Abstract
AIM: To investigate the association between statin use and colorectal cancer risk, we conducted an updated meta-analysis of published studies.
METHODS: We performed a comprehensive search for studies published up to July 2013. Eligible studies for this meta-analysis were either randomized controlled trials (RCTs) or observational studies (case-control or cohort) evaluating any exposure to statins and the risk of colorectal cancer. Two reviewers selected studies based on predefined inclusion criteria, and abstracted the data. Pooled relative risk (RR) estimates with their 95%CI were calculated using fixed- and random-effects models. Then, we assessed the potential presence of publication bias and between-studies heterogeneity. To evaluate the results, we also performed a “leave-one-out” sensitivity analysis.
RESULTS: A total of 40 studies, involving more than eight million subjects, contributed to the analysis. They were grouped on the basis of study design and, consequently, three separate meta-analyses were conducted. A similar modest reduction in the risk of colorectal cancer with statin use was observed, which was not statistically significant among RCTs (RR = 0.89, 95%CI: 0.74-1.07; n = 8), but reached statistical significance among cohort studies (RR = 0.91, 95%CI: 0.83-1.00; n = 13) and case-control studies (RR = 0.92, 95%CI: 0.87-0.98; n = 19). While we did not find significant evidence of selective outcome reporting or publication bias, substantial heterogeneity was detected, mainly among the observational studies. The sensitivity analysis confirmed the stability of our results.
CONCLUSION: A modest reduction in risk of colorectal cancer among statin users cannot be disproved. Further targeted research is warranted.
Keywords: 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, Statins, Colorectal cancer, Systematic review, Meta-analysis, Cancer chemoprevention
Core tip: To investigate the association between statin use and colorectal cancer risk, we conducted a systematic review and meta-analysis of published studies. A total of 40 studies, involving more than eight million subjects, contributed to our analysis. A modest reduction in the risk of colorectal cancer with statin use was observed, which was not statistically significant among RCTs (RR = 0.89, 95%CI: 0.74-1.07; n = 8), but reached statistical significance among cohort studies (RR = 0.91, 95%CI: 0.83-1.00; n = 13) and case-control studies (RR = 0.92, 95%CI: 0.87-0.98; n = 19). Further targeted research is warranted.
INTRODUCTION
Statins are some of the most widely prescribed drugs worldwide[1], as a result of their proven efficacy in the primary and secondary prevention of cardiovascular morbidity and mortality, in a variety of populations[2-7]. Their main mechanism of action is the reduction of serum cholesterol, by means of competitive inhibition of hepatic 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, which is the rate-limiting enzyme in the mevalonate synthesis pathway[8]; this reduces endogenous cholesterol biosynthesis, leading to decreased levels of low-density lipoprotein (LDL), a major risk factor for atherosclerosis[9]. In addition, statins exert a variety of so-called “pleiotropic” effects on human physiology[10]; these are thought to contribute to their principal cardiovascular benefit[11,12], although this has not as yet been reflected in clinical trial data[13].
Because of their pleiotropic effects, it has been suggested that statins might have an effect on cancer risk and play a role in cancer chemoprevention[10,14-16]. Data from in vitro and animal model studies have been encouraging[17], but the epidemiological evidence remains inconclusive[18]. In addition, several meta-analyses of randomized controlled trials (RCTs) of statins for cardiovascular outcomes failed to find an association between statin use and overall cancer risk[19-22]. Cancer is not a homogenous disease entity however, and the effects of statins might significantly differ according to anatomical site and molecular type. Thus, overall cancer risk is not a very sensitive outcome, and may mask important effects of statins at particular sites.
The relation between statins and colorectal cancer has been the focus of a growing body of both basic and epidemiological research[23,24]. A fair amount of epidemiological studies have examined the effect of statins on colorectal cancer risk, with often inconsistent results ranging from very protective[25] (47% risk reduction) to moderately harmful[26] (7% risk increase). In 2007, we undertook a meta-analysis of published studies reporting on statin use and colorectal cancer risk[27]; at that time we identified 18 studies (6 RCTs and 12 observational) and concluded that the evidence did not support a strong reduction in colorectal cancer risk by the use of statins in usual dosage, although a modest protective effect or an effect at higher doses could not be excluded. Since then, many additional studies have been published, and therefore we sought to update our previous systematic review and meta-analysis to reflect the current totality of evidence on statins and colorectal cancer risk.
MATERIALS AND METHODS
Search strategy
We identified studies by a systematic literature search of MEDLINE electronic database (1966 through July 2013). We ran two queries, one aimed at RCTs and one aimed at observational studies (case-control or cohort). Query I was: [“Randomized Controlled Trial” (ptyp)] AND (“HMG-CoA reductase inhibitor” OR “HMG-CoA reductase inhibitors” OR “HMG-CoA reductase inhibitor” OR “HMG-CoA reductase inhibitors” OR statin OR statins OR atorvastatin OR cerivastatin OR fluvastatin OR lovastatin OR mevastatin OR pravastatin OR rivastatin OR rosuvastatin OR simvastatin). Query II was: (“HMG-CoA reductase inhibitor” OR “HMG-CoA reductase inhibitors” OR statin OR statins OR atorvastatin OR cerivastatin OR fluvastatin OR lovastatin OR mevastatin OR pravastatin OR rivastatin OR rosuvastatin OR simvastatin OR pitavastatin) AND (cancer OR cancers OR neoplasm OR neoplasms OR malignancy OR malignancies).
In addition, we browsed the reference lists of relevant narrative and systematic reviews, and asked a knowledgeable expert to identify any additional studies. We browsed the title and abstract of all identified studies to exclude any that were clearly irrelevant. The full text of the remaining articles was read to determine whether it contained information on the topic of interest.
Selection criteria
Eligible studies for this meta-analysis were either RCTs or observational studies (case-control or cohort) evaluating any exposure to statins and the risk of colorectal cancer. In order to be included in the meta-analysis, studies had to report an estimated measure of effect size (risk ratio, rate ratio, hazard ratio or odds ratio) and its associated CI, or had to provide enough data to calculate such an effect measure and CI. In cases of multiple publications from the same population, only data from the most recent report were included.
In particular, RCTs were considered eligible if they (1) evaluated a statin therapy compared with placebo or no treatment; (2) had no other intervention difference between the experimental and the control group; (3) enrolled at least 2000 participants; (4) had a minimum duration of 2 years; and (5) reported the incidence of colorectal cancer in both arms during the trial period. The fourth criterion was used because the effects of the intervention may require long-term exposure. In addition, as colorectal cancer is a rare disease, RCTs of short duration are unlikely to register any significant number of cases.
We did not assess the methodological quality of the primary studies, since quality scoring in meta-analyses of observational studies is controversial, as it is for RCTs[28,29]. Instead, we performed subgroup and sensitivity analyses, as is recommended.
Data extraction
Two reviewers (Lytras T, Bonovas S) abstracted the data independently. The following information was collected from each study: (1) citation data, first author’s last name, year of publication, and country of the population studied; (2) study design; (3) number of subjects; (4) relative risk (RR) and 95%CI; (5) for RCTs, the number of events (colorectal cancer cases) in the statin and control groups; (6) definition of statin exposure; and (7) control for confounding factors by matching or adjustments, if applicable.
Risk ratios and 95%CI were calculated for each RCT by reconstructing contingency tables based on the number of subjects randomly assigned and the number of subjects with incident colorectal cancer (analysis in accordance with the intention-to-treat principle). In observational studies, we extracted the RR estimates that reflected the greatest degree of control for potential confounders. Differences in data extraction were resolved by consensus, referring back to the original article.
Statistical analysis
We included in this meta-analysis studies reporting different measures of relative risk: RCTs (risk ratio), cohort studies (rate ratio, hazard ratio), and case-control studies (odds ratio). In practice, these measures of effect yield very similar RR estimates, since the absolute risk of colorectal cancer is very low[30].
Studies were grouped on the basis of study design, and three separate meta-analyses were conducted: one each for RCTs, cohort studies and case-control studies, using both fixed-effects and random-effects models. This was done to examine consistency of results across varying study designs with different potential biases. We also compared the summary RR estimates derived from the three separate meta-analyses with a test of interaction[31].
Each meta-analysis was performed twice, assuming either a fixed-effects or a random-effects model. In the absence of heterogeneity, the fixed-effects and the random-effects models provide similar results. When heterogeneity is found, the random-effects model is considered to be more appropriate, though both models may be biased[32].
For all statistical analyses we used the R software environment[33], version 3.0.1, and the “meta” package for R[34], version 2.3-0. For RCTs we used the function “metabin” to perform meta-analysis of binary outcome data, using as input the number of colorectal cases per group in each study; the Mantel-Haenszel method[35] was used to calculate pooled estimates, and the DerSimonian-Laird method[36] to estimate between-study variance in the random-effects model. For cohort and case-control studies we used the function “metagen”, inputting the log RR and its standard error (SE) for each study; the inverse variance weighting method was used to calculate pooled estimates, and the DerSimonian-Laird method[36] to estimate between-studies variance in the random-effects model. The standard error of the log RR was calculated from the upper (U) and lower (L) limits of the 95%CI published in each study, using the formula SE = (logU-logL)/(2 × 1.96).
Selective outcome reporting or publication bias was assessed using the Begg and Mazumdar adjusted rank correlation test[37] and the Egger regression asymmetry test[38]. To evaluate whether the results of the studies were homogeneous, we used the Cochran’s Q test with a 0.10 level of significance. We also calculated the I2 statistic[39] that describes the percentage variation across studies that is due to heterogeneity rather than chance. Negative values of I2 were put equal to zero, so that I2 lies between 0% (i.e., no observed heterogeneity) and 100%. We regarded an I2 value less than 40% as indicative of “not important heterogeneity” and a value higher than 75% as indicative of “considerable heterogeneity”[40].
All P-values are two-tailed. For all tests (except for heterogeneity), a probability level less than 0.05 was considered statistically significant. This work was performed according to the guidelines proposed by the Meta-analysis of Observational Studies in Epidemiology (MOOSE) group[41], and the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) statement[42].
RESULTS
Search results
The results of our search process are presented on Figure 1. We identified and analyzed 40 independent studies that met the predefined inclusion criteria[4,5,24,25,39-74]. Eight out of 40 were RCTs of statins for cardiovascular outcomes[4,5,43-48], 13 were cohort studies[49-61], and 19 were case-control studies[25,26,62-78]. Sixteen studies had been included in the previous meta-analysis[4,5,25,45-48,60,61,72-78] while 24 were newly published[26,43,44,49-59,62-71], of which 2 RCTs[43,44], 11 cohort studies[49-59] and 11 case-control studies[26,62-71].
Figure 1.
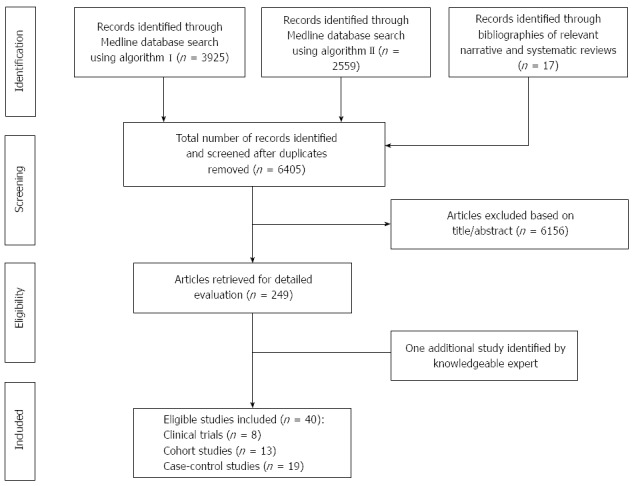
Summary of evidence search and selection.
In total, the 40 studies involved more than 8.2 million subjects. The number of colorectal cancer cases ranged from 32 to 245 in the RCTs, from 76 to 6637 in the cohort studies, and from 56 to 36736 in the case-control studies. Three out of the 40 studies had been published solely in abstract form[73-75], and one was an academic dissertation[62]. One study from Japan[54] was a pooled analysis of two randomized trials and one cohort study, providing a single summary RR for statin use and colorectal cancer; we treated this as a single cohort study.
Seven out of eight RCTs were placebo-controlled[4,5,43-45,47,48], while one RCT was a non-blinded trial comparing statin treatment with a usual care control group[46]. All RCTs reported site-specific cancer outcomes, including colorectal cancer, as secondary endpoints. We were thus able to conduct a post hoc analysis of these trials and calculate risk ratios for colorectal cancer in an intention-to-treat analysis. All observational studies[24,25,47-76] evaluated exposure to statins and risk of colorectal cancer, and were controlled for various potential confounding factors by matching or adjustments. The publication dates of the studies included in the meta-analysis ranged between 1996 and 2013. Study designs, along with the RR estimates and 95%CI are shown in Table 1 for the RCTs, in Table 2 for the cohort studies, and in Table 3 for the case-control studies.
Table 1.
Randomized controlled trials included in the meta-analysis
| Study | Agent | All subjects | Duration (yr) |
Incident colorectal cancer cases |
RR | 95%CI | |
| Statin group | Control group | ||||||
| JUPITER[43] | Rosuvastatin | 16304 | Median: 2.0 | 16 of 8154 | 28 of 8150 | 0.57 | 0.31-1.05 |
| WOSCOPS[44] | Pravastatin | 6577 | Mean: 4.9 | 12 of 3291 | 20 of 3286 | 0.60 | 0.29-1.23 |
| 4S[47] | Simvastatin | 4444 | Median: 10.4 | 25 of 2221 | 32 of 2223 | 0.78 | 0.46-1.32 |
| ALLHAT-LLT[46] | Pravastatin | 10355 | Mean: 4.8 | 461 of 5170 | 381 of 5185 | 1.21 | 0.79-1.86 |
| HPS[45] | Simvastatin | 20536 | Mean: 5.0 | 114 of 10269 | 131 of 10267 | 0.87 | 0.68-1.12 |
| LIPID[48] | Pravastatin | 9014 | Mean: 8.0 | 75 of 4512 | 71 of 4502 | 1.05 | 0.76-1.45 |
| AFCAPS[5] | Lovastatin | 6605 | Mean: 5.2 | 251 of 3304 | 201 of 3301 | 1.25 | 0.70-2.24 |
| CARE[4] | Pravastatin | 4159 | Mean: 4.8 | 12 of 2081 | 21 of 2078 | 0.57 | 0.28-1.16 |
Figures for colon cancer rather than colorectal cancer.
Table 2.
Cohort studies included in the meta-analysis
| Study | Study location | All subjects | CRC cases | RR | 95%CI | Control for potential confounding factors2 |
| Clancy et al[50], 2013 | Italy | 266109 | 2420 | 0.84 | 0.76-0.93 | 1-9 |
| Leung et al[49], 2013 | Taiwan | 34205 | 654 | 0.57 | 0.45-0.721 | - |
| Simon et al[51], 2012 | United States | 159219 | 2000 | 0.99 | 0.83-1.20 | 1, 4, 10-27 |
| Jacobs et al[53], 2011 | United States | 133255 | 1739 | 1.03 | 0.94-1.141 | - |
| Lee et al[52], 2011 | United States | 131922 | 1680 | 0.97 | 0.84-1.12 | 1, 6, 10, 12, 13, 28-34 |
| Haukka et al[55], 2010 | Finland | 944962 | 5016 | 1.04 | 0.98-1.101 | - |
| Matsushita et al[54], 2010 | Japan | 13724 | 76 | 1.22 | 0.77-1.94 | - |
| Flick et al[57], 2009 | United States | 69115 | 171 | 0.89 | 0.61-1.30 | 1, 4, 6, 10-13, 15, 18, 19, 28, 35-38 |
| Singh et al[56], 2009 | Canada | 413271 | 6637 | 1.13 | 1.02-1.25 | 1-4, 6, 7, 16, 39-42 |
| Friedman et al[58], 2008 | United States | 4243067 | 5684 | 0.88 | 0.82-0.961 | - |
| Farwell et al[59], 2008 | United States | 62842 | 687 | 0.65 | 0.55-0.78 | 1, 6, 11, 13, 19, 29, 43-53 |
| Setoguchi et al[60], 2007 | United States | 31723 | 249 | 0.96 | 0.70-1.31 | 1-9, 39, 43, 54-64 |
| Friis et al[61], 2005 | Denmark | 334754 | 3006 | 0.85 | 0.65-1.11 | 1, 2, 4, 16, 66 |
Calculated crude relative risk (RR);
1: Age; 2: Sex; 3: Inflammatory bowel disease; 4: Use of nonsteroidal anti-inflammatory drugs; 5: Obesity; 6: Colonoscopy; 7: Comorbidity score; 8: Distinct generic medicines taken; 9: Prior hospitalizations; 10: Body mass index; 11: Smoking status; 12: Family History of colorectal cancer; 13: Alcohol use; 14: Education; 15: Physical activity level; 16: Hormone replacement therapy; 17: Ethnic group; 18: Colorectal polyps; 19: Cardiovascular disease; 20: Calcium intake; 21: Percent energy from fat; 22: Fruit and vegetable intake; 23: Calcium supplement use; 24: Selenium supplement use; 25: Current healthcare provider; 26: Last medical visit within one year; 27: Colon screening; 28: Red meat consumption; 29: Use of aspirin; 30: Calendar year; 31: Study; 32: Pack-years of smoking before age 30; 33: Height; 34: Total energy intake; 35: Hypercholesterolemia; 36: Multivitamin use; 37: Energy-adjusted fibre intake; 38: Folate intake; 39: Benign mammary dysplasia; 40: Coronary heart disease; 41: Resective colorectal surgery; 42: Socioeconomic status; 43: Diabetes; 44: Weight; 45: Thyroid disease; 46: Hypertension; 47: Renal failure; 48: Chest pain; 49: Mental illness; 50: Lung disease; 51: Gastrointestinal Disease; 52: Prostate disease; 53: Total cholesterol; 54: Race; 55: Arthritis; 56: Use of gastroprotective drugs; 57: Estrogen use; 58: Tobacco abuse; 59: Mammography; 60: Gynecologic examination; 61: Pap smear; 62: Fecal occult blood; 63: Number of physician visits; 64: Prior nursing home stay; 65: History of heart attack; 66: Use of cardiovascular drugs. CRC: Colorectal cancer.
Table 3.
Case-control studies included in the meta-analysis
| Study | Study location | All subjects | CRC cases | OR | 95%CI | Control for potential confounding factors2 |
| Deshpande[62], 2013 | United States | 73472 | 36736 | 0.96 | 0.89-1.05 | 1-4 |
| Broughton et al[64], 2012 | United Kingdom | 233 | 101 | 0.43 | 0.25-0.80 | 1, 2, 5-8 |
| Lakha et al[63], 2012 | United Kingdom | 603 | 309 | 0.33 | 0.15-0.69 | 1-3, 9-15 |
| Cheng et al[65], 2011 | Taiwan | 5780 | 1156 | 1.09 | 0.91-1.30 | 1-3, 5, 8, 9, 16-21 |
| Vinogradova et al[26], 2011 | United Kingdom | 60373 | 11749 | 1.07 | 1.00-1.15 | 1-3, 7, 9, 11, 12, 22-27 |
| Robertson et al[66], 2010 | Denmark | 109769 | 9979 | 0.87 | 0.80-0.96 | 1, 2, 5-7, 9, 10, 19 |
| Hachem et al[68], 2009 | United States | 30400 | 6080 | 0.91 | 0.86-0.96 | 1-4, 9, 16, 19, 20, 28, 29 |
| Shadman et al[67], 2009 | United States | 2044 | 669 | 1.17 | 0.74-1.85 | 1, 4, 11, 12, 30-33 |
| Boudreau et al[70], 2008 | United States | 1330 | 665 | 1.02 | 0.65-1.59 | 1, 2, 5, 9, 11, 12, 32 |
| Yang et al[69], 2008 | United Kingdom | 48724 | 4432 | 1.10 | 0.50-2.20 | 1, 2, 4, 6, 7, 9, 11, 12, 32, 34 |
| Hoffmeister et al[71], 2007 | Germany | 1154 | 540 | 0.69 | 0.45-1.06 | 1, 2, 4-7, 9, 11, 12, 30-32, 35-37 |
| Coogan et al[72], 2007 | United States | 3618 | 1809 | 0.92 | 0.78-1.09 | 1, 2, 4, 9, 10 |
| Li et al[73], 2006 | United States | 741 | 3391 | 0.8 | 0.34-1.87 | 1, 2, 5, 6, 9, 11, 12, 30, 38 |
| Poynter et al[25], 2005 | Israel | 3968 | 1953 | 0.53 | 0.38-0.74 | 1, 2, 9, 30, 36, 39-41 |
| Rubin et al[74], 2005 | United States | 387240 | 18440 | 0.92 | 0.89-0.96 | 1, 2, 4, 9, 17, 42-45 |
| Kaye et al[76], 2004 | United Kingdom | 18088 | 3291 | 1.0 | 0.6-1.7 | 1, 2, 11, 12, 42 |
| Graaf et al[77], 2004 | Netherlands | 20105 | 2921 | 0.87 | 0.48-1.57 | 1, 2, 5, 9, 17, 18, 32, 34, 46-49 |
| Khurana et al[75], 2004 | United States | 534273 | 53391 | 0.94 | 0.89-1.00 | 1, 2, 6, 9, 12, 38, 50 |
| Blais et al[78], 2000 | Canada | 5962 | 561 | 0.83 | 0.37-1.89 | 1, 2, 14, 18, 34, 46 |
Figures for colon cancer rather than colorectal cancer;
1: Age; 2: Sex; 3: Inflammatory bowel disease; 4: Colonoscopy; 5: Diabetes; 6: Alcohol use; 7: Use of aspirin; 8: Use of metformin; 9: Use of NSAIDs; 10: Precinct of residence; 11: Body mass index; 12: Smoking status; 13: Physical activity level; 14: History of neoplasia; 15: Family history of cancer; 16: Fecal occult blood; 17: Prior hospitalizations; 18: Other lipid-lowering therapy; 19: Cholecystectomy; 20: Liver disease; 21: Colorectal polyps; 22: History of heart attack; 23: Hypertension; 24: Coronary heart disease; 25: Socioeconomic status; 26: Rheumatoid arthritis; 27: Use of COX-2 inhibitors; 28: Diabetic nephropathy; 29: Use of sulfonylurea; 30: Family history of colorectal cancer; 31: Education; 32: Hormone replacement therapy; 33: Calendar year; 34: Duration of follow-up; 35: Red meat consumption; 36: Hypercholesterolemia; 37: History of rheumatic disease; 38: Race; 39: Ethnic group; 40: Sports participation; 41: Level of vegetable consumption; 42: Number of physician visits; 43: Use of glucocorticosteroids; 44: Use of immunomodulators; 45: Use of 5-aminosalicylic acids; 46: Comorbidity score; 47: Use of diuretics; 48: Use of ACE inhibitors; 49: Use of CCBs; 50: Obesity. CRC: Colorectal cancer.
Meta-analysis of RCTs
Eight large RCTs contributed to the analysis[4,5,43-48]. A total of 77994 individuals participated in these trials; 39002 in treatment groups and 38992 in control groups. The participants had a mean follow-up of approximately 5.0 years and a total experience of 390000 person-years. Five trials[4,43-45,47] reported a lower risk of colorectal cancer in the treatment group, while the other three trials[5,46,48] reported a higher risk (Table 1). None was statistically significant. The overall rate of colorectal cancer on all 8 RCTs was 0.83% in the statin group (325 incident cases) and 0.93% in the control group (361 incident cases). We found a modest but not statistically significant protective association ( about 10% risk reduction) of statin use against colorectal cancer, both under the assumption of a fixed-effects model (RR = 0.90, 95%CI: 0.78-1.04), or a random-effects model (RR = 0.89, 95%CI: 0.74-1.07) (Table 4). Figure 2 shows the forest plot of the RR estimates and 95%CI from the individual trials and the pooled results. The Cochran’s Q test had a P-value of 0.23 and the corresponding I2 statistic was 25%, both indicating little variability between studies that cannot be explained by chance. The P-values for the Begg’s and the Egger’s tests were P = 0.22 and P = 0.31, respectively, both suggesting a low probability of selective outcome reporting or publication bias. Figure 3 shows the contribution of the randomized studies (represented by the blue points) in the overall funnel plot, while Figure 4 shows the incidence of colorectal cancer in the statin group against the incidence in the control group, across the 8 RCTs (L’Abbé plot)[79]. This plot demonstrates no substantial heterogeneity between studies, and no relation of statin effect to baseline risk of colorectal cancer.
Table 4.
Meta-analysis results
| No. of studies |
Fixed-effects model |
Random-effects model |
Tests of homogeneity |
Tests of publication bias |
||||||
| RR | 95%CI | RR | 95%CI | Q value (df) | P value | I2 | Begg’s P value | Egger’s P value | ||
| Randomized Controlled Trials | 8 | 0.90 | 0.78-1.04 | 0.89 | 0.74-1.07 | 9.31 (7) | 0.23 | 25% | 0.22 | 0.31 |
| Placebo-controlled RCTs | 7 | 0.86 | 0.74-1.01 | 0.85 | 0.71-1.03 | 7.21 (6) | 0.30 | 17% | 0.18 | 0.24 |
| RCTs of lipophilic statins | 3 | 0.90 | 0.73-1.10 | 0.90 | 0.73-1.10 | 1.55 (2) | 0.46 | 0% | 0.60 | 0.70 |
| RCTs of lipophobic statins | 5 | 0.90 | 0.73-1.12 | 0.83 | 0.60-1.15 | 7.75 (4) | 0.10 | 48% | 0.62 | 0.05 |
| Cohort Studies | 13 | 0.96 | 0.93-0.99 | 0.91 | 0.83-1.00 | 70.85 (12) | < 0.001 | 83% | 0.54 | 0.22 |
| Case-control studies | 19 | 0.93 | 0.91-0.96 | 0.92 | 0.87-0.98 | 50.31 (18) | < 0.001 | 64% | 0.46 | 0.27 |
| Published in full text form | 16 | 0.94 | 0.91-0.98 | 0.90 | 0.83-0.99 | 49.12 (15) | < 0.001 | 69% | 0.47 | 0.19 |
| Observational studies | 32 | 0.94 | 0.92-0.96 | 0.92 | 0.87-0.96 | 122.68 (31) | < 0.001 | 75% | 0.36 | 0.16 |
| All studies | 40 | 0.94 | 0.92-0.96 | 0.91 | 0.87-0.96 | 132.3 (39) | < 0.001 | 71% | 0.33 | 0.11 |
RCT: Randomized controlled trials.
Figure 2.
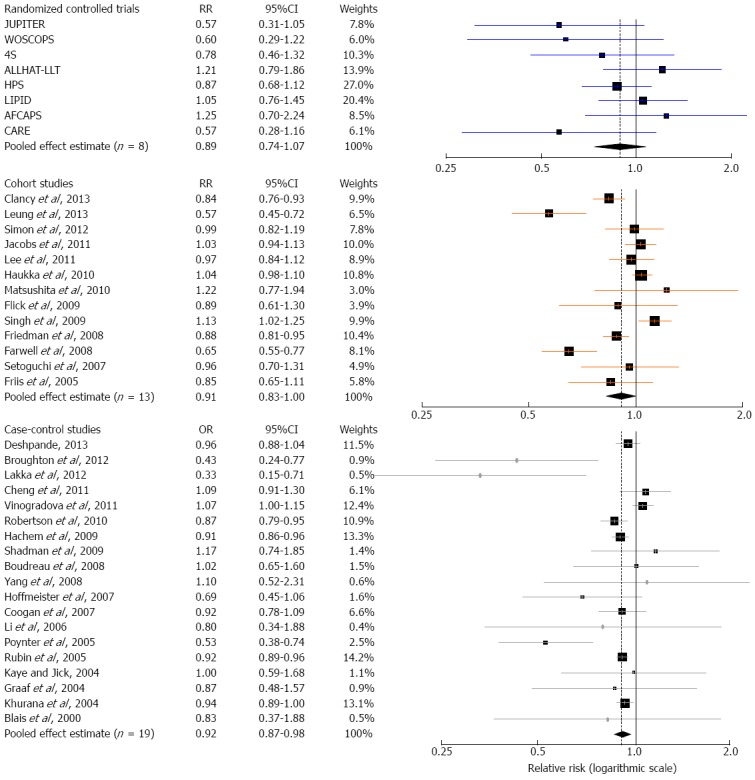
Forest plot: results from individual studies and meta-analyses. The RR and 95%CI for each study are displayed on a logarithmic scale. Pooled estimates are from a random-effects model.
Figure 3.
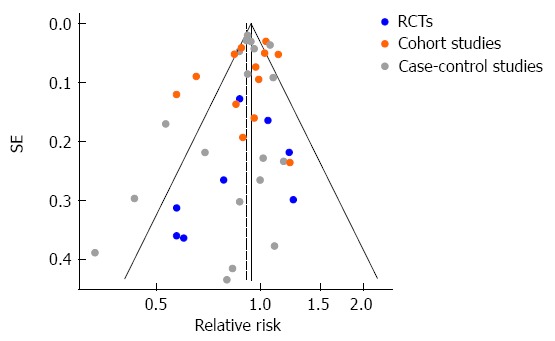
Funnel plot of observed relative risk against standard error (as a surrogate of study size) for all studies analyzed. RCT: Randomized controlled trials.
Figure 4.
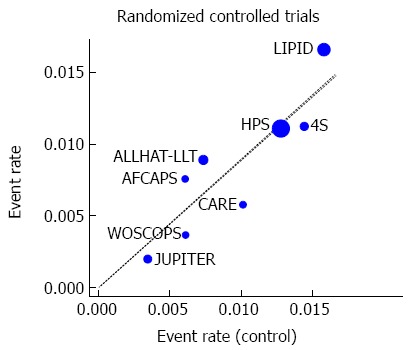
L’Abbé plot of the incidence of colorectal cancer in the experimental (statin) group, against the incidence in the control group, across the analyzed randomized controlled trials (n = 8).
When the analysis was restricted to trials that evaluated statin therapy compared with placebo[4,5,43-45,47,48], the results did not substantially change (fixed-effects model: RR = 0.86, 95%CI: 0.74-1.01; random-effects model: RR = 0.85, 95%CI: 0.71-1.03; Cochran’s P = 0.30 and I2 = 17%; Begg’s P = 0.18 and Egger’s P = 0.24). Similarly, after stratifying the data in two subgroups (lipophilic[5,45,47] vs lipophobic statins[4,43,44,46,48]), we did not find any statistically significant association between lipophilic or lipophobic statins and risk of colorectal cancer (Table 4). Last, to explore whether the results were dominated by a single study, we performed a “leave-one-out” sensitivity analysis, removing one study at a time (Figure 5A). This approach confirmed the stability of our results.
Figure 5.
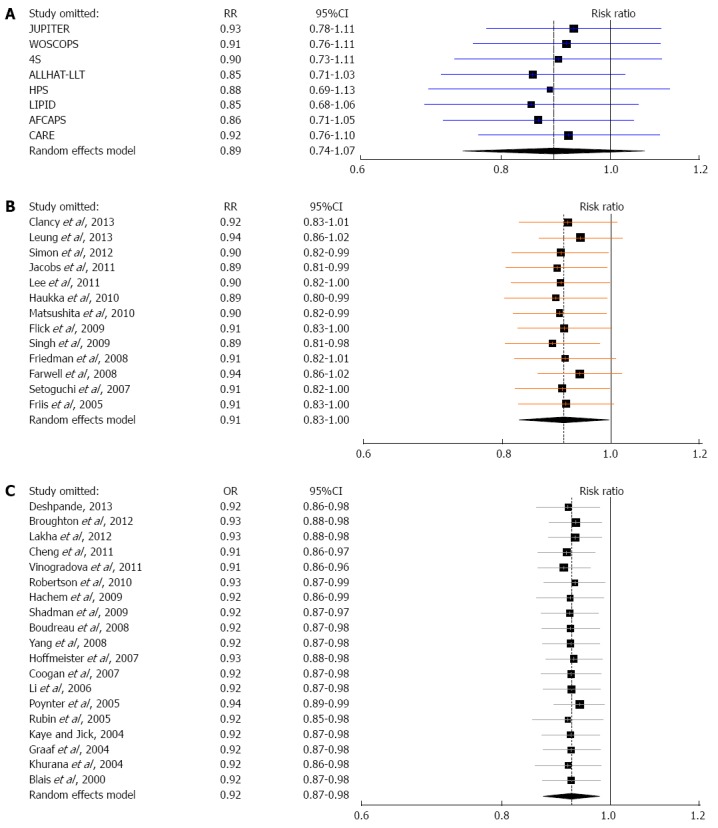
‘‘Leave-one-out” sensitivity analysis for the three meta-analyses: pooled estimates are from random-effects models with one study omitted at a time. A: Randomized controlled trials, B: Cohort studies, C: Case-control studies.
Meta-analysis of cohort studies
Thirteen cohort studies[49-61] evaluating exposure to statins and colorectal cancer risk were included in the meta-analysis (Table 2, Figure 2). Approximately seven million patients participated in these studies, with the occurrence of 30019 colorectal cancer cases. Four cohort studies reported not an overall, but two or more “correlated” subgroup effect-estimates[49,53,55,58]; however, based on the available data, we were able to calculate study-specific crude RR estimates for these four studies for the purpose of our meta-analysis (Table 2).
Statin use was associated with a modest reduction in the risk of colorectal cancer, and this association reached statistical significance both under a fixed-effects model (RR = 0.96, 95%CI: 0.93-0.99) and under a random-effects model (RR = 0.91, 95%CI: 0.83-1.00) (Table 4, Figure 2). However, the Cochran’s Q test had a P-value lower than 0.001 and the corresponding I2 statistic was 83%, indicating substantial heterogeneity between studies. In the sensitivity analysis (Figure 5B), omitting any single study did not lower the I2 further than 78%. The P-values for the Begg’s and the Egger’s tests were P = 0.54 and P = 0.22, respectively, suggesting a low probability of publication bias.
Meta-analysis of case-control studies
Nineteen case-control studies[25,26,62-78] evaluated exposure to statins and colorectal cancer risk (Table 3). A total of 1.3 million patients participated in these studies, of which 100000 were colorectal cancer cases. Once again, statin use was associated with a similar modest reduction in the risk of colorectal cancer, which was statistically significant under both a fixed-effects model (RR = 0.93, 95%CI: 0.91-0.96) and a random-effects model (RR = 0.92, 95%CI: 0.87-0.98) (Table 4, Figure 2). We found substantial heterogeneity between studies; the Cochran’s Q test had a P-value lower than 0.001 and the corresponding I2 statistic was 64%. In the “leave-one-out” sensitivity analysis, we identified the study by Vinogradova et al[26] as contributing most to the between-studies variability, but not to a crucial degree; excluding this study from the analysis lowered the I2 to 50%. The P-values for the Begg’s and the Egger’s tests were P = 0.46 and P = 0.27, respectively, suggesting a low probability of publication bias.
When the analysis was limited to studies published in full-text, i.e., excluding those published solely in abstract form[73-75], the results did not appreciably change (Table 4). The association between statins and colorectal cancer risk remained statistically significant assuming either a fixed-effects model (RR = 0.94, 95%CI: 0.91-0.98), or a random-effects model (RR = 0.90, 95%CI: 0.83-0.99). The Cochran’s Q test had a P-value lower than 0.001, and the corresponding I2 was 69%. The P-values for the Begg’s and the Egger’s tests were P = 0.47 and P = 0.19, respectively, but the funnel plot was slightly asymmetric, indicating a small likelihood of publication bias. Thus, selective publication of smaller case-control studies with statistically significant results might have occurred to some extent. It should be noted, however, that the result of our meta-analysis of case-control studies was fairly robust in the “leave-one-out” sensitivity analysis (Figure 5C); removal of any single study did not alter the statistical significance of the pooled estimate.
Combined analysis
We compared pairwise the pooled RR estimates derived from the three separate analyses with a test of interaction[31]. We found no statistically significant differences between estimates, either between those assuming a fixed-effects model (RCTs vs cohort studies, z = 0.81, P = 0.42; RCTs vs case-control studies, z = 0.49, P = 0.62; cohort studies vs case-control studies, z = 1.23, P = 0.22) or those assuming a random-effects model (RCTs vs cohort studies, z = 0.18, P = 0.86; RCTs vs case-control studies, z = 0.36, P = 0.72; cohort studies vs case-control studies, z = 0.30, P = 0.76).
In addition, we performed a combined analysis of observational studies, i.e., cohort and case-control studies (Table 4). Statin use was again associated with a modest reduction in the risk of colorectal cancer, which was statistically significant assuming either a fixed-effects model (RR = 0.94, 95%CI: 0.92-0.96) or a random-effects model (RR = 0.92, 95%CI: 0.87-0.96; n = 32). The Cochran’s Q test had a P-value lower than 0.001 and the corresponding I2 statistic was 75%, indicating substantial between-studies variability. The P-values for the Begg’s and the Egger’s tests were P = 0.36 and P = 0.16, respectively, both suggesting a very low probability of publication bias.
Combining all 40 studies for analysis yielded very similar results (Table 4). This is expected, as this particular analysis was dominated by the observational studies (36 studies; 8.1 million participants). These studies accounted for 92.3% and the 98.5% of the weight in the fixed- and the random-effects model, respectively.
Finally, we attempted to analyze the effect of statins separately in colon and rectal cancer. Six cohort studies (out of 13) and five case-control studies (out of 19) provided results by colorectal cancer subsite. In these 11 studies, statins did not appear to have an effect on colon cancer, assuming either a fixed-effects model (RR = 0.97, 95%CI: 0.93-1.02), or a random-effects model (RR = 0.95, 95%CI: 0.87-1.04). The Cochran’s Q test had a P-value of 0.02, and the corresponding I2 was 54%. The p-values for the Begg’s and the Egger’s tests were P = 0.31 and P = 0.39, respectively. As regards rectal cancer, the fixed-effects model suggested no effect of statins (RR = 0.98, 95%CI: 0.91-1.05) but the random-effects model suggested a statistically significant effect (RR = 0.78, 95%CI: 0.62-0.97). The Cochran’s Q test had a P-value lower than 0.001 and the corresponding I2 was 79%, indicating substantial heterogeneity. The P-values for the Begg’s and the Egger’s tests were P = 0.59 and P = 0.06, respectively, which highlights a significant potential for selective outcome reporting bias in this analysis.
DISCUSSION
Cancer chemoprevention is an area of research that focuses on cancer prevention through pharmacological, biological, and nutritional interventions[80,81]. In recent years, a growing body of studies suggests that statins may have chemopreventive potential against cancer[14,15]. However, these hypotheses have not been confirmed by meta-analyses on the association between statin use and most site-specific cancers[82-86]. On the other hand, concerns have also been raised about the safety of statins, especially among elderly patients[87-89].
Meta-analysis is a systematic and quantitative integration of the results of a set of independent studies. It allows for an objective appraisal of the epidemiological evidence, which may lead to resolution of uncertainty and disagreement[90]. We undertook this updated meta-analysis to examine the latest evidence on the association of statin use and colorectal cancer risk. Our results again exclude the strong protective effect (47% risk reduction) of statins first noted in the study by Poynter et al[25]. However, a more mixed picture emerges from the 40 studies included in the analysis; a modest (on the order of 10% risk reduction) protective effect of statin use at therapeutic doses against colorectal cancer cannot be excluded by these data.
The point estimates from the three individual meta-analyses were almost identical (RCTs, RR = 0.89; cohort studies, RR = 0.91; case-control studies, OR = 0.92; results from random-effects models), with the effect reaching statistical significance for cohort and case-control studies, but not for RCTs. This is not unexpected, as these were RCTs with cardiovascular primary outcomes and mean follow-up was short (range: 2.0-10.4 years) with only two studies exceeding 6 years; in comparison, a single pre-existing adenomatous colorectal polyp typically requires 10-15 years to evolve into clinically invasive cancer[91]. As a result, there were few incident colorectal cancer cases and low statistical power in these trials to detect any effect of statin use. More importantly, however, the follow-up time may be insufficient in order for statins to meaningfully affect the neoplastic process and demonstrate an effect on colorectal cancer incidence. For this reason, any potential such effect of statins in these trials might reflect a slower evolution of pre-existing pre-malignant lesions, rather than a lower incidence of new lesions.
Our result for RCTs is not inconsistent with that of a recent individual patient data meta-analysis from the Cholesterol Treatment Trialists’ (CTT) Collaboration[22], which showed a lack of effect of statin use on colorectal cancer incidence (RR = 0.97, 95%CI: 0.87-1.09). That study included 27 RCTs that had the same limitations as the eight we included, i.e., short follow-up time (mean: 4.1 years), few colorectal cancer cases (1114 in total, overall rate of 0.64%) and ascertainment of colorectal cancer as a secondary outcome. It should also be noted that our findings for RCTs are consistent with those corresponding to the association between fibrates and colorectal cancer risk (RR = 0.98, 95%CI: 0.71-1.34), reported in a recent systematic review and meta-analysis of our research group[92].
Because of the limitations of RCTs, it is important to also examine the association of statin use and colorectal cancer risk through observational evidence. In our study, meta-analysis of both cohort and case-control studies revealed statistically significant effects, but high between-studies heterogeneity. This heterogeneity is important to consider, because it may point to a variable effect of statin use in different populations and in different colorectal cancer subtypes. Colorectal cancer is a heterogeneous disease in terms of molecular subtypes[93], and inherited genetic susceptibility plays a role in a significant proportion of cases[94]. The observational studies analyzed were performed in diverse populations from eleven countries, each of whom might have a different susceptibility profile and different molecular epidemiology of colorectal cancer. Pharmacogenomics might play an important role, and indeed evidence has emerged about a particular gene polymorphism that modifies the effect of statins on colorectal cancer[95]. Therefore, substantial differences may underlie the overall effect observed in our meta-analysis.
When undertaking a meta-analysis of observational studies, bias and confounding may always be an alternative explanation for the results. The 13 cohort and 19 case-control studies were statistically controlled for a large number of potential confounders, but adjustment for too many factors can itself introduce bias[96], and adjustment for different factors in different studies may also explain the substantial heterogeneity observed. In any event, the possibility of residual confounding cannot be excluded, either from unknown or unmeasured factors, or from imperfectly adjusted real confounders.
In this context, one such confounder of particular interest is the socioeconomic status of patients[97], which may underlie important differences between statin users and non-users as regards lifestyle choices and health-seeking behaviors. Notably, despite the large number of potential confounders controlled for by the 32 observational studies included in our analysis, only two studies[26,56] adjusted their results for socioeconomic status.
Selection bias and publication bias is another possibility that could affect both randomized and observational studies. Our literature search was as fully inclusive as possible, and we did not exclude any study because of methodological characteristics or subjective quality criteria. Nevertheless, we did not search for unpublished studies or original data. The Begg’s and the Egger’s tests for all three study types did not show a high likelihood of selective outcome reporting or publication bias, and the funnel plot showed no obvious asymmetry for RCTs and cohort studies, but a slight asymmetry for case-control studies. Thus selective publication of smaller case-control studies with statistically significant results might have occurred to some extent. It should be noted, however, that the result of our meta-analysis of case-control studies was fairly robust in the sensitivity analysis.
By similar reasoning, our sub-analyses of the effect of statins on colon and rectal cancer should be interpreted with caution. A differential effect of statins by colorectal cancer subsite is biologically plausible, due to differences in embryology and physiology. In our analysis, however, available data were scarce (only 11 of 32 observational studies) and there is no way to evaluate and control for outcome reporting bias.
In conclusion, it is safe to say that statins do not appear to strongly reduce the overall risk of colorectal cancer in the general population, at the low doses for managing hypercholesterolemia. However, there is some evidence to suggest a modest overall risk reduction, which could be a composite of an effect of statins in some populations and some colorectal cancer types, and lack of effect in others. Therefore, we believe it is not the end of the road, as has been suggested[98], for statins and colorectal cancer; rather a new approach is needed. One needs to focus more on basic research and pharmacogenomics, and perform epidemiological studies and clinical trials on high risk populations that might be more likely to benefit from statins, either as primary chemoprevention or as an adjuvant to treatment. In the meantime, the use of statins should remain restricted to the approved indications.
COMMENTS
Background
Statins are some of the most widely prescribed drugs worldwide, as a result of their proven efficacy in the primary and secondary prevention of cardiovascular morbidity and mortality. Besides their main mechanism of action through the reduction of serum cholesterol, by means of competitive inhibition of hepatic 3-hydroxy-3-methylglutaryl-coenzyme A reductase, statins exert a variety of “pleiotropic” effects. It has been suggested that statins might play a role in cancer chemoprevention, and data from in vitro and animal model studies have been encouraging.
Research frontiers
The relation between statins and colorectal cancer has been the focus of a growing body of epidemiological research, with often inconsistent results ranging from very protective to moderately harmful.
Innovations and breakthroughs
The authors sought to update their previous systematic review and meta-analysis to reflect the current totality of evidence on statins and colorectal cancer risk.
Applications
The authors believe it is not the end of the road, as has been suggested, for statins and colorectal cancer; rather a new approach is needed. One needs to focus more on basic research and pharmacogenomics, and perform epidemiological studies and clinical trials on high risk populations that might be more likely to benefit from statins, either as primary chemoprevention or as an adjuvant to treatment. In the meantime, the use of statins should remain restricted to the approved indications.
Terminology
Cancer chemoprevention is an area of research that focuses on cancer prevention through pharmacological, biological, and nutritional interventions. Meta-analysis is a systematic and quantitative integration of the results of a set of independent studies. It allows for an objective appraisal of the epidemiological evidence, which may lead to resolution of uncertainty and disagreement.
Peer review
The article is interesting and welcome. It is written by a team with experience in the meta-analysis and who has published scientific papers in the field of colorectal cancer. Choice trials were correctly made and motivated. Statistical analysis is laborious and thorough. The results are analyzed and discussed with competence. The risk reduction of colon cancer is properly justified, as the need for future studies. Figures are properly made, and the bibliography is correctly written.
Footnotes
P- Reviewers: Friis S, Mihaila RG S- Editor: Zhai HH L- Editor: A E- Editor: Liu XM
References
- 1.Walley T, Folino-Gallo P, Schwabe U, van Ganse E. Variations and increase in use of statins across Europe: data from administrative databases. BMJ. 2004;328:385–386. doi: 10.1136/bmj.328.7436.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 3.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 4.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 5.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 6.Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998;339:1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 9.Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991;88:1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gazzerro P, Proto MC, Gangemi G, Malfitano AM, Ciaglia E, Pisanti S, Santoro A, Laezza C, Bifulco M. Pharmacological actions of statins: a critical appraisal in the management of cancer. Pharmacol Rev. 2012;64:102–146. doi: 10.1124/pr.111.004994. [DOI] [PubMed] [Google Scholar]
- 11.Bonetti PO, Lerman LO, Napoli C, Lerman A. Statin effects beyond lipid lowering--are they clinically relevant? Eur Heart J. 2003;24:225–248. doi: 10.1016/s0195-668x(02)00419-0. [DOI] [PubMed] [Google Scholar]
- 12.Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109:III39–III43. doi: 10.1161/01.CIR.0000131517.20177.5a. [DOI] [PubMed] [Google Scholar]
- 13.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 14.Katz MS. Therapy insight: Potential of statins for cancer chemoprevention and therapy. Nat Clin Pract Oncol. 2005;2:82–89. doi: 10.1038/ncponc0097. [DOI] [PubMed] [Google Scholar]
- 15.Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 16.Hindler K, Cleeland CS, Rivera E, Collard CD. The role of statins in cancer therapy. Oncologist. 2006;11:306–315. doi: 10.1634/theoncologist.11-3-306. [DOI] [PubMed] [Google Scholar]
- 17.Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res. 2003;9:10–19. [PubMed] [Google Scholar]
- 18.Boudreau DM, Yu O, Johnson J. Statin use and cancer risk: a comprehensive review. Expert Opin Drug Saf. 2010;9:603–621. doi: 10.1517/14740331003662620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjerre LM, LeLorier J. Do statins cause cancer? A meta-analysis of large randomized clinical trials. Am J Med. 2001;110:716–723. doi: 10.1016/s0002-9343(01)00705-7. [DOI] [PubMed] [Google Scholar]
- 20.Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: a meta-analysis. JAMA. 2006;295:74–80. doi: 10.1001/jama.295.1.74. [DOI] [PubMed] [Google Scholar]
- 21.Bonovas S, Filioussi K, Tsavaris N, Sitaras NM. Statins and cancer risk: a literature-based meta-analysis and meta-regression analysis of 35 randomized controlled trials. J Clin Oncol. 2006;24:4808–4817. doi: 10.1200/JCO.2006.06.3560. [DOI] [PubMed] [Google Scholar]
- 22.Emberson JR, Kearney PM, Blackwell L, Newman C, Reith C, Bhala N, Holland L, Peto R, Keech A, Collins R, et al. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7:e29849. doi: 10.1371/journal.pone.0029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lochhead P, Chan AT. Statins and colorectal cancer. Clin Gastroenterol Hepatol. 2013;11:109–118; quiz e13-14. doi: 10.1016/j.cgh.2012.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonovas S, Tsantes A, Drosos T, Sitaras NM. Cancer chemoprevention: a summary of the current evidence. Anticancer Res. 2008;28:1857–1866. [PubMed] [Google Scholar]
- 25.Poynter JN, Gruber SB, Higgins PD, Almog R, Bonner JD, Rennert HS, Low M, Greenson JK, Rennert G. Statins and the risk of colorectal cancer. N Engl J Med. 2005;352:2184–2192. doi: 10.1056/NEJMoa043792. [DOI] [PubMed] [Google Scholar]
- 26.Vinogradova Y, Coupland C, Hippisley-Cox J. Exposure to statins and risk of common cancers: a series of nested case-control studies. BMC Cancer. 2011;11:409. doi: 10.1186/1471-2407-11-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonovas S, Filioussi K, Flordellis CS, Sitaras NM. Statins and the risk of colorectal cancer: a meta-analysis of 18 studies involving more than 1.5 million patients. J Clin Oncol. 2007;25:3462–3468. doi: 10.1200/JCO.2007.10.8936. [DOI] [PubMed] [Google Scholar]
- 28.Greenland S. Invited commentary: a critical look at some popular meta-analytic methods. Am J Epidemiol. 1994;140:290–296. doi: 10.1093/oxfordjournals.aje.a117248. [DOI] [PubMed] [Google Scholar]
- 29.Emerson JD, Burdick E, Hoaglin DC, Mosteller F, Chalmers TC. An empirical study of the possible relation of treatment differences to quality scores in controlled randomized clinical trials. Control Clin Trials. 1990;11:339–352. doi: 10.1016/0197-2456(90)90175-2. [DOI] [PubMed] [Google Scholar]
- 30.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 31.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petitti DB. Statistical methods in meta-analysis. In: Petitti DB, editor. Meta-Analysis, Decision Analysis, and Cost-Effectiveness Analysis. New York: Oxford University Press; 1999. [Google Scholar]
- 33.Team RDC. R: A Language and Environment for Statistical Computing [Internet] Vienna, Austria: R Foundation for Statistical Computing; 2013. Available from: http://www.R-project.org/ [Google Scholar]
- 34.Schwarzer G. Meta: An R package for meta-analysis [Internet] 2013. Available from: http://cran.r-project.org/package=meta.
- 35.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 36.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 37.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 38.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Chichester: The Cochrane Collaboration; 2008. [Google Scholar]
- 41.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 42.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsia J, MacFadyen JG, Monyak J, Ridker PM. Cardiovascular event reduction and adverse events among subjects attaining low-density lipoprotein cholesterol & lt; 50 mg/dl with rosuvastatin. The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) J Am Coll Cardiol. 2011;57:1666–1675. doi: 10.1016/j.jacc.2010.09.082. [DOI] [PubMed] [Google Scholar]
- 44.Ford I, Murray H, Packard CJ, Shepherd J, Macfarlane PW, Cobbe SM. Long-term follow-up of the West of Scotland Coronary Prevention Study. N Engl J Med. 2007;357:1477–1486. doi: 10.1056/NEJMoa065994. [DOI] [PubMed] [Google Scholar]
- 45.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 46.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT) JAMA. 2002;288:2998–3007. doi: 10.1001/jama.288.23.2998. [DOI] [PubMed] [Google Scholar]
- 47.Strandberg TE, Pyörälä K, Cook TJ, Wilhelmsen L, Faergeman O, Thorgeirsson G, Pedersen TR, Kjekshus J. Mortality and incidence of cancer during 10-year follow-up of the Scandinavian Simvastatin Survival Study (4S) Lancet. 2004;364:771–777. doi: 10.1016/S0140-6736(04)16936-5. [DOI] [PubMed] [Google Scholar]
- 48.LIPID Study Group (Long-term Intervention with Pravastatin in Ischaemic Disease) Long-term effectiveness and safety of pravastatin in 9014 patients with coronary heart disease and average cholesterol concentrations: the LIPID trial follow-up. Lancet. 2002;359:1379–1387. doi: 10.1016/S0140-6736(02)08351-4. [DOI] [PubMed] [Google Scholar]
- 49.Leung HW, Chan AL, Lo D, Leung JH, Chen HL. Common cancer risk and statins: a population-based case-control study in a Chinese population. Expert Opin Drug Saf. 2013;12:19–27. doi: 10.1517/14740338.2013.744392. [DOI] [PubMed] [Google Scholar]
- 50.Clancy Z, Keith SW, Rabinowitz C, Ceccarelli M, Gagne JJ, Maio V. Statins and colorectal cancer risk: a longitudinal study. Cancer Causes Control. 2013;24:777–782. doi: 10.1007/s10552-013-0160-x. [DOI] [PubMed] [Google Scholar]
- 51.Simon MS, Rosenberg CA, Rodabough RJ, Greenland P, Ockene I, Roy HK, Lane DS, Cauley JA, Khandekar J. Prospective analysis of association between use of statins or other lipid-lowering agents and colorectal cancer risk. Ann Epidemiol. 2012;22:17–27. doi: 10.1016/j.annepidem.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee JE, Baba Y, Ng K, Giovannucci E, Fuchs CS, Ogino S, Chan AT. Statin use and colorectal cancer risk according to molecular subtypes in two large prospective cohort studies. Cancer Prev Res (Phila) 2011;4:1808–1815. doi: 10.1158/1940-6207.CAPR-11-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacobs EJ, Newton CC, Thun MJ, Gapstur SM. Long-term use of cholesterol-lowering drugs and cancer incidence in a large United States cohort. Cancer Res. 2011;71:1763–1771. doi: 10.1158/0008-5472.CAN-10-2953. [DOI] [PubMed] [Google Scholar]
- 54.Matsushita Y, Sugihara M, Kaburagi J, Ozawa M, Iwashita M, Yoshida S, Saito H, Hattori Y. Pravastatin use and cancer risk: a meta-analysis of individual patient data from long-term prospective controlled trials in Japan. Pharmacoepidemiol Drug Saf. 2010;19:196–202. doi: 10.1002/pds.1870. [DOI] [PubMed] [Google Scholar]
- 55.Haukka J, Sankila R, Klaukka T, Lonnqvist J, Niskanen L, Tanskanen A, Wahlbeck K, Tiihonen J. Incidence of cancer and statin usage--record linkage study. Int J Cancer. 2010;126:279–284. doi: 10.1002/ijc.24536. [DOI] [PubMed] [Google Scholar]
- 56.Singh H, Mahmud SM, Turner D, Xue L, Demers AA, Bernstein CN. Long-term use of statins and risk of colorectal cancer: a population-based study. Am J Gastroenterol. 2009;104:3015–3023. doi: 10.1038/ajg.2009.574. [DOI] [PubMed] [Google Scholar]
- 57.Flick ED, Habel LA, Chan KA, Haque R, Quinn VP, Van Den Eeden SK, Sternfeld B, Orav EJ, Seeger JD, Quesenberry CP, et al. Statin use and risk of colorectal cancer in a cohort of middle-aged men in the US: a prospective cohort study. Drugs. 2009;69:1445–1457. doi: 10.2165/00003495-200969110-00004. [DOI] [PubMed] [Google Scholar]
- 58.Friedman GD, Flick ED, Udaltsova N, Chan J, Quesenberry CP, Habel LA. Screening statins for possible carcinogenic risk: up to 9 years of follow-up of 361,859 recipients. Pharmacoepidemiol Drug Saf. 2008;17:27–36. doi: 10.1002/pds.1507. [DOI] [PubMed] [Google Scholar]
- 59.Farwell WR, Scranton RE, Lawler EV, Lew RA, Brophy MT, Fiore LD, Gaziano JM. The association between statins and cancer incidence in a veterans population. J Natl Cancer Inst. 2008;100:134–139. doi: 10.1093/jnci/djm286. [DOI] [PubMed] [Google Scholar]
- 60.Setoguchi S, Glynn RJ, Avorn J, Mogun H, Schneeweiss S. Statins and the risk of lung, breast, and colorectal cancer in the elderly. Circulation. 2007;115:27–33. doi: 10.1161/CIRCULATIONAHA.106.650176. [DOI] [PubMed] [Google Scholar]
- 61.Friis S, Poulsen AH, Johnsen SP, McLaughlin JK, Fryzek JP, Dalton SO, Sørensen HT, Olsen JH. Cancer risk among statin users: a population-based cohort study. Int J Cancer. 2005;114:643–647. doi: 10.1002/ijc.20758. [DOI] [PubMed] [Google Scholar]
- 62.Deshpande G. Association between cardiovascular drugs and colon cancer [Internet] [Cited Aug 13 2013]. Available from: http://archive.hshsl.umaryland.edu/handle/10713/2752.
- 63.Lakha F, Theodoratou E, Farrington SM, Tenesa A, Cetnarskyj R, Din FV, Porteous ME, Dunlop MG, Campbell H. Statin use and association with colorectal cancer survival and risk: case control study with prescription data linkage. BMC Cancer. 2012;12:487. doi: 10.1186/1471-2407-12-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Broughton T, Sington J, Beales IL. Statin use is associated with a reduced incidence of colorectal cancer: a colonoscopy-controlled case-control study. BMC Gastroenterol. 2012;12:36. doi: 10.1186/1471-230X-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng MH, Chiu HF, Ho SC, Tsai SS, Wu TN, Yang CY. Statin use and the risk of colorectal cancer: a population-based case-control study. World J Gastroenterol. 2011;17:5197–5202. doi: 10.3748/wjg.v17.i47.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robertson DJ, Riis AH, Friis S, Pedersen L, Baron JA, Sørensen HT. Neither long-term statin use nor atherosclerotic disease is associated with risk of colorectal cancer. Clin Gastroenterol Hepatol. 2010;8:1056–1061. doi: 10.1016/j.cgh.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 67.Shadman M, Newcomb PA, Hampton JM, Wernli KJ, Trentham-Dietz A. Non-steroidal anti-inflammatory drugs and statins in relation to colorectal cancer risk. World J Gastroenterol. 2009;15:2336–2339. doi: 10.3748/wjg.15.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hachem C, Morgan R, Johnson M, Kuebeler M, El-Serag H. Statins and the risk of colorectal carcinoma: a nested case-control study in veterans with diabetes. Am J Gastroenterol. 2009;104:1241–1248. doi: 10.1038/ajg.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang YX, Hennessy S, Propert K, Hwang WT, Sarkar M, Lewis JD. Chronic statin therapy and the risk of colorectal cancer. Pharmacoepidemiol Drug Saf. 2008;17:869–876. doi: 10.1002/pds.1599. [DOI] [PubMed] [Google Scholar]
- 70.Boudreau DM, Koehler E, Rulyak SJ, Haneuse S, Harrison R, Mandelson MT. Cardiovascular medication use and risk for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:3076–3080. doi: 10.1158/1055-9965.EPI-08-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoffmeister M, Chang-Claude J, Brenner H. Individual and joint use of statins and low-dose aspirin and risk of colorectal cancer: a population-based case-control study. Int J Cancer. 2007;121:1325–1330. doi: 10.1002/ijc.22796. [DOI] [PubMed] [Google Scholar]
- 72.Coogan PF, Smith J, Rosenberg L. Statin use and risk of colorectal cancer. J Natl Cancer Inst. 2007;99:32–40. doi: 10.1093/jnci/djk003. [DOI] [PubMed] [Google Scholar]
- 73.Li L, Thompson C, Tucker T. No association between lipid-lowering statin use and risk of colon cancer (abstract CC7) In: Proceedings of the 34th Annual Meeting of the North American Primary Care Research Group (NAPCRG), editor. AZ: Tucson; 2006. [Google Scholar]
- 74.Rubin DT, Blumentals WA, Sheer RL, Steinbuch M, Law L. Statins and risk of colorectal cancer: Results from a large case-control study. Am J Gastroenterol. 2005;100:S394. [Google Scholar]
- 75.Khurana V, Jaganmohan S, Chalasani R, Singh T, Roy P, Caldito G, Fort C. Statins do not reduce colon cancer risk in humans: A case-control study in half million veterans. Am J Gastroenterol. 2004;99:S242. [Google Scholar]
- 76.Kaye JA, Jick H. Statin use and cancer risk in the General Practice Research Database. Br J Cancer. 2004;90:635–637. doi: 10.1038/sj.bjc.6601566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Graaf MR, Beiderbeck AB, Egberts AC, Richel DJ, Guchelaar HJ. The risk of cancer in users of statins. J Clin Oncol. 2004;22:2388–2394. doi: 10.1200/JCO.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 78.Blais L, Desgagné A, LeLorier J. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and the risk of cancer: a nested case-control study. Arch Intern Med. 2000;160:2363–2368. doi: 10.1001/archinte.160.15.2363. [DOI] [PubMed] [Google Scholar]
- 79.L'Abbé KA, Detsky AS, O’Rourke K. Meta-analysis in clinical research. Ann Intern Med. 1987;107:224–233. doi: 10.7326/0003-4819-107-2-224. [DOI] [PubMed] [Google Scholar]
- 80.Sporn MB. Approaches to prevention of epithelial cancer during the preneoplastic period. Cancer Res. 1976;36:2699–2702. [PubMed] [Google Scholar]
- 81.Bonovas S. Cancer chemoprevention: progress and perspectives. Curr Drug Targets. 2011;12:1871–1873. doi: 10.2174/138945011798184137. [DOI] [PubMed] [Google Scholar]
- 82.Bonovas S, Nikolopoulos G, Filioussi K, Peponi E, Bagos P, Sitaras NM. Can statin therapy reduce the risk of melanoma? A meta-analysis of randomized controlled trials. Eur J Epidemiol. 2010;25:29–35. doi: 10.1007/s10654-009-9396-x. [DOI] [PubMed] [Google Scholar]
- 83.Bonovas S, Filioussi K, Sitaras NM. Statins are not associated with a reduced risk of pancreatic cancer at the population level, when taken at low doses for managing hypercholesterolemia: evidence from a meta-analysis of 12 studies. Am J Gastroenterol. 2008;103:2646–2651. doi: 10.1111/j.1572-0241.2008.02051.x. [DOI] [PubMed] [Google Scholar]
- 84.Bonovas S, Filioussi K, Sitaras NM. Statin use and the risk of prostate cancer: A metaanalysis of 6 randomized clinical trials and 13 observational studies. Int J Cancer. 2008;123:899–904. doi: 10.1002/ijc.23550. [DOI] [PubMed] [Google Scholar]
- 85.Bonovas S, Filioussi K, Tsantes A, Sitaras NM. Use of statins and risk of haematological malignancies: a meta-analysis of six randomized clinical trials and eight observational studies. Br J Clin Pharmacol. 2007;64:255–262. doi: 10.1111/j.1365-2125.2007.02959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bonovas S, Filioussi K, Tsavaris N, Sitaras NM. Use of statins and breast cancer: a meta-analysis of seven randomized clinical trials and nine observational studies. J Clin Oncol. 2005;23:8606–8612. doi: 10.1200/JCO.2005.02.7045. [DOI] [PubMed] [Google Scholar]
- 87.Bonovas S, Sitaras NM. Does pravastatin promote cancer in elderly patients? A meta-analysis. CMAJ. 2007;176:649–654. doi: 10.1503/cmaj.060803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weverling-Rijnsburger AW, Blauw GJ, Lagaay AM, Knook DL, Meinders AE, Westendorp RG. Total cholesterol and risk of mortality in the oldest old. Lancet. 1997;350:1119–1123. doi: 10.1016/s0140-6736(97)04430-9. [DOI] [PubMed] [Google Scholar]
- 89.Bonovas S, Nikolopoulos G, Sitaras NM. Efficacy and safety of more intensive lowering of LDL cholesterol. Lancet. 2011;377:715; author reply 715–716. doi: 10.1016/S0140-6736(11)60261-4. [DOI] [PubMed] [Google Scholar]
- 90.Nikolopoulos GK, Bagos PG, Bonovas S. Developing the evidence base for cancer chemoprevention: use of meta-analysis. Curr Drug Targets. 2011;12:1989–1997. doi: 10.2174/138945011798184191. [DOI] [PubMed] [Google Scholar]
- 91.Jänne PA, Mayer RJ. Chemoprevention of colorectal cancer. N Engl J Med. 2000;342:1960–1968. doi: 10.1056/NEJM200006293422606. [DOI] [PubMed] [Google Scholar]
- 92.Bonovas S, Nikolopoulos GK, Bagos PG. Use of fibrates and cancer risk: a systematic review and meta-analysis of 17 long-term randomized placebo-controlled trials. PLoS One. 2012;7:e45259. doi: 10.1371/journal.pone.0045259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shen L, Toyota M, Kondo Y, Lin E, Zhang L, Guo Y, Hernandez NS, Chen X, Ahmed S, Konishi K, et al. Integrated genetic and epigenetic analysis identifies three different subclasses of colon cancer. Proc Natl Acad Sci U S A. 2007;104:18654–18659. doi: 10.1073/pnas.0704652104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lipkin SM, Chao EC, Moreno V, Rozek LS, Rennert H, Pinchev M, Dizon D, Rennert G, Kopelovich L, Gruber SB. Genetic variation in 3-hydroxy-3-methylglutaryl CoA reductase modifies the chemopreventive activity of statins for colorectal cancer. Cancer Prev Res (Phila) 2010;3:597–603. doi: 10.1158/1940-6207.CAPR-10-0007. [DOI] [PubMed] [Google Scholar]
- 96.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20:488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bonovas S, Sitaras NM. Statins and cancer risk: a confounded association. Gastroenterology. 2009;137:740; author reply 740–741. doi: 10.1053/j.gastro.2009.02.088. [DOI] [PubMed] [Google Scholar]
- 98.Ahnen DJ, Byers T. Editorial: Colorectal cancer and statins: reflections from the end of the road. Am J Gastroenterol. 2009;104:3024–3026. doi: 10.1038/ajg.2009.572. [DOI] [PubMed] [Google Scholar]


