Abstract
Around 40% of cirrhotic patients show minimal hepatic encephalopathy (MHE), with mild cognitive impairment which reduces their quality of life and life span. Treatment of MHE is unsatisfactory, and there are no specific treatments for the neurological alterations in MHE. Hyperammonemia is the main contributor to neurological alterations in MHE. New agents acting on molecular targets involved in brain mechanisms leading to neurological alterations are needed to treat MHE. Chronic hyperammonemia impairs learning of a Y-maze task by impairing the glutamate-nitric-oxide (NO)-cGMP pathway in cerebellum, in part by enhancing GABAA receptor activation, which also induces motor in-coordination. Acute pregnenolone sulfate (PregS) restores the glutamate-NO-cGMP pathway in hyperammonemic rats. This work aimed to assess whether chronic treatment of hyperammonemic rats with PregS restores (1) motor coordination; (2) extracellular GABA in cerebellum; (3) learning of the Y-maze task; (4) the glutamate-NO-cGMP pathway in cerebellum. Chronic intracerebral administration of PregS normalizes motor coordination likely due to extracellular GABA reduction. PregS restores learning ability by restoring the glutamate-NO-cGMP pathway, likely due to both enhanced NMDA receptor activation and reduced GABAA receptor activation. Similar treatments would improve cognitive and motor alterations in patients with MHE.
Keywords: Pregnenolone sulfate, hyperammonemia, minimal hepatic encephalopathy, motor coordination, learning
Hepatic encephalopathy (HE) is a neuropsychiatric syndrome present in patients with liver disease. Around 40% of cirrhotic patients show minimal hepatic encephalopathy (MHE), with attention deficits, psychomotor slowing, mild cognitive impairment and visuomotor and bimanual coordination deficits.1−3 MHE reduces their quality of life and life span and increases falls, domestic, work, and driving accidents, and risk of death.4,5 MHE may progress to clinical hepatic encephalopathy (HE), with stronger alterations in cognitive and motor function and in consciousness, which can lead to coma and death. MHE affects around two million people in the United States and Europe,6 and is an important clinical, social, and economic problem. However, treatment of MHE is unsatisfactory and there are no specific treatments for the neurological alterations.
Hyperammonemia is the main contributor to neurological alterations in MHE and HE.7,8 Inflammation plays a synergistic role with hyperammonemia in cognitive and motor alterations.9−11 Current treatments aim to reduce ammonia levels by reducing protein intake and ammonia production by intestinal flora using nonabsorbable antibiotics (rifaximin) or disaccharides (lactulose). These treatments are of limited value. New psychopharmacological agents acting on molecular targets involved in brain mechanisms leading to neurological alterations are needed to treat successfully cognitive and motor alterations in MHE.
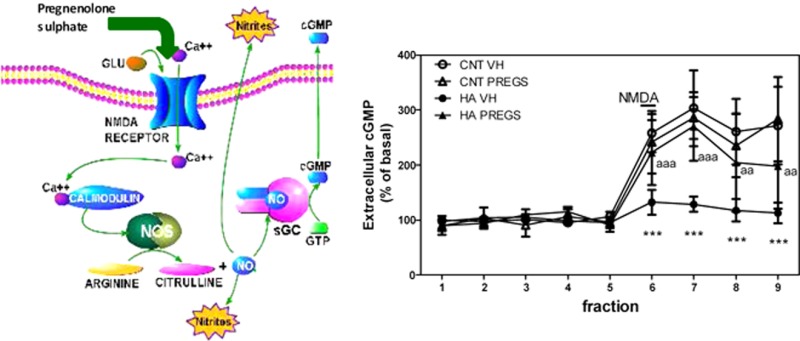
The mechanisms leading to cognitive and motor alterations are beginning to be understood from studies in animal models of chronic hyperammonemia and MHE. One of the models recommended by the International Society for Hepatic Encephalopathy to study the effects of hyperammonemia on brain function are rats fed high-ammonia diets.12 Studies using this model show that chronic hyperammonemia impairs learning of a Y-maze conditional discrimination task by impairing the glutamate-nitric-oxide (NO)-cGMP pathway in cerebellum. NMDA receptors activation increases Ca2+, leading to nitric oxide synthase activation and enhanced NO production which activates guanylate cyclase and increases cGMP. This NMDA-induced formation of cGMP is necessary for learning the Y maze task. This glutamate-NO-cGMP pathway is impaired in cerebellum of rats with chronic hyperammonemia without (13) or with (14) liver failure.
Restoring the pathway and cGMP formation with sildenafil, a phosphodiesterase 5 inhibitor,14 ibuprofen, an anti-inflammatory,15 or antagonists of GABAA receptors as bicuculline,16 restore learning in hyperammonemic rats. However, these treatments may have secondary effects in cirrhotic patients and new psychopharmacological agents are necessary.
A main mechanism by which hyperammonemia impairs the glutamate-NO-cGMP pathway is by enhancing tonic activation of GABAA receptors (GABAergic tone).16 GABAA receptors antagonists reduce GABAergic tone and restores the pathway and learning.16 However, they may have secondary effects. It would be useful to find agents which modulate GABAergic tone and the glutamate-NO-cGMP pathway without secondary effects.
Neurosteroids are a family of compounds which modulate GABAA and/or NMDA receptors and the function of the glutamate-NO-cGMP pathway.17 Acute administration of pregnenolone sulfate (PregS) through microdialysis probes in vivo restores the glutamate-NO-cGMP pathway in hyperammonemic rats.18 This lead us to hypothesize that chronic treatment with PregS would restore learning ability in hyperammonemic rats.
Increased GABAergic tone in cerebellum induces motor in-coordination and extracellular GABA in cerebellum correlates with motor in-coordination in rats.19,20 Hyperammonemic rats show increased extracellular GABA in cerebellum.16 It is likely that treatment with PregS, by modulating GABAergic tone, could also improve motor in-coordination in hyperammonemic rats.
This work aimed to assess whether chronic treatment of hyperammonemic rats with PregS restore (1) The glutamate-NO-cGMP pathway function in cerebellum; (2) learning of the Y-maze task; (3) extracellular GABA in cerebellum; and (4) motor coordination.
Results and Discussion
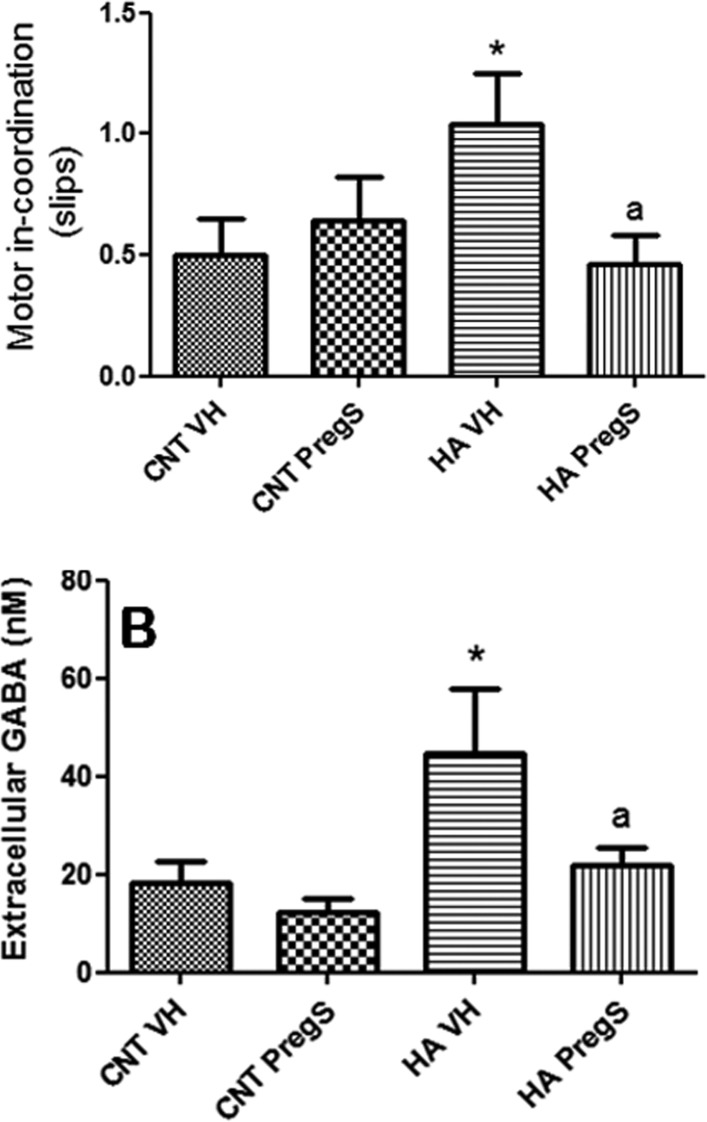
Hyperammonemic rats show motor in-coordination, with more foot faults (slips) in the beam walking (1.04 ± 021 slips, p < 0.05) than controls (0.50 ± 0.15 slips). PregS normalizes motor coordination in hyperammonemic rats (0.46 ± 0.12 slips) and does not affect control rats (Figure 1A).
Figure 1.
PregS restores motor coordination and extracellular GABA in cerebellum in hyperammonemic rats. (A) Motor coordination was assessed in the beam walking as described in methods. The number of slips is shown. Values are the mean ± SEM of 13 rats. (B) Extracellular GABA in cerebellum was measured by microdialysis. Values are the mean ± SEM of the 5 initial fractions from 6 rats. Values significantly different from controls are indicated by asterisks (*p < 0.05). Values different from hyperammonemic rats are indicated by “a”, ap < 0.05.
Hyperammonemic rats show increased extracellular GABA in cerebellum (45 ± 13 nM) compared to controls (18 ± 5 nM). PregS normalizes extracellular GABA in hyperammonemic rats (22 ± 4 nM) and tended to reduce GABA in controls (12 ± 3 nM), but the reduction was not significant (Figure 1B).
There was a significant correlation (r = 0.880; p < 0.001) between extracellular GABA in cerebellum and motor in-coordination expressed as number of slips (Figure 2).
Figure 2.
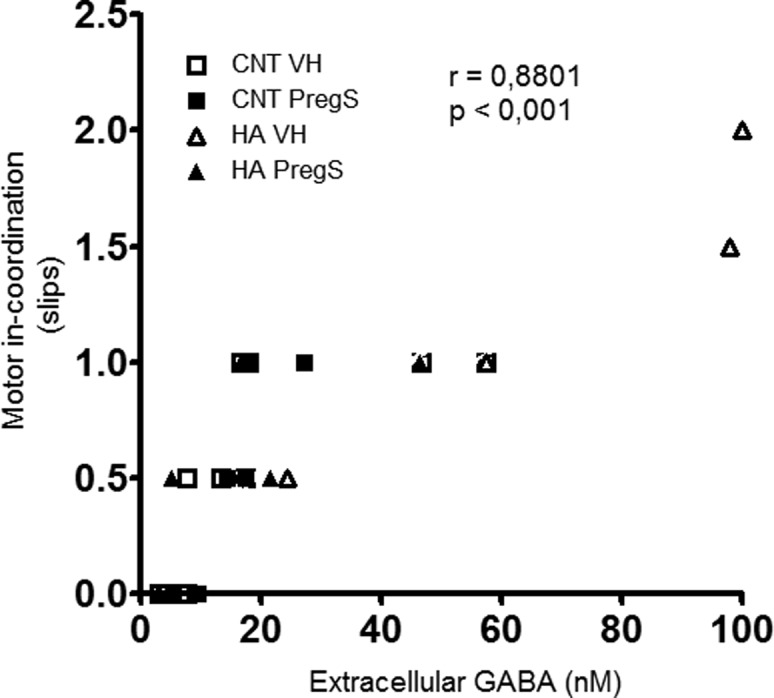
There is an excellent correlation between extracellular GABA in cerebellum and motor in-coordination. The figure shows, for each rat, motor in-coordination (expressed as the number of slips) and extracellular GABA. There was a significant correlation (r = 0.8801; p ≤ 0.001) between both parameters.
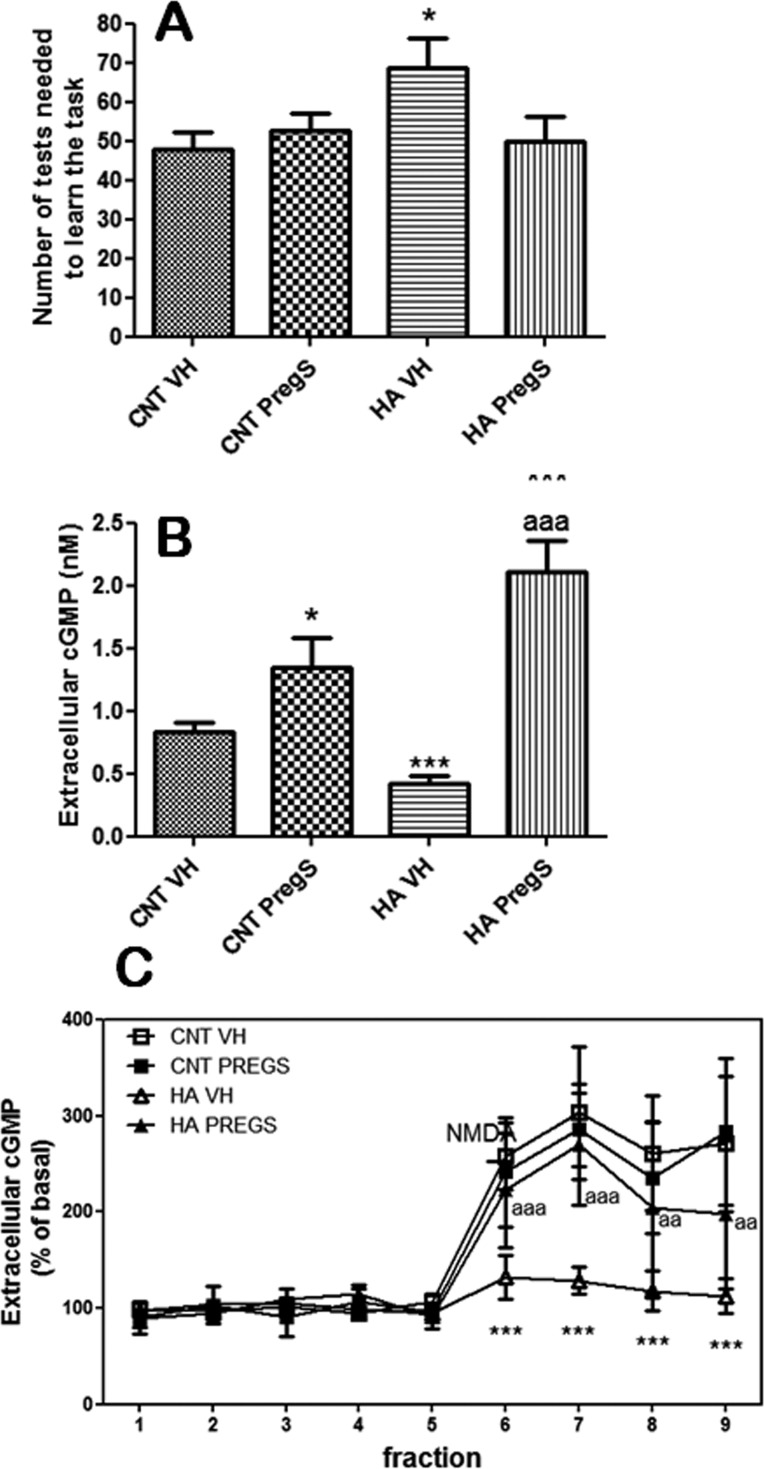
Hyperammonemic rats show reduced ability to learn the Y-maze task, and they need more trials (69 ± 7, p < 0,05) than control rats (48 ± 4) to learn the task (Figure 3A). PregS completely normalizes learning ability in hyperammonemic rats (50 ± 6 trials) and does not affect control rats (53 ± 4 trials) (Figure 3A).
Figure 3.
PregS increases extracellular cGMP and restores the function of the glutamate-NO-cGMP pathway and learning ability in hyperammonemic rats. (A) Learning ability is expressed as the number of trials needed to learn the task. Values are the mean ± SEM of 10 rats. (B) Extracellular cGMP in cerebellum was measured. Values are the mean ± SEM of the 5 initial fractions from 6 rats. (C) The glutamate-NO-cGMP pathway function was assessed by microdialysis. The time course of extracellular cGMP changes is shown. Values are the mean ± SEM of 6 rats. Values significantly different from controls are indicated by asterisks (*p < 0.05; ***p < 0.001). Values different from hyperammonemic rats are indicated by “a”, ap < 0.05; aap < 0.01; aaap < 0.001).
Hyperammonemic rats show lower basal levels of extracellular cGMP in cerebellum (0.42 ± 0.06 nM, p < 0,001) than controls (0.83 ± 0.08nM). PregS increased extracellular cGMP in control (1.34 ± 0.24 nM, p < 0.05) and, even more, in hyperammonemic rats (2.11 ± 0.25 nM, p < 0.001) (Figure 3B).
Hyperammonemic rats show reduced function of the glutamate-NO-cGMP pathway in cerebellum. The increase in cGMP induced by NMDA is significantly lower than that in controls (Figure 3C). PregS normalizes the glutamate-NO-cGMP pathway in hyperammonemic rats. The increase in cGMP induced by NMDA in hyperammonemic rats treated with PregS is similar to that of controls. PregS did not affect the pathway in control rats (Figure 3C).
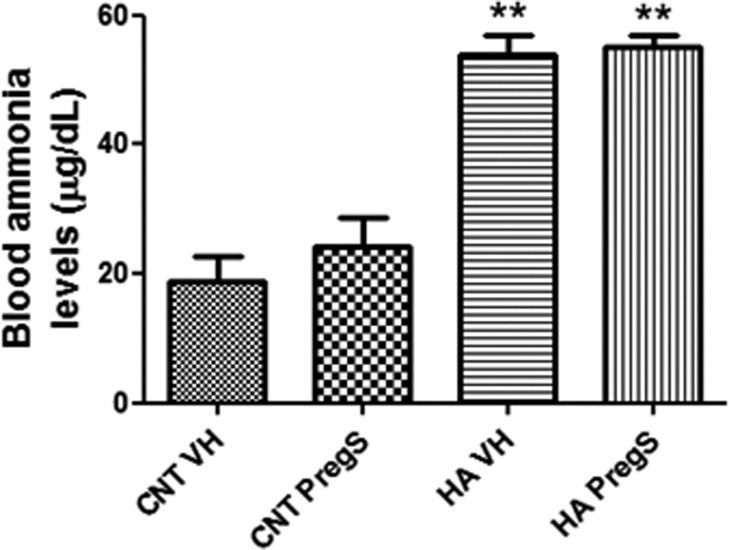
Blood ammonia levels are higher in hyperammonemic rats (54 ± 3 μg/dL, p < 0.01) than in control rats (19 ± 4 μg/dL). PregS did not affect ammonia levels in control or hyperammonemic rats (Figure 4).
Figure 4.
PregS does not affect ammonia levels in blood. Values are the mean ± SEM of 6 rats per group. Values significantly different from controls are indicated by asterisks (**p < 0.01).
The results reported show that chronic intracerebral administration of PregS normalizes extracellular GABA and the glutamate-NO-cGMP pathway function in cerebellum in vivo in hyperammonemic rats. These changes are associated with restoration of motor coordination and of the ability to learn a Y-maze task.
It is well-known that enhanced GABAergic tone (increased activation of GABAA receptors) in cerebellum induces motor in-coordination and that increased extracellular GABA in cerebellum correlates with motor in-coordination in rats.19,20 This and the excellent correlation between extracellular GABA and motor in-coordination found here support that recovery of motor coordination by PregS would be a consequence of normalization of extracellular GABA in cerebellum. PregS also normalizes cGMP levels; however, there was no significant correlation (r = 0.229; p = 0.260) between cGMP levels and motor in-coordiantion.
A possible mechanism by which PregS could reduce extracellular GABA is by reducing its release. This has been shown to happen in hippocampus. Teschemacher et al.21 showed that PregS in hippocampal cultures reduces spontaneous GABAergic inhibitory postsynaptic currents (sIPSCs) by reducing presynaptic release of GABA. Similar effects were reported by Mtchedlishvili and Kapur,22 also in hippocampus. This suggests that PregS could reduce extracellular GABA in cerebellum of hyperammonemic rats by reducing its (possibly enhanced) release from presynaptic neurons. This will be the mechanism by which PregS improves motor coordination in hyperammonemia.
The cellular components involved in the alterations in GABAergic neurotransmission and in the glutamate-NO-cGMP pathway cannot be assessed in vivo by microdialysis. We take the extracellular fluid, but is not possible to assess from which cell type cGMP or GABA has been released. It is also impossible to know if there is a blockade of GABA uptake in some specific cell type. It is likely that granular neurons are main players. We have shown that the effects of hyperammonemia on the glutamate-NO-cGMP pathway are reproduced in primary cultures of cerebellar granular cells.13
Hyperammonemic rats show reduced ability to learn the Y-maze task. In this task the rats must learn to associate the color of the maze wall with the position (left or right arms) of the food. Impairment of learning in hyperammonemic rats is due to a delay in the ability to make this association and not to motor alterations. Hyperammonemic rats reach the end of the arms at the same time than control rats, but they go to the wrong arm.
PregS also restores the ability of hyperammonemic rats to learn the Y-maze task. As discussed in detail by Monfort et al.,23 the glutamate-NO-cGMP pathway modulates different forms of learning and memory. The function of this pathway in hippocampus modulates long-term potentiation and spatial learning in the Morris water maze or object recognition.24 The ability to learn a Y-maze conditional discrimination task is also modulated by the function of the glutamate-NO-cGMP pathway function in cerebellum. This was shown by Yamada et al.25 who showed that blocking NMDA receptors or inhibiting nitric oxide synthase impaired memory in a Y-maze in mice. Moreover, they showed that dizocilpine reduced the concentration of cGMP in cerebellum but not in cerebral cortex or in hippocampus and dizocilpine-induced impairment in learning was ameliorated by cGMP analogues. Yamada et al.25 proposed that the cerebellum, but not the cerebral cortex or hippocampus, is the main brain region modulating kind of learning. This has been repeatedly confirmed by our group in studies showing an excellent correlation between the function of the pathway in cerebellum and the ability to learn the Y-maze task.26
Reduced function of the pathway in cerebellum is associated with reduced learning of the Y-maze in many pathological situations in rats: developmental exposure to methylmercury or polychlorinated biphenyls,27,28 rats with chronic hyperammonemia13 or with minimal hepatic encephalopathy (MHE) due to portacaval shunt14 or bile duct ligation29 or in aging of the rats.30 Also, developmental exposure to PBDE99 enhances the function of the pathway and learning ability.31 Moreover, in rats with hyperammonemia or MHE, restoring the function of the pathway also restores learning ability. This can be achieved with sildenafil;14 ibuprofen,15,29 bicuculline (16) or inhibitors of p38.32 This clearly support that PregS restores learning in hyperammonemic rats because it restores the function of the glutamate-NO-cGMP pathway. Although the above studies support a correlation between the function of the glutamate-NO-cGMP pathway in cerebellum and the ability to learn the Y-maze task, these studies do not exclude a role for hippocampus, as only the cerebellum was investigated.
The reduced function of the glutamate-NO-cGMP pathway in chronic hyperammonemia seems to be an adaptive compensatory mechanism to prevent excitotoxic damage. Acute hyperammonemia with large ammonia levels leads to excessive activation of NMDA receptors and of the glutamate-NO-cGMP pathway.33 This is responsible for animal death, which can be prevented by blocking NMDA receptors.34 In chronic hyperammonemia the brain engages neural mechanisms that allow for compensation of these toxic effects. Activation of nitric oxide synthase (35) and, subsequently, of the glutamate-NO-cGMP pathway is reduced. This prevents excitotoxicity associated to excessive activation of NMDA receptors, but results in reduced ability to learn tasks requiring the function of the pathway, including the Y-maze.
The mechanism by which PregS restores the pathway would include two components: enhanced activation of NMDA receptors and reduced activation of GABAA receptors.
Enhancement of NMDA receptors activation by PregS has been already reported in other systems by Wu et al.36 We have also shown that acute administration of PregS through the microdialysis probe enhances activation of NMDA receptors in cerebellum of control or hyperammonemic rats.18 The enhanced activation of NMDA receptors in control rats is reflected in an increase in basal cGMP, but not in potentiation of NMDA-induced increase in cGMP because in these rats, activation of the pathway by 0.5 mM NMDA is already maximal.18 In hyperammonemic rats, PregS enhances both basal extracellular cGMP and NMDA-induced increase in cGMP. This supports that, in hyperammonemic rats, PregS enhances activation of NMDA receptors and, subsequently, of the glutamate-NO-cGMP pathway, which contributes to restore NMDA-induced increase in cGMP to levels similar to control rats.
PregS would also enhance NMDA-induced cGMP formation in hyperammonemic rats by reducing GABAergic tone and the associated inhibition of the glutamate-NO-cGMP pathway. Activation of GABAA receptors reduces the function of the pathway in cerebellum.16,37 Extracellular GABA and tonic activation of GABAA receptors are enhanced in hyperammonemia, contributing to reduced function of the glutamate-NO-cGMP pathway and to impaired learning ability. Blocking GABAA receptors with bicuculline restores the function of the pathway and learning ability.16 PregS would reduce GABAergic tone in cerebellum of hyperammonemic rats by two complementary mechanisms: reduction of extracellular GABA concentration and a possible direct effect of PregS on GABAA receptor. We show that PregS reduces extracellular GABA in hyperammonemic rats to levels similar to controls. It has been also reported that PregS may bind directly to GABAA receptors, acting as an antagonist.38 Both reduction of extracellular GABA and a direct antagonistic effect of PregS would reduce tonic activation of GABAA receptors and enhance the function of the pathway similarly to bicuculline. This would also explain the higher effect of PregS on basal extracellular cGMP in hyperammonemic rats shown in Figure 3B. Tonic activation of GABAA receptors is enhanced in hyperammonemic rats but is low in control rats.16 As bicuculline does, PregS reduces GABAergic tone and the associated inhibition of the glutamate-NO-cGMP pathway strongly in hyperammonemic rats but only mildly in control rats resulting in a strong increase in basal extracellular cGMP.
Although PregS could be desulphated to Preg or converted to other neurosteroids, the effects reported here seem to be due to a direct effect of PregS. This is supported by the fact that Preg does not affect the function of the glutamate-NO-cGMP pathway while other neurosteroids affect it in opposite way, reducing its function.17
The results presented support that PregS would be a good pharmacological agent to improve cognitive and motor coordination alterations in patients with hyperammonemia and minimal hepatic encephalopathy.
A limitation of PregS is that it does not cross the blood-brain barrier, so that its direct utility is limited. However, the results reported serve as the proof-of-concept that new psychopharmacological agents modulating both GABAergic tone and NMDA receptor activation in cerebellum would be of high benefit to improve neurological status and quality of life in patients with MHE. This new pharmacological agents could be obtained by introducing PregS in systems for transport through the blood-brain barrier (liposomes or nanoconjugates) or by modifying PregS or other neurosteroids to allow them to reach the brain and act as PregS.
In summary, we have identified an agent, PregS, which is able to restore motor coordination and learning ability in hyperammonemic rats. Improvement of motor coordination seems to be due to PregS-induced reduction in extracellular GABA concentration. Recovery of learning ability is due to restoration by PregS of the function of the glutamate-NO-cGMP pathway in cerebellum. This in turn seems to be due to both enhanced activation of NMDA receptors and reduced activation of GABAA receptors. This indicates that PregS modified to cross the blood brain barrier and/or derivatives of this or other neurosteroids reaching the brain would be of great benefit to treat the cognitive and motor alterations and improve quality of life in patients with minimal hepatic encephalopathy. This is especially relevant considering the large incidence of MHE and the lack of specific treatments for its cognitive and motor deficits.
Methods
Rats
Male Wistar rats (140–160 g) were made hyperammonemic by feeding them a diet containing ammonium acetate (30% by weight).39 Animal experiments were approved by the Center and were performed in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Continuous Intracerebral Administration of Pregnenolone Sulfate (PregS)
5-Pregnen-3β-ol-20-one sulfate (pregnenolone sulfate, PregS) was from Sigma-Aldrich (St. Louis, MO). Two weeks after starting the ammonia diet, osmotic pumps with a brain infusion kit (ALZET mini-osmotic pump, model 2004, ALZET brain infusion hit II) were implanted in the back of the rats and connected to a cannula implanted in the cerebral ventricle as in Agusti et al.32 These pumps released 0.25 μL/h during 28 days. Rats were divided in four groups, two groups were fed the control diet and the other two ammonia-containing diet. For one group of rats on control diet (control + PregS) and one of hyperammonemic rats (ammonia + PregS), osmotic pumps were filled with 250 μL of PregS (0.667 mg/mL) in 10% dimethyl sulfoxide in sterile saline. For the other two groups (control and ammonia), pumps were filled with 10% dimethyl sulfoxide in sterile saline. PregS is administered intracerebrally because it does not cross the blood-brain barrier.
Blood Ammonia Determination
Blood ammonia was measured using the kit II Ammonia Arkray test (PocketChem BA, Arkray) using 20 μL of fresh blood.
In Vivo Microdialysis
Rats were anesthetized using isoflurane, and a microdialysis guide (MD-2251, Omega-ring Intracerebral Guide Cannula and Stylet, 6/pkg. BASi) was implanted in cerebellum (AP −10.2, ML −1.6, and DV −1.2), as in ref (13). After 48 h, a microdialysis probe (MD-2200, Brain Microdialysis Probes) was implanted in the freely moving rat. Probes were perfused (3 μL/min) with artificial cerebrospinal fluid (in mM): NaCl, 145; KCl, 3.0; CaCl2, 2.26; buffered at pH 7.4 with 2 mM phosphate. After a 2–3 h stabilization period, samples were collected every 30 min. When indicated, NMDA (0.5 mM) was administered to activate the glutamate-NO-cGMP pathway.13 Samples were made 4 mM in EDTA and stored at −80 °C until analysis of cGMP or GABA content.
cGMP Determination
cGMP was measured with an enzyme immunoassay kit from Amersham (Amersham Biotrak GE Healthcare) using 50 μL of dialysate from cerebellar microdialysis experiments. The amount of cGMP was detected by absorbance at 450 nm and calculated according the standard curve generated in the same assay.
GABA Determination
Extracellular GABA concentration was measured in the microdialysis samples by HPLC as in ref (40).
Learning of a Conditional Discrimination Task in a Y-Maze
Learning ability was tested 1 week after implantation of osmotic pumps as in Aguilar et al.41 in a wooden Y-shaped maze. Rats must learn where is the food depending on the color of the walls. Rats performed 10 trials per day, until the completion of a criterion of 10 correct responses in the same day or a maximum of 250 trials.
Motor Coordination: Beam Walking Test
Motor coordination was tested 3 weeks after implantation of mini osmotic pumps as described by Carter et al (42) using a wood strip (20 mm diameter). Rats are made to go through a 1 m length wooden stick located approximately 1 m above the ground, and two observers count the number of slips committed by the rats. At the end of the stick there is a closed box which provides a pleasant environment for rodents. The diameter of wood stick used is 20 mm. Before the test, we made the rats get used to going through the stick (five times at maximum). The number of foot faults (slips) is recorded as a measure of in-coordination.
Statistical Analysis
Results are expressed as mean ± SEM. Data were analyzed by analysis of variance (ANOVA) followed by Dunnett post hoc test. Significance levels were set at p = 0.05.
Glossary
Abbreviations
- HE
hepatic encephalopathy
- MHE
minimal hepatic encephalopathy
- NO
nitirc oxide
- PregS
pregnenolone sulfate
Author Contributions
A.G.U. performed most microdialysis experiments and learning tests and determined GABA and cGMP concentrations. O.C. and A.A. performed learning tests and some of the microdialysis experiments. V.F. designed the study, obtained funding, analyzed the results, and wrote the article.
Supported in part by grants from Ministerio de Ciencia Innovacion Spain (SAF2011-23051; CSD2008-00005) and Consellería Educación (PROMETEO-2009-027; ACOMP/2012/066; ACOMP/2013/101).
The authors declare no competing financial interest.
References
- Amodio P.; Montagnese S.; Gatta A.; Morgan M. Y. (2004) Characteristics of minimal hepatic encephalopathy. Metab. Brain Dis. 193, 253–267. [DOI] [PubMed] [Google Scholar]
- Weissenborn K.; Giewekemeyer K.; Heidenreich S.; Bokemeyer M.; Berding G.; Ahl B. (2005) Attention, memory, and cognitive function in hepatic encephalopathy. Metab. Brain Dis. 204, 359–367. [DOI] [PubMed] [Google Scholar]
- Felipo V.; Ordoño J. F.; Urios A.; El Mlili N.; Giménez-Garzó C.; Aguado C.; González-Lopez O.; Giner-Duran R.; Serra M. A.; Wassel A.; Rodrigo J. M.; Salazar J.; Montoliu C. (2012) Patients with minimal hepatic encephalopathy show impaired mismatch negativity correlating with reduced performance in attention tests. Hepatology 552, 530–539. [DOI] [PubMed] [Google Scholar]
- Román E.; Córdoba J.; Torrens M.; Torras X.; Villanueva C.; Vargas V.; Guarner C.; Soriano G. (2011) Minimal hepatic encephalopathy is associated with falls. Am. J. Gastroenterol. 1063, 476–482. [DOI] [PubMed] [Google Scholar]
- Bajaj J. S.; Saeian K.; Schubert C. M.; Hafeezullah M.; Franco J.; Varma R. R.; Gibson D. P.; Hoffmann R. G.; Stravitz R. T.; Heuman D. M.; Sterling R. K.; Shiffman M.; Topaz A.; Boyett S.; Bell D.; Sanyal A. J. (2009) Minimal hepatic encephalopathy is associated with motor vehicle crashes: the reality beyond the driving test. Hepatology 504, 1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leevy C. B.; Phillips J. A. (2007) Hospitalizations During the Use of Rifaximin Versus Lactulose for the Treatment of Hepatic Encephalopathy. Dig. Dis. Sci. 52, 737–741. [DOI] [PubMed] [Google Scholar]
- Felipo V.; Butterworth R. F. (2002) Neurobiology of Ammonia. Prog. Neurobiol. 67, 259–279. [DOI] [PubMed] [Google Scholar]
- Cauli O.; Rodrigo R.; Llansola M.; Montoliu C.; Monfort P.; Piedrafita B.; El Mlili N.; Boix J.; Agustí A.; Felipo V. (2009) Glutamatergic and GABAergic neurotransmission and neuronal circuits in hepatic encephalopathy. Metab. Brain Dis. 24, 69–80. [DOI] [PubMed] [Google Scholar]
- Shawcross D. L.; Davies N. A.; Williams R.; Jalan R. (2004) Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis. J. Hepatol. 40, 247–254. [DOI] [PubMed] [Google Scholar]
- Montoliu C.; Piedrafita B.; Serra M. A.; del Olmo J. A.; Urios A.; Rodrigo J. M.; Felipo V. (2009) IL-6 and IL-18 in blood may discriminate cirrhotic patients with and without minimal hepatic encephalopathy. J. Clin. Gastroenterol. 43, 272–279. [DOI] [PubMed] [Google Scholar]
- Felipo V.; Urios A.; Montesinos E.; Molina I.; El Mlili N.; Garcia-Torres M. L.; Civera M.; Olmo J. A.; Ortega J.; Martinez-Valls J.; Serra M. A.; Cassinello N.; Wassel A.; Jordá E.; Montoliu C. (2012) Contribution of hyperammonemia and inflammatory factors to cognitive impairment in minimal hepatic encephalopathy. Metab. Brain Dis. 271, 51–58. [DOI] [PubMed] [Google Scholar]
- Butterworth R. F.; Norenberg M. D.; Felipo V.; Ferenci P.; Albrecht J.; Blei A. T. (2009) Group Authors: ISHEN Commission Expt Models HE. Experimental models of hepatic encephalopathy: ISHEN guidelines. Liver Int. 29, 783–788. [DOI] [PubMed] [Google Scholar]
- Hermenegildo C.; Montoliu C.; Llansola M.; Muñoz M. D.; Gaztelu J. M.; Miñana M. D.; Felipo V. (1998) Chronic hyperammonemia impairs the glutamate-nitric oxide-cyclic GMP pathway in cerebellar neurons in culture and in the rat in vivo. Eur. J. Neurosci.. 10, 3201–3209. [DOI] [PubMed] [Google Scholar]
- Erceg S.; Monfort P.; Hernández-Viadel M.; Rodrigo R.; Montoliu C.; Felipo V. (2005) Oral administration of sildenafil restores learning ability in rats with hyperammonemia and with portacaval shunt. Hepatology 45, 2–10. [DOI] [PubMed] [Google Scholar]
- Cauli O.; Rodrigo R.; Piedrafita B.; Boix J.; Felipo V. (2007) Inflammation and hepatic encephalopathy: ibuprofen restores learning ability in rats with portacaval shunts. Hepatology 46, 514–519. [DOI] [PubMed] [Google Scholar]
- Cauli O.; Mansouri M. T.; Agusti A.; Felipo V. (2009) Hyperammonemia increases GABAergic tone in the cerebellum but decreases it in the rat cortex. Gastroenterology 136, 1359–67. [DOI] [PubMed] [Google Scholar]
- Cauli O.; González-Usano A.; Agustí A.; Felipo V. (2011) Differential modulation of the glutamate-nitric oxide- cyclic GMP pathway by distinct neurosteroids in cerebellum in vivo. Neuroscience 190, 27–36. [DOI] [PubMed] [Google Scholar]
- González-Usano A.; Cauli O.; Agustí A.; Felipo V. (2013) Hyperamonemia alters the modulation by different neurosteroids of the glutamate-nitric oxide-cyclic GMP pathway through NMDA- GABAA- or sigma receptors in cerebellum in vivo. J. Neurochem. 1251, 133–143. [DOI] [PubMed] [Google Scholar]
- Hanchar H. J.; Dodson P. D.; Olsen R. W.; Otis T. S.; Wallner M. (2005) Alcohol-induced motor impairment caused by increased extrasynaptic GABAA. receptor activity. Nat. Neurosci. 8, 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boix J.; Cauli O.; Felipo V. (2010) Developmental exposure to polychlorinated biphenyls 52, 138 or 180 affects differentially learning or motor coordination in adult rats. Mechanisms involved. Neuroscience 167, 994–03. [DOI] [PubMed] [Google Scholar]
- Teschemacher A.; Kasparov S.; Kravitz E. A.; Rahamimoff R. (1997) Presynaptic action of the neurosteroidpregnenolone sulfate on inhibitory transmitter release in cultured hippocampal neurons. Brain Res. 772, 226–232. [DOI] [PubMed] [Google Scholar]
- Mtchedlishvili Z.; Kapur J. (2003) A presynaptic action of the neurosteroidpregnenolone sulfate on GABAergic synaptic transmission. Mol. Pharmacol. 64, 857–864. [DOI] [PubMed] [Google Scholar]
- Monfort P.; Cauli O.; Montoliu C.; Rodrigo R.; Llansola M.; Piedrafita B.; El Mlili N.; Boix J.; Agustí A.; Felipo V. (2009) Mechanisms of cognitive alterations in hyperammonemia and hepatic encephalopathy. Therapeutical implications. Neurochem. Int. 55, 106–112. [DOI] [PubMed] [Google Scholar]
- Rutten K.; Vente J. D.; Sik A.; Ittersum M. M.; Prickaerts J.; Blokland A. (2005) The selective PDE5 inhibitor, sildenafil, improves object memory in Swiss mice and increases cGMP levels in hippocampal slices. Behav. Brain Res. 164(1), 11–16. [DOI] [PubMed] [Google Scholar]
- Yamada K.; Hiramatsu M.; Noda Y.; Mamiya T.; Murai M.; Kameyama T.; Komori Y.; Nikai T.; Sugihara H.; Nabeshima T. (1996) Role of nitric oxide and cyclic GMP in the dizocilpine-induced impairment of spontaneous alternation behavior in mice. Neuroscience 74, 365–374. [DOI] [PubMed] [Google Scholar]
- Felipo V. (2013) Hepatic encephalopathy: effects of liver failure on brain function. Nat. Rev. Neurosci. 14(12), 851–858. [DOI] [PubMed] [Google Scholar]
- Piedrafita B.; Erceg S.; Cauli O.; Monfort P.; Felipo V. (2008) Developmental exposure to polychlorinated biphenyls PCB153 or PCB126 impairs learning ability in young but not in adult rats. Eur. J. Neurosci. 27, 177–182. [DOI] [PubMed] [Google Scholar]
- Piedrafita B.; Erceg S.; Cauli O.; Felipo V. (2008) Developmental exposure to polychlorinated biphenyls or methylmercury, but not to its combination, impairs the glutamate-nitric oxide-cyclic GMP pathway and learning in 3 months-old rats. Neuroscience 154(4), 1408–1416. [DOI] [PubMed] [Google Scholar]
- Rodrigo R.; Cauli O.; Gomez-Pinedo U.; Agusti A.; Hernandez-Rabaza V.; Garcia-Verdugo J. M.; Felipo V. (2010) Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology 1392, 675–684. [DOI] [PubMed] [Google Scholar]
- Piedrafita B.; Cauli O.; Montoliu C.; Felipo V. (2007) The function of the glutamate-nitric oxide -cGMP pathway in brain in vivo and learning ability decrease in parallel in mature compared to young rats. Learn. Mem. 14, 254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llansola M.; Hernandez-Viadel M.; Erceg S.; Montoliu C.; Felipo V. (2009) Increasing the function of the glutamate-nitric oxide-cGMP pathway increases the ability to learn a Y maze task. J. Neurosci. Res. 87, 2351–2355. [DOI] [PubMed] [Google Scholar]
- Agusti A.; Cauli O.; Rodrigo R.; Llansola M.; Hernández-Rabaza V.; Felipo V. (2011) p38 MAP kinase is a therapeutic target for hepatic encephalopathy in rats withportacaval shunts. Gut 60, 1572–9. [DOI] [PubMed] [Google Scholar]
- Hermenegildo C.; Monfort P.; Felipo V. (2000) Activation of NMDA receptors in rat brain in vivo following acute ammonia intoxication. Characterization by in vivo brain microdialysis. Hepatology 31, 709–715. [DOI] [PubMed] [Google Scholar]
- Hermenegildo C.; Marcaida G.; Montoliu C.; Grisolía S.; Miñana M. D.; Felipo V. (1996) NMDA receptor antagonists prevent acute ammonia toxicity in mice. Neurochem. Res. 21, 1237–1244. [DOI] [PubMed] [Google Scholar]
- El-Mlili N.; Rodrigo R.; Naghizadeh B.; Cauli O.; Felipo V. (2008) Chronic hyperammonemia reduces the activity of neuronal nitric oxide synthase in cerebellum by altering its localization and increasing its phosphorylation by calcium-calmodulin kinase II. J. Neurochem. 106(3), 1440–1449. [DOI] [PubMed] [Google Scholar]
- Wu F. S.; Gibbs T. T.; Farb D. H. (1991) Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-D-aspartate receptor. Mol. Pharmacol. 40, 333–336. [PubMed] [Google Scholar]
- Majewska M. D. (1990) Steroid regulation of the GABAA receptor: ligand binding, chloride transport and behaviour. Ciba Found. Symp. 153, 83–97. [DOI] [PubMed] [Google Scholar]
- Fedele E.; Ansaldo M. A.; Varnier G.; Raiteri M. (2000) Benzodiazepine-sensitive GABAA.receptors limit the activity of the NMDA/NO/cyclic GMP pathway: a microdialysis study in the cerebellum of freely moving rats. J. Neurochem. 75, 782–787. [DOI] [PubMed] [Google Scholar]
- Felipo V.; Miñana M. D.; Grisolía S. (1988) Long term ingestion of ammonium increases acetylglutamate and urea levels without affecting the amount of carbamyl phosphate synthase. Eur. J. Biochem. 176, 567–571. [DOI] [PubMed] [Google Scholar]
- Canales J. J.; Elayadi A.; Errami M.; Llansola M.; Cauli O.; Felipo V. (2003) Chronic hyperammonemia alters motor and neurochemical responses to activation of group I metabotropic glutamate receptors in the nucleus accumbens in rats in vivo. Neurobiol. Dis. 14, 380–390. [DOI] [PubMed] [Google Scholar]
- Aguilar M. A.; Miñarro J.; Felipo V. (2000) Chronic moderate hyperammonemia impairs active and passive avoidance behavior and conditional discrimination learning in rats. Exp. Neurol. 161, 704–713. [DOI] [PubMed] [Google Scholar]
- Carter R. J.; Morton J.; Dunnett S. B. (2001) Motor coordination and balance in rodents. Curr. Protoc. Neurosci. 15, 8.12.1–8.12.14. [DOI] [PubMed] [Google Scholar]