Abstract
Purpose
Scoring of radiation pneumonitis (RP), a dose-limiting toxicity after thoracic radiochemotherapy, is subjective and thus inconsistent among studies. Here we investigated if the extent of change in DLCO after radiation therapy (RT) for non-small cell lung cancer (NSCLC) could be used as an objective means of quantifying RP.
Methods and Materials
We analyzed potential correlations between the diffusing capacity of the lung for carbon monoxide (DLCO) and RP in 140 patients who received definitive RT (≥60 Gy) with or without chemotherapy for primary NSCLC. All underwent DLCO analysis before and after RT. Post-RT DLCO values within 1 week of the RP diagnosis (grade 0, 1, 2, or 3) were selected and compared with that individual’s preradiation values. Percent reductions in DLCO and RP grade were compared by point biserial correlation in the entire patient group and in subgroups stratified according to various clinical factors.
Results
Patients experiencing grade 0, 1, 2, or 3 RP had median percentage changes in DLCO after RT of 10.7%, 13%, 22.1%, or 35.2%. Percent reduction in DLCO correlated with RP grade ≤1 vs. ≥2 (P = 0.0004). This association held for the following subgroups: age ≥65 years, advanced stage, smokers, use of chemotherapy, volume of normal lung receiving at least 20 Gy ≥30%, and baseline DLCO or forced expiratory volume in 1 second (FEV1) ≥60%.
Conclusions
By correlating percent change in DLCO from pretreatment values at the time of diagnosis of RP with RP grade, we were able to identify categories of RP based on the change in DLCO. These criteria provide a basis for an objective scoring system for RP based on change in DLCO.
Keywords: non–small cell lung cancer, radiation therapy, diffusing capacity of the lung for carbon monoxide
INTRODUCTION
Thoracic RT is associated with significant alterations in lung function as assessed by objective pulmonary function tests (PFTs) (1, 2). The extent of residual lung function is a major determinant of a patient’s functional status after treatment, particularly for patients with lung cancer, who often have pretreatment pulmonary compromise secondary to both malignancy and coexisting lung disease (3). It is increasingly important to understand the relationship between thoracic RT and the decline in lung function in the setting of aggressive concurrent chemoradiation. Studies at MD Anderson (4) and elsewhere (1, 5) have shown that the largest and most consistent changes in PFT values after definitive RT for NSCLC occur in diffusing capacity of the lung for carbon monoxide (DLCO). However, data regarding the extent of radiation-induced change in DLCO and symptomatic pulmonary toxicity are minimal, particularly specific quantitative changes in DLCO in the setting of RP.
The primary aim of the current study was thus to assess possible associations between the extent of change in DLCO after RT and RP, with the hypothesis that patients with symptomatic RP will experience a significant decrease in pulmonary function that can be quantified in terms of DLCO and used as an objective scoring system for RP.
PATIENTS AND METHODS
Selection criteria
This investigation was approved by the MD Anderson Cancer Center institutional review board and was in compliance with the Health Insurance Portability and Accountability Act regulations. Patients had locally advanced NSCLC treated with RT and PFTs from 1998 through 2010. We chose this period because MD Anderson began using conformal radiation techniques in 1998 (6-8) and thus all patients were treated with conformal RT. Inclusion criteria were having a primary diagnosis of NSCLC, DLCO analysis within 6 months before RT and again up to 1 year after RT, no prior history of thoracic surgery or RT, and receiving a total radiation dose of ≥60 Gy at 1.2 to 2.5 Gy/fraction. Besides fulfilling these criteria, patients experiencing RP had to have also the post-RT PFT test within 1 week of the date of diagnosis of RP. Data on previous respiratory or cardiovascular disease were collected for all patients. Respiratory disease was defined as a history of at least one of the following factors: chronic pulmonary disease, history of pulmonary embolism, asthma, oxygen dependence, or recent respiratory infection (within 1 month before radiation). Cardiovascular disease was defined as a history of angina, coronary bypass surgery, stroke, congestive heart failure, deep vein thrombosis, transient ischemic attack, coronary angioplasty, myocardial infarction or hypertension.
Patients were evaluated at approximately 1-3 months after completion of therapy and then every 3 months thereafter. Follow-up evaluations consisted of an interval history and physical examination. Computed tomography (CT) scans were obtained at intervals of 3-6 months. PFTs were performed at intervals of 3-6 months or more frequently if clinically indicated.
Symptom endpoints
RP was scored according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 (9). To avoid inter-clinician variability, all RP endpoints were evaluated and confirmed by investigators in this study (JLG and DG). The time to RP was measured relative to the completion of RT. Patients who did not develop symptomatic RP had had a minimum of 12 months of follow-up. The 12-month cut-point was chosen based on a widely accepted definition of the typical interval for the development of RP (10).
Pulmonary function tests
On the basis of recommendations from the American Thoracic Society and the European Respiratory Society (11), we chose the DLCO as a measure of diffusion capacity. Pre- and posttreatment DLCO values were compared to assess the effect of treatment. All values were recorded as a percentage of predicted level (determined by sex, height, and weight). Declines in DLCO were described as the percent reduction from the pre-RT value (i.e., percent reduction in DLCO = (1 − post/pre) × 100). For example, a patient with a pre-RT DLCO of 80% (percentage of predicted) and a post-RT DLCO of 60% (percentage of predicted) at the time of RP was documented as having a 25% reduction in DLCO. Patients had variable numbers of follow-up PFTs depending on survival, duration of follow-up, and the number of return visits to MD Anderson. We selected the PFT performed within 1 week of the date of diagnosis of RP. For patients with grade 0 pneumonitis and several PFTs after treatment, we used the average post-radiation DLCO change for the analysis.
Statistical methods
Stata/SE 11.1 (Stata Corp LP, College Station, Texas) was used for data analyses. The primary endpoint of the study was to investigate whether the post-RT change in DLCO rate within one week of the diagnosis of RP is associated with the RP severity and therefore could be an objective scoring system for RP. The significance of potential associations between the change in DLCO after treatment and the RP grade was assessed by the Mann–Whitney and Wilcoxon tests. The correlation between postradiation DLCO change and development of symptomatic pneumonitis (grade ≥2) versus no pneumonitis or asymptomatic pneumonitis (grade ≤1) was assessed by point biserial correlation. Our initial analyses were done with the entire patient population. Then, to account for possible confounding effects of clinical factors on lung function, we repeated the analyses with subgroups stratified by patient factors (age, smoking habits, Karnofsky performance status, and respiratory comorbidities), tumor factors (disease stage), treatment factors (the use of chemotherapy, fractionation, lung dosimetric parameters), and pretreatment PFT values. In addition, a multivariate Cox regression was performed to adjust for all covariates. For the dosimetric factors, the median values were used as cut-off points in the analysis. In contrast, the analyses of pretreatment PFTs were performed using recognized PFT cut-off values (< 60% vs. ≥ 60% predicted) (12), so that these pretreatment PFT results could potentially be considered in clinical decisions. The percent reduction in the DLCO and the RP grade were compared (by point biserial correlation) in the overall group and in subgroups stratified according to various clinical factors.
RESULTS
Of the 140 patients with NSCLC selected for this study, complete lung dosimetric data were available for 132 (94.3%). Patient characteristics, treatment characteristics, and dosimetric information for all patients are shown in Table 1. The total radiation dose for all patients ranged from 60 to 87.5 Gy (median 69.6 Gy); 23 patients had 1.2 Gy/fraction twice a day to 69.6 Gy in 58 fractions. One hundred twenty-two patients (87%) received platinum and taxane-based concurrent chemotherapy. One hundred four of the 140 patients (74%) experienced RP (24% grade 1, 34% grade 2, and 16% grade 3), with a median interval of 3 months (range 0.5-10 months) between the end of RT and the RP date.
Table 1. Patient characteristics.
| Characteristic | No. of Patients (%) (n = 140) |
|---|---|
| Sex | |
| Male | 75 (54) |
| Female | 65 (46) |
| Age, years | |
| Median | 70 |
| Range | 40-87 |
| Race | |
| White | 134 (96) |
| Other | 6 (4) |
| Chronic respiratory disease | |
| Yes | 51 (36) |
| No | 89 (64) |
| Disease stage | |
| I | 16 (11) |
| II | 15 (11) |
| III | 106 (76) |
| IV | 3 (2) |
| Histology | |
| Squamous cell Adenocarcinoma | 60 (43) |
| NSCLC, NOS | 46 (33) |
| 34 (24) | |
| Karnofsky performance status score | |
| 90-100 | 49 (35) |
| 80 | 69 (49) |
| <80 | 22 (16) |
| Smoking status | |
| Current | 26 (19) |
| Former | 106 (76) |
| Never | 8 (6) |
| Treatment | |
| Concurrent chemoradiation | 64 (46) |
| Chemotherapy+concurrent chemoradiation | 47 (34) |
| Radiation therapy | 18 (13) |
| Sequencial chemoradiation therapy | 11 (8) |
| Radiation total dose, Gy (GyE for protons) | |
| Median | 69.6 |
| Range | 60-87.5 |
| Radiation technique | |
| 3D-CRT | 44 (31) |
| IMRT | 64 (46) |
| PBT | 32 (23) |
| Radiotherapy fractionation | |
| Once a day | 117 (84) |
| Twice a day | 23 (16) |
| Mean lung dose, Gy (GyE for protons) (n=132) | |
| Median | 17 |
| Range | 3-67 |
| V20, %(n=132) | |
| Median | 30 |
| Range | 4-44 |
| Baseline DLCO (percentage of predicted), % | |
| Median | 68 |
| Range | 26-126 |
| Baseline FEV1 (percentage of predicted), % | |
| Median | 67 |
| Range | 20-115 |
Abbreviations: NSCLC, NOS, non–small-cell lung cancer, not otherwise specified; 3D-CRT, 3-dimensional conformal radiation therapy; IMRT, intensity-modulated radiation therapy; PBT, proton beam radiation therapy; GyE, cobalt-Gray equivalent; V20, volume of normal lung receiving ≥20 Gy; DLCO, capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 second.
Diffusing capacity
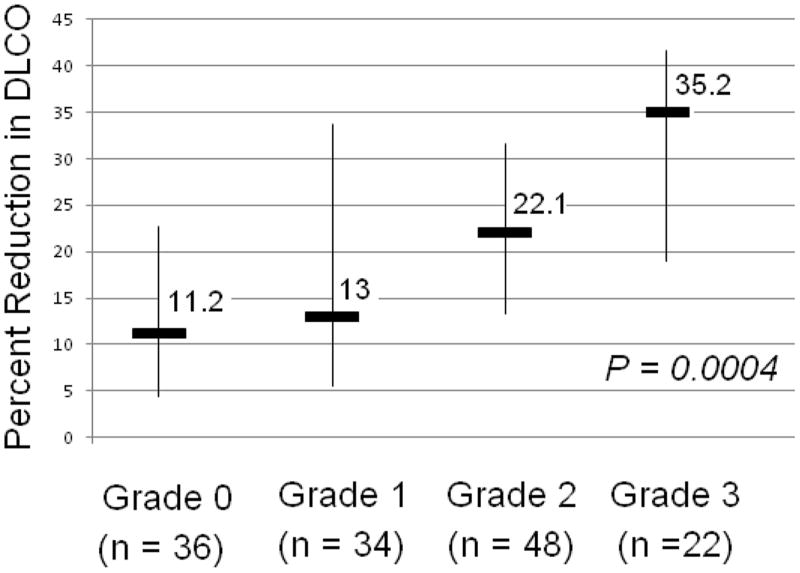
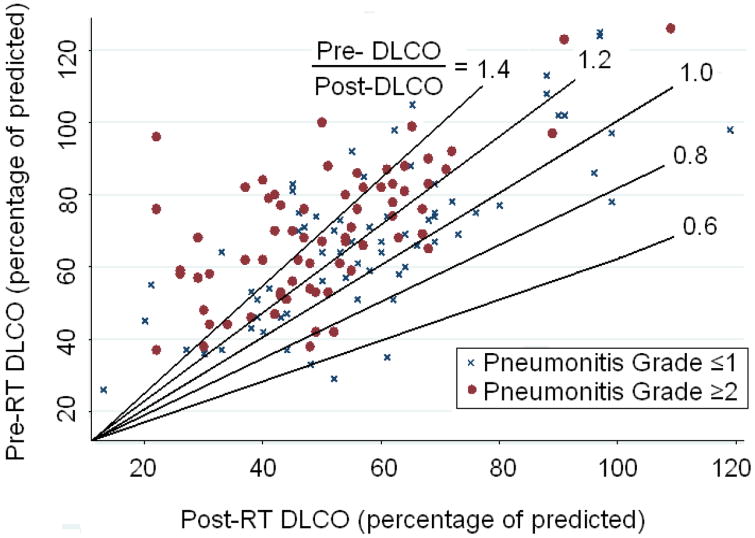
Pretreatment DLCO was not associated with RP (RP grade ≤1 vs. grade ≥2, P=0.095). Eighty-five percent of patients (n=119) were found to have decreased DLCO after RT, with a median reduction of 20% in the percent predicted value. Those patients who had grade 0, 1, 2, or 3 RP experienced median change in DLCO of 11%, 13%, 22%, or 35% (P=0.0004; Fig. 1). Percentage reduction in DLCO was associated with RP severity (P=0.0004, r=0.30 for grade ≤1 vs. grade ≤2). Patients who experienced higher proportional reductions in DLCO had greater rates of high-grade RP (Fig. 2).
Figure 1.
Radiation-induced pneumonitis grade versus median percent reductions in diffusing capacity of the lung for carbon monoxide (DLCO). Thin lines indicate 25th and 75th percentile ranges.
Figure 2.
Scatter plot of pretreatment (pre-RT) versus post-RT diffusing capacity of the lung for carbon monoxide (DLCO) for patients with symptomatic radiation pneumonitis (RP) (grade ≥2) or asymptomatic RP (grade ≤1). The lines represent ‘iso-percent reduction’ in DLCO (each line represents the same percent change in pre:post radiation DLCO). The predominance of data points above these iso-percent lines for patients with high-grade RP indicates that patients with high-grade RP had greater reductions in DLCO after treatment.
Univariate Cox proportional hazard analyses of the data for grade ≥ 2 RP showed a significant association between the DLCO reduction and symptomatic RP. Those patients experiencing a greater DLCO reduction within one week of the diagnosis of RP had a higher incidence of grade ≥ 2 RP (hazard ratio [HR] = 1.03, 95% confidence interval [CI], 1.01-1.05, P = 0.001; Table 2). This effect was virtually unchanged after adjustment for other covariates by multivariate analysis (HR = 1.02, 95% CI, 1.002-1.05, P = 0.030).
Table 2.
Associations between patient-, tumor-, therapy-, and pulmonary function tests-related characteristics and grade ≥ 2 radiation pneumonitis
| Univariate Analysis | Multivariate Analysis** | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Parameter | HR | 95% CI | P | HR | 95% CI | P |
| Age, years | 1.04 | 1.01-1.08 | 0.023 | 1.05 | 0.99-1.09 | 0.055 |
| Karnofsky performance status | ||||||
| ≥80 | 1.00 | 1.00 | ||||
| <80 | 1.93 | 0.75-4.96 | 0.168 | 4.03 | 1.05-15.35 | 0.041 |
| Disease stage | ||||||
| I,II | 1.00 | 1.00 | ||||
| III,IV | 2.57 | 1.11-5.97 | 0.028 | 4.82 | 1.27-18.19 | 0.020 |
| Smoking status | ||||||
| Nonsmoker/Former | 1.00 | 1.00 | ||||
| Current smoker | 1.46 | 0.61-3.46 | 0.386 | 1.87 | 0.66-5.33 | 0.237 |
| Chronic respiratory disease | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 0.83 | 0.41-1.65 | 0.598 | 1.01 | 0.41-2.51 | 0.969 |
| Chemotherapy | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 2.20 | 0.77-6.25 | 0.137 | 1.86 | 0.39-8.82 | 0.434 |
| Radiation fractionation | ||||||
| Once daily | 1.00 | 1.00 | ||||
| Twice daily | 1.11 | 0.45-2.71 | 0.820 | 0.33 | 0.92-1.22 | 0.099 |
| V20 | ||||||
| ≥30% | 1.00 | 1.00 | ||||
| <30% | 1.73 | 0.87-3.45 | 0.202 | 0.88 | 0.21-3.80 | 0.869 |
| Mean lung dose, Gy (or GyE) | ||||||
| ≥17 | 1.00 | 1.00 | ||||
| <17 | 1.00 | 0.99-1.00 | 0.357 | 1.64 | 0.39-6.73 | 0.492 |
| Baseline DLCO (% of predicted) | ||||||
| ≥60% | 1.00 | 1.00 | ||||
| <60% | 1.20 | 0.60-2.42 | 0.595 | 0.69 | 0.27-1.81 | 0.460 |
| Baseline FEV1 (% of predicted) | ||||||
| ≥60% | 1.00 | 1.00 | ||||
| <60% | 1.88 | 0.96-3.68 | 0.064 | 2.29 | 0.97-5.36 | 0.056 |
| DLCO reduction (%)* | 1.03 | 1.01-1.05 | 0.001 | 1.02 | 1.002-1.05 | 0.030 |
Abbreviations: HR, hazard ratio; V20, volume of normal lung receiving 20 Gy or more radiation; DLCO, diffusion capacity for carbon monoxide of the lung; FEV1, forced expiratory volume in 1 second.
Percent reduction in DLCO = (1 − post/pre) × 100
Multivariate analyses were adjusted for all factors listed in Table.
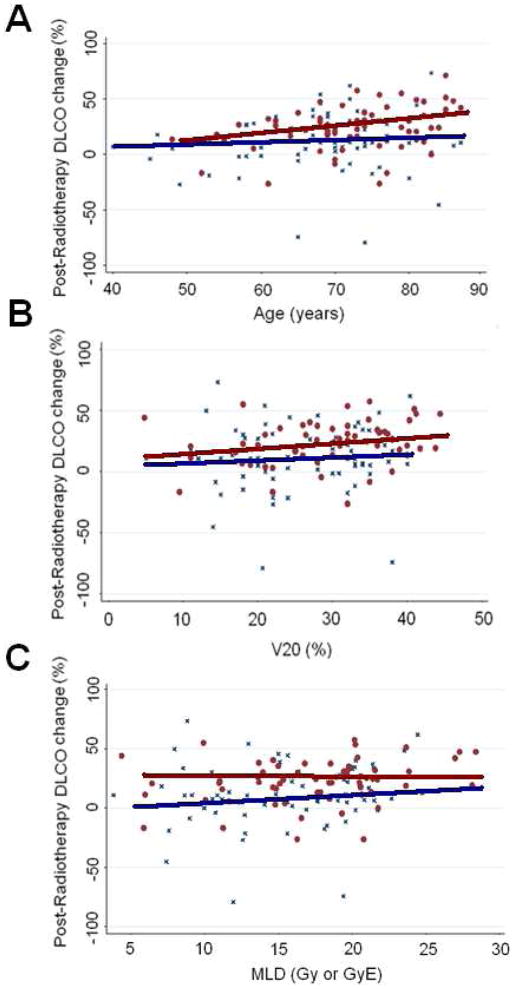
In a subgroup analysis, we observed a statistically significant association between the percentage of reduction in DLCO and RP (grade ≤1 vs. grade ≥2) for the following subgroups (Table 3): age ≥65 years, advanced stage, Karnofsky performance score (at any level), smokers, the use of chemotherapy, volume of normal lung receiving 20 Gy or more radiation (V20) ≥30% pretreatment DLCO ≥60%, and pretreatment forced expiratory volume in 1 second (FEV1) ≥60%. When the analysis was limited to those patients with a pretreatment DLCO <60%, the median change in DLCO for patients with RP grade ≤1 RP was 8.4% and that for grade ≥2 RP was 17.4% (P=0.064). Similar results were observed for patients with a pretreatment FEV1 <60% Gy (median DLCO reduction of 10.8% for those with RP grade ≤1 and 24.2% for those with grade ≥2 RP (P=0.051). In addition, when the analysis was restricted to patients with V20 and MLD below the median values, the extent of DLCO reduction appeared to be larger for symptomatic (grade ≥2) cases (10% grade ≥1 v s. 20% for grade ≥2, P=0.11 for both V20 <30% and MLD <17 Gy cases). Correlations of DLCO change with RP in the three subgroup categories (age, V20, and MLD) are shown graphically in Figure 3.
Table 3.
Correlations between percentage reduction in diffusing capacity of the lung for carbon monoxide after radiation therapy and pneumonitis (grade ≤1 vs. grade ≥2) for patient subgroups
| Factor | No. of Patients | r | P |
|---|---|---|---|
| Age, years | |||
| ≥65 | 106 | 0.31 | 0.0015 |
| <65 | 34 | 0.15 | 0.38 |
| Karnofsky performance status | |||
| ≥80 | 118 | 0.43 | 0.0471 |
| <80 | 22 | 0.28 | 0.0022 |
| Disease stage | |||
| I,II | 31 | 0.14 | 0.45 |
| III,IV | 109 | 0.32 | 0.0008 |
| Smoking status | |||
| Smoker | 132 | 0.30 | 0.0004 |
| Nonsmoker | 8 | 0.15 | 0.71 |
| Chronic respiratory disease | |||
| Yes | 51 | 0.33 | 0.0175 |
| No | 89 | 0.27 | 0.0104 |
| Chemotherapy | |||
| Yes | 122 | 0.28 | 0.0015 |
| No | 18 | 0.31 | 0.20 |
| Radiation fractionation | |||
| Once daily | 117 | 0.27 | 0.003 |
| Twice daily | 23 | 0.41 | 0.054 |
| V20 | |||
| ≥30% | 67 | 0.30 | 0.0128 |
| <30% | 65 | 0.19 | 0.11 |
| Mean lung dose, Gy (or GyE) | |||
| ≥17 | 66 | 0.30 | 0.0147 |
| <17 | 66 | 0.20 | 0.11 |
| Baseline DLCO (% of predicted) | |||
| ≥60% | 91 | 0.37 | 0.0003 |
| <60% | 49 | 0.22 | 0.1134 |
| Baseline FEV1 (% of predicted) | |||
| ≥60% | 81 | 0.37 | 0.0007 |
| <60% | 59 | 0.22 | 0.10 |
Abbreviations: V20, percentage of lung exposed to ≥20 Gy; DLCO, diffusing capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 second
Figure 3.
Scatter plot of percent changes in diffusing capacity of the lung for carbon monoxide (DLCO) versus age (A) and lung dosimetric parameters (volume of normal lung receiving ≥20 Gy [V20] [B] and mean lung dose [MLD] [C]) for patients with grade ≥2 RP (red dots) or grade ≤1 RP (blue crosses). A positive slope represents a greater change in DLCO, which appeared to be more pronounced for those with high-grade RP.
DISCUSSION
To our knowledge, this is the largest study assessing quantitative changes in pulmonary diffusing capacity and RP after definitive RT for NSCLC. Our study was also 3 novel in that all post-RT DLCO values were measured within 1 week of the time of diagnosis of RP, so that confounding factors, such as a potential lag time in clinical changes from DLCO measurement and the effects of treatment for RP on the DLCO value, did not apply. In doing so, we found that, in both the entire patient cohort and in subgroup analysis, while the pretreatment DLCO did not have predictive value, the change in DLCO could be quantitavely associated with the risk and grade of RP.
Although many studies (13-15) have investigated the correlation between baseline PFTs and RP, few have examined the effect of changes in PFT values after RT on RP. For instance, Marks et al. (10) reported that by 6 months, 81% of patients had a reduction in DLCO compared with pretreatment values. Our study is consistent with these results. However, contrary to our findings, no clear difference was evident in the Marks study between patients with symptomatic and asymptomatic RP. One potential reason for this divergence with our results could be that the Marks study included several tumor sites (lung, breast, lymphoma and other) and a relatively low rate of symptomatic RP (10%) compared with RP rates reported elsewhere (16, 17), particularly for patients receiving concurrent chemotherapy (18). The rate of RP observed in that study may have been too low to show any significant difference between patients with and without symptomatic RP. Furthermore, in the Marks study the post-RT DLCO values were not measured within the strict timeframe used in our study, bringing forth the issues noted above that treatment- and disease-related factors may influence the results when PFTs are taken several weeks before or after the onset of symptoms.
The implications of discovering a correlation between a quantitative change in DLCO and RP grade are significant. Currently, the criteria for grading RP in the CTCAE are based on clinical outcomes, which can lead to subjectivity and inconsistency among physicians and institutions. For instance, categorizing RP in patients who are oxygen-dependent at the start of RT can be difficult and subjective, given that grade 3 toxicity is defined by the indication for oxygen. If the putative association between percent change in DLCO and RP grade can be verified, these findings could provide an objective scoring system that would be much more reproducible in clinical trials. To further develop this concept, we recommend that these findings be applied to future trials examining the characteristics and pulmonary sequelae of RP to determine if, with larger patient numbers, reliable cut-off points can be validated and ultimately put into use in clinical practice.
With regard to our second finding that pretreatment DLCO level was not related to RP, our results are consistent with those of other investigators (19, 20), who also found no correlation between pretreatment PFT values and RP. Several authors (13, 14) have also attempted to develop models that used pretreatment PFTs to predict the onset of pulmonary symptoms after RT. For example, Kocak et al. (13) tested a method of identifying patients who were deemed to be at high risk of developing clinically relevant pulmonary symptoms, based on dosimetric and functional parameters and pretreatment pulmonary function. The authors found interactions between pretreatment PFT values, overall lung perfusion (measured by single photon emission CT), and the subsequent risk of pneumonitis. However, unlike the findings in the current study, the model could not accurately segregate patients into high- vs. low-risk groups.
We observed that some subgroups of patients did not show significant associations between the extent of change in DLCO after treatment and RP. These subgroups included patients who did not smoke, were <65 years old, had early-stage disease, did not receive chemotherapy, and received twice-daily radiation fractionation. Because the proportions of such patients were small, the small patient numbers may have precluded full statistical analysis. For example, no associations were found between DLCO impairment and RP grade in those patients who had an MLD <17 Gy, a V20 < 30%, or a pretreatment DLCO <60% of predicted values. However, in analyzing the incidence of symptomatic RP in these subgroups, we found that patients with RP grade ≥2 had DLCO reductions twice as large as those patients who had grade ≤1 RP. Thus, larger studies may be able to further characterize the importance of these patient and treatment characteristics.
We acknowledge several limitations of our study. First, the study included a heterogeneous group of patients who had been treated with thoracic RT over a period of 12 years and had received different combinations of RT and chemotherapy, rather than being treated prospectively on a well-defined treatment protocol. Nevertheless, we observed a significant association between DLCO reduction and symptomatic RP after adjustment of these and other confounding factors. Second, the DLCO values used for this study were not corrected for hemoglobin concentration because of missing data for several patients; however, this limitation probably had a modest influence at most on the current results, as the correction factor is generally relatively small. Third, although we assessed changes in DLCO in relation to RP, we did not assess other variables such as lung perfusion or CT density, which may allow us to expand on our current results to create an objective model for evaluating pulmonary injury based on biological or functional findings. We agree with Lind et al. (14) that multidimensional models generally provide greater sensitivity and specificity, and therefore this investigation is ongoing in our department. Fourth, our study did not provide information as to whether the changes in DLCO at the time of RP are temporary or permanent, and if temporary what timeframe is needed to recover a significant degree of lung function. This analysis would be an excellent correlative study to the current findings.
Finally, from the reported correlation between RP and DLCO we cannot definitively determine which of these two variables is the causative parameter in this relationship (e.g. does RP cause decreases in DLCO or vice-versa). Our assessment is that it is more reasonable for RP to cause changes in DLCO. Specifically, that radiation causes direct toxic injury to endothelial and epithelial cells resulting in an acute inflammatory process within the alveolar walls (RP), thereby causing reductions in DLCO. However, this mechanism has not been established and it is possible that in this complex process, there is no single causative factor with changes in each parameter influencing the other. Furthermore, from a standpoint of clinical implications, which of these two factors is the causative process is not as important as the finding that the two are closely interrelated, as this close correlation (regardless of causation) could be applied to scoring systems for RP.
SUMMARY.
We investigated the possible association between the extent of change in DLCO after radiation therapy and radiation pneumonitis (RP), with the hypothesis that patients with symptomatic RP will experience a significant decrease in pulmonary function that can be quantified in terms of diffusing capacity of the lung for carbon monoxide (DLCO) and used as an objective scoring system for RP. We found that patients who experienced higher proportional reductions in DLCO had greater rates of high-grade RP. This finding retained significance after adjustment by other confounding factors. Our findings are significant in that they provide a basis for developing a quantitative, objective scoring system for RP. Such a system, if validated with larger numbers of patients, preferably treated on a prospective basis, could improve upon the current scoring system and provide greater reproducibility amongst physicians in the clinic and researchers in the setting of clinical trials.
Acknowledgments
Supported in part by MD Anderson’s Cancer Center Support (Core) Grant CA16672.
Footnotes
Disclaimers: The authors declare no conflicts of interest regarding this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cerfolio RJ, Talati A, Bryant AS. Changes in pulmonary function tests after neoadjuvant therapy predict postoperative complications. Ann Thorac Surg. 2009;88:930–935. doi: 10.1016/j.athoracsur.2009.06.013. discussion 935-936. [DOI] [PubMed] [Google Scholar]
- 2.Kepka L, Bujko K, Orlowski TM, et al. Cardiopulmonary morbidity and quality of life in non-small cell lung cancer patients treated with or without postoperative radiotherapy. Radiother Oncol. 98:238–243. doi: 10.1016/j.radonc.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Sundar IK, Mullapudi N, Yao H, et al. Lung cancer and its association with chronic obstructive pulmonary disease: update on nexus of epigenetics. Curr Opin Pulm Med. 2011 doi: 10.1097/MCP.0b013e3283477533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gopal R, Starkschall G, Tucker SL, et al. Effects of radiotherapy and chemotherapy on lung function in patients with non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2003;56:114–120. doi: 10.1016/s0360-3016(03)00077-4. [DOI] [PubMed] [Google Scholar]
- 5.Takeda S, Funakoshi Y, Kadota Y, et al. Fall in diffusing capacity associated with induction therapy for lung cancer: a predictor of postoperative complication? Ann Thorac Surg. 2006;82:232–236. doi: 10.1016/j.athoracsur.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 6.Fang LC, Komaki R, Allen P, et al. Comparison of outcomes for patients with medically inoperable Stage I non-small-cell lung cancer treated with two-dimensional vs. three-dimensional radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:108–116. doi: 10.1016/j.ijrobp.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Liao ZX, Komaki RR, Thames HD, Jr, et al. Influence of technologic advances on outcomes in patients with unresectable, locally advanced non-small-cell lung cancer receiving concomitant chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:775–781. doi: 10.1016/j.ijrobp.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 8.Zinner RG, Komaki R, Cox JD, et al. Dose escalation of gemcitabine is possible with concurrent chest three-dimensional rather than two-dimensional radiotherapy: a phase I trial in patients with stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;73:119–127. doi: 10.1016/j.ijrobp.2008.03.069. [DOI] [PubMed] [Google Scholar]
- 9.Cancer Therapy Evaluation Program: Common terminology criteria for adverse events, version 3. Bethesda, MD: National Cancer Institute; 2003. [Google Scholar]
- 10.Marks LB, Fan M, Clough R, et al. Radiation-induced pulmonary injury: symptomatic versus subclinical endpoints. Int J Radiat Biol. 2000;76:469–475. doi: 10.1080/095530000138466. [DOI] [PubMed] [Google Scholar]
- 11.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. European Respiratory Journal. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 12.Videtic GM, Stitt LW, Ash RB, et al. Impaired diffusion capacity predicts for decreased treatment tolerance and survival in limited stage small cell lung cancer patients treated with concurrent chemoradiation. Lung Cancer. 2004;43:159–166. doi: 10.1016/j.lungcan.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 13.Kocak Z, Borst GR, Zeng J, et al. Prospective assessment of dosimetric/physiologic-based models for predicting radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2007;67:178–186. doi: 10.1016/j.ijrobp.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lind PA, Marks LB, Hollis D, et al. Receiver operating characteristic curves to assess predictors of radiation-induced symptomatic lung injury. Int J Radiat Oncol Biol Phys. 2002;54:340–347. doi: 10.1016/s0360-3016(02)02932-2. [DOI] [PubMed] [Google Scholar]
- 15.Robnett TJ, Machtay M, Vines EF, et al. Factors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 2000;48:89–94. doi: 10.1016/s0360-3016(00)00648-9. [DOI] [PubMed] [Google Scholar]
- 16.Sejpal S, Komaki R, Tsao A, et al. Early findings on toxicity of proton beam therapy with concurrent chemotherapy for nonsmall cell lung cancer. Cancer. 2011 doi: 10.1002/cncr.25848. [DOI] [PubMed] [Google Scholar]
- 17.Wang S, Liao Z, Wei X, et al. Analysis of clinical and dosimetric factors associated with treatment-related pneumonitis (TRP) in patients with non-small-cell lung cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT) Int J Radiat Oncol Biol Phys. 2006;66:1399–1407. doi: 10.1016/j.ijrobp.2006.07.1337. [DOI] [PubMed] [Google Scholar]
- 18.Parashar B, Edwards A, Mehta R, et al. Chemotherapy significantly increases the risk of radiation pneumonitis in radiation therapy of advanced lung cancer. Am J Clin Oncol. 2011;34:160–164. doi: 10.1097/COC.0b013e3181d6b40f. [DOI] [PubMed] [Google Scholar]
- 19.Inoue A, Kunitoh H, Sekine I, et al. Radiation pneumonitis in lung cancer patients: a retrospective study of risk factors and the long-term prognosis. Int J Radiat Oncol Biol Phys. 2001;49:649–655. doi: 10.1016/s0360-3016(00)00783-5. [DOI] [PubMed] [Google Scholar]
- 20.Claude L, Perol D, Ginestet C, et al. A prospective study on radiation pneumonitis following conformal radiation therapy in non-small-cell lung cancer: clinical and dosimetric factors analysis. Radiother Oncol. 2004;71:175–181. doi: 10.1016/j.radonc.2004.02.005. [DOI] [PubMed] [Google Scholar]