use of antibiotics. Fungal blood cultures have previously been positive for Candida spp. in about 50–60% of patients [3,4]. As a complication of the candidemia episode the infection can spread to the spine and develop into clinical illness over several months—the time to clinical symptoms ranging from 2 to 15 months [7].
Candida spondylodiscitis is a subacute condition and has vague symptoms. This is due to a low degree of virulence of Candida and poor vascularization of the disc space where the infection causes narrowing of the cartilage followed by destruction and lysis of the vertebral endplates and underlying vertebral bones. Patients often present with symptoms several weeks or months before diagnosis. Back pain is the most common presenting complaint and, unlike pyogenic spondylodiscitis, fever and malaise are quite rare. Neurological complications occur in 20% of the patients with Candida spondylodiscitis [3]. These may be consequences of spinal epidural abscess, vertebral collapse, spinal cord infarction or meningitis.
The differential diagnosis of Candida spondylodiscitis includes infections caused by pyogenic bacteria, mycobacteria, other fungi and malignancies, including multiple myeloma and metastatic disease. Laboratory testing gives generally nonspecific results. As in our patient, the markers of inflammation, ESR and CRP, are usually only slightly elevated. If a suspicion of spondylodiscitis arises, MRI is the most sensitive and specific method for early detection of the infection. Because clinical, laboratory and radiological findings of spondylodiscitis are non-specific, percutaneous CT guided biopsy, direct aspiration or open biopsy is necessary to confirm the diagnosis of fungal infection. A thorough microbiological examination of the biopsy specimen is required to identify the causative organism and sensitivities of the organism to antifungal agents.
In more than half of cases with Candida spondylodiscitis the lumbar spine is involved. Among Candida spp. C. albicans accounts for nearly two-thirds of infections, followed by Candida tropicalis, Candida glabrata and Candida parapsilosis [3]. No case of spondylodiscitis with C. dubliniensis has been reported to date.
C. dubliniensis was first identified in Dublin in 1995 in oral isolates recovered from HIV-infected individuals. Because of the difficulty of readily distinguishing this species from C. albicans, which is morphologically similar, the true prevalence of C. dubliniensis fungemia is unknown [8,9]. In recent years, however, C. dubliniensis has been reported as a causative agent of candidemia at increasing frequency, but mainly in immunocompromised patients [10,11]. There is only one case report of osteomyelitis caused by C. dubliniensis in a patient after hematopoietic stem cell transplantation [12].
The optimal treatment of Candida spondylodiscitis is not known and the organism is variably sensitive to antifungal drugs. Amphotericin B has been considered as the first treatment of choice, but the azoles have also been used with good results to treat patients with isolated Candida strains found susceptible to azoles [3]. In certain patient populations resistance of Candida spp. to azoles may be evolving—the risk of resistance increases if a patient has been on azole prophylaxis or, e.g., on fluconazole treatment for oral or esophageal candidiasis. The ideal duration of treatment is not known. Miller and coworkers recommend 4–6 weeks of intravenous amphotericin B therapy followed by oral fluconazole for 2–6 months [3]. The Infectious Diseases Society of America (IDSA) recommendations for vertebral Candida osteomyelitis include surgical debridement and an initial course of amphotericin B for 2–3 weeks, followed by fluconazole for a total duration of 6–12 months [13]. The European Society for Clinical Microbiology and Infectious Disease (ESCMID) guidelines for treating Candida spondylodiscitis include a strong recommendation to use fluconazole if the species is susceptible, but fluconazole may be preceded by a course of lipid-based amphotericin B [14]. Antifungal treatment is usually continued until resolution of clinical symptoms and normalization of inflammation markers. Slenker and coworkers reported a series of 211 patients, some of whom were published patient reports, with Candida osteomyelitis and found that most initial antifungal regimens included amphotericin B (59%), with an increasing use of fluconazole (26%). Echinocandins were used infrequently (4%). Seventy five percent of the patients were cured by 6 months of antifungal treatment [15].
Candida spondylosdiscitis can be treated conservatively but surgery may be required. Indications for surgical treatment are progressive neurological impairment, spinal instability and persistent infection despite antifungal chemotherapy [6,7]. The prognosis of Candida spondylodiscitis is good, with an overall 85% cure, when the diagnosis is made in a timely manner [3].
In conclusion, Candida spondylodiscitis is a rare and difficult-to-diagnose infection. Indeed, the condition appears to be more difficult to diagnose than to treat, since cure with appropriate therapy is common. Our case highlights that the physician needs to keep an open mind and to consider the possibility of a fungal infection in an immunocompetent patient who is an intravenous drug abuser. It is of note that our patient had inflammatory markers within the reference range. Our case shows the crucial importance of taking fungal (as well as bacterial) cultures of biopsy specimens in patients with spondylodiscitis. This first case of C. dubliniensis spondylodiscitis adds to the current literature highlighting the importance of this pathogen as a cause of fungemia and subsequent deep-seated infection.
Conflict of interest statement
J. O. is a member at an advisory board for fungal diseases (MSD Finland). All other authors have no conflict of interest. No financial support was received for this work.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Zimmerli W. Clinical practice. Vertebral osteomyelitis. N Engl J Med. 2010;362:1022–1029. doi: 10.1056/NEJMcp0910753. [DOI] [PubMed] [Google Scholar]
- 2.Aagaard T., Roed C., Dragsted C., Skinhøj P. Microbiological and therapeutic challenges in infectious spondylodiscitis: a cohort study of 100 cases, 2006–2011. Scand J Infect Dis. 2013;45:417–424. doi: 10.3109/00365548.2012.753160. [DOI] [PubMed] [Google Scholar]
- 3.Miller D.J., Mejicano G.C. Vertebral osteomyelitis due to Candida species: case report and literature review. Clin Infect Dis. 2001;33:523–530. doi: 10.1086/322634. [DOI] [PubMed] [Google Scholar]
- 4.Hendrickx L., van Wijngaerden E., Samson I., Peetermans W.E. Candidal vertebral osteomyelitis: report of 6 patients, and a review. Clin Infect Dis. 2001;32:527–533. doi: 10.1086/318714. [DOI] [PubMed] [Google Scholar]
- 5.Skaf G.S., Kanafani Z.A., Araj G.F., Kanj S.S. Non-pyogenic infections of the spine. Int J Antimicrob Agents. 2010;36:99–105. doi: 10.1016/j.ijantimicag.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Moon H.H., Kim J.H., Moon B.G., Kim J.S. Cervical spondylodiscitis caused by Candida albicans in non-immunocompromised patient. J Korean Neurosurg Soc. 2008;43:45–47. doi: 10.3340/jkns.2008.43.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chia S.L., Tan B.H., Tan C.T., Tan S.B. Candida spondylodiscitis and epidural abscess: management with shorter courses of anti-fungal therapy in combination with surgical debridement. J Infect. 2005;51:17–23. doi: 10.1016/j.jinf.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Ells R., Kock J.L., Pohl C.H. Candida albicans or Candida dubliniensis? Mycoses. 2011;54:1–16. doi: 10.1111/j.1439-0507.2009.01759.x. [DOI] [PubMed] [Google Scholar]
- 9.Khan Z., Ahmad S., Joseph L., Chandy R. Candida dubliniensis: an appraisal of its clinical significance as a bloodstream pathogen. PLoS One. 2012;7(3):e32952. doi: 10.1371/journal.pone.0032952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jabra-Rizk M.A., Johnson J.K., Forrest G., Mankes K., Meiller T.F., Venezia R.A. Prevalence of Candida dubliniensis fungemia at a large teaching hospital. Clin Infect Dis. 2005;41:1064–1067. doi: 10.1086/432943. [DOI] [PubMed] [Google Scholar]
- 11.Horn D.L., Neofytos D., Anaissie E.J., Fishman J.A., Steinbach W.J., Olyaei A.J. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis. 2009;48:1695–1703. doi: 10.1086/599039. [DOI] [PubMed] [Google Scholar]
- 12.Wellinghausen N., Moericke A., Bundschuh S., Friedrich W., Schulz A.S., Gatz S.A. Multifocal osteomyelitis caused by Candida dubliniensis. J Med Microbiol. 2009;58:386–390. doi: 10.1099/jmm.0.003970-0. [DOI] [PubMed] [Google Scholar]
- 13.Pappas P.G., Kauffman C.A., Andes D., Benjamin D.K., Jr, Calandra T.F., Edwards J.E., Jr Infectious Diseases Society of America. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornely O.A., Bassetti M., Calandra T., Garbino J., Kullberg B.J., Lortholary O. ESCMID Fungal Infection Study Group. ESCMID guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18(Suppl. 7):19–37. doi: 10.1111/1469-0691.12039. [DOI] [PubMed] [Google Scholar]
- 15.Slenker A.K., Keith S.W., Horn D.L. Two hundred and eleven cases of Candida osteomyelitis: 17 case reports and a review of the literature. Diagn Microbiol Infect Dis. 2012;73:89–93. doi: 10.1016/j.diagmicrobio.2012.02.004. [DOI] [PubMed] [Google Scholar]


