Abstract
Cell-mediated nanoparticle delivery has recently emerged as an efficacious method of delivering therapeutic agents across physiological barriers. Use of cells as nanodelivery vehicles requires accurate assessment of their loading capacity and identification of intracellular compartments where nanoparticles are sequestered. This is of great interest since specific endocytic trafficking routes can ultimately influence the mode of nanoparticle release and their efficacy and function. Here, we describe a technique that allows for the isolation of individual populations of nanoparticle-containing endosomes for subsequent quantitative analysis and more accurate description of where nanoparticles are stored on a subcellular level.
Keywords: Nanoparticles, Immunoisolation, Endosomes, Subcellular trafficking, Macrophage
1 Introduction
For over 20 years, nanoparticles (NP) have been researched for their use in drug delivery (1, 2). Drug-loaded NP have the potential to increase efficacy, reduce toxicity, and improve clinical outcomes of diseases. These nanoparticles tend to be designed to deliver drugs or other therapeutic compounds, such as protein or DNA, to specific cell populations. One of the important questions when researching drug-carrying nanoparticles is determining precisely how much drug the target cells are able to take up. Generally, this question is not difficult to answer. However, of even greater importance than how much drug the target cells are able to pick up is where within the cells are the nanoparticles being trafficked and stored.
2 Materials
Prepare all solutions using ultrapure water (prepared by purifying deionized water to attain a sensitivity of 18 MΩ cm at 25°C) and analytical grade reagents. Prepare and store all reagents at room temperature (unless indicated otherwise). Diligently follow all waste disposal regulations when disposing waste materials.
2.1 Conjugation of Magnetic Beads to Antibodies
PureProteome Protein A and Protein G Paramagnetic Beads (Millipore).
Antibodies to endosome surface markers of interest (see Note 1).
Bovine serum albumin fraction V (10%).
Sterile 1× phosphate-buffered saline (PBS).
Microcentrifuge tubes (1.7 mL).
Microcentrifuge tube tumbler rotator.
Magnetic separator rack.
Refrigerated tabletop centrifuge.
2.2 Cellular Treatment Components
2.3 Homogenization of Nanoparticle-Loaded Cells
Homogenization buffer: 10 mM HEPES–KOH, pH 7.2, 250 mM sucrose, 1 mM EDTA, and 1 mM Mg(OAc)2.
Cell scrapers.
Dounce homogenizer (7 mL).
15 mL centrifuge tubes.
Refrigerated centrifuge.
2.4 Isolation of Nanoparticle-Containing Endosomes
Homogenate from nanoparticle-treated cells (from Subheading 2.3).
Magnetic beads with attached antibodies (from Subheading 2.1).
Magnetic separator rack.
Sterile PBS.
Refrigerated tabletop centrifuge.
2.5 Quantification of Drug Content by HPLC
HPLC-grade methanol.
Sonicator disruptor with probe tip.
Refrigerated tabletop centrifuge.
0.5 mL microcentrifuge tubes.
HPLC autoinjector vials with low-volume inserts.
3 Methods
3.1 Conjugate Antibodies to Magnetic Beads
In a 1.7-mL microcentrifuge tube, combine 1 mL of 10% bovine serum albumin in PBS with 20 μL of magnetic bead slurry and 20 μg of antibody of interest (see Note 1).
Place tubes on a microcentrifuge tube tumbler rotator and rotate at 15 rpm for 12 h at 4°C.
Place tubes in the magnetic separator rack for up to 1 h at 4°C. Remove supernatant and resuspend antibody-bead conjugates in sterile PBS. Repeat wash cycle two more times. Finally, resuspend antibody-bead conjugates in 500 μL of sterile PBS and use within 24 h.
3.2 Treat Cells with Nanoparticles
Wash cells three times for 10 min with 37°C serum-free medium to remove residual serum protein.
Add nanoparticles in sterile serum-free DMEM (or other appropriate cell medium) to cells at desired concentration (Fig. 1a).
Incubate at 37°C to allow cells to take up nanoparticles (see Note 4).
Once maximum nanoparticle uptake has been reached, wash the cells three times with 37°C sterile PBS to remove any nano-particles that have not been taken up. Keep cells in PBS and immediately begin homogenization.
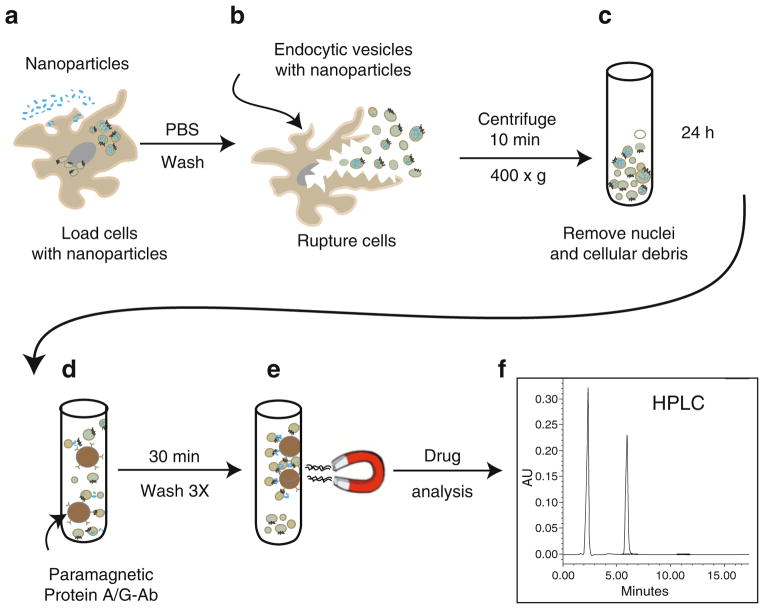
Fig. 1.
Schematic diagram of immunoisolation of nanoparticle-containing endosomes. Cells are first treated with nanoparticles (a). After maximum nanoparticle endocytosis, cells are ruptured in homogenization buffer using a Dounce tissue homogenizer (b). Cell homogenate is then slowly centrifuged in order to remove nuclei, organelles, and cellular debris (c). Following enrichment, the endosome fraction is exposed to protein A/G paramagnetic beads conjugated to antibodies and allowed to bind with the endosomes carrying the surface markers of interest. (d) The endosome population bound to the beads is isolated by magnetic separation (e). The isolated fraction undergoes several washes with sterile cold PBS prior to drug quantitation (f)
3.3 Homogenization of Nanoparticle-Loaded Cells
Remove PBS and add enough homogenization buffer to cover the bottom of each well or flask being used.
Detach cells from bottom of well or flask using cell lifter.
Add scraped cells to Dounce homogenizer and grind cells with 15 strokes (Fig. 1b) (see Note 5).
Add entire volume to a 15 mL centrifuge tube and remove nuclei and unbroken cells by centrifuging at 400 × g for 10 min at 4°C (Fig. 1c).
Remove supernatant, place in 1.7 mL microcentrifuge tubes, and use for immune isolation of endocytic compartments.
3.4 Isolate Nanoparticle-Containing Endosomes
In a 1.7-mL microcentrifuge tube, combine 1 mL of the cellular homogenate from Subheading 3.3, step 5, and the entire volume (500 μL) of one antibody-bead combination from Subheading 3.1, step 3 (Fig. 1d). Be sure to include one tube with blank beads (i.e., fresh beads with no antibody conjugate) as a control.
Place tubes on a microcentrifuge tube tumbler rotator and rotate at 15 rpm for 18–24 h at 4°C.
Place tubes on microcentrifuge tube magnetic separator and allow for magnetic separation for 1 h at 4°C (Fig. 1e). The solution should become clear as the beads form a layer in the tube wall facing the magnet.
Carefully remove the solution without disturbing the beads. Add 1 mL of cold sterile PBS and resuspend the beads (see Note 6). Repeat this wash cycle two more times. Endosomes are now ready for quantitative analysis (store at 4°C until ready for analysis).
3.5 Quantification of Drug Content of Nanoparticle-Containing Endosomes by HPLC
Take samples from Subheading 3.4, step 4, and centrifuge at 10,000×g for 10 min at 4°C. Remove the supernatant and add 400 μL of 100% methanol (see Note 7).
Sonicate solution with sonicator probe for 3 s at 20% amplitude.
Centrifuge solutions at 20,000 × g for 10 min at 4°C. Remove supernatant and add to a clean 0.5 mL microcentrifuge tube.
Transfer 70 μL to an HPLC autosampler vial and inject three 20 μL aliquots onto the HPLC for drug quantitation (see Notes 8 and 9).
Determine drug content by comparing peak area of drug in sample to peak areas of known concentrations of drug standards.
Acknowledgments
The work was supported by the National Institutes of Health grants 1P01 DA028555, 2R01 NS034239, 2R37 NS36126, P01 NS31492, P20RR 15635, P01MH64570, and P01 NS43985 (to H.E.G.) and a research grant from Baxter Healthcare. The authors thank Ms. Robin Taylor for critical reading of the manuscript and outstanding graphic and literary support.
Footnotes
There are hundreds of potential endosomal markers to choose from, which can be very overwhelming. When first performing this experiment, it may be wise to select an antibody to isolate each of the major endosomal compartments, for example, early endosomes (early endosome antigen-1), recycling endosomes (Rab-11), late endosomes (Rab-7), and lysosomes (lysosome-associated membrane protein-1). In this way one can find out which major endosomal compartment the nanoparticles are trafficked to. Afterwards, a more thorough search of that specific endosomal compartment can be performed.
This protocol has been used primarily with primary human monocytes and human monocyte-derived macrophages. However, any cells that will take up the nanoparticle being tested could be used. Adjust the protocol appropriately to accommodate for cell type being used. It is suggested to use at least 10×106 cells in order to isolate enough nanoparticle- containing endosomes for drug analysis.
This protocol has been used only for crystalline antiretroviral nanoparticles coated in lipophilic surfactants (3). For this method drug-containing nanoparticles are being used because the final step of the protocol involves quantitation of drug levels by HPLC. However, this protocol could easily be used to isolate nanoparticle-containing endosomes for any type of nanoparticle. Although, if the nanoparticle contains a nondrug therapeutic compound (such as protein or DNA), a method other than HPLC will be necessary to quantify the amount of therapeutic material contained within each endosomal compartment.
Both the amount of nanoparticles to be added to serum-free DMEM (or other appropriate cell medium) and the duration of treatment must be determined ahead of time by previous experiments. It is suggested to allow the cells enough time for maximum nanoparticle uptake before harvesting for endosomal isolation in order to ensure that sufficient material will be available.
When using the Dounce homogenizer, press and pull the piston hard enough to keep the solution flowing past the glass rod at a steady rate. Only light force is necessary to effectively homogenize the cells and excess force could break the homogenizer.
Be very careful not to disturb the beads when removing the solution otherwise endosomes will be lost during the wash process. Place the pipette tip on the wall of the tube opposite from the side with the beads just beneath the surface and remove the solution from the top down. If the beads are disturbed, place the magnetic rack back in the refrigerator for about 10 min to allow the beads to gather at the magnet again.
An internal standard of known quantity may be added to the methanol (or other extraction solvent) prior to addition to the samples. Acetonitrile extraction of drug may also be used. To concentrate samples prior to HPLC, sample extracts can be evaporated to dryness using a SpeedVac concentrator and resuspended in methanol or mobile phase.
Low-volume inserts for autosampler vials are available for many 1–4 mL vial types. Vial types appropriate for your system will be identified in the autoinjector user manual. Glass inserts are preferred because of their inertness to solvents used for drug extraction. Add enough volume to the autosampler vials to provide for at least two injections of sample onto the HPLC system, although three injections are preferred.
Depending on the drug of interest, HPLC with UV/Vis or mass spectrometry detection may be used.
References
- 1.Speiser PP. Nanoparticles and liposomes: a state of the art. Methods Find Exp Clin Pharmacol. 1991;13:337–342. [PubMed] [Google Scholar]
- 2.Douglas SJ, Davis SS, Illum L. Nanoparticles in drug delivery. Crit Rev Ther Drug Carrier Syst. 1987;3:233–261. [PubMed] [Google Scholar]
- 3.Kadiu I, Nowacek A, McMillan J, Gendelman HE. Macrophage endocytic trafficking of antiretroviral nanoparticles. Nanomedicine (Lond) 2011;6:975–994. doi: 10.2217/nnm.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]