Abstract
AIM: To explore the effect of sophocarpine on experimental liver fibrosis and the potential mechanism involved.
METHODS: Sophocarpine was injected intraperitoneally in two distinct rat hepatic fibrosis models induced either by dimethylnitrosamine or bile duct ligation. Masson’s trichrome staining, Sirius red staining and hepatic hydroxyproline level were used for collagen determination. Primary hepatic stellate cells (HSCs) were isolated and treated with different concentrations of sophocarpine. Real-time reverse transcription-polymerase chain reaction was used to detect the mRNA levels of fibrotic markers and cytokines. The expression of pathway proteins was measured by Western blot. The Cell Counting Kit-8 test was used to detect the proliferation rate of activated HSCs treated with a gradient concentration of sophocarpine.
RESULTS: Sophocarpine decreased serum levels of aminotransferases and total bilirubin in rats under chronic insult. Moreover, administration of sophocarpine suppressed extracellular matrix deposition and prevented the development of hepatic fibrosis. Furthermore, sophocarpine inhibited the expression of α-smooth muscle actin (SMA), interleukin (IL)-6, transforming growth factor-β1 (TGF-β1), Toll-like receptor 4 (TLR4), and extracellular-related kinase (ERK) in rats. Sophocarpine also down-regulated the mRNA expression of α-SMA, collagen I, collagen III, TGF-β1, IL-6, tumor necrosis factor-α and monocyte chemoattractant protein-1, and decreased protein levels of TLR4, p-ERK, p-JNK, p-P38 and p-IKK in vitro after Lipopolysaccharide induction. In addition, sophocarpine inhibited the proliferation of HSCs accompanied by a decrease in the expression of Cyclin D1. The protein level of proliferating cell nuclear antigen was decreased in activated HSCs following a gradient concentration of sophocarpine.
CONCLUSION: Sophocarpine can alleviate liver fibrosis mainly by inhibiting the TLR4 pathway. Sophocarpine may be a potential chemotherapeutic agent for chronic liver diseases.
Keywords: Liver fibrosis, Sophocarpine, Toll-like receptor 4, Hepatic stellate cells, Cytokines
Core tip: Sophocarpine significantly ameliorated liver function and hepatic fibrosis in both the dimethylnitrosamine and bile duct ligation models. In addition, sophocarpine inhibited the activation and proliferation of hepatic stellate cells in vitro, which contributed to the anti-fibrotic effect of sophocarpine in vivo. Toll-like receptor 4 signaling was blocked by sophocarpine in vivo and in vitro, accompanied by a reduction in pro-inflammatory and fibrotic cytokines, as well a decrease in the expression of Cyclin D1 and proliferating cell nuclear antigen.
INTRODUCTION
Liver fibrosis is a wound-healing response to chronic liver injury and is characterized by the accumulation of extracellular matrix (ECM), which depends on the balance between ECM synthesis and degradation. Following liver injury, hepatic stellate cells (HSCs) are the predominant ECM-producing cell type in the liver[1,2]. The activation and proliferation of HSCs, characterized by the morphological transition to myofibroblast-like cells, have been well established as the central events in the pathogenesis of hepatic fibrosis[3]. Previous studies have suggested that inhibition of the activation, proliferation and migration of HSCs may be an attractive anti-fibrotic therapy[4].
In cirrhotic rats and patients, intestinal bacterial overgrowth can occur, including Gram-negative and -positive bacteria[5]. Lipopolysaccharide (LPS), a major cellular component of Gram-negative bacteria, aggravates liver cirrhosis with increased permeability of the intestinal mucosal barrier. LPS-induced HSC activation has been proved to be an important mechanism in liver injury[6]. Quiescent murine HSCs responsive to LPS can express Toll-like receptor 4 (TLR4) similar to in vivo-activated HSCs, even at the low LPS dose of 1 ng/mL[7]. In addition, activated murine HSCs express TLR4 and respond to LPS with up-regulation of extracellular-related kinase (ERK) phosphorylation, interleukin (IL)-6 and transforming growth factor-β1 (TGF-β1)[7,8]. TLR4-mutant mice displayed a profound reduction in hepatic fibrogenesis in three different experimental models of biliary or toxic fibrosis[7]. These results confirm the critical role of TLR4 signaling in regulating HSC activation which affects the risk of hepatic fibrosis progression.
Sophocarpine is a matrine-type quinolizidine alkaloid widespread in the genus Sophora. Basic and clinical studies have shown that sophocarpine possesses a variety of pharmacological effects, such as anti-inflammatory, immuno-regulatory, anti-virus and anti-tumor[9-11]. Moreover, our previous research proved that sophocarpine alleviated the progression of non-alcohol steatohepatitis through the down-regulation of inflammatory cytokines in vivo[12]. More importantly, sophocarpine has minor toxic side effects and significant potential for clinical application.
In this study, we demonstrated that sophocarpine ameliorated liver fibrosis by inhibiting the activation and proliferation of HSCs in rats. In addition, negative regulation of the TLR4 signaling pathway contributed to the effects of sophocarpine which decreased the expression of profibrotic and inflammatory cytokines such as tumor necrosis factor (TNF)-α, TGF-β1 and IL-6, and reduced Cyclin D1 and proliferating cell nuclear antigen (PCNA).
MATERIALS AND METHODS
Sophocarpine
Sophocarpine (High Performance Liquid Chromatography purity > 98%) was obtained from Winherb Medical Science Co., Ltd (Shanghai, China). The sophocarpine used was endotoxin-free, as determined by the limulus lysate assay, with a minimum detectable level of 8 pg/L.
Animal fibrosis models and sophocarpine administration in vivo
Male Sprague-Dawley rats (190 ± 15 g) were housed in cages with stainless-steel wire tops under standard animal laboratory conditions in the specific pathogen-free-grade animal room of the Experimental Animal Center of the Second Military Medical University. The rats had free access to standard rat chow and water. This study was approved by the Ethics Committee of the Second Military Medical University, Shanghai, China. Two distinct models of hepatic fibrosis were induced in rats using dimethylnitrosamine (DMN) injection (10 mg/kg, three injections per week for 4 wk) or bile duct ligation (BDL) as described previously[13]. For the BDL model, seven rats served as controls and underwent sham surgery. Three days after surgery, 24 BDL rats were randomly divided into two groups and treated as follows: the model group (n = 12, Ringer’s solution) and sophocarpine treatment group (n = 12, 20 mg/kg sophocarpine dissolved in Ringer’s solution). For the DMN model, seven rats were included as controls. Fourteen days after DMN injection, the 24 DMN rats were divided as described above (12 in the model group and 12 in the sophocarpine treatment group). Sophocarpine and placebo solution were injected intraperitoneally once a day. The animals were sacrificed 3 wk after BDL or 4 wk after DMN administration.
Serum biochemical analysis
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin (TB) were determined in all experimental rats using the appropriate kits from Sigma-Aldrich.
Histological examination and immunohistological staining
All paraffin-embedded liver samples were stained with hematoxylin and eosin for histopathological examination. Masson’s trichrome staining and Sirius red staining were used for collagen determination. For the semiquantitative analysis, connective tissues stained with Sirius red were measured on an image analyzer (Image-Pro Plus, Media Cybernetics) by a technician blinded to the samples. The intensity of collagen deposition or protein expression was calculated as the percentage of the positive area in the corresponding field of liver tissue. Immunohistochemical examinations were carried out to determine the expression of α-smooth muscle actin (SMA) (BM0002, Boster, Wuhan, China), TGF-β1 (sc-146, Santa Cruz), IL-6 (ab6672, Abcam), TLR4 (ab13556, Abcam) and ERK1/2 (4695, Cell Signaling).
Measurement of hepatic hydroxyproline content
Total hepatic hydroxyproline levels in all experimental rats were determined in the hydrolysates of liver samples as previously described[13]. One hundred mg of wet liver samples were subjected to acid hydrolysis to determine the amount of hydroxyproline according to the protocol outlined in the Hydroxyproline Testing Kit (A030-2, Jiancheng, Nanjing, China).
Cell culture and treatment
Primary HSCs were freshly isolated as previously described[14]. The primary HSCs were cultured in plastic cell culture dishes. Forty-eight hours later, sophocarpine was added at the concentrations of 25, 50 or 200 mg/mL for 48 or 72 h, and the cells were then treated with LPS (Sigma, 2 ng/mL) for 30 min (Western blotting) or 12 h [Real-time reverse transcription-polymerase chain reaction (RT-PCR)]. Activated HSCs were derived from the primary HSCs which were cultured for approximately 14 d and passaged for 2-3 passages, the cells were then treated with sophocarpine (100-1000 μg/mL) for 0-5 d. These cells were cultured in Dulbecco’s modified medium containing 10% fetal bovine serum.
RT-PCR
Total RNA was extracted from the cells and liver tissues (3 animals representative of each group) with Trizol reagent (Invitrogen, Carlsbad, CA, United States), and cDNA was synthesized according to the manufacturer’s instructions (TAKARA, Japan). Transcription levels were detected by real-time RT-PCR with a SYBR Green PCR Kit (Applied Biosystems, Foster City, CA, United States). Primer sequences are listed in Table 1.
Table 1.
Primers used for detection of gene transcription
| Gene | Forward | Reverse |
| α-SMA | 5'-ACTGGGACGACATGGAAAAG-3' | 5'-CATCTCCAGAGTCCAGCACA-3' |
| Pro-collagen I | 5'-AGGCATAAAGGGTCATCGTG-3' | 5'-ACCGTTGAGTCCATCTTTGC-3' |
| Collagen III | 5'-GTCCACGAGGTGACAAAGGT-3' | 5'-CATCTTTTCCAGGAGGTCCA-3' |
| TNFα | 5'-AGATGTGGAACTGGCAGAGG-3' | 5'-CCCATTTGGGAACTTCTCCT-3' |
| IL-6 | 5'-CCGGAGAGGAGACTTCACAG-3' | 5'-ACAGTGCATCATCGCTGTTC-3' |
| TGFβ1 | 5'-ATACGCCTGAGTGGCTGTCT-3' | 5'-TGGGACTGATCCCATTGATT-3' |
| MCP-1 | 5'-ATGCAGTTAATGCCCCACTC-3' | 5'-TTCCTTATTGGGGTCAGCAC-3' |
| TLR4 | 5'-TGCTCAGACATGGCAGTTTC-3' | 5'-TCAAGGCTTTTCCATCCAAC-3' |
| Myd88 | 5'-GAGATCCGCGAGTTTGAGAC-3' | 5'-CTGTTTCTGCTGGTTGCGTA-3' |
| TRAF6 | 5'-AGGGTACAATACGCCTCACG-3' | 5'-GCGGGTAGAGACTTCACAGC-3' |
| ERK1 | 5'-TCCAAGGGCTACACCAAATC-3' | 5'-AGGTAGTTTCGGGCCTTCAT-3' |
| JNK1 | 5'-GCCACAAAATCCTCTTTCCA-3' | 5'-CACATCGGGGAACAGTTTCT-3' |
| Cyclin D1 | 5'-GCGTACCCTGACACCAATCT-3' | 5'-GGCTCCAGAGACAAGAAACG-3' |
| β-actin | 5'-GCCAACACAGTGCTGTCTGG-3' | 5'-TGATCCACATCTGCTGGAAGG-3' |
Western blot analysis
Western blot analyses of α-SMA (ab5694, Abcam), Collagen I (ab6308, Abcam), TLR4 (ab13556, Abcam), PCNA (ab29, Abcam), GAPDH (BSAP0063, Bioworld), p-JNK (4668, Cell Signaling), JNK (9258, Cell Signaling), p-ERK1/2 (4370, Cell Signaling), ERK1/2 (4695, Cell Signaling), p-P38 (4511, Cell Signaling), P38 (8690, Cell Signaling), p-IKKα/β (2697, Cell Signaling) and IKKα/β (sc-7607, Santa Cruz) were performed according to the manufacturer’s instructions (Bio-Rad Laboratories, Hercules, CA, United States).
Measurement of HSC proliferation
Activated HSCs were plated in triplicate wells on a 96-well plate at 3 × 103 cells/well and cultured for 24 h. These cells were then treated with sophocarpine at the concentrations of 0, 100, 200, 400, 600 and 1000 μg/mL. The number of metabolically active and viable cells was detected colorimetrically at 450 nm using the Cell Counting Kit-8 (DOJINDO, Tokyo, Japan) assays.
Statistical analysis
Results are presented as the mean of three independent experiments (mean ± SD). The two-sided independent Student’s t test was performed to analyze the differences in gene expression levels, hydroxyproline content and histology data. P < 0.05 was considered statistically significant.
RESULTS
Sophocarpine ameliorates liver function in fibrotic rats
To assess the effect of sophocarpine on liver function, serum aminotransferases and total bilirubin were determined. Liver function tests showed that sophocarpine significantly down-regulated the concentrations of aminotransferases and total bilirubin in both the DMN and BDL models (Table 2, Figure 1A and B). TB concentration in sophocarpine-treated rats showed a decrease by approximately 70% compared with the DMN rat model, however, there was no significant decrease in the sophocarpine-treated BDL group (Table 2, Figure 1C).
Table 2.
Effect of sophocarpine on the improvement in serum alanine aminotransferase, aspartate aminotransferase and total bilirubin levels in both models of liver fibrosis
| Group | ALT (U/L) | AST (U/L) | TB (mmol/L) |
| Normal | 29.29 ± 1.76 | 123.67 ± 29.06 | 1.29 ± 0.28 |
| DMN model | 68.27 ± 3.43 | 176.9 ± 8.99 | 8.09 ± 1.35 |
| DMN + sophocarpine | 48.58 ± 4.521 | 108.42 ± 15.461 | 3.00 ± 0.481 |
| Sham operation | 28.83 ± 2.22 | 121.00 ± 36.72 | 2.00 ± 0.63 |
| BDL model | 150.50 ± 23.6 | 959.50 ± 255 | 139.67 ± 16.23 |
| BDL + sophocarpine | 91.86 ± 6.711 | 464.14 ± 182.61 | 123.86 ± 9.69 |
Compared with the model group, P < 0.05. ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; TB: Total bilirubin; DMN: Dimethylnitrosamine; BDL: Bile duct ligation.
Figure 1.
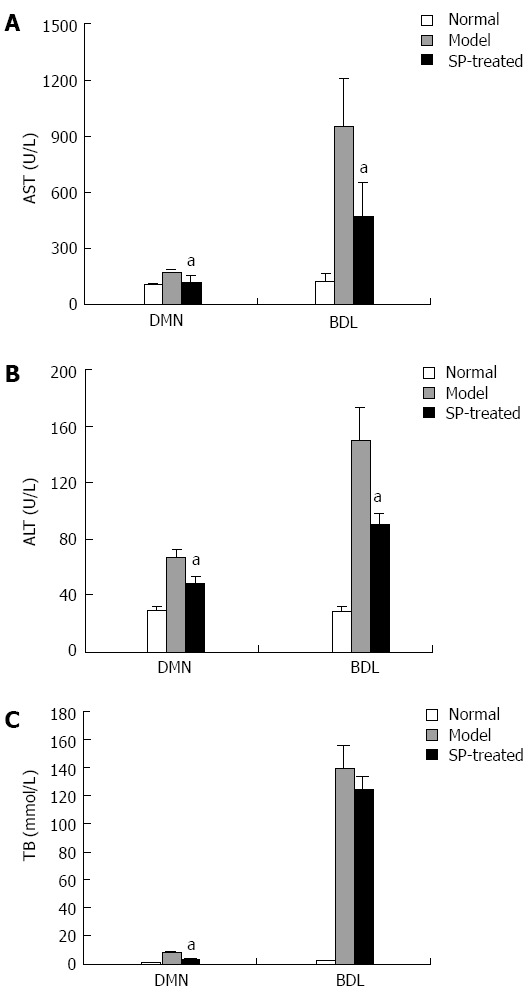
Sophocarpine ameliorates liver function in fibrotic rats. Serum was collected from each group of rats. AST (A), ALT (B) and TB (C) levels were determined to assess liver function in the sophocarpine-treated group compared to each model group (aP < 0.05, by two-tailed Student’s t test). ALT: Alanine aminotransfer; AST: Aspartate aminotransferase; TB: Total bilirubin; DMN: Dimethylnitrosamine; BDL: Bile duct ligation.
Sophocarpine attenuates hepatic fibrosis induced by DMN or BDL in rats
We next examined the potential effect of sophocarpine on the two distinct fibrotic models. Liver sections stained with Masson’s trichrome stain showed that periportal fibrosis with fibrous septa extended to adjacent portal tracts and the terminal hepatic venue in the DMN model; in the BDL model, livers showed extensive bile duct proliferation, a detrimental collapse of liver parenchyma and overt ECM deposition around the reactive bile ductile (Figure 2A). Following administration of sophocarpine, the ECM area (Masson’s staining) was reduced by 55.6% and 39.3% (P < 0.05) in DMN and BDL rats, respectively (Figure 2B). The contents of hydroxyproline in the sophocarpine-treated rats were lower than those in the model rats (261.17 ± 20.45 μg/g in the sophocarpine-treated DMN group vs 361.17 ± 20.55 μg/g in the DMN model group, P < 0.05; 178 ± 15.89 μg/g in the sophocarpine-treated BDL group vs 259.33 ± 23.18 μg/g in the BDL model group, P < 0.05) (Figure 2C). The mRNA expression of α-SMA, TGF-β1 and alpha 1 type I procollagen detected by RT-PCR was reduced significantly in the sophocarpine-treated groups in both the DMN and BDL models (P < 0.05) (Figure 2D and E).
Figure 2.
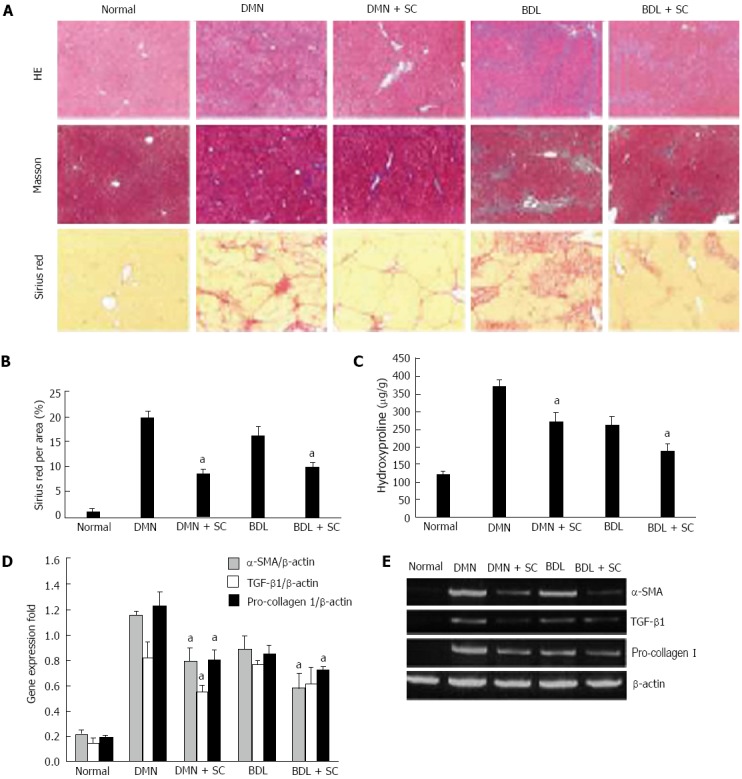
Sophocarpine attenuates hepatic fibrosis induced by dimethylnitrosamine or bile duct ligation in rats. DMN and BDL were used to construct two types of hepatic fibrosis models to evaluate the therapeutic effect of sophocarpine. A: Liver fibrosis in each group was assessed by HE (× 40), Masson’s trichrome (× 40) and Sirius red staining (× 40); B: The percentage of Sirius-red in fibrotic livers was quantified using an image analysis system (aP < 0.05); C: The amount of hydroxyproline in fibrotic livers was detected in the sophocarpine-treated group compared with each model group (aP < 0.05); D, E: Real-time RT-PCR was employed to examine the expression of α-SMA, TGF-β and pro-collagen I in fibrotic livers following sophocarpine administration compared with the control models (aP < 0.05 by two-tailed Student’s t test). DMN: Dimethylnitrosamine; BDL: Bile duct ligation; TGF-β: Transforming growth factor-β; RT-PCR: Reverse transcription-polymerase chain reaction; SMA: Smooth muscle actin.
Expression of pro-fibrotic cytokines and TLR4 signaling pathway-related proteins is suppressed in sophocarpine-treated rats
We subsequently determined the effects of sophocarpine on the expression of pro-fibrotic cytokines. Immunohistochemical examination revealed that α-SMA, IL-6 and TGF-β1 protein expression was significantly elevated in both the DMN and BDL models, and was suppressed following sophocarpine administration (Figure 3). Furthermore, from the observed effects of sophocarpine on HSCs in vitro, the expression of TLR4 signaling pathway-related proteins, such as TLR4 and ERK1/2, was also increased in the DMN and BDL models. Sophocarpine down-regulated these proteins in vivo as shown by immunohistochemistry (Figure 3). These results indicated that sophocarpine blocked the TLR4 signaling pathway which aggravated the progression of liver fibrosis and reduced pro-fibrotic cytokine expression.
Figure 3.
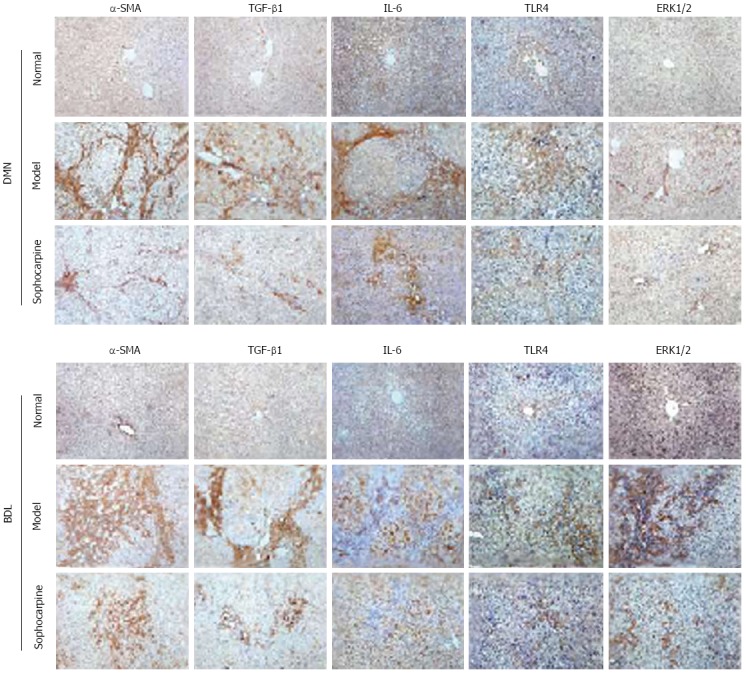
Expression of pro-fibrotic cytokines and toll-like receptor 4 signaling pathway related-proteins is suppressed in sophocarpine-treated rats. Immunochemical analysis of the protein expression of α-SMA, TGF-β1, IL-6, TLR4 and ERK1/2 in the liver tissue of each group was performed as described in Materials and Methods. The results show the protein expression of α-SMA (× 200), TGF-β1 (× 200), IL-6 (× 200), TLR4 (× 200) and ERK1/2 (× 200) in the fibrotic livers of each group. TLR4: Toll-like receptor 4; IL-6: Interleukin-6; TGF-β: Transforming growth factor-β; SMA: Smooth muscle actin.
Sophocarpine inhibits the activation of hepatic stellate cells
RT-PCR was performed to determine the gene expression of inflammatory and fibrotic markers in HSCs treated with a gradient concentration of sophocarpine at 48 and 72 h to observe the effect of sophocarpine during activation of HSCs. In contrast to the freshly isolated and self-activated HSCs, dose gradient sophocarpine-treated cells showed significantly lower expression of α-SMA, collagen I, collagen III, TGF-β1 and inflammatory cytokines, including IL-6, TNF-α and monocyte chemoattractant protein-1 (MCP-1) (Figure 4A). Furthermore, the protein expression of α-SMA and collagen I was depressed by sophocarpine in HSCs (Figure 4B). We also measured the above-mentioned gene expression at different culture times. The mRNA expression of α-SMA, collagen III, IL-6, TNF-α and MCP-1 decreased by 38.6%, 43.7%, 59.5%, 45.2% and 55.6% at 72 h compared to 48 h, respectively (Figure 4C). These results confirmed that sophocarpine had a dose- and time-dependent inhibitory effect on the activation of HSCs.
Figure 4.
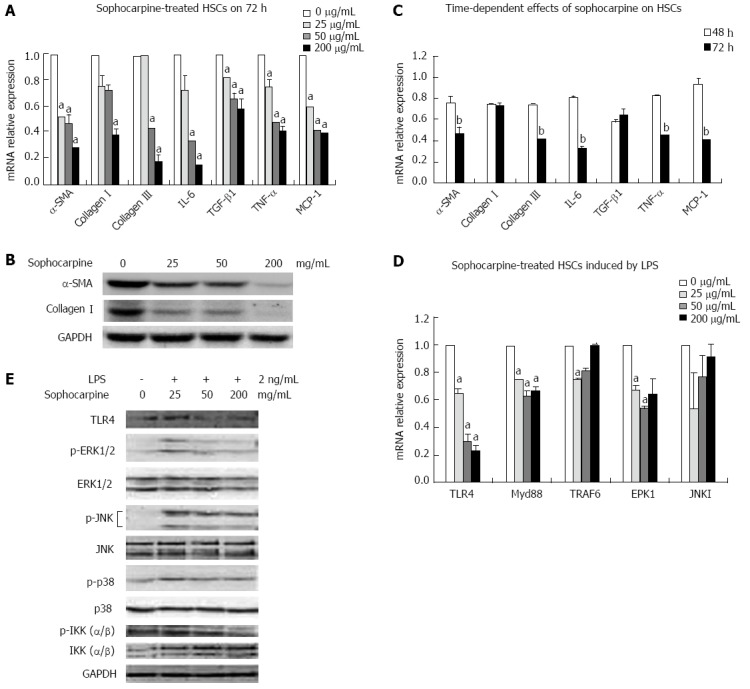
Sophocarpine inhibits the activation of hepatic stellate cells by blocking the lipopolysaccharide-induced toll-like receptor 4 signaling pathway. Primary HSCs were isolated and plated in 6-well plates (1 × 106 cells/well). Forty-eight hours later, the HSCs were treated with a gradient concentration of sophocarpine for 48 or 72 h. A: Real-time reverse transcription-polymerase chain reaction (RT-PCR) was performed to analyze the mRNA levels of α-SMA, collagen I, collagen III, IL-6, TGF-β1, TNF-α and MCP-1 in HSCs treated with a gradient concentration of sophocarpine for 72 h (compared to 0 μg/mL, aP < 0.05); B: Immunoblots of α-SMA, collagen I and GAPDH were detected by Western blot from HSCs treated with a gradient concentration of sophocarpine for 72 h; C: The mRNA expression of the above genes was detected in HSCs treated with sophocarpine (50 mg/mL) at 72 h compared to that at 48 h, bP < 0.05; D, E: Gradient concentration sophocarpine-treated HSCs were incubated with LPS (2 ng/mL), and real-time RT-PCR (compared to 0 μg/mL, aP < 0.05, D) and Western blot analysis (E) were employed to detect the expression of TLR4 pathway-related genes at the gene and protein levels (P value by two-tailed Student’s t test). LPS: Lipopolysaccharide; HSCs: Hepatic stellate cells; TGF-β: Transforming growth factor-β; IL-6: Interleukin-6; SMA: Smooth muscle actin; TNF: Tumor necrosis factor; MCP: Monocyte chemoattractant protein.
Sophocarpine blocks the LPS-induced TLR4 signaling pathway affecting the activation of HSCs
We then detected mRNA expression of TLR4 pathway-related genes induced by LPS using RT-PCR at 72 h. The gene expression of TLR4 and Myd88 showed a significant dose-dependent decrease in sophocarpine-treated HSCs (Figure 4D). Subsequently, we found that the protein expression of TLR4, p-ERK1, p-JNK1, p-P38 and p-IKK decreased markedly in sophocarpine-treated HSCs compared with control cells induced by LPS at 72 h. Thus, the Myd88-dependent TLR4 signaling pathway was blocked by sophocarpine which inhibited the activation of HSCs (Figure 4E).
Sophocarpine suppresses the proliferation of HSCs
To determine the effects of sophocarpine on inhibition of HSC proliferation, CCK-8 assays were performed. The results of CCK-8 measurement showed that sophocarpine at doses ≥ 200 μL/mL significantly suppressed the proliferation of HSCs. These inhibitory effects were dose- and time-dependent (Figure 5A). We also detected mRNA expression of Cyclin D1 in self-activated HSCs treated with sophocarpine and in control cells, and sophocarpine significantly down-regulated the expression of Cyclin D1 at 72 h (Figure 5B). The inhibitory effect of sophocarpine on the proliferation of HSCs was confirmed by decreased protein expression of PCNA in HSCs (Figure 5C).
Figure 5.
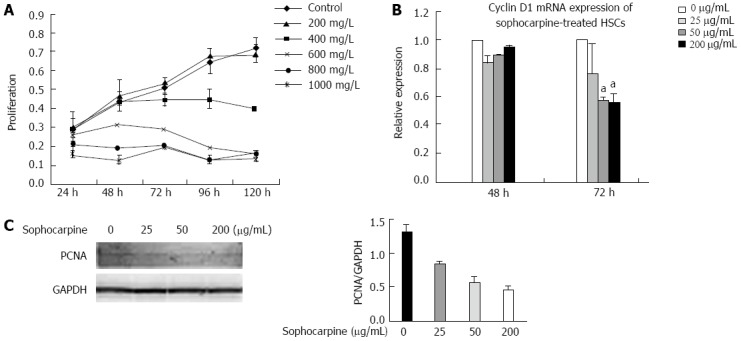
Sophocarpine suppresses the proliferation of hepatic stellate cells. A: Activated HSCs were treated with a gradient concentration of sophocarpine and the proliferation of HSCs was assessed using the CCK-8 kit; B: Real-time polymerase chain reaction was performed to examine the expression of Cyclin D1 in HSCs after treatment with a gradient concentration of sophocarpine (aP < 0.05); C: Western blot was employed to detect PCNA expressed in HSCs after treatment with sophocarpine. HSCs: Hepatic stellate cells; PCNA: Proliferating cell nuclear antigen.
DISCUSSION
Liver fibrosis occurs as a wound-healing scar response following acute and chronic inflammation, including viral hepatitis, alcohol consumption, autoimmune and metabolic liver diseases[15]. Liver inflammation results in the activation of HSCs through various inflammatory or fibrogenic mediators including TNF-α, IL-1β, IL-6 and TGF-β1[16-21]. In recent studies, sophocarpine, a monomer used in traditional Chinese medicine, inhibited the production of inflammatory cytokines IL-6 and TNF-α in murine macrophages[11]. In addition, sophocarpine prevented cachexia-related symptoms induced by adenocarcinoma and alleviated non-alcoholic steatohepatitis in rats[11,12]. Based on the validated effects of sophocarpine on inflammation regulation, we hypothesized that sophocarpine may have a restorative effect on liver fibrosis.
In this study, we used sophocarpine to treat hepatic fibrosis induced by two mechanistically different fibrosis models: DMN administration and BDL. Based on the histopathological and immunohistochemical results, hepatocellular injury and HSC activation improved following sophocarpine administration in both models. More importantly, it was shown for the first time that sophocarpine administration attenuated ECM deposition and hydroxyproline content in liver fibrosis induced by DMN and BDL, indicating that sophocarpine suppressed hepatic fibrosis in these rat models. Furthermore, we demonstrated that reduced production of inflammatory cytokines, such as IL-6 and TNF-α, contributed to the anti-fibrotic effect of sophocarpine. This was accompanied by alleviation of liver fibrosis and a reduction in pro-fibrotic cytokines such as TGF-β1 in vivo[22,23]. The expression of α-SMA which is considered a marker of myofibroblasts[24] decreased significantly in fibrotic rats treated with sophocarpine. Based on these findings, we hypothesize that sophocarpine inhibited the activation and proliferation of HSCs which mediate the central pathological effects in the progression of liver fibrosis.
During hepatic fibrosis, HSCs undergo activation and conversion to myofibroblast-like cells which secrete collagens and aggravate the deposition of ECM. Reversal of these processes is critical in the treatment of liver fibrosis. In the present study, sophocarpine decreased the expression of α-SMA, collagen I and III in vitro, which indicated that sophocarpine inhibited the activation and conversion of HSCs. Moreover, sophocarpine reduced the expression of TGF-β1 which is a major pro-fibrogenic molecule. Furthermore, sophocarpine attenuated the hepatic inflammation reaction and reduced the expression of IL-6, MCP-1 and TNF-α during the in vivo activation of HSCs. Sophocarpine also inhibited the proliferation of HSCs with a reduction in Cyclin D1 which participates in cell cycle regulation. These results demonstrated that the inhibition of activation and proliferation of HSCs was the major cytological mechanism involved in the alleviation of liver fibrosis by sophocarpine.
Toll-like receptors (TLRs) play an important role in the regulation of inflammation, even under sterile conditions, such as injury and wound healing. The healthy liver contains lower mRNA levels of TLRs and signaling molecules such as MD-2 and MyD88 than other organs, suggesting that the low expression of TLR signaling molecules may contribute to the high tolerance of the liver to TLR ligands from the intestinal microbiota to which the liver is constantly exposed[25-28]. Numerous studies have demonstrated that LPS is elevated in experimental models of hepatic fibrosis[7,29,30] and in cirrhotic patients[31-33]. Cirrhotic patients have markedly elevated endotoxin levels compared with healthy subjects[33]. In view of the critical role of the intestinal microbiota in hepatic fibrogenesis, the LPS-induced TLR4 signaling pathway contributes significantly to the progression of liver cirrhosis[34]. Activated human HSCs expressed TLR4 and its coreceptors MD-2 and CD-14 in vitro[35]. LPS treatment can induce strong activation of the nuclear factor kappa B (NF-κB) and JNK/AP-1 pathways as well as the secretion of pro-inflammatory cytokines in activated HSCs[36]. Activated murine HSCs expressed TLR4 and responded to LPS with an up-regulation of extracellular-related kinase (ERK) phosphorylation and IL-6, TGF-β1 and MCP-1 secretion[7,8].
As IL-6, TNF-α, TGF-β1 and MCP-1, whose expression decreased after sophocarpine administration, are all regulated by the LPS-induced TLR4 signal pathway, we suspected that sophocarpine may affect the TLR4 signal pathway and inhibit liver fibrosis. We found that sophocarpine reduced the expression of TLR4 and Myd88, but not TLR2 or TLR9 which can also mediate the progression of liver fibrosis[37,38]. During TLR4 signaling, the MyD88-dependent pathway mediates the up-regulation of inflammatory cytokines through activation of NF-κB and mitogen-activated protein kinases (MAPK)[39]. The MAPK signal pathway involves ERK, JNK, p38 and their phosphorylation in the pathogenesis of liver fibrosis[40-42]. SiRNAs or selective inhibitors targeting these molecules reduced their expression or activity, and alleviated liver fibrosis in vivo[13,43]. We found that the phosphorylation of ERK, JNK, p38 and IKK was significantly down-regulated after sophocarpine administration, which indicated that sophocarpine suppressed the MyD88-dependent TLR4 pathway and inhibited the activation of HSCs. The ERK/AP-1 pathway also induces c-Myc and Cyclin D1 expression which facilitates the proliferation of HSCs[14,44,45]. Sophocarpine inhibited the phosphorylation of ERK, and then down-regulated the expression of Cyclin D1, which may contribute to the inhibitory effect of sophocarpine on the proliferation of HSCs.
In our study, sophocarpine exhibited potent control of liver inflammation, which mainly contributed to the inhibition of hepatic fibrosis and HSC activation. For decades many researchers have investigated many stimuli, with the exception of inflammatory cytokines, that can drive the activation of HSCs including hepatocellular necrosis due to oxidative stress and apoptosis[46-48]. The TLR4 and complements also play important roles in oxidative stress and hepatotoxicity, especially in the initiation of alcoholic steatohepatitis and fibrosis[49,50]. It is likely that sophocarpine has an impact on suppressing oxidative stress and subsequently protecting hepatocytes from necrosis or apoptosis, which merits investigation. Moreover, as a monomer derived from matrine, although sophocarpine blocked the TLR4 pathway which was confirmed by our investigation, the direct target molecules of sophocarpine in the LPS-induced TLR4 pathway are unknown and require further study.
In summary, our investigation provides strong evidence for a suppressive effect of sophocarpine on hepatic fibrosis through inhibition of the activation and proliferation of HSCs. Moreover, sophocarpine exhibited potent blockage of the TLR4 signaling pathway and subsequently decreased the expression of pro-inflammatory and fibrotic cytokines. Based on the present study, sophocarpine may emerge as a novel option for the clinical therapy of chronic liver diseases.
COMMENTS
Background
The activation and proliferation of hepatic stellate cells (HSCs) are central events in the pathogenesis of hepatic fibrosis. However, there is no efficient treatment for chronic liver diseases in clinical practice.
Research frontiers
Previous studies have suggested that inhibition of the activation, proliferation and migration of HSCs may be an attractive anti-fibrotic therapy. Inflammatory cells and the inflammatory response are involved in driving the activation of HSCs through various inflammatory or fibrogenic mediators and pathways. Lipopolysaccharide toll-like receptor 4 (LPS-TLR4) signaling plays a critical role in regulating HSC activation and affects the risk of hepatic fibrosis progression.
Innovations and breakthroughs
Sophocarpine is a matrine-type quinolizidine alkaloid and exhibits a variety of pharmacological effects including anti-inflammatory, immuno-regulatory, anti-virus and anti-tumor. In this study, the authors demonstrated that sophocarpine administration ameliorated liver fibrosis by inhibiting the activation and proliferation of HSCs in rats. Moreover, blockage of the TLR4 signaling pathway contributed to the effects of sophocarpine by inhibiting the expression of fibrotic cytokines.
Applications
Due to the inhibitory effect of sophocarpine on hepatic fibrosis, it is likely that sophocarpine may have potential application in the clinical treatment of chronic liver diseases.
Terminology
HSCs are pericytes found in the perisinusoidal space (a small area between the sinusoids and hepatocytes) of the liver also known as the space of Disse. The stellate cell is the major cell type involved in liver fibrosis, which is the formation of scar tissue in response to liver damage.
Peer review
This study suggests that sophocarpine can alleviate liver fibrosis mainly through inhibiting the TLR4 signaling pathway. Sophocarpine might be present as a potential agent for chronic liver diseases.
Footnotes
Supported by The National Natural Science Foundation of China, Nos. 30971343, 81270486, 81000167 and 81370009
P- Reviewers: Apte MV, Abdel-Raheem IT, Trapero-Marugan M S- Editor: Cui XM L- Editor: Wang TQ E- Editor: Wu HL
References
- 1.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 2.Yang C, Zeisberg M, Mosterman B, Sudhakar A, Yerramalla U, Holthaus K, Xu L, Eng F, Afdhal N, Kalluri R. Liver fibrosis: insights into migration of hepatic stellate cells in response to extracellular matrix and growth factors. Gastroenterology. 2003;124:147–159. doi: 10.1053/gast.2003.50012. [DOI] [PubMed] [Google Scholar]
- 3.Sato M, Suzuki S, Senoo H. Hepatic stellate cells: unique characteristics in cell biology and phenotype. Cell Struct Funct. 2003;28:105–112. doi: 10.1247/csf.28.105. [DOI] [PubMed] [Google Scholar]
- 4.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nolan JP. The role of endotoxin in liver injury. Gastroenterology. 1975;69:1346–1356. [PubMed] [Google Scholar]
- 6.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 8.Brun P, Castagliuolo I, Pinzani M, Palù G, Martines D. Exposure to bacterial cell wall products triggers an inflammatory phenotype in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G571–G578. doi: 10.1152/ajpgi.00537.2004. [DOI] [PubMed] [Google Scholar]
- 9.Lin Z, Huang CF, Liu XS, Jiang J. In vitro anti-tumour activities of quinolizidine alkaloids derived from Sophora flavescens Ait. Basic Clin Pharmacol Toxicol. 2011;108:304–309. doi: 10.1111/j.1742-7843.2010.00653.x. [DOI] [PubMed] [Google Scholar]
- 10.Gao Y, Li G, Li C, Zhu X, Li M, Fu C, Li B. Anti-nociceptive and anti-inflammatory activity of sophocarpine. J Ethnopharmacol. 2009;125:324–329. doi: 10.1016/j.jep.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Wang S, Li Y, Xiao Z, Hu Z, Zhang J. Sophocarpine and matrine inhibit the production of TNF-alpha and IL-6 in murine macrophages and prevent cachexia-related symptoms induced by colon26 adenocarcinoma in mice. Int Immunopharmacol. 2008;8:1767–1772. doi: 10.1016/j.intimp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Song CY, Zeng X, Chen SW, Hu PF, Zheng ZW, Ning BF, Shi J, Xie WF, Chen YX. Sophocarpine alleviates non-alcoholic steatohepatitis in rats. J Gastroenterol Hepatol. 2011;26:765–774. doi: 10.1111/j.1440-1746.2010.06561.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhong W, Shen WF, Ning BF, Hu PF, Lin Y, Yue HY, Yin C, Hou JL, Chen YX, Zhang JP, et al. Inhibition of extracellular signal-regulated kinase 1 by adenovirus mediated small interfering RNA attenuates hepatic fibrosis in rats. Hepatology. 2009;50:1524–1536. doi: 10.1002/hep.23189. [DOI] [PubMed] [Google Scholar]
- 14.Marra F, Efsen E, Romanelli RG, Caligiuri A, Pastacaldi S, Batignani G, Bonacchi A, Caporale R, Laffi G, Pinzani M, et al. Ligands of peroxisome proliferator-activated receptor gamma modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology. 2000;119:466–478. doi: 10.1053/gast.2000.9365. [DOI] [PubMed] [Google Scholar]
- 15.Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- 16.Byl B, Roucloux I, Crusiaux A, Dupont E, Devière J. Tumor necrosis factor alpha and interleukin 6 plasma levels in infected cirrhotic patients. Gastroenterology. 1993;104:1492–1497. doi: 10.1016/0016-5085(93)90361-f. [DOI] [PubMed] [Google Scholar]
- 17.Spirlì C, Nathanson MH, Fiorotto R, Duner E, Denson LA, Sanz JM, Di Virgilio F, Okolicsanyi L, Casagrande F, Strazzabosco M. Proinflammatory cytokines inhibit secretion in rat bile duct epithelium. Gastroenterology. 2001;121:156–169. doi: 10.1053/gast.2001.25516. [DOI] [PubMed] [Google Scholar]
- 18.Albillos A, Muñoz L, Nieto M, Ubeda M, de-la-Hera A, Alvarez-Mon M. Systemic effects of TNF-alpha secreted by circulating monocytes and fatigue in cirrhosis. Hepatology. 2006;43:1399; author reply 1399–1400. doi: 10.1002/hep.21205. [DOI] [PubMed] [Google Scholar]
- 19.Tiggelman AM, Boers W, Linthorst C, Sala M, Chamuleau RA. Collagen synthesis by human liver (myo)fibroblasts in culture: evidence for a regulatory role of IL-1 beta, IL-4, TGF beta and IFN gamma. J Hepatol. 1995;23:307–317. [PubMed] [Google Scholar]
- 20.Napoli J, Bishop GA, McCaughan GW. Increased intrahepatic messenger RNA expression of interleukins 2, 6, and 8 in human cirrhosis. Gastroenterology. 1994;107:789–798. doi: 10.1016/0016-5085(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 21.Tilg H, Wilmer A, Vogel W, Herold M, Nölchen B, Judmaier G, Huber C. Serum levels of cytokines in chronic liver diseases. Gastroenterology. 1992;103:264–274. doi: 10.1016/0016-5085(92)91122-k. [DOI] [PubMed] [Google Scholar]
- 22.Koff RS. Transforming growth factors in human chronic hepatitis and cirrhosis: correlations with fibrogenesis and hepatic regeneration. Gastroenterology. 1991;101:1445–1446. doi: 10.1016/0016-5085(91)90104-s. [DOI] [PubMed] [Google Scholar]
- 23.Milani S, Herbst H, Schuppan D, Stein H, Surrenti C. Transforming growth factors beta 1 and beta 2 are differentially expressed in fibrotic liver disease. Am J Pathol. 1991;139:1221–1229. [PMC free article] [PubMed] [Google Scholar]
- 24.Cassiman D, Libbrecht L, Desmet V, Denef C, Roskams T. Hepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat livers. J Hepatol. 2002;36:200–209. doi: 10.1016/s0168-8278(01)00260-4. [DOI] [PubMed] [Google Scholar]
- 25.De Creus A, Abe M, Lau AH, Hackstein H, Raimondi G, Thomson AW. Low TLR4 expression by liver dendritic cells correlates with reduced capacity to activate allogeneic T cells in response to endotoxin. J Immunol. 2005;174:2037–2045. doi: 10.4049/jimmunol.174.4.2037. [DOI] [PubMed] [Google Scholar]
- 26.Lichtman SN, Wang J, Lemasters JJ. LPS receptor CD14 participates in release of TNF-alpha in RAW 264.7 and peritoneal cells but not in kupffer cells. Am J Physiol. 1998;275:G39–G46. doi: 10.1152/ajpgi.1998.275.1.G39. [DOI] [PubMed] [Google Scholar]
- 27.Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–561. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 28.Mencin A, Kluwe J, Schwabe RF. Toll-like receptors as targets in chronic liver diseases. Gut. 2009;58:704–720. doi: 10.1136/gut.2008.156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nolan JP, Leibowitz AI. Endotoxin and the liver. III. Modification of acute carbon tetrachloride injury by polymyxin b--an antiendotoxin. Gastroenterology. 1978;75:445–449. [PubMed] [Google Scholar]
- 30.Grinko I, Geerts A, Wisse E. Experimental biliary fibrosis correlates with increased numbers of fat-storing and Kupffer cells, and portal endotoxemia. J Hepatol. 1995;23:449–458. doi: 10.1016/0168-8278(95)80204-5. [DOI] [PubMed] [Google Scholar]
- 31.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 32.Chan CC, Hwang SJ, Lee FY, Wang SS, Chang FY, Li CP, Chu CJ, Lu RH, Lee SD. Prognostic value of plasma endotoxin levels in patients with cirrhosis. Scand J Gastroenterol. 1997;32:942–946. doi: 10.3109/00365529709011206. [DOI] [PubMed] [Google Scholar]
- 33.Lin RS, Lee FY, Lee SD, Tsai YT, Lin HC, Lu RH, Hsu WC, Huang CC, Wang SS, Lo KJ. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995;22:165–172. doi: 10.1016/0168-8278(95)80424-2. [DOI] [PubMed] [Google Scholar]
- 34.Aoyama T, Paik YH, Seki E. Toll-like receptor signaling and liver fibrosis. Gastroenterol Res Pract. 2010;2010:pii:192543. doi: 10.1155/2010/192543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott MJ, Billiar TR. Beta2-integrin-induced p38 MAPK activation is a key mediator in the CD14/TLR4/MD2-dependent uptake of lipopolysaccharide by hepatocytes. J Biol Chem. 2008;283:29433–29446. doi: 10.1074/jbc.M803905200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37:1043–1055. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- 37.Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48:322–335. doi: 10.1002/hep.22306. [DOI] [PubMed] [Google Scholar]
- 38.Gäbele E, Mühlbauer M, Dorn C, Weiss TS, Froh M, Schnabl B, Wiest R, Schölmerich J, Obermeier F, Hellerbrand C. Role of TLR9 in hepatic stellate cells and experimental liver fibrosis. Biochem Biophys Res Commun. 2008;376:271–276. doi: 10.1016/j.bbrc.2008.08.096. [DOI] [PubMed] [Google Scholar]
- 39.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 40.Davis BH, Chen A, Beno DW. Raf and mitogen-activated protein kinase regulate stellate cell collagen gene expression. J Biol Chem. 1996;271:11039–11042. doi: 10.1074/jbc.271.19.11039. [DOI] [PubMed] [Google Scholar]
- 41.Marra F, Arrighi MC, Fazi M, Caligiuri A, Pinzani M, Romanelli RG, Efsen E, Laffi G, Gentilini P. Extracellular signal-regulated kinase activation differentially regulates platelet-derived growth factor’s actions in hepatic stellate cells, and is induced by in vivo liver injury in the rat. Hepatology. 1999;30:951–958. doi: 10.1002/hep.510300406. [DOI] [PubMed] [Google Scholar]
- 42.Svegliati-Baroni G, Ridolfi F, Caradonna Z, Alvaro D, Marzioni M, Saccomanno S, Candelaresi C, Trozzi L, Macarri G, Benedetti A, et al. Regulation of ERK/JNK/p70S6K in two rat models of liver injury and fibrosis. J Hepatol. 2003;39:528–537. doi: 10.1016/s0168-8278(03)00291-5. [DOI] [PubMed] [Google Scholar]
- 43.Kluwe J, Pradere JP, Gwak GY, Mencin A, De Minicis S, Osterreicher CH, Colmenero J, Bataller R, Schwabe RF. Modulation of hepatic fibrosis by c-Jun-N-terminal kinase inhibition. Gastroenterology. 2010;138:347–359. doi: 10.1053/j.gastro.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Gao J, Zhang D, Zhang J, Ma J, Jiang H. New insights into the antifibrotic effects of sorafenib on hepatic stellate cells and liver fibrosis. J Hepatol. 2010;53:132–144. doi: 10.1016/j.jhep.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Wang Z, Kwong SQ, Lui EL, Friedman SL, Li FR, Lam RW, Zhang GC, Zhang H, Ye T. Inhibition of PDGF, TGF-β, and Abl signaling and reduction of liver fibrosis by the small molecule Bcr-Abl tyrosine kinase antagonist Nilotinib. J Hepatol. 2011;55:612–625. doi: 10.1016/j.jhep.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 46.Iacobini C, Menini S, Ricci C, Blasetti Fantauzzi C, Scipioni A, Salvi L, Cordone S, Delucchi F, Serino M, Federici M, et al. Galectin-3 ablation protects mice from diet-induced NASH: a major scavenging role for galectin-3 in liver. J Hepatol. 2011;54:975–983. doi: 10.1016/j.jhep.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 47.Bellafante E, Murzilli S, Salvatore L, Latorre D, Villani G, Moschetta A. Hepatic-specific activation of peroxisome proliferator-activated receptor γ coactivator-1β protects against steatohepatitis. Hepatology. 2013;57:1343–1356. doi: 10.1002/hep.26222. [DOI] [PubMed] [Google Scholar]
- 48.Robert K, Nehmé J, Bourdon E, Pivert G, Friguet B, Delcayre C, Delabar JM, Janel N. Cystathionine beta synthase deficiency promotes oxidative stress, fibrosis, and steatosis in mice liver. Gastroenterology. 2005;128:1405–1415. doi: 10.1053/j.gastro.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 49.Suliman HB, Welty-Wolf KE, Carraway MS, Schwartz DA, Hollingsworth JW, Piantadosi CA. Toll-like receptor 4 mediates mitochondrial DNA damage and biogenic responses after heat-inactivated E. coli. FASEB J. 2005;19:1531–1533. doi: 10.1096/fj.04-3500fje. [DOI] [PubMed] [Google Scholar]
- 50.Gao B, Seki E, Brenner DA, Friedman S, Cohen JI, Nagy L, Szabo G, Zakhari S. Innate immunity in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2011;300:G516–G525. doi: 10.1152/ajpgi.00537.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


