Abstract
Objectives
To compare echocardiographic findings in patients with critical aortic stenosis following surgical (SAVR) or transcatheter aortic valve replacement (TAVR
Background
The Placement of Aortic Transcatheter Valves trial randomized patients 1:1 to SAVR or TAVR
Methods
Echocardiograms were obtained at baseline, discharge, 30 days, 6 months, 1 year, and 2 years post procedure and analyzed in a core laboratory. For the analysis of post-implant variables, the first interpretable study (≤ 6 mos) was used.
Results
Both groups showed a decrease in aortic valve gradients and increase in effective orifice area (EOA) (p < 0.0001) which remained stable over 2 years. Compared to SAVR, TAVR resulted in: larger indexed EOA (p = 0.038), less prosthesis-patient mismatch (p = 0.019), and more total and paravalvular aortic regurgitation (AR) (p < 0.0001). Baseline echocardiographic univariate predictors of death were: lower peak transaortic gradient in TAVR patients; low left ventricular diastolic volume (LVDV), low stroke volume, and greater severity of mitral regurgitation in SAVR patients. Post-implantation echocardiographic univariate predictors of death were: larger LVDV, systolic volume (LVSV) and EOA, decreased ejection fraction, and greater AR in TAVR patients; smaller LVSV and LVDV, low stroke volume, smaller EOA and prosthesis-patient mismatch in SAVR patients.
Conclusions
Patients randomized to either SAVR or TAVR experience enduring, significant reductions in transaortic gradients and increase in EOA. Compared to SAVR, TAVR patients had higher indexed EOA, lower prosthesis-patient mismatch and more AR. Univariate predictors of death for the TAVR group and SAVR groups differed and may allow future refinement in patient selection.
Keywords: transcatheter aortic valve replacement, aortic stenosis, echocardiography
Introduction
Transcatheter aortic valve replacement (TAVR) has emerged as a reasonable alternative to surgical aortic valve replacement (SAVR) (1–5). The Placement of Aortic Transcatheter Valves (Partner) trial was the first randomized trial comparing TAVR to standard-of-care therapies in a rigorous fashion. Two-year clinical outcomes in high risk, operable patients with severe aortic stenosis (Partner Cohort A) showed TAVR was non-inferior to SAVR without significant differences in all-cause mortality or cardiovascular mortality or evidence for structural valve failure.
Echocardiography is the recommended imaging modality for the assessment of aortic valve stenosis and prosthetic valve function (6–8) and was used for patient selection, valve sizing, and extended follow up (1, 2). In contrast to previous reports relying on site interpretations of images, the trial core laboratory provided rigorous quality control of the image acquisition and analysis process (9). The current investigation reports the complete, centrally analyzed echocardiographic findings from the high risk, operable patient population (Cohort A).
Methods
Patient Selection, Study Design and Management
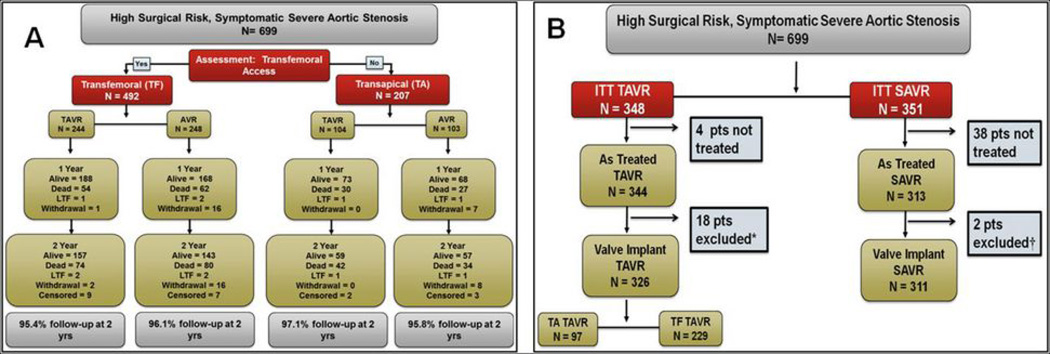
Cohort A of the Placement of Aortic Transcatheter Valves trial (2) randomized 699 high surgical risk patients (mortality of ≥ 15%) with severe, symptomatic aortic stenosis, between SAVR and TAVR with the Edwards SAPIEN™ valve (in a 1:1 ratio) (Figure 1). All patients enrolled had site determined, severe native tricuspid aortic stenosis defined by echocardiographically-determined aortic valve area of ≤ 0.8cm2 plus either a peak velocity ≥ 4 m/s or a mean gradient ≥ 40 mmHg at rest or during dobutamine infusion. Study design and complete inclusion and exclusion criteria are presented in a previous publication (2).
Figure 1.
Flow chart of high risk Placement of Aortic Transcatheter (PARTNER) Valves trial. Figure 1A shows the flow chart of patient randomization and follow-up for Cohort A of the PARTNER trial. Figure 1B outlines the intention-to-treat (ITT) and as-treated patient population of Cohort A.
Randomization to SAVR or TAVR was stratified by feasibility of transapical or transfemoral access. Echocardiograms were obtained at baseline, and at 7 days, 30 days, 6 months, 1 year, and 2 years post procedure.
Echocardiography Core Laboratory Analysis
All echocardiograms were analyzed at an independent core lab which followed the American Society of Echocardiography (ASE) standards for echocardiography core laboratories.(10) Image acquisition quality was assured by: use of a detailed acquisition protocol, site qualification and training with quality feedback at regular intervals, and retraining of sites with unacceptable image quality. Image analysis quality was assured by: reader qualification, detailed analysis instructions, group and individual training, regular intra- and inter- observer variability testing, retraining and coaching when indicated (11). All measurements and analyses were performed without knowledge of clinical or other laboratory data including prior echo results, group assignment, and timing of the assessment.
Reproducibility was determined on 649–1360 pair wise comparisons among readers for each of eight critical variables on 30 echoes (total number of comparisons =8,031). Intra-class correlation coefficients were 0.92–0.99 for physician over readers and 0.89–0.97 for sonographers. Kappa statistics for agreement for categorical variables calculated for physician readers were 0.56–0.85.
Ventricular size and function and valvular function were measured according to previously published guidelines (7, 8, 12). An integrative, semi-quantitative approach was used to assess the severity of valvular regurgitation. Both qualitative (visual) and quantitative (biplane Simpson’s method of discs) were used to report ejection fraction. Relative wall thickness (RWT) was calculated as 2x posterior wall thickness/LVED (RWTp) and also using the posterior wall thickness plus septal wall thickness as (septal wall thickness + posterior wall thickness)/LVED, or RWTm. Site-reported systolic annulus diameters were derived from long axis views. The effective orifice area (EOA) is calculated as the Doppler stroke volume ÷ aortic velocity time integral. The cover index was determined as (13): [Prosthesis diameter – annular diameter] /prosthesis diameter. The severity of prosthesis-patient mismatch was graded using EOA indexed to body surface area (7) with absence defined as > 0.85 cm2/m2, moderate ≥ 0.65 and ≤ 0.85 cm2/m2, and <0.65 cm2/m2.
Paravalvular regurgitation after TAVR/SAVR was graded in accordance with the ASE recommendations for native valves (14) and adoption of the 2009 prosthetic valve guidelines (7) with the following exception. Because of the eccentric, irregular, jet and the frequent noncylindrical ‘spray’ of the paravalvular jet contour, the parasternal short axis view(s) was weighted more heavily than other signals in providing an integrated assessment, as follows:
None - no regurgitant color flow
Trace - pinpoint jet in AV
Mild – jet arc length is < 10% of the annulus circumference
Moderate - jet arc length is 10–30% of the annulus circumference
Severe - jet arc length is > 30% of the annulus circumference
Statistical Methods
Analysis is based on the actual valve implant patients who received and retained either a surgical or transcatheter valve, as this group is most appropriate for studying the echocardiographic measurements and outcomes. Intention to treat analysis (ITT) for evaluating trial endpoints has previously been reported (2, 5). Because of the difficulty in imaging patients immediately following intervention, the first post-implant values are obtained from the first available value at discharge, 30 days or 6 months.
Categorical variables were compared using Fisher’s exact test. Since regurgitation and prosthesis-patient mismatch are ordinal variables, comparisons involving these variables use the exact Jonckheere-Terpstra test. It should be noted that when one of the variables has two levels the test is equivalent to the exact Mann-Whitney U-test; where both have > two levels the use of the Jonckheere-Terpstra test is important. Continuous variables were presented as means (± SD) and compared using Student’s t-test; comparisons with baseline values use the paired sample ttest. Survival curves for time-to-event variables were constructed using Kaplan-Meier estimates based on all available data and were compared using the log-rank test. To study the impact of risk factors on mortality, Cox proportional hazards regression was performed.
Imputation was not performed for missing baseline or first post-implant variables except in the multivariable models. The effect is that patients whose values are missing for a particular analysis are removed from that analysis.
Data are based on an extract date of February 13, 2012. All statistical analyses were performed in SAS®, version 9.2.
Results
In the ITT TAVR arm there were 348 randomized patients; 344 were As Treated TAVR and 326 were Valve Implant of which 97 used transapical and 229 used transfemoral approaches. In the ITT SAVR arm there were 351 randomized patients; of these 313 were As Treated SAVR and 310 were Valve Implant (Figure 1). Patients’ baseline clinical demographics using the ITT populations are listed in Table 1 (online supplement). There were no statistically significant differences between the groups, except there were more patient with high creatinine in the TAVR group.
Baseline Echocardiographic Parameters
There were no baseline differences in LV size, geometry and function between as treated SAVR and TAVR groups (Table 2). The two groups were similar in LVED, LV end-systolic dimensions (LVES), RWTp and RWTm, left ventricular mass, left ventricular mass index, LVDV, LVSV and left ventricular stroke volume as well as calculated ejection fraction.
Table 2.
Baseline Echocardiographic Findings TAVR vs. SAVR (Valve Implant Population) *
| Echocardiographic Parameter | SAVR | TAVR | Pr > |t| |
|---|---|---|---|
| LVED Dimension (cm) | (n = 267) 4.5 ± 0.8 | (n = 277) 4.5 ± 0.8 | 0.9620 |
| LVES Dimension (cm) | (n = 259) 3.3 ± 1.0 | (n = 266) 3.3 ± 0.9 | 0.7410 |
| Concentric Remodeling(RWTm) | (n = 265) 0.68 ± 0.19 | (n = 276) 0.69 ± 0.20 | 0.4723 |
| Concentric Remodeling (RWTp) | (n = 267) 0.61 ± 0.18 | (n = 277) 0.62 ± 0.20 | 0.4340 |
| LV Mass(gm) | (n = 265) 277.6 ± 86.2 | (n = 276) 283.7 ± 84.7 | 0.4061 |
| LV Mass index (gm/m2) | (n = 264) 153.2 ± 44.0 | (n = 275) 155.6 ± 40.6 | 0.5085 |
| LVDV (mL) | (n = 179) 118.5 ± 46.9 | (n = 176) 122.8 ± 48.4 | 0.3958 |
| LVSV (mL) | (n = 179) 57.9 ± 35.1 | (n = 176) 62.5 ± 38.2 | 0.2361 |
| Stroke Volume 2DE (mL) | (n = 179) 60.5 ± 20.4 | (n = 176) 60.3 ± 22.7 | 0.9323 |
| Doppler Stroke Volume (mL) | (n = 290) 62.9 ± 18.4 | (n = 303) 63.5 ± 19.6 | 0.7222 |
| Ejection Fraction (%) | (n = 295) 53.4 ± 12.6 | (n = 313) 52.6 ± 13.4 | 0.4602 |
| AV Annulus Diameter (cm) | (n = 252) 2.00 ± 0.22 | (n = 263) 2.01 ± 0.24 | 0.7983 |
| Ascending Aorta (cm) | (n = 170) 3.0 ± 0.4 | (n = 163) 3.1 ± 0.4 | 0.5284 |
| AV Peak Velocity (cm/sec) | (n = 293) 4.22 ± 0.70 | (n = 305) 4.19 ± 0.69 | 0.5308 |
| AV Peak Gradient (mmHg) | (n = 295) 73.3 ± 24.2 | (n = 307) 71.7 ± 23.5 | 0.4349 |
| AV Mean Gradient (mmHg) | (n = 295) 43.4 ± 14.3 | (n = 307) 43.1 ± 14.5 | 0.7929 |
| AV Area (cm2) | (n = 290) 0.64 ± 0.19 | (n = 301) 0.66 ± 0.20 | 0.3212 |
| AV Area Index (cm2/m2) | (n = 288) 0.35 ± 0.10 | (n = 300) 0.36 ± 0.11 | 0.3760 |
| LVOT VTI (cm) | (n = 293) 19.6 ± 5.7 | (n = 306) 19.5 ± 5.7 | 0.8654 |
| AV VTI (cm) | (n = 293) 100.8 ± 23.4 | (n = 305) 99.3 ± 22.5 | 0.4062 |
| Doppler Velocity Index | (n = 292) 0.2 ± 0.1 | (n = 304) 0.2 ± 0.1 | 0.5383 |
| Aortic Regurgitation** | (n = 295) 1.59 ± 0.95 | (n = 312) 1.56 ± 0.81 | 0.7188 |
| Mitral Regurgitation** | (n = 292) 1.94 ± 0.78 | (n = 311) 1.91 ± 0.82 | 0.6611 |
Plus-minus values are mean ± SD.
Aortic and Mitral regurgitation expressed as categorical variables with the following values assigned to visual grades: 0 = none, 1 = trace, 2 = mild, 3 = moderate, 4 = severe.
2DE = two-dimensional echo, AT = as-treated, AV = aortic valve, DVI =Doppler velocity index, LV= left ventricle, LVDV = left ventricular diastolic volume, LVED = left ventricular end diastolic dimension, LVES= left ventricular end systolic dimension, LVOT =left ventricular outflow tract, LVSV = left ventricular systolic volume, MR =mitral regurgitation, RWTm= mean relative wall thickness, RWTp =posterior wall relative thickness, TAVR = transcatheter aortic valve replacement, VTI =velocity time integral
Baseline valvular hemodynamics have previously been reported (4) and are summarized in Table 2. There were no significant differences between SAVR and TAVR groups for baseline peak velocity, peak gradient, mean gradient, stroke volume (by any method) calculated aortic valve area, or aortic valve area index. There was no significant difference in the severity of mitral or aortic regurgitation.
Ventricular and Valvular Changes Immediately Following Intervention
In the TAVR cohort (Table 3, online supplement), neither LVED nor LVDV changed immediately post intervention; however, LVES (p = 0.0005) and LVSV (p = 0.0016) were significantly smaller and ejection fraction higher (p < 0.0001). Peak and mean aortic valve gradients decreased (p < 0.0001) and EOA increased (p < 0.0001). There was a significant reduction in mitral regurgitation (p<0.0001) with no change in total aortic regurgitation (p = 0.649).
In the SAVR cohort there was a significant decrease in LVED, LVDV, LVES and LVSV immediately following intervention (Table 3, online supplement), associated with reduced stroke volume by 2DE (p<0.0001) and Doppler (p<0.0001), but no change in ejection fraction. Peak and mean aortic valve gradients decreased (p < 0.0001) and an increase in EOA (p < 0.0001). There was a significant reduction in both mitral regurgitation and aortic regurgitation (p < 0.0001).
Comparison of SAVR and TAVR
Table 4 compares TAVR and SAVR baseline and post-implant echocardiographic variables of ventricular size and function. TAVR as compared to SAVR had a significantly larger LVED up to 6 months following valve replacement, but not after 1 or 2 years of follow-up. LVDV showed inconsistent differences, neither LVES nor LVSV showed between group differences throughout follow-up. LV mass and LV mass index were larger in the TAVR patients at discharge, 6 months and 1 yr, but not at 2 yrs. The percent LV mass reduction was initially greater for the SAVR group, but was not significantly different after 6 months. RWTp and RWTm following valve replacement was initially higher for the SAVR group but progressively decreased for both groups.
Table 4.
Post-intervention Echocardiographic Findings for Ventricular Structure and Function in the TAVR vs. SAVR (Valve Implant Population)
| Echocardiographic Parameter | SAVR | TAVR | P-value for comparison of trial arms |
|---|---|---|---|
| LVED Dimension (cm) | |||
| Baseline | (n = 267) 4.5 ± 0.8 | (n = 277) 4.5 ± 0.8 | 0.9620 |
| Discharge | (n = 204) 4.3 ± 0.8 | (n = 266) 4.6 ± 0.8 | 0.0002 |
| 30 Day | (n = 201) 4.3 ± 0.7 | (n = 248) 4.6 ± 0.8 | 0.0003 |
| 6 Month | (n = 159) 4.3 ± 0.7 | (n = 204) 4.5 ± 0.8 | 0.0101 |
| 1 Year | (n = 144) 4.4 ± 0.7 | (n = 193) 4.5 ± 0.8 | 0.4101 |
| 2 Year | (n = 105) 4.4 ± 0.7 | (n = 132) 4.5 ± 0.8 | 0.2488 |
| P-value for change from baseline to first post-implant | <.0001 | 0.5253 | |
| P-value for change from first post-implant to 2 years | 0.3893 | 0.1940 | |
| LVES Dimension (cm) | |||
| Baseline | (n = 259) 3.3 ± 1.0 | (n = 266) 3.3 ± 0.9 | 0.7410 |
| Discharge | (n = 191) 3.1 ± 0.9 | (n = 258) 3.2 ± 0.9 | 0.4025 |
| 30 Day | (n = 184) 3.2 ± 0.8 | (n = 241) 3.2 ± 0.9 | 0.2609 |
| 6 Month | (n = 155) 3.0 ± 0.8 | (n = 193) 3.1 ± 0.8 | 0.3529 |
| 1 Year | (n = 139) 3.0 ± 0.9 | (n = 183) 3.1 ± 0.9 | 0.4200 |
| 2 Year | (n = 103) 3.1 ± 0.8 | (n = 122) 3.2 ± 0.9 | 0.2195 |
| P-value for change from baseline to first post-implant | 0.0019 | 0.0005 | |
| P-value for change from first post-implant to 2 years | 0.0045 | 0.8793 | |
| Concentric Remodeling (RWTm) | |||
| Baseline | (n = 265) 0.68 ± 0.19 | (n = 276) 0.69 ± 0.20 | 0.4723 |
| Discharge | (n = 203) 0.73 ± 0.23 | (n = 264) 0.67 ± 0.21 | 0.0034 |
| 30 Day | (n = 199) 0.68 ± 0.18 | (n = 246) 0.65 ± 0.20 | 0.1229 |
| 6 Month | (n = 158) 0.66 ± 0.18 | (n = 203) 0.65 ± 0.19 | 0.5777 |
| 1 Year | (n = 144) 0.64 ± 0.19 | (n = 193) 0.65 ± 0.21 | 0.4413 |
| 2 Year | (n = 105) 0.60 ± 0.17 | (n = 132) 0.60 ± 0.20 | 0.9679 |
| P-value for continuous change from baseline | <.0001 | <.0001 | |
| Concentric Remodeling (RWTp) | |||
| Baseline | (n = 267) 0.61 ± 0.18 | (n = 277) 0.62 ± 0.20 | 0.4340 |
| Discharge | (n = 203) 0.66 ± 0.22 | (n = 265) 0.59 ± 0.20 | 0.0006 |
| 30 Day | (n = 200) 0.61 ± 0.17 | (n = 247) 0.58 ± 0.18 | 0.1353 |
| 6 Month | (n = 158) 0.59 ± 0.16 | (n = 204) 0.58 ± 0.18 | 0.3650 |
| 1 Year | (n = 144) 0.55 ± 0.18 | (n = 193) 0.57 ± 0.18 | 0.3319 |
| 2 Year | (n = 105) 0.50 ± 0.14 | (n = 132) 0.50 ± 0.18 | 0.9996 |
| P-value for continuous change from baseline | <.0001 | <.0001 | |
| LV Mass (gm) | |||
| Baseline | (n = 265) 277.6 ± 86.2 | (n = 276) 283.7 ± 84.7 | 0.4061 |
| Discharge | (n = 203) 253.1 ± 75.0 | (n = 264) 274.0 ± 80.1 | 0.0042 |
| 30 Day | (n = 200) 246.6 ± 77.8 | (n = 246) 269.9 ± 80.1 | 0.0021 |
| 6 Month | (n = 158) 233.7 ± 74.1 | (n = 203) 257.4 ± 79.0 | 0.0039 |
| 1 Year | (n = 144) 232.9 ± 69.5 | (n = 193) 250.7 ± 83.9 | 0.0384 |
| 2 Year | (n = 105) 213.7 ± 60.7 | (n = 132) 226.7 ± 73.8 | 0.1474 |
| P-value for continuous change from baseline | <.0001 | <.0001 | |
| LV mass index (gm/m2) | |||
| Baseline | (n = 264) 153.2 ± 44.0 | (n = 275) 155.6 ± 40.6 | 0.5085 |
| Discharge | (n = 178) 140.5 ± 36.0 | (n = 228) 150.8 ± 37.5 | 0.0055 |
| 30 Day | (n = 180) 139.1 ± 39.4 | (n = 236) 148.6 ± 38.3 | 0.0140 |
| 6 Month | (n = 152) 128.6 ± 37.5 | (n = 200) 140.4 ± 38.1 | 0.0041 |
| 1 Year | (n = 135) 127.5 ± 36.3 | (n = 186) 135.7 ± 37.9 | 0.0521 |
| 2 Year | (n = 98) 116.4 ± 30.2 | (n = 126) 124.7 ± 35.6 | 0.0669 |
| P-value for continuous change from baseline | <.0001 | <.0001 | |
| LV mass index regression (%) | |||
| Discharge | (n = 159) −3.9% ± 22.4% | (n = 203) 0.4% ± 19.4% | 0.0506 |
| 30 Day | (n = 159) −6.7% ± 22.9% | (n = 210) −0.7% ± 23.5% | 0.0153 |
| 6 Month | (n = 140) −12.6% ± 25.3% | (n = 176) −8.8% ± 23.0% | 0.1651 |
| 1 Year | (n = 122) −13.3% ± 25.2% | (n = 167) −9.4% ± 21.6% | 0.1596 |
| 2 Year | (n = 92) −21.9% ± 25.7% | (n = 112) −17.3% ± 21.2% | 0.1608 |
| P-value for continuous change from baseline | <.0001 | <.0001 | |
| LVDV (mL) | |||
| Baseline | (n = 179) 118.5 ± 46.9 | (n = 176) 122.8 ± 48.4 | 0.3958 |
| Discharge | (n = 117) 106.7 ± 41.3 | (n = 142) 123.3 ± 43.4 | 0.0019 |
| 30 Day | (n = 103) 111.1 ± 38.1 | (n = 125) 117.9 ± 50.5 | 0.2562 |
| 6 Month | (n = 82) 106.2 ± 31.5 | (n = 107) 116.8 ± 44.4 | 0.0681 |
| 1 Year | (n = 84) 101.0 ± 34.5 | (n = 103) 113.9 ± 49.6 | 0.0456 |
| 2 Year | (n = 53) 117.0 ± 43.7 | (n = 56) 118.6 ± 52.3 | 0.8670 |
| P-value for change from baseline to first post-implant | <.0001 | 0.3433 | |
| P-value for change from first post-implant to 2 years | 0.9873 | 0.3232 | |
| LVSV (ml) | |||
| Baseline | (n = 179) 57.9 ± 35.1 | (n = 176) 62.5 ± 38.2 | 0.2361 |
| Discharge | (n = 117) 52.8 ± 30.9 | (n = 142) 57.9 ± 32.8 | 0.2015 |
| 30 Day | (n = 103) 51.5 ± 28.8 | (n = 125) 53.3 ± 33.6 | 0.6807 |
| 6 Month | (n = 82) 47.3 ± 21.7 | (n = 107) 52.1 ± 30.2 | 0.2253 |
| 1 Year | (n = 84) 44.7 ± 24.7 | (n = 103) 52.6 ± 36.1 | 0.0896 |
| 2 Year | (n = 53) 50.9 ± 29.5 | (n = 56) 54.0 ± 33.6 | 0.6123 |
| P-value for change from baseline to first post-implant | <.0001 | 0.0016 | |
| P-value for change from first post-implant to 2 years | 0.0417 | 0.1587 | |
| Stroke Volume 2DE (cm2) | |||
| Baseline | (n = 179) 60.5 ± 20.4 | (n = 176) 60.3 ± 22.7 | 0.9323 |
| Discharge | (n = 117) 53.8 ± 18.4 | (n = 142) 65.4 ± 19.7 | <.0001 |
| 30 Day | (n = 103) 59.5 ± 20.9 | (n = 125) 64.5 ± 23.2 | 0.0921 |
| 6 Month | (n = 82) 58.7 ± 16.9 | (n = 107) 64.7 ± 21.0 | 0.0383 |
| 1 Year | (n = 84) 56.3 ± 16.7 | (n = 103) 61.3 ± 20.5 | 0.0763 |
| 2 Year | (n = 53) 65.9 ± 19.5 | (n = 56) 64.5 ± 23.6 | 0.7379 |
| P-value for change from baseline to first post-implant | <.0001 | 0.0670 | |
| P-value for change from first post-implant to 2 years | 0.0076 | 0.8734 | |
| Doppler Stroke Volume (cm2) | |||
| Baseline | (n = 290) 62.9 ± 18.4 | (n = 303) 63.5 ± 19.6 | 0.7222 |
| Discharge | (n = 239) 54.4 ± 16.6 | (n = 278) 64.8 ± 18.7 | <.0001 |
| 30 Day | (n = 224) 60.7 ± 18.0 | (n = 270) 67.6 ± 21.2 | 0.0001 |
| 6 Month | (n = 163) 66.0 ± 20.1 | (n = 224) 72.4 ± 24.4 | 0.0067 |
| 1 Year | (n = 151) 64.7 ± 19.9 | (n = 209) 70.2 ± 21.4 | 0.0132 |
| 2 Year | (n = 110) 65.1 ± 18.9 | (n = 139) 69.0 ± 22.5 | 0.1418 |
| P-value for change from baseline to first post-implant | <.0001 | 0.4642 | |
| P-value for change from first post-implant to 2 years | <.0001 | 0.0088 | |
| Ejection Fraction (%) | |||
| Baseline | (n = 295) 53.4 ± 12.6 | (n = 313) 52.6 ± 13.4 | 0.4602 |
| Discharge | (n = 257) 53.8 ± 12.1 | (n = 305) 55.4 ± 11.0 | 0.1064 |
| 30 Day | (n = 227) 56.2 ± 11.3 | (n = 275) 56.0 ± 11.2 | 0.8290 |
| 6 Month | (n = 171) 57.0 ± 9.8 | (n = 231) 56.7 ± 10.2 | 0.7596 |
| 1 Year | (n = 155) 57.1 ± 10.4 | (n = 215) 56.6 ± 10.4 | 0.7018 |
| 2 Year | (n = 114) 57.4 ± 10.4 | (n = 145) 56.0 ± 10.0 | 0.2902 |
| P-value for change from baseline to first post-implant value | 0.4872 | <.0001 | |
| P-value for change from first post-implant to 2 years | <.0001 | 0.9708 | |
Aortic and Mitral regurgitation expressed as categorical variables with the following values assigned to visual grades: 0 = none, 1 = trace, 2 = mild, 3 = moderate, 4 = severe.
2DE = two-dimensional echo, AT = as-treated, AV = aortic valve, DVI =Doppler velocity index, EOA = effective orifice area, iEOA = indexed effective orifice area, LV= left ventricle, LVDV = left ventricular diastolic volume, LVED = left ventricular end diastolic dimension, LVES= left ventricular end systolic dimension, LVOT =left ventricular outflow tract, LVSV = left ventricular systolic volume, MR =mitral regurgitation, RWTm= mean relative wall thickness, RWTp =posterior wall relative thickness, TAVR = transcatheter aortic valve replacement, VTI =velocity time integral
Although 2DE stroke volume showed inconsistent differences at various time points, Doppler stroke volume was significantly larger in TAVR patients up to 2 years at which time there was no significant difference. There was no significant between-group difference in ejection fraction throughout follow-up.
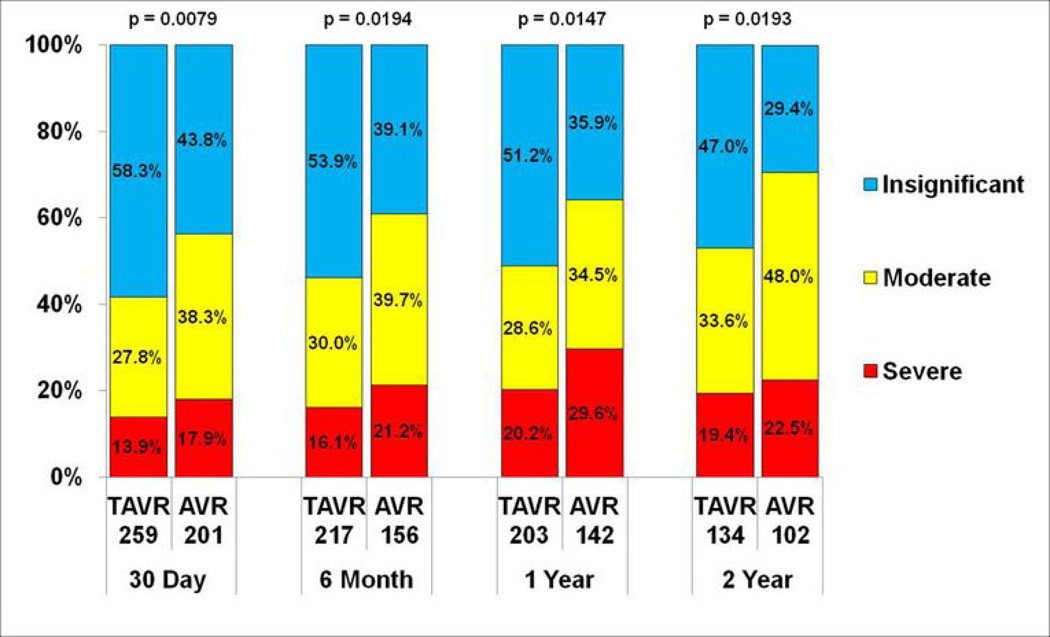
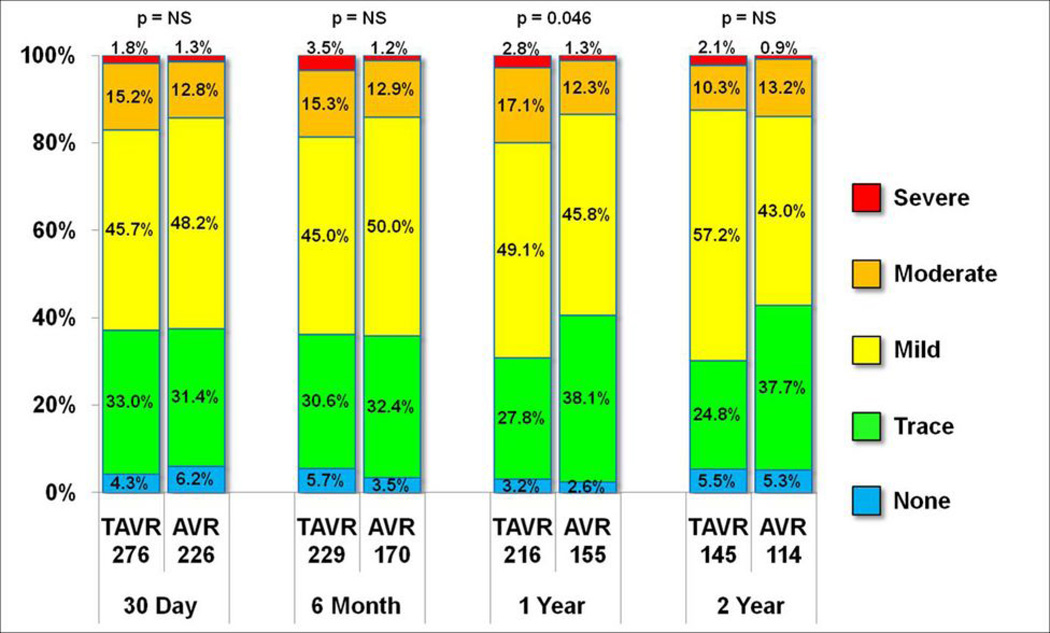
Table 5 compares the baseline and post-implant echocardiographic variables of valvular function, for TAVR versus SAVR groups. Peak and mean transaortic gradients were significantly lower in the TAVR group for most time points. EOA and indexed EOA were significantly larger in TAVR at all follow-up time points. Prosthesis-patient mismatch was more common in SAVR throughout follow-up (Table 6 online supplement, and Figure 2). There were no significant differences in mitral regurgitation between TAVR and SAVR groups up to 2 years (Table 5, Figure 3).
Table 5.
Post-intervention Echocardiographic Findings for Valvular Function in the TAVR vs. SAVR (Valve Implant Population)
| Echocardiographic Parameter | SAVR | TAVR | P-value for comparison of trial arms |
|---|---|---|---|
| AV Peak Velocity (cm/s) | |||
| Baseline | (n = 293) 4.22 ± 0.70 | (n = 305) 4.19 ± 0.69 | 0.5308 |
| Discharge | (n = 253) 2.35 ± 0.52 | (n = 290) 2.22 ± 0.46 | 0.0029 |
| 30 Day | (n = 228) 2.21 ± 0.47 | (n = 274) 2.16 ± 0.43 | 0.1599 |
| 6 Month | (n = 166) 2.20 ± 0.47 | (n = 227) 2.16 ± 0.41 | 0.3423 |
| 1 Year | (n = 155) 2.24 ± 0.48 | (n = 216) 2.14 ± 0.41 | 0.0471 |
| 2 Year | (n = 112) 2.20 ± 0.52 | (n = 144) 2.13 ± 0.44 | 0.2451 |
| P-value for continuous change from baseline | <.0001 | <.0001 | |
| P-value for change from first post-implant to 2 years | <.0001 | <.0001 | |
| AV Peak Gradient (mmHg) | |||
| Baseline | (n = 295) 73.3 ± 24.2 | (n = 307) 71.7 ± 23.5 | 0.4349 |
| Discharge | (n = 255) 23.3 ± 10.2 | (n = 291) 20.8 ± 8.4 | 0.0014 |
| 30 Day | (n = 228) 20.6 ± 8.9 | (n = 275) 19.3 ± 7.8 | 0.0815 |
| 6 Month | (n = 168) 20.4 ± 8.7 | (n = 233) 19.4 ± 7.8 | 0.2506 |
| 1 Year | (n = 155) 21.2 ± 9.5 | (n = 218) 19.1 ± 7.4 | 0.0172 |
| 2 Year | (n = 112) 20.5 ± 9.8 | (n = 144) 19.0 ± 8.2 | 0.1788 |
| P-value for change from baseline to first post-implant | <.0001 | <.0001 | |
| P-value for change from first post-implant to 2 years | <.0001 | <.0001 | |
| AV Mean Gradient (mmHg) | |||
| Baseline | (n = 295) 43.4 ± 14.3 | (n = 307) 43.1 ± 14.5 | 0.7929 |
| Discharge | (n = 255) 11.9 ± 5.3 | (n = 291) 10.8 ± 4.5 | 0.0066 |
| 30 Day | (n = 228) 10.8 ± 5.0 | (n = 275) 9.8 ± 4.1 | 0.0139 |
| 6 Month | (n = 168) 10.8 ± 4.8 | (n = 233) 10.1 ± 4.1 | 0.0784 |
| 1 Year | (n = 155) 11.4 ± 5.3 | (n = 218) 10.1 ± 3.9 | 0.0051 |
| 2 Year | (n = 112) 11.1 ± 5.2 | (n = 144) 10.2 ± 4.7 | 0.1611 |
| P-value for change from baseline to first post-implant | <.0001 | <.0001 | |
| P-value for change from first post-implant to 2 years | 0.0003 | 0.0009 | |
| AV Area (EOA) (cm2) | |||
| Baseline | (n = 290) 0.64 ± 0.19 | (n = 301) 0.66 ± 0.20 | 0.3212 |
| Discharge | (n = 238) 1.47 ± 0.46 | (n = 279) 1.62 ± 0.50 | 0.0003 |
| 30 Day | (n = 224) 1.52 ± 0.43 | (n = 269) 1.66 ± 0.48 | 0.0010 |
| 6 Month | (n = 162) 1.50 ± 0.48 | (n = 223) 1.67 ± 0.50 | 0.0015 |
| 1 Year | (n = 151) 1.44 ± 0.47 | (n = 210) 1.59 ± 0.47 | 0.0026 |
| 2 Year | (n = 110) 1.50 ± 0.46 | (n = 139) 1.57 ± 0.42 | 0.1607 |
| P-value for change from baseline to first post-implant | <.0001 | <.0001 | |
| P-value for change from first post-implant to 2 years | 0.9434 | 0.8212 | |
| AV Area index (iEOA) (cm2/m2) | |||
| Baseline | (n = 288) 0.35 ± 0.10 | (n = 300) 0.36 ± 0.11 | 0.3760 |
| Discharge | (n = 210) 0.81 ± 0.26 | (n = 244) 0.90 ± 0.29 | 0.0006 |
| 30 Day | (n = 201) 0.84 ± 0.24 | (n = 259) 0.92 ± 0.27 | 0.0009 |
| 6 Month | (n = 156) 0.82 ± 0.25 | (n = 217) 0.92 ± 0.28 | 0.0008 |
| 1 Year | (n = 142) 0.80 ± 0.25 | (n = 203) 0.89 ± 0.27 | 0.0022 |
| 2 Year | (n = 102) 0.81 ± 0.24 | (n = 134) 0.87 ± 0.24 | 0.0379 |
| P-value for change from baseline to first post-implant value | <.0001 | <.0001 | |
| P-value for change from first post-implant to 2 years | 0.7019 | 0.6669 | |
| Doppler Velocity Index | |||
| Baseline | (n = 292) 0.20 ± 0.05 | (n = 304) 0.20 ± 0.06 | 0.5383 |
| Discharge | (n = 247) 0.49 ± 0.12 | (n = 282) 0.51 ± 0.15 | 0.0910 |
| 30 Day | (n = 225) 0.51 ± 0.12 | (n = 270) 0.51 ± 0.12 | 0.5854 |
| 6 Month | (n = 164) 0.49 ± 0.14 | (n = 226) 0.51 ± 0.12 | 0.0947 |
| 1 Year | (n = 153) 0.48 ± 0.12 | (n = 214) 0.51 ± 0.13 | 0.0544 |
| 2 Year | (n = 111) 0.48 ± 0.12 | (n = 141) 0.49 ± 0.11 | 0.3003 |
| P-value for continuous change from baseline | <.0001 | <.0001 | |
| P-value for change from first post-implant to 2 years | 0.5609 | 0.5756 | |
| Total Aortic Regurgitation* | |||
| Baseline | (n = 295) 1.59 ± 0.95 | (n = 312) 1.56 ± 0.81 | 0.7188 |
| Discharge | (n = 258) 0.58 ± 0.71 | (n = 309) 1.59 ± 0.87 | <.0001 |
| 30 Day | (n = 228) 0.68 ± 0.77 | (n = 279) 1.70 ± 0.87 | <.0001 |
| 6 Month | (n = 173) 0.65 ± 0.73 | (n = 231) 1.64 ± 0.88 | <.0001 |
| 1 Year | (n = 156) 0.71 ± 0.76 | (n = 217) 1.55 ± 0.85 | <.0001 |
| 2 Year | (n = 113) 0.67 ± 0.73 | (n = 145) 1.54 ± 0.87 | <.0001 |
| P-value for change from baseline to first post-implant | <.0001 | 0.6489 | |
| P-value for change from first post-implant to 2 years | 0.0055 | 0.8061 | |
| Mitral Regurgitation* | |||
| Baseline | (n = 292) 1.94 ± 0.78 | (n = 311) 1.91 ± 0.82 | 0.6611 |
| Discharge | (n = 253) 1.65 ± 0.84 | (n = 299) 1.65 ± 0.80 | 0.9931 |
| 30 Day | (n = 226) 1.72 ± 0.82 | (n = 276) 1.77 ± 0.82 | 0.4558 |
| 6 Month | (n = 170) 1.76 ± 0.77 | (n = 229) 1.80 ± 0.89 | 0.5991 |
| 1 Year | (n = 155) 1.72 ± 0.76 | (n = 216) 1.88 ± 0.82 | 0.0464 |
| 2 Year | (n = 114) 1.67 ± 0.81 | (n = 145) 1.79 ± 0.78 | 0.2297 |
| P-value for change from baseline to first post-implant | <.0001 | <.0001 | |
| P-value for change from first post-implant to 2 years | 0.0304 | <.0001 | |
Aortic and Mitral regurgitation expressed as categorical variables with the following values assigned to visual grades: 0 = none, 1 = trace, 2 = mild, 3 = moderate, 4 = severe.
2DE = two-dimensional echo, AT = as-treated, AV = aortic valve, DVI =Doppler velocity index, EOA = effective orifice area, iEOA = indexed effective orifice area, LV= left ventricle, LVDV = left ventricular diastolic volume, LVED = left ventricular end diastolic dimension, LVES= left ventricular end systolic dimension, LVOT =left ventricular outflow tract, LVSV = left ventricular systolic volume, MR =mitral regurgitation, RWTm= mean relative wall thickness, RWTp =posterior wall relative thickness, TAVR = transcatheter aortic valve replacement, VTI =velocity time integral
Figure 2.
Incidence of prosthesis-patient mismatch. The incidence of prosthesis-patient mismatch (%) in the TAVR and SAVR patient populations is shown. The definition for prosthesis-patient mismatch is as follows: Insignificant is an indexed EOA > 0.85 cm2/m2; Moderate is an indexed EOA of ≥ 0.65 cm2/m2 and ≤ 0.85 cm2/m2; Severe is an indexed EOA of < 0.65 cm2/m2
Figure 3.
Mitral regurgitation prevalence. The comparison of mitral regurgitation severity in the TAVR versus SAVR patient populations is shown at various follow-up time points. The only significant between-group difference was seen at the 1-year time point with no significant difference at any other time point.
TAVR patients had significantly more total aortic regurgitation at every post-implant time point compared to the SAVR cohort (Table 5 and 7). Mild, moderate or severe paravalvular aortic regurgitation (Table 8 online supplement, and Figure 4) was more common in the TAVR group at every follow-up time point (p <0.0001).
Table 7.
Total Aortic Regurgitation: SAVR vs. TAVR (Valve Implant Population)
| Treatment | Total (n) | None (%) | Trace (%) | Mild (%) | Moderate (%) | Severe (%) | P-value |
|---|---|---|---|---|---|---|---|
| Baseline | 0.7188 | ||||||
| SAVR | 295 | 14.6% | 28.1% | 42.7% | 12.9% | 1.7% | |
| TAVR | 312 | 10.9% | 30.1% | 51.9% | 5.8% | 1.3% | |
| 6 Month | <.0001 | ||||||
| SAVR | 173 | 49.7% | 36.4% | 13.3% | 0.6% | 0.0% | |
| TAVR | 231 | 11.7% | 27.3% | 47.2% | 13.0% | 0.9% | |
| 1 Year | <.0001 | ||||||
| SAVR | 156 | 45.5% | 41.0% | 10.9% | 2.6% | 0.0% | |
| TAVR | 217 | 12.0% | 31.3% | 47.5% | 7.8% | 1.4% | |
| 2 Year | <.0001 | ||||||
| SAVR | 113 | 46.9% | 39.8% | 12.4% | 0.9% | 0.0% | |
| TAVR | 145 | 13.8% | 29.7% | 45.5% | 11.0% | 0.0% | |
SAVR = surgical aortic valve replacement, TAVR = transcatheter aortic valve replacement
Figure 4.
Paravalvular aortic regurgitation incidence. The incidence of paravalvular aortic regurgitation in the TAVR versus SAVR patient populations is shown at various follow-up time points with p-values for between group differences noted. There is a significantly more aortic regurgitation in the TAVR group at every time point.
There were no cases of structural valve failure or migration of the valve after initially successful implantation in either group.
Within Group Changes over Time
Tables 4 and 5 show within-group changes over the 2 year follow-up period. Both groups showed a continuous reduction in RWT and left ventricular mass or left ventricular mass index over time. After an initial fall in Doppler stroke volume in the SAVR group, both groups showed significant increases in stroke volume over time. The TAVR group showed an immediate increase in ejection fraction following intervention with no change up to 2 years. The SAVR group showed no immediate increase in ejection fraction but a significant increase at 2years. Both groups showed immediate reduction in peak and mean transaortic gradients with slight further reductions over time. Both groups showed a sustained, stable increase in EOA and indexed EOA.
Aortic Regurgitation
Following intervention, there was no significant change in total aortic regurgitation in the TAVR group, and an immediate reduction in the SAVR group followed by a slight increase in aortic regurgitation over time (Table 5). Mitral regurgitation severity was reduced in both groups following intervention but showed a slight increase from immediate post-intervention levels by 2 years.
In the TAVR group, paravalvular aortic regurgitation remained unchanged in 45.4% of patients, improved in 31.9% and worsened in 22.7% of patients. There were 7 patients who worsened from none/trace/mild to moderate following intervention. Conversely, there were 8 patients who improved from moderate to none/trace/mild. No patient developed new severe aortic regurgitation. There was no significant baseline patient clinical differences between patients with none/trace or ≥ mild paravalvular aortic regurgitation (Table 9) however, there were significant differences in echocardiographic measures of ventricular size and function. Patients with ≥ mild paravalvular aortic regurgitation had larger baseline measurements of aortic annular dimensions (p = 0.040), LVED (p =0.008), LVDV (p = 0.019), left ventricular mass (p = 0.003) and left ventricular mass index (p = 0.007). Baseline ejection fraction was also lower in the ≥ mild paravalvular aortic regurgitation group. A greater number of patients with ≥ mild paravalvular aortic regurgitation had low stroke volume (≤ 35 ml/m2) at baseline. There were no significant differences in baseline gradients or valve area. There was no impact of the prosthesis size on severity of total aortic regurgitation (none, trace, mild, moderate or severe) (p = 0.38, not shown) or paravalvular aortic regurgitation following TAVR (Table 9).
Table 9.
TAVR subgroup analysis for baseline characteristics of patients with paravalvular regurgitation.
| Echocardiographic Parameter | None/Trace | Mild/Mod/Severe | P-value |
|---|---|---|---|
| Age at Screening (years) | (n = 158) 83.4 ± 6.7 | (n = 160) 83.7 ± 7.2 | 0.7519 |
| Gender | 0.0696 | ||
| Female | 76 (55.9%) | 60 (44.1%) | . |
| Male | 82 (45.1%) | 100 (54.9%) | . |
| Body Surface Area (m2) | (n = 158) 1.8 ± 0.3 | (n = 159) 1.8 ± 0.2 | 0.1133 |
| STS Risk Score | (n = 158) 11.6 ± 3.0 | (n = 160) 12.0 ± 3.6 | 0.2508 |
| Native Annular Diameter (mm) | (n = 158) 21.3 ± 1.8 | (n = 160) 21.7 ± 1.7 | 0.0404 |
| Baseline Echocardiographic Variables | |||
| LVED (cm) | (n = 135) 4.4 ± 0.8 | (n = 137) 4.6 ± 0.7 | 0.0084 |
| LVES (cm) | (n = 129) 3.2 ± 1.0 | (n = 132) 3.4 ± 0.9 | 0.0849 |
| LVDV (mL) | (n = 86) 114.9 ± 46.0 | (n = 85) 132.2 ± 49.7 | 0.0190 |
| LVDV Index (mL/m2) | (n = 86) 64.5 ± 23.7 | (n = 84) 71.9 ± 23.9 | 0.0437 |
| LVSV (mL) | (n = 86) 57.3 ± 37.1 | (n = 85) 68.4 ± 39.3 | 0.0598 |
| LVSV Index ((mL/m2) | (n = 86) 32.2 ± 20.3 | (n = 84) 37.0 ± 20.3 | 0.1199 |
| LV Mass (gm) | (n = 135) 268.9 ± 85.1 | (n = 136) 299.0 ± 81.5 | 0.0032 |
| LV Mass Index (gm/m2) | (n = 135) 149.2 ± 40.3 | (n = 135) 162.3 ± 39.3 | 0.0074 |
| Ejection fraction (%) | (n = 152) 54.4 ± 12.8 | (n = 155) 51.1 ± 13.9 | 0.0348 |
| AV Peak Gradient (mmHg) | (n = 152) 70.4 ± 21.2 | (n = 149) 73.9 ± 25.4 | 0.1939 |
| AV Mean Gradient (mmHg) | (n = 152) 42.4 ± 13.2 | (n = 149) 44.3 ± 15.6 | 0.2373 |
| AV Area (cm2) | (n = 149) 0.67 ± 0.20 | (n = 147) 0.65 ± 0.21 | 0.2984 |
| AV Area Index (cm/m2) | (n = 149) 0.4 ± 0.1 | (n = 146) 0.4 ± 0.1 | 0.0651 |
| DVI at baseline | (n = 149) 0.2 ± 0.1 | (n = 149) 0.2 ± 0.1 | 0.0009 |
| Stroke Volume 2DE (mL) | (n = 86) 57.7 ± 21.1 | (n = 85) 63.9 ± 23.9 | 0.0752 |
| Doppler Stroke Volume (mL) | (n = 149) 64.6 ± 19.9 | (n = 149) 62.7 ± 19.3 | 0.4166 |
| Doppler Stroke Volume Index (mL/m2) | (n = 149) 36.2 ± 11.3 | (n = 148) 34.3 ± 10.6 | 0.1467 |
| Low Doppler Stroke Volume at baseline* | 0.0106 | ||
| No | 83 (58.0%) | 60 (42.0%) | |
| Yes | 66 (42.9%) | 88 (57.1%) | |
| Total AR ** | (n = 152) 1.5 ± 0.8 | (n = 154) 1.6 ± 0.8 | 0.3637 |
| Mitral Regurgitation ** | (n = 152) 1.8 ± 0.9 | (n = 153) 2.0 ± 0.8 | 0.0791 |
| Post-Implant Echocardiographic Variables | |||
| First post implant LVED (cm) | (n = 149) 4.4 ± 0.8 | (n = 154) 4.7 ± 0.7 | 0.0091 |
| First post implant LVES (cm) | (n = 148) 3.1 ± 1.0 | (n = 153) 3.3 ± 0.8 | 0.0992 |
| First post implant LVDV (mL) | (n = 104) 110.0 ± 43.1 | (n = 114) 128.1 ± 45.4 | 0.0029 |
| First post implant LVDV Index (mL/m2) | (n = 101) 61.8 ± 22.3 | (n = 109) 70.2 ± 22.8 | 0.0076 |
| First post implant LVSV (mL) | (n = 104) 50.6 ± 30.6 | (n = 114) 60.2 ± 33.9 | 0.0300 |
| First post implant LVSV Index ((mL/m2) | (n = 101) 28.3 ± 16.9 | (n = 109) 33.2 ± 17.6 | 0.0443 |
| First post implant LV Mass (gm) | (n = 149) 259.3 ± 80.2 | (n = 153) 289.0 ± 77.7 | 0.0012 |
| First post implant LV Mass Index (gm/m2) | (n = 145) 144.4 ± 35.0 | (n = 146) 155.9 ± 37.7 | 0.0073 |
| First post implant Ejection Fraction (%) | (n = 157) 55.4 ± 10.1 | (n = 160) 55.6 ± 11.6 | 0.8716 |
| First post implant AV Peak Gradient (mmHg) | (n = 157) 20.4 ± 8.6 | (n = 157) 20.5 ± 7.7 | 0.9632 |
| First post implant AV Mean Gradient (mmHg) | (n = 157) 10.6 ± 4.5 | (n = 157) 10.6 ± 4.1 | 0.8799 |
| First post implant EOA (cm2) | (n = 155) 1.6 ± 0.5 | (n = 156) 1.7 ± 0.5 | 0.2895 |
| First post implant EOA Index (cm/m2) | (n = 151) 0.9 ± 0.3 | (n = 150) 0.9 ± 0.3 | 0.7496 |
| First post implant DVI | (n = 156) 0.5 ± 0.2 | (n = 156) 0.5 ± 0.1 | 0.8859 |
| First Stroke Volume 2DE (mL) | (n = 104) 59.4 ± 18.9 | (n = 114) 67.9 ± 20.4 | 0.0017 |
| First Doppler Stroke Volume (mL) | (n = 155) 62.2 ± 19.3 | (n = 156) 68.0 ± 20.2 | 0.0110 |
| First post implant Doppler Stroke Volume Index (mL/m2) | (n = 151) 35.5 ± 11.8 | (n = 150) 37.3 ± 11.3 | 0.1918 |
| First post implant Low Stroke Volume* | 0.4203 | ||
| No | 74 (47.7%) | 81 (52.3%) | |
| Yes | 77 (52.7%) | 69 (47.3%) | |
| First post implant PP Mismatch | 0.6602 | ||
| Insignificant (Area Index > 0.85 cm/m2) | 84 (51.5%) | 79 (48.5%) | |
| Moderate (0.65 ≤ Area Index ≤ 0.85 cm/m2) | 38 (48.1%) | 41 (51.9%) | |
| Severe (Area Index < 0.65 cm/m2) | 29 (49.2%) | 30 (50.8%) | |
| Implanted Valve size | 1.0000 | ||
| Size 23 mm | 75 (49.7%) | 76 (50.3%) | |
| Size 26 mm | 83 (49.7%) | 84 (50.3%) | |
| Cover Index | 0.0123 | ||
| < 8% | 28 (36.8%) | 48 (63.2%) | |
| ≥ 8% | 130 (53.7%) | 112 (46.3%) | |
Low stroke volume defined as SV index ≤ 35 ml/m2
Aortic and Mitral regurgitation expressed as categorical variables with the following values assigned to visual grades: 0 = none, 1 = trace, 2 = mild, 3 = moderate, 4 = severe.
2DE = two-dimensional echo, AT = as-treated, AV = aortic valve, DVI =Doppler velocity index, EOA = effective orifice area, iEOA = indexed effective orifice area, LV= left ventricle, LVDV = left ventricular diastolic volume, LVED = left ventricular end diastolic dimension, LVES= left ventricular end systolic dimension, LVOT =left ventricular outflow tract, LVSV = left ventricular systolic volume, MR =mitral regurgitation, RWTm= mean relative wall thickness, RWTp =posterior wall relative thickness, TAVR = transcatheter aortic valve replacement, VTI =velocity time integral
Following TAVR, patients with ≥ mild paravalvular aortic regurgitation had larger first post-implant LVED (p =0.009), LVDV (p = 0.003), LV mass (p = 0.001) and LV mass index (p = 0.007). There was no difference in first post-implant ejection fraction, transaortic gradients, EOA, prosthesis-patient mismatch or implanted valve size. There was a higher frequency of low cover index (< 8%) in patients with ≥ mild paravalvular aortic regurgitation (p = 0.012). There was a significantly higher 2DE and Doppler stroke volume in the ≥ mild paravalvular aortic regurgitation group.
Echocardiographic Variables Associated with Outcome
Univariate echocardiographic determinants of mortality in the TAVR group were evaluated (Table 10). The only baseline echocardiographic univariate predictor of death in the TAVR group was baseline peak gradient (HR [95%CI] = 0.94 [0.90, 0.99] per 5 mmHg increase, p = 0.010). After implantation, there were a number of echocardiographic univariate predictors of death in the TAVR group, the strongest of which was ≥ mild paravalvular aortic regurgitation (HR [95%CI] = 2.11 [1.43, 3.10], p = 0.0002). Other post-TAVR predictors of mortality include: larger LVDV, LVSV and indexed EOA, and lower ejection fraction. The presence of prosthesis-patient mismatch (indexed EOA<0.85 cm2/m2) was a predictor of decreased mortality (HR [95%CI] = 0.736 [0.57, 0.96], p = 0.024).
Table 10.
Univariate Proportional Hazards regression analysis of predictors of Death in the TAVR patients
| Predictor Variable | Number of non-missing observations |
Scale Factor (continuous variables) |
Log hazard ratio |
P-value | Hazard ratio |
Lower confidence limit for hazard ratio |
Upper confidence limit for hazard ratio |
|
|---|---|---|---|---|---|---|---|---|
| Baseline Variables | ||||||||
| Body Mass Index | 325 | 1 lbs/in2 | −0.0605 | 0.0005 | 0.941 | 0.910 | 0.974 | |
| LVED | 277 | 1 cm | 0.0323 | 0.8015 | 1.033 | 0.803 | 1.329 | |
| LVES | 266 | 1 cm | 0.1586 | 0.1525 | 1.172 | 0.943 | 1.456 | |
| LVDV | 176 | 5 ml | 0.0180 | 0.1503 | 1.018 | 0.994 | 1.043 | |
| LVDV index | 276 | 5 ml/m2 | 0.0378 | 0.4402 | 1.039 | 0.988 | 1.091 | |
| LVSV | 176 | 5 ml | 0.0248 | 0.1021 | 1.025 | 0.995 | 1.056 | |
| LVSV index | 175 | 5 ml/m2 | 0.0440 | 0.1273 | 1.045 | 0.988 | 1.106 | |
| LV Mass | 276 | 10 gm | 0.0023 | 0.8377 | 1.002 | 0.981 | 1.0251 | |
| LV Mass Index | 276 | 10 gm/m2 | 0.0174 | 0.8377 | 1.003 | 0.971 | 1.067 | |
| EF | 313 | 5% | −0.0373 | 0.2547 | 0.963 | 0.904 | 1.027 | |
| Peak AV gradient | 307 | 5 mmHg | −0.0580 | 0.0102 | 0.944 | 0.903 | 0.986 | |
| Mean AV gradient | 300 | 5 mmHg | −0.1045 | 0.4511 | 0.901 | 0.837 | 0.969 | |
| AV Area | 301 | 0.1 cm2 | 0.0149 | 0.7544 | 1.015 | 0.924 | 1.115 | |
| AV Area Index | 301 | 0.1 cm2/m2 | 0.0669 | 0.7544 | 1.068 | 0.899 | 1.272 | |
| DVI | 304 | 0.01 | 0.0094 | 1.0094 | 1.009 | 0.977 | 1.044 | |
| Stroke Volume (2DE) | 176 | 5 ml | 0.0081 | 0.7583 | 1.0081 | 0.957 | 1.062 | |
| Doppler Stroke Volume | 303 | 5 ml | −-0.0441 | 0.0785 | 0.957 | 0.911 | 1.005 | |
| Doppler Stroke Volume Index | 302 | 5 ml/m2 | −0.0592 | 0.1906 | 0.863 | 1.0300 | 1.006 | |
| Low Stroke Volume* | 302 | 0.3596 | 0.0649 | 1.433 | 0.978 | 2.099 | ||
| Aortic Regurgitation | 312 | −0.0191 | 0.8684 | 0.981 | 0.782 | 1.230 | ||
| Mitral Regurgitation | 311 | 0.0739 | 0.5201 | 1.077 | 0.860 | 1.349 | ||
| Post-Implant Echocardiographic Variables | ||||||||
| First post implant LVED | 304 | 1 cm | 0.0882 | 0.4724 | 1.092 | 0.859 | 1.389 | |
| First post implant LVES | 302 | 1 cm | 0.1972 | 0.0682 | 1.218 | 0.985 | 1.506 | |
| First post implant LVDV | 220 | 5 ml | 0.0279 | 0.0196 | 1.028 | 1.005 | 1.053 | |
| First post implant LVDV index | 212 | 5 cc/m2 | 0.0611 | 0.0093 | 1.063 | 1.015 | 1.113 | |
| First post implant LVSV | 220 | 5 ml | 0.0463 | 0.0023 | 1.047 | 1.017 | 1.079 | |
| First post- implant LVSV index | 212 | 5 ml/m2 | 0.0906 | 0.0017 | 1.095 | 1.035 | 1.158 | |
| First post implant LV Mass | 303 | 10 gm | 0.007 | 0.5674 | 1.007 | 0.983 | 1.031 | |
| First post implant LV Mass Index | 292 | 10 gm/m2 | 0.0319 | 0.2469 | 1.032 | 0.978 | 1.090 | |
| First LV Mass Regression | 256 | 1% | −0.3055 | 0.5851 | 0.737 | 0.246 | 2.206 | |
| First post implant EF | 319 | 5% | 0.0951 | 0.0188 | 0.909 | 0.840 | 0.984 | |
| First post implant peak gradient | 316 | 5 mmHg | −0.0959 | 0.1128 | 0.909 | 0.807 | 1.023 | |
| First post implant mean gradient | 316 | 5 mmHg | −0.2219 | 0.0581 | 0.801 | 0.637 | 1.008 | |
| First post implant EOA | 313 | 0.1 cm2 | 0.4065 | 0.0291 | 1.042 | 1.004 | 1.080 | |
| First post implant EOA Index | 303 | 0.1 cm2/m2 | 0.0770 | 0.0244 | 1.080 | 1.010 | 1.155 | |
| First post implant DVI | 314 | 0.01 | 0.0077 | 0.2240 | 1.007 | 0.995 | 1.020 | |
| First post implant Stroke Volume 2DE | 220 | 5 ml | 0.0079 | 0.7919 | 1.008 | 0.995 | 1.068 | |
| First post implant Doppler Stroke Volume | 313 | 5 ml | −0.0046 | 0.8468 | 0.995 | 0.950 | 1.043 | |
| First post implant Doppler Stroke Volume index | 303 | 5 ml/m2 | 0.0134 | 0.7420 | 1.014 | 0.936 | 1.043 | |
| First post implant Low Stroke Volume* | 303 | 0.1813 | 0.3579 | 1.199 | 0.814 | 1.765 | ||
| First post implant PPM** | 303 | −0.3059 | 0.0235 | 0.736 | 0.565 | 0.960 | ||
| First Post-Implant Total AR | 321 | 0.3311 | 0.0028 | 1.392 | 1.120 | 1.730 | ||
| First Total AR Mild-Severe | 321 | 0.5573 | 0.0063 | 1.746 | 1.170 | 2.605 | ||
| First Post-Implant any PAR | 318 | 0.2762 | 0.0063 | 1.318 | 1.081 | 1.607 | ||
| First PAR Mild-Severe | 318 | 0.7461 | 0.0002 | 2.109 | 1.433 | 3.103 | ||
| First post-implant MR | 318 | 0.1267 | 0.2871 | 1.135 | 0.899 | 1.433 | ||
Low stroke volume defined as SV index ≤ 35 ml/m2
Any PPM (AVA ≤ 0.85 cm2/m2)
2DE = two-dimensional echo, AT = as-treated, AV = aortic valve, DVI =Doppler velocity index, EOA = effective orifice area, iEOA = indexed effective orifice area, LV= left ventricle, LVDV = left ventricular diastolic volume, LVED = left ventricular end diastolic dimension, LVES= left ventricular end systolic dimension, LVOT =left ventricular outflow tract, LVSV = left ventricular systolic volume, MR =mitral regurgitation, RWTm= mean relative wall thickness, RWTp =posterior wall relative thickness, TAVR = transcatheter aortic valve replacement, VTI =velocity time integral
There were many baseline univariate echocardiographic determinants of mortality in the SAVR group (Table 11), the strongest of which was mitral regurgitation (HR [95%CI] = 1.49[1.17, 1.90] per 1 grade, p = 0.001). After valve replacement, the strongest univariate echocardiographic predictors of death were the presence of low stroke volume (≤ 35 ml/m2) (HR [95%CI] = 1.97 [1.16, 3.33], p = 0012) and prosthesis-patient mismatch (HR [95%CI] = 1.43[1.11, 1.84], p = 0.005). Other post-SAVR predictors of mortality include: smaller LVDV, LVSV, EOA, and stroke volume.
Table 11.
Univariate Proportional Hazards regression analysis of predictors of Death in the SAVR patients
| Predictor Variable | Number of non-missing observations |
Scale Factor (continuous variables) |
Log hazard ratio |
P-value | Hazard ratio |
Lower confidence limit for hazard ratio |
Upper confidence limit for hazard ratio |
|
|---|---|---|---|---|---|---|---|---|
| Baseline Variables | ||||||||
| Body Mass Index (lbs/in2) | 308 | 1 lbs/in2 | −0.0232 | 0.1778 | 0.977 | 0.945 | 1.011 | |
| LVED | 267 | 1 cm | −0.0245 | 0.8400 | 0.976 | 0.769 | 1.238 | |
| LVES | 259 | 1 cm | −0.0427 | 0.6739 | 0.958 | 0.785 | 1.169 | |
| LVDV | 179 | 5 ml | −0.0300 | 0.0407 | 0.970 | 0.943 | 0.999 | |
| LVDV index | 177 | 5 ml/m2 | −0.0486 | 0.953 | 0.990 | 0.902 | 1.006 | |
| LVSV | 179 | 5 ml | −0.0360 | 0.0689 | 0.965 | 0.928 | 1.003 | |
| LVSV index | 177 | 5 ml/m2 | −0.0570 | 0.945 | 0.989 | 0.880 | 1.014 | |
| LV Mass | 265 | 10 gm | −0.018 | 0.1120 | 0.982 | 0.960 | 1.004 | |
| LV Mass Index | 264 | 10 gm/m2 | −0.0294 | 0.1866 | 0.971 | 0.930 | 1.014 | |
| EF | 295 | 5% | −0.0030 | 0.9332 | 0.997 | 0.929 | 1.070 | |
| Peak AV gradient | 295 | 5 mmHg | −0.0248 | 0.2210 | 0.976 | 0.938 | 1.015 | |
| Mean AV gradient | 295 | 5 mmHg | −0.0438 | 0.1950 | 0.957 | 0.896 | 1.023 | |
| AV Area | 290 | 0.1cm2 | −0.0478 | 0.3680 | 0.953 | 0.859 | 1.058 | |
| AV Area Index | 288 | 0.1 cm2/m2 | −0.025 | 0.7861 | 0.975 | 0.814 | 1.015 | |
| DVI | 292 | 0.01 | −0.0004 | 0.9835 | 1.000 | 0.965 | 1.036 | |
| Stroke Volume (2DE) | 179 | 5 ml | −0.0503 | 0.1260 | 0.951 | 0.892 | 1.014 | |
| Doppler Stroke Volume | 290 | 5 ml | −0.0681 | 0.0116 | 0.934 | 0.886 | 0.985 | |
| Doppler Stroke Volume Index | 288 | 5 ml/m2 | −0.0844 | 0.0644 | 0.919 | 0.840 | 1.005 | |
| Low Stroke Volume* | 288 | 0.1697 | 0.3819 | 1.185 | 0.810 | 1.734 | ||
| Aortic Regurgitation | 295 | −0.0896 | 0.3790 | 0.914 | 0.749 | 1.116 | ||
| Mitral Regurgitation | 292 | 0.3997 | 0.0011 | 1.491 | 1.173 | 1.897 | ||
| Post-Implant Echocardiographic Variables | ||||||||
| First post implant LVED | 276 | 1 cm | −0.1765 | 0.1766 | 0.838 | 0.649 | 1.083 | |
| First post implant LVES | 267 | 1 cm | −0.0769 | 0.5181 | 0.926 | 0.733 | 1.169 | |
| First post implant LVDV Volume | 188 | 5 cc | −0.0536 | 0.0094 | 0.948 | 0.910 | 0.987 | |
| First post implant LVDV index | 175 | 5 cc/m2 | −0.1308 | 0.0048 | 0.877 | 0.801 | 0.961 | |
| First post implant LVSV | 188 | 5 cc | −0.988 | 0.0492 | 0.906 | 0.832 | 0.986 | |
| First post- implant LVSV index | 175 | 5 cc/m2 | −0.1332 | 0.0760 | 0.875 | 0.818 | 0.936 | |
| First post implant LV Mass | 275 | 10 gm | −0.0268 | 0.0454 | 0.974 | 0.948 | 0.999 | |
| First post implant LV Mass Index | 260 | 10 gm/m2 | −0.0429 | 0.1134 | 0.958 | 0.909 | 1.010 | |
| First LV Mass Regression | 230 | 1% | 0.1362 | 0.7621 | 1.146 | 0.474 | 2.768 | |
| First post implant EF | 287 | 5% | 0.0288 | 0.4970 | 1.029 | 0.947 | 1.118 | |
| First post implant peak gradient | 286 | 5 mmHg | 0.0155 | 0.7537 | 1.016 | 0.922 | 1.119 | |
| First post implant mean gradient | 286 | 5 mmHg | 0.0090 | 0.9257 | 1.009 | 0.835 | 1.219 | |
| First post implant EOA | 283 | 0.1 cm2 | −0.0743 | 0.0033 | 0.928 | 0.884 | 0.976 | |
| First post implant EOA Index | 268 | 0.1 cm2/m2 | −0.1328 | 0.0036 | 0.876 | 0.801 | 0.958 | |
| First post implant DVI | 285 | 0.01 | −0.0065 | 0.4629 | 0.994 | 0.976 | 1.011 | |
| First post implant Stroke Volume (2DE) | 188 | 5 ml | −0.0988 | 0.0223 | 0.906 | 0.832 | 0.986 | |
| First post implant Doppler Stroke Volume | 283 | 5 ml | −0.1332 | 0.0001 | 0.875 | 0.818 | 0.936 | |
| First post implant Doppler Stroke Volume Index | 268 | 5 ml/m2 | −0.2369 | 0.0001 | 0.789 | 0.700 | 0.900 | |
| First post implant Low Stroke Volume* | 268 | 0.6769 | 0.0118 | 1.968 | 1.162 | 3.333 | ||
| First post implant PPM ** | 268 | 0.3580 | 0.0051 | 1.430 | 1.114 | 1.837 | ||
| First Post-Implant Total AR | 289 | −0.0098 | 0.9435 | 0.990 | 0.756 | 1.298 | ||
| First Total AR Mild-Severe | 289 | 0.0565 | 0.8492 | 1.058 | 0.591 | 1.894 | ||
| First Post-Implant PAR | 285 | 0.0357 | 0.8484 | 1.036 | 0.719 | 1.494 | ||
| First PAR Mild-Severe | 285 | −0.1323 | 0.7956 | 0.876 | 0.322 | 2.384 | ||
| First post-implant MR | 288 | −0.0932 | 0.4145 | 0.911 | 0.728 | 1.140 | ||
Low stroke volume defined as SV index ≤ 35 ml/m2
Any PPM (AVA ≤ 0.85 cm2/m2)
2DE = two-dimensional echo, AT = as-treated, AV = aortic valve, DVI =Doppler velocity index, EOA = effective orifice area, iEOA = indexed effective orifice area, LV= left ventricle, LVDV = left ventricular diastolic volume, LVED = left ventricular end diastolic dimension, LVES= left ventricular end systolic dimension, LVOT =left ventricular outflow tract, LVSV = left ventricular systolic volume, MR =mitral regurgitation, RWTm= mean relative wall thickness, RWTp =posterior wall relative thickness, TAVR = transcatheter aortic valve replacement, VTI =velocity time integral
Discussion
Cohort A of the PARTNER trial is the first large randomized trial showing comparable outcomes of SAVR and TAVR in high risk, operable patients with severe symptomatic aortic stenosis (4). The present study reports the centrally analyzed echocardiographic data comparing SAVR and TAVR, which documents early and sustained hemodynamic improvements with both therapies, and freedom from structural valve deterioration, while highlighting the differences in therapeutic groups, and presenting echocardiographic determinants of outcome.
Valve Performance
Improvements in valve area and mean gradients are sustained over the two year interval reported with no evidence of stent recoil in the TAVR group. No structural valve dysfunction was found out to 2 years in either arm of this study. The current study thus highlights the twoyear durability of the Edwards SAPIEN balloon-expandable valve.
Ventricular remodeling and function
Following valve replacement, there is substantial ventricular remodeling with reduction in RWT and left ventricular mass in both groups. The SAVR group had more absolute LV mass regression early however there was no difference in mass regression rates over the follow-up period between SAVR and TAVR. Factors other than valve area may adversely influence left ventricular remodeling including: more aortic regurgitation (in the TAVR population), or concomitant surgical procedures (MV repair/replacement or coronary bypass performed as protocol violations in the SAVR population).
Ejection fraction improves early following TAVR and later during follow-up in the SAVR group with no significant difference by 2 years. Because LVED, LVES and mitral regurgitation are not significantly different between groups, the larger stroke volumes in the TAVR group support the qualitative finding of greater aortic regurgitation. Other factors which could influence LV remodeling (i.e.: hypertension, renal dysfunction, sex) and ventricular function warrant further study.
Factors Associated with Outcome
The TAVR and SAVR groups had different univariate factors associated with outcome. In the TAVR group only baseline low peak gradients, possibly in the setting of low stroke volume, predicted worse outcome. Post-TAVR, larger LV volumes (either systolic or diastolic) and lower EF determined worse outcome; how this may be related to aortic regurgitation is unknown. In the SAVR group, baseline or post-SAVR small LVEDV and low stroke volume, are associated with increased mortality. The strongest predictor of mortality was baseline severity of mitral regurgitation. Some patients (6) had concomitant mitral surgery (a protocol violation) and how this may influence outcomes require further investigation.
Similar to previous studies (25), our study confirms that prosthesis-patient mismatch has a negative effect on survival in the SAVR population. Also similar to prior studies (15), indexed EOA in the TAVR groups is larger compared to the SAVR group despite comparable annular dimensions. Counter-intuitively, univariate analysis of the TAVR group had a lower mortality in the presence of prosthesis-patient mismatch. This finding may be driven by body size, since in the TAVR cohort; body mass index was also associated with lower mortality (Table 10). Conversely, this finding may also be driven by a population of patients with a small annulus; patients with a small aortic annulus have better outcomes with TAVR compared to SAVR (16, 17). In addition, a small annulus in the TAVR population has been associated with less paravalvular aortic regurgitation (13). Further multivariable modeling should be performed.
Aortic Regurgitation
Recent surgical studies suggest paravalvular regurgitation following SAVR occurs in 10–48% (18–23) and even mild paravalvular regurgitation has a negative impact on survival (24). The current study confirms a high incidence of aortic regurgitation in the SAVR group (50%) however only 14% have ≥ mild paravalvular aortic regurgitation.
Our study supports other studies (25–28) showing a significant relationship between mortality and paravalvular aortic regurgitation in TAVR patients. Despite the inherent limitations of the current grading method for paravalvular regurgitation, it has been shown to predict outcomes (5) which provides indirect validation. Further validation of this grading scheme against other quantitative assessments of severity of regurgitation should be performed. Finally, it is possible that differences in the baseline characteristics between groups with paravalvular aortic regurgitation may have contributed to the increased mortality.
Limitations
We used the site-specified systolic annular measurement since: 1) most were from intraprocedural transesophageal echocardiograms and likely more accurate than transthoracic measurements and 2) the measurement was performed in systole (whereas as per previously published guidelines (12) the ECL performed aorta measurements in diastole). In the PARTNER trial, a systolic, sagittal plane systolic annulus was used for sizing the transcatheter heart valve. Whenever possible, we evaluated paired echocardiographic data; however, longitudinal differences between groups with high mortality rates may introduce survivorship bias. Finally, echocardiographic measurements are dependent on image quality as well as inter- and intraobserver variability. Use of an echocardiographic Core Laboratory should limit this variability as has been demonstrated previously for the PARTNER trial (9) however limited echocardiographic measurability may introduce another form of bias. In addition, a large number of variable comparisons have been made in the manuscript, and no adjustment has been made for the multiple comparisons.
For the “first post-implant” measurement discharge or 30 day values were used in 90–96% of ventricular size and function measurements, and 99% of Doppler measurements; 6 month data was used rarely.
The limitations of paravalvular aortic regurgitation grading have been discussed but remain a significant issue for both surgical and transcatheter valves. It is important to note that the circumferential extent grading scheme used differs slightly from the upper cut point of 20% for moderate paravalvular regurgitation recommended by the 2009 ASE/EAE guidelines (7) which were published after the PARTNER analysis plan was established.
Advanced echo techniques, such as three-dimensional color Doppler echocardiography or quantitative methods, as well as intra-procedural studies were not evaluated.
Conclusions
This study is the first to use centrally analyzed echocardiographic data to compare the structural and hemodynamic results in high-risk patients with symptomatic severe aortic stenosis randomized to SAVR or TAVR. Immediately following valve replacement, both groups showed a significant reduction in transaortic gradients and increase in EOA with similar LV mass regression and remodeling over time. However, indexed EOA was consistently higher and prosthesis-patient mismatch lower in the TAVR group throughout the follow-up period. The intermediate-term durability of the Edwards-SAPIEN valve is comparable to SAVR. Survival was strongly affected by low stroke volume and prosthesis-patient mismatch in the SAVR cohort and post-intervention aortic regurgitation in the TAVR cohort. A complete understanding of these differences may allow future refinement in patient selection.
Supplementary Material
Acknowledgments
Howard C. Herrmann has received consulting fees from St. Jude Medical and Paieon and holds equity in Microinterventional Devices. Susheel K. Kodali has received consulting fees from Edwards Lifesciences and Medtronic, and is a member of the Scientific Advisory Board of Thubrikar Aortic Valve, Inc., the Medical Advisory Board of Paieon Medical, and the TAVI Advisory Board of St. Jude Medical. Lars Svensson has received travel reimbursement from Edwards Lifesciences for activities related to his participation on the Executive Committee of the PARTNER Trial. Vinod Thourani has received consulting fees from Edwards LifeSciences, Sorin Medical, St. Jude Medical, and DirectFlow. Jodi J. Akin is a salaried employee of Edwards Lifesciences. William N. Anderson is a consultant for Edwards Lifesciences. Martin B. Leon is a nonpaid member of the Scientific Advisory Board of Edwards Lifesciences and has received travel reimbursement from Edwards for activities related to his participation on the Executive Committee of the PARTNER Trial. Pamela S. Douglas has received institutional research support from Edwards Lifesciences.
Abbreviations
- 2DE
two-dimensional echocardiography
- EOA
effective orifice area
- LVDV
left ventricular diastolic volume
- LVED
left ventricular end-diastolic dimensions
- LVES
left ventricular end-systolic dimensions
- LVSV
left ventricular systolic volume
- RWTm
relative wall thickness the septal and posterior wall thickness
- RWTp
relative wall thickness using formula twice the posterior wall thickness
- SAVR
surgical aortic valve replacement
- TAVR
transcatheter aortic valve replacement
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The other authors report no financial conflicts of interest.
Clinical trial info: PARTNER; http://clinicaltrials.gov/show/NCT00530894; NCT00530894
References
- 1.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 2.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 3.Makkar RR, Fontana GP, Jilaihawi H, et al. Transcatheter Aortic-Valve Replacement for Inoperable Severe Aortic Stenosis. N Engl J Med. 2012 doi: 10.1056/NEJMoa1202277. [DOI] [PubMed] [Google Scholar]
- 4.Kodali SK, Williams MR, Smith CR, et al. Two-Year Outcomes after Transcatheter or Surgical Aortic-Valve Replacement. N Engl J Med. 2012 doi: 10.1056/NEJMoa1200384. [DOI] [PubMed] [Google Scholar]
- 5.Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. The New England journal of medicine. 2012;366:1686–1695. doi: 10.1056/NEJMoa1200384. [DOI] [PubMed] [Google Scholar]
- 6.Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84–e231. doi: 10.1161/CIRCULATIONAHA.106.176857. [DOI] [PubMed] [Google Scholar]
- 7.Zoghbi WA, Chambers JB, Dumesnil JG, et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report From the American Society of Echocardiography's Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2009;22:975–1014. doi: 10.1016/j.echo.2009.07.013. quiz 1082-4. [DOI] [PubMed] [Google Scholar]
- 8.Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23. doi: 10.1016/j.echo.2008.11.029. quiz 101-2. [DOI] [PubMed] [Google Scholar]
- 9.Douglas PSWR, Bloomfield G, Dunn G, et al. Implementation of Echocardiography Core Laboratory Best Practices: A Case Study of the PARTNER I Trial. J Am Soc Echocardiography. 2013 doi: 10.1016/j.echo.2013.01.013. in press. [DOI] [PubMed] [Google Scholar]
- 10.Douglas PS, DeCara JM, Devereux RB, et al. Echocardiographic imaging in clinical trials: American Society of Echocardiography Standards for echocardiography core laboratories: endorsed by the American College of Cardiology Foundation. J Am Soc Echocardiogr. 2009;22:755–765. doi: 10.1016/j.echo.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 11.Douglas PSWR, Bloomfield G, Dunn G, Davis L, Hahn RT, Pibarot P, Stewart WJ, Weissman NJ, Hueter I, Siegel R, Lerakis S, Miller DC, Smith CR, Leon MB . Implementation of Echocardiography Core Laboratory Best Practices: A Case Study of the PARTNER I Trial. J Am Soc Echocardiography. 2013 doi: 10.1016/j.echo.2013.01.013. in press. [DOI] [PubMed] [Google Scholar]
- 12.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Detaint D, Lepage L, Himbert D, et al. Determinants of significant paravalvular regurgitation after transcatheter aortic valve: implantation impact of device and annulus discongruence. JACC Cardiovascular interventions. 2009;2:821–827. doi: 10.1016/j.jcin.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 15.Clavel MA, Webb JG, Pibarot P, et al. Comparison of the hemodynamic performance of percutaneous and surgical bioprostheses for the treatment of severe aortic stenosis. J Am Coll Cardiol. 2009;53:1883–1891. doi: 10.1016/j.jacc.2009.01.060. [DOI] [PubMed] [Google Scholar]
- 16.Clavel MA, Webb JG, Rodes-Cabau J, et al. Comparison between transcatheter and surgical prosthetic valve implantation in patients with severe aortic stenosis and reduced left ventricular ejection fraction. Circulation. 2010;122:1928–1936. doi: 10.1161/CIRCULATIONAHA.109.929893. [DOI] [PubMed] [Google Scholar]
- 17.Kalavrouziotis D, Rodes-Cabau J, Bagur R, et al. Transcatheter aortic valve implantation in patients with severe aortic stenosis and small aortic annulus. J Am Coll Cardiol. 2011;58:1016–1024. doi: 10.1016/j.jacc.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 18.O'Rourke DJ, Palac RT, Malenka DJ, Marrin CA, Arbuckle BE, Plehn JF. Outcome of mild periprosthetic regurgitation detected by intraoperative transesophageal echocardiography. J Am Coll Cardiol. 2001;38:163–166. doi: 10.1016/s0735-1097(01)01361-4. [DOI] [PubMed] [Google Scholar]
- 19.Rallidis LS, Moyssakis IE, Ikonomidis I, Nihoyannopoulos P. Natural history of early aortic paraprosthetic regurgitation: a five-year follow-up. Am Heart J. 1999;138:351–357. doi: 10.1016/s0002-8703(99)70124-9. [DOI] [PubMed] [Google Scholar]
- 20.Davila-Roman VG, Waggoner AD, Kennard ED, et al. Prevalence and severity of paravalvular regurgitation in the Artificial Valve Endocarditis Reduction Trial (AVERT) echocardiography study. J Am Coll Cardiol. 2004;44:1467–1472. doi: 10.1016/j.jacc.2003.12.060. [DOI] [PubMed] [Google Scholar]
- 21.Safi AM, Kwan T, Afflu E, Al Kamme A, Salciccioli L. Paravalvular regurgitation: a rare complication following valve replacement surgery. Angiology. 2000;51:479–487. doi: 10.1177/000331970005100605. [DOI] [PubMed] [Google Scholar]
- 22.Ionescu A, Fraser AG, Butchart EG. Prevalence and clinical significance of incidental paraprosthetic valvar regurgitation: a prospective study using transoesophageal echocardiography. Heart. 2003;89:1316–1321. doi: 10.1136/heart.89.11.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chambers J, Monaghan M, Jackson G. Colour flow Doppler mapping in the assessment of prosthetic valve regurgitation. Br Heart J. 1989;62:1–8. doi: 10.1136/hrt.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sponga S, Perron J, Dagenais F, et al. Impact of residual regurgitation after aortic valve replacement. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2012;42:486–492. doi: 10.1093/ejcts/ezs083. [DOI] [PubMed] [Google Scholar]
- 25.Tamburino C, Barbanti M, Capodanno D, et al. Early- and mid-term outcomes of transcatheter aortic valve implantation in patients with logistic EuroSCORE less than 20%: a comparative analysis between different risk strata. Catheter Cardiovasc Interv. 2012;79:132–140. doi: 10.1002/ccd.23100. [DOI] [PubMed] [Google Scholar]
- 26.Abdel-Wahab M, Zahn R, Horack M, et al. Aortic regurgitation after transcatheter aortic valve implantation: incidence and early outcome. Results from the German transcatheter aortic valve interventions registry. Heart. 2011;97:899–906. doi: 10.1136/hrt.2010.217158. [DOI] [PubMed] [Google Scholar]
- 27.Moat NE, Ludman P, de Belder MA, et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Am Coll Cardiol. 2011;58:2130–2138. doi: 10.1016/j.jacc.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 28.Ussia GP, Barbanti M, Petronio AS, et al. Transcatheter aortic valve implantation: 3-year outcomes of self-expanding CoreValve prosthesis. Eur Heart J. 2012 doi: 10.1093/eurheartj/ehr491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.