Abstract
Toxicology has long relied on animal models in a tedious approach to understanding risk of exposure to an uncharacterized molecule. Stem cell-derived tissues can be made in high purity, quality, and quantity to enable a new approach to this problem. Currently, stem cell-derived tissues are primarily “generic” genetic backgrounds; the future will see the integration of various genetic backgrounds and complex three-dimensional models to create truly unique in vitro organoids. This minireview focuses on the state of the art of a number of stem cell-derived tissues and details their application in toxicology.
Keywords: Cardiovascular, Drug Development, Drug Discovery, Embryonic Stem Cell, Hepatocyte, Induced Pluripotent Stem Cells, Neurons, Stem Cells, Toxicology
Introduction
Delineating the toxicological profile of a new molecular entity is an incremental investigation across in silico, in vitro, and in vivo models that culminates in an informed opinion of risk. Many of these assessments are conducted to comply with regulatory guidance documents and rely heavily on the use of animal toxicology studies. This approach is slow and costly and still results in unappreciated “surprises” when preclinical animal data uncover intractable toxicity that has an unclear correlation with the drug's effect in humans. Primary cell culture has long been the preferred cell-based system for in vitro research; however, ample and consistent supply, uncontrolled phenotypic variation in viability and function, and a progressive loss of in vivo properties when cultured among other constraints provide room for improved models of predicting human adverse responses. One potential opportunity is seen in the chemical industry in the European Union, where the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) mandates safety testing for all high production volume chemicals and soon lower production volume entities as well, requiring a new standard of product safety (1). Accordingly, cost-conscience improvements to the early safety screening would align with the 3Rs principles of refine, reduce, and replace the use of animals in research, which occurs in concert with integrating innovative technologies and approaches into all areas of safety assessment (2).
No field better highlights the opportunity to embrace the 3Rs principles while improving human safety than human pluripotent stem cell (hPSC)2-derived tissues. Advances in recent years, in particular the ability to reprogram adult cells such that they acquire an embryonic stem cell (ESC)-like phenotype, not only alter the ethical debate related to the harvest of ESCs but open brand new avenues of research on the path to true personalized medicine. One can envision a day when personalized induced pluripotent stem cells (iPSCs) turned into tissues of interest can assess drug safety and efficacy prior to exposing a patient. Although the discovery of iPSC reprogramming was a watershed moment, the true potential still resides in the ability to differentiate those cells down a lineage of interest in high quality, purity, and quantity to enable research and ultimately demonstrate clinical benefit. For a number of tissues, including cardiomyocytes, hepatocytes, endothelial cells, hematopoietic precursors, and different types of neurons, high quality cells are available currently, yet a long road is ahead to manufacture other cell types such as podocytes, keratinocytes, pancreatic beta islet cells, and tissue-specific endothelium before making true in vitro “organoids.” Although personalized cell models and therapy represent the future, current stem cell research in toxicology primarily uses “normal” genetic background cells and thus will be the focus of this minireview.
The majority of this minireview is focused on differentiated tissues; however, it is important to pay tribute to the first stem cell-based toxicology assay, designed to predict embryonic-fetal developmental toxicity using mouse ESCs. The regulatory attitude toward assessing teratogenicity changed abruptly after the thalidomide disaster, when thousands of children were born with severe defects (phocomelia, or shortening of the limbs). Fast forward 30 years, and European Centre for the Validation of Alternative Methods validated a number of in vitro teratogenicity testing models, including the rat whole embryo culture, the limb bud micromass assay, and the mouse embryonic stem cell test (mEST), and deemed these assays as suitable for consideration for regulatory acceptance and submission documents (3, 4).
The mEST assesses whether or not a drug impairs differentiation of mouse ESCs to form beating cardiomyocytes and includes cytotoxicity as a secondary end point (5, 6). The validation set of 20 chemicals was reproducible among different laboratories, and the overall correlation between in vitro and in vivo data was >75%. Broader testing of the assay beyond the initial validation set of compounds revealed lower performance (7). Subsequent publications improved the algorithm by developing models that use selected gene expression profiling to enhance the discrimination between embryotoxicants and non-embryotoxicants (8) One publication using 77 compounds identified molecular differentiation markers, reduced the assay to a single sampling time point, shortened the cytotoxicity assay (which allowed the definitive test to be only a single test concentration), and revealed an overall accuracy of ∼72% (8).
Despite optimization, automation, and molecular improvements, use of a mouse cell assay will have issues of cross-species predictivity when one is ultimately concerned with identifying human teratogens. Classic examples of human-specific teratogens such as thalidomide and isotretinoin come to mind (9) but also coumarin and streptomycin (10). In addition, a number of drugs and chemicals show discordant responses in the target organ of teratogenic response from mice to humans (lithium, valproic acid, busulfan, chlorambucil, and cytarabine). Finally, the concordance across 289 compounds for mouse teratogenicity results to humans is 59% (10). Although caveats exist for in vitro models, the importance in developing a stem cell-based embryo toxicity assay that is relevant to humans is summarized succinctly by the senior author of the original mEST: “The ultimate and most challenging goal in improving the mEST is to use human ESCs in a hEST, thus avoiding the extrapolation of responses from animals to humans … ” (11).
The number of compounds evaluated in human EST assays is limited. Challenges (such as the low rate of embryoid body formation with human cells and hPSCs is developmentally more similar to later stage murine post-implantation-derived epiblast stem cells than to blastocyst-derived murine ESCs (12–14)) suggest that changes in assay conduct from the mEST approach are necessary. For example, instead of embryoid body formation, gene expression analysis can be used, as ethanol increased endodermal differentiation, retinoic acid (RA) altered genes involved in neural development, and thalidomide affected both endodermal and neural genes (15). RA, a well known teratogenic agent, affected neural rosette formation with concentration-dependent morphological alteration and gene expression changes similar to those induced in vivo by RA exposure (16). A study examining a large set of compounds in human pluripotent cells used metabolomics as the discriminating variable and had ∼70–80% prediction of reproductive toxicity (17). Early expression analysis of differentiating human ESCs revealed a robust correlation with mesendoderm markers such as T-Brachyury, EOMES, and SOX-17 and correctly classified 55 of 59 compounds with their appropriate teratogenic response in vivo (18). Taken together, these data confirm an improvement for human over mouse models.
Cardiomyocytes
The first intact organ to form, the heart, was also the first organ to be used extensively in hPSC-derived research. Disruption of cardiac function, due to either overt cellular damage/loss or alteration of cardiac action potentials, is a major toxicological concern and has halted a number of drugs in development or led to their removal from the marketplace (19). Thus, cardiovascular safety pharmacology is evaluated early and often in drug development, often starting with heterologous expression systems evaluating isolated cardiac ion channels (e.g. hERG (human ether-à-go-go-related gene)). This system is sensitive and amenable to high throughput screening, but the obvious lack of the remaining complement of cardiac ion channels involved in the concert of voltage-dependent contraction results in loss of accuracy (20). The hERG channel is of particular interest, as inhibition of this channel causes delayed repolarization and prolonged QT and may lead to ventricular tachyarrhythmia such as life-threatening torsades de pointes. The correlation at each step from hERG inhibition to QT prolongation to arrhythmia to torsades de pointes is far from perfect, and better tools to understand the molecular cascade of events are needed. Subsequent models such as isolated cardiac tissues, primary cells, and perfused intact hearts are labor-intensive and suffer from preparation variability, limited time frame of suitable experimentation, expense, and need to coordinate tissue collection from living organisms, resulting in a suboptimal predictive milieu (21). Thus, cardiac safety pharmacology has a deficiency in the early prediction of human electrophysiological events, and studies with hPSC-derived cardiomyocytes have promise in filling this gap.
hPSC-derived cardiomyocytes are similar to their in vivo cardiac counterparts. hPSC-derived cardiomyocytes express the major cardiac ion channels, demonstrate cardiac action potentials, and respond in identifiable and anticipated ways to known cardioactive molecules using a number of approaches, including patch clamping, multielectrode arrays (MEAs), and impedance (22, 23). hPSC-derived cardiomyocytes exhibit cardiomyocyte morphology, including sarcomeric structures, and express a fetal gene expression pattern (24). In terms of drug response, both MEA and patch clamping revealed that hPSC-derived cardiomyocytes have the anticipated dose-responsive effects on beat rate and electrophysiological changes across a set of 43 compounds (25) and across 19 different compounds (26). MEA and impedance across 28 cardioactive drugs indicated a strong correlation between in vitro findings and known in vivo arrhythmia and QT prolongation effects (27). An in vitro surrogate for drug-induced arrhythmia was described and shown to be attenuated with the calcium channel blocker nifedipine, suggesting a molecular relationship with early afterdepolarization and arrhythmia. Importantly, the abovementioned studies demonstrate the robust utility of hPSC-derived cardiomyocytes to predict QT prolongation and arrhythmia over rote hERG screening and make them an excellent complement to drug discovery and safety pharmacology, potentially reducing the need for extensive QT evaluation in clinical development.
Although safety assessment is a slowly changing field, an opportunity exists for hPSC-derived cardiomyocytes across the entire landscape of drug discovery and development. In addition, to improve early safety screening, a larger opportunity downstream in clinical development is obviating the need for extensive QT evaluation. The E14 clinical guidance document issued by ICH in 2005 details conducting a dedicated study to quantify the QT interval effect of a new molecular entity, the thorough QT/QTc (TQT) study. This study is designed to confidently identify compounds that do not prolong the QTc by 10 ms and thus reduce or eliminate additional electrocardiogram monitoring. The underlying hypothesis is that QT prolongation would create a pro-arrhythmic environment and consequently put exposed patients at risk of arrhythmia. At present, “clean” preclinical data as mandated by ICH7A and ICH7B do not automatically eliminate the need for a TQT trial. A large sample set of compounds has been tested in TQT, so it would be an opportune testing set for determining if hPSC-derived models could provide equivalent decision-making information.
An area of research for hPSC-derived tissues is obtaining a mature adult phenotype. hPSC-derived cardiomyocytes resemble a fetal, relatively immature cardiac phenotype. The exact degree of maturation required will vary with the end point of evaluation. For example, hPSC-derived cardiomyocytes for QT and arrhythmia detection show a strong correlation with intact adult responses, even though the cells exhibit a less than fully mature phenotype. In contrast, hypertrophy, where injury response is predicated on the re-expression of fetal marker genes such as brain natriuretic peptide or β-myosin heavy chain (28), will require maturation to decrease background levels of marker genes to detect an increase in response to stimuli. Efforts to develop a more mature phenotype include three-dimensional and patterned microenvironment (29). In addition, because small molecules are often added to directed differentiation protocols, they could be used to encourage maturation (30). Alternatively, hPSC-derived cardiomyocytes at the current maturity could be studied and the cardiac channels deconstructed such that a mature phenotype could be generated via computer-based modeling (31). A less optimal approach to maturity is to culture for extended periods of time, as 1 year in culture led to myosin and band changes indicative of adult cardiomyocytes (32, 33). Thus, there are a multitude of avenues to explore maturing cardiomyocytes should the scientific need arise.
Although much emphasis has been placed on electrophysiological applications, hPSC-derived cardiomyocytes are also useful to study cardiotoxicity. hPSC-derived cardiomyocytes are distinct from other in vitro cardiac models because they maintain voltage-dependent contractions for indefinite periods of time, have a maturing phenotype, and exhibit in vivo relevant drug responses (19, 27, 34, 35). The voltage-dependent contractile properties exhibited in vitro are essential and necessary to replicate the primary in vivo role of cardiomyocytes. For mechanistic toxicology questions, one must assess rhythmic perturbations and consequences of energy production as they relate to drug-induced cardiotoxicity simultaneously. hPSC-derived cardiomyocytes were used to help tease out the mechanism of cardiotoxicity of a drug development compound that induced mild cardiac myofiber loss and also had extensive electrophysiological effects, the latter of which were determined as the driving factor (27, 36). These types of studies guide subsequent mechanistic efforts to the true mechanistic cause, e.g. overt cardiac injury leading to electrophysiological abnormalities or vice versa. hPSC-derived cardiomyocytes were used to interrogate potential kinases, including AMP-activated protein kinase, ribosomal S6 kinase, and aurora kinase, involved in the cardiotoxicity of sunitinib. Again, the mechanistic evaluation of cardiotoxicity in a beating model was crucial, as sunitinib was discovered to inhibit hERG as well as other cardiac ion channels in addition to potent cytotoxicity (37). hPSC-derived models of cardiac injury have shown that cardiac troponins and cardiac natriuretic peptides can be useful biomarkers of cardiac damage induced by chemotherapeutic agents (38), as can the release of troponin T and FABP3 (fatty acid-binding protein 3) following doxorubicin-induced cardiac necrosis (39) Using a variety of molecular markers, including mitochondrial membrane potential, endoplasmic reticulum integrity, Ca2+ mobilization, and membrane permeability, combined with an assessment of cell viability, hPSC-derived cardiomyocytes were shown to be more predictive than primary dog cardiomyocytes and H9c2 cells when taking therapeutic concentration into consideration (40, 41). Across a panel of cardiotoxic and non-cardiotoxic kinase inhibitors, mitochondrial dysfunction was identified as a critical component of injury of many of these compounds (40).
hPSC-derived cardiomyocytes are valuable in the pursuit to assess electrophysiological and cardiac toxicity. hPSC-derived cardiomyocytes provide a direct assessment of human responses without concern of cross-species extrapolation, and electrophysiological models are being developed to obviate extensive and potentially redundant clinical assessments of cardiac safety pharmacology. In any event, the production of consistent, genetically similar (or diverse as specific questions arise) cells with consistent electrophysiological responses to treatment across and within experiments has provided stability to hPSC-derived model research. Although not the scope of this minireview, iPSC-derived cardiomyocytes from a range of patients with a diversity of cardiac abnormalities, including long QT1, long QT2, long QT3/Brugada syndrome, long QT8/Timothy syndrome, and catecholaminergic polymorphic ventricular tachycardia, have already been created and can be used to help draw clear associations with genetic information and functional consequences, a step that will be paramount to optimizing and personalizing drug therapy in the future.
Neurons
Although not as extensive as hPSC-derived cardiomyocytes, hPSC-derived neurons have similarly offered a novel platform for research into the neurotoxicity of drugs and chemicals. The neonatal development status of hPSC-derived neurons has been leveraged to understand the deleterious drug effects on the process of neurodevelopment (42, 43). Because cultured hPSC-derived neurons rapidly and continually develop neurites to enable cell-cell communication, the ability to demonstrate long-term potentiation in vitro is a possibility (44). The in vivo-like properties of hPSC-derived neurons are also being used to study phenotypic end points beyond neurite outgrowth, including electrical activity, migration, and overt cellular morphology, which can be coupled with traditional measurements such as biochemical assays and gene expression analysis (45, 46).
hPSC-derived neurons have shown specific applications in toxicology. Because hPSC-derived neurons are sensitive to botulinum toxin, an assay has been developed that can effectively replace animal testing for botulinum toxicity assays (47). The authors demonstrated that molecular targets of botulinum toxin uptake and toxicity are expressed in hPSC-derived neurons; thus, the assay replicates appropriate in vivo toxicity mechanisms. MEA was used to demonstrate subtle perturbations in electrical activity in ESC-derived neurospheres and was more sensitive than measurements of cellular injury and cytotoxicity (48, 49). By way of example, hPSC-derived neurons were detectably sensitive to known neurite outgrowth inhibitors such as bisindolylmaleimide I, U0126, lithium, sodium orthovanadate, and brefeldin A; thus, the end point of neurite outgrowth could be an in vitro hallmark of neurotoxicity (45).
hPSC-derived neurons have been used in conjunction with chemical libraries to inform and investigate mechanism of action. In a discovery pharmacology screening model with toxicological underpinnings, hPSC-derived neurons were used to determine the mechanism of toxicity of amyloid-β42 accumulation (a biomarker of Alzheimer disease) as a re-entry into the cell cycle, as specific Cdk2 inhibitors attenuated toxicity. In addition, the model of amyloid-β-induced cytotoxicity in hPSC-derived neurons was used to screen a compound library to identify novel drug targets (50). Using dopaminergic neurons derived from human ESCs, a collection of 720 Food and Drug Administration-approved drugs was examined for molecules that were selectively cytotoxic to progenitor neurons but non-cytotoxic to differentiated neurons. Although this experiment was designed with aspirations to assist in purifying a cell therapy product of contaminating proliferative cells, one could envision using this type of library screening approach to identify molecules that are potently toxic to the neuronal progenitor population (51). The anesthetic ketamine has been shown to be neurotoxic, and hPSC-derived neurons were used to study the mechanism. Ketamine increased neural stem cell proliferation and caused neuronal apoptosis in a mitochondrion-dependent mechanism (52).
Neurotoxicology models have benefited from access to hPSC-derived neurons. For example, iPSC lines have been made from Parkinson disease, Alzheimer disease, spinal muscular atrophy, familial dysautonomia, Rett syndrome, and schizophrenia and have been differentiated into neurons. These models recapitulate neurodegenerative disease and allow one to predict the beneficial and deleterious effects of pharmaceutical agents prior to exposing patients. As with many other in vitro studies, strictly relying on cell viability assays is limited in predictive power, and the integration of functional end points that underlie the sophisticated events of neuropharmacology, electrical activity, cell signaling, and mechanistic cascade following injury alongside morphologic assessment such as neurite outgrowth and cell replication will greatly expand the utility of hPSC-derived neurons in the field of toxicology.
Hepatocytes
To an in vitro toxicologist, hepatocytes are the most sought after cell type. Primary cultured hepatocytes, the current gold standard for in vitro liver-based questions, are an integral part of early safety assays, including generic cellular toxicity signals, metabolic drug activation, P450 induction signals, transporter activity, formation of toxic drug metabolites, and impairment of mitochondrial function, to name just a few. Parenchymal hepatocytes are the major cell type of the liver and, when cultured, can retain many physiological functions of intact liver, albeit only for a limited time, and exhibit cytochrome P450 levels that vary greatly from preparation to preparation (53). Consequently, there is a strong need for a human-specific in vitro test system that mirrors the critical characteristics of the liver, and extensive efforts are being directed toward the establishment of hPSC-derived hepatocytes.
To date, hPSC-derived hepatocytes with robust properties have been challenging to efficiently produce, largely due to difficulties in obtaining a mature metabolic phenotype. For full implementation of hPSC-derived hepatocytes in toxicology, the cells need to have the ability to metabolize drugs via the CYP450 family of phase I enzymes, as drug-induced liver injury is in part due to CYP450-dependent formation of reactive metabolites that damage cellular macromolecules, exert hepatotoxicity, and potentially form immunogenic liver protein adducts. In hPSC-derived hepatocytes, the expression of CYP3A4, the most abundant cytochrome P450 in the liver, was present but at lower levels in stem cell-derived hepatocytes compared with human liver or primary human hepatocytes even after induction with xenobiotics (54–56). In contrast, other P450s such as CYP1A2 demonstrate comparable levels in hPSC-derived hepatocytes as primary human hepatocytes (57, 58). The activity of four major human liver cytochromes (CYP1A2, CYP2C9, CYP3A4, and CYP2D6), as well as metabolite profiling of bufuralol, a nonselective β-adrenoreceptor-blocking agent, indicated comparable metabolic activity of ESC-derived hepatocyte-like cells compared with primary human hepatocytes. In addition, four new metabolic pathways of bufuralol were identified, expanding the total number of known metabolic pathways for this molecule (59). The activity of GSTs has been shown to also be comparable to that of primary human hepatocytes, despite differential and, in some cases, lower expression of the various GST subunits (60). Although limited, these examples highlight the potential for the current version of hPSC-derived hepatocytes to be at least equivalent to that of primary human hepatocytes.
Similar to other hPSC-derived tissues and hepatocytes from pluripotent cells, efforts have been taken to identify a means to produce a more mature phenotype. Extended culturing has been shown to lead to a more mature phenotype; for example, α1-antitrypsin secretion increased by nearly 6-fold after 6–7 weeks of culturing, although it was still less than primary human hepatocyte levels (61). Additionally, α-fetoprotein levels dropped and CYP3A4 levels increased after 8 weeks of culture of hPSC-derived hepatocytes (62). The re-creation of a three-dimensional matrix environment and the use of co-culture with stromal cells also appeared to increase the differentiation and function of hPSC-derived hepatocytes (63, 64). Although others have used genetic engineering to express hepatic transcription factors such as HNF4α to drive maturation (65), the use of small molecule “modifiers” is common in differentiation protocols and could similarly be used to enhance the maturation status of hPSC-derived hepatocytes (66).
Limited publications exist currently on the use of hPSC-derived hepatocytes in toxicology. However, a few publications have shown promising data. Across a panel of 15 compounds that were known non-carcinogens, genotoxic carcinogens, and non-genotoxic carcinogens, gene expression analysis identified a subset of ∼600 genes that separated the classes with 95% confidence and yielded biologically meaningful classification. The gene set identified also highly correlated with expression profiles from primary human hepatocytes (63). Another publication showed that hPSC-derived hepatocytes were sensitive to d-galactosamine hepatotoxicity, which could be attenuated by prostaglandin E1 (67). Although there are limited data on the utility of hPSC-derived hepatocytes in toxicology, the true assessment of their value will be determined once more adult-like metabolic expression is achieved.
Conclusions
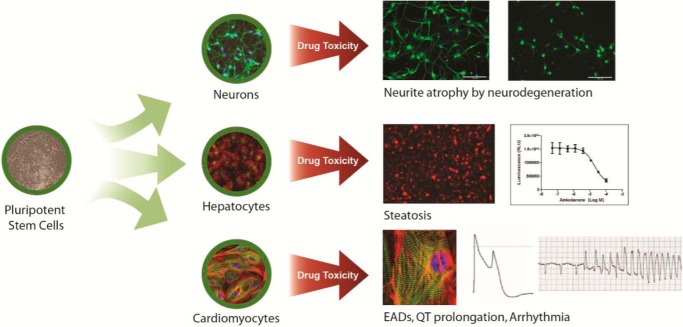
hPSC-derived tissues have only recently been produced in sufficient quality and quantity to enable basic drug research in pharmacology and toxicology (Fig. 1). However, the field is moving rapidly past the initial curiosity stage, where more reviews are written than primary data publications, into the brass tacks of true research investigations. By example, many public and private laboratories are using hPSC-derived tissues for screening to identify new targets and drug molecules. The improved biological properties of hPSC-derived tissues become even more useful when combined with an increasing number of sophisticated tools to introduce precise genetic, gene, and chromosomal alterations; stem cell-derived tissues can be engineered to replicate a disease, express factors to enable differentiation or maturation, or introduce biomarker-reporting constructs. Similarly, hPSC banking efforts such as those announced by the California Institute for Regenerative Medicine and the Innovative Medicines Initiative will collect thousands of samples from diverse patient genetic and disease backgrounds, which, coupled with high quality manufacturing of hPSC-derived cells, will shed much light on the connection of genotype to phenotype.
FIGURE 1.
Potential of iPSC-derived tissues in safety assessment. Drug-induced injury models have been developed in a number of target organs. Examples from neurons, hepatocytes, and cardiomyocytes have demonstrated physiologically relevant disease phenotypes as well as drug-induced toxicities. In hPSC-derived neurons, amyloid-β accumulation and neurotoxic drugs will induce neurite atrophy prior to neuronal cell death. In hPSC-derived hepatocytes, the steatosis-inducing drug amiodarone recapitulates this finding in vitro. Finally, hPSC-derived cardiomyocytes accurately reproduce and can be used to predict drug-induced arrhythmias. RLU, relative light units; EADs, early afterdepolarizations.
Although the seminal work on the embryonic stem cell test provided a clear example of the in vitro to in vivo correlative power of stem cells, the safety pharmacology applications of hPSC-derived cardiomyocytes could have a major impact on pharmaceutical drug development. The cost impact of hERG signals and QT prolongation has cast a long shadow from preclinical research into late stage drug development, and the data generated from hPSC-derived cardiomyocytes not only can help in selecting better candidates at the outset and provide context to the high false positive rate of hERG channel inhibition but may also be suitable in obviating the need for costly clinical safety trials such as the TQT study. hPSC-derived neurons and hepatocytes are just beginning to be investigated but have already provided a more substantial human model for research. Although it is still early days, substantial financial effort has been placed on creating so-called “man-on-a-chip” models, where hPSC-derived tissues will be the primary cell format of choice given their robust reproducible manufacturing coupled with the ability to make cells from patients with known medical conditions. The challenge to use this technology as a suitable replacement for animal models is enormous, yet if these approaches produce better multicellular in vitro organoids that can improve our understanding of human disease as well as beneficial and deleterious drug responses, the effort will be warranted. Many technology improvements have been full of promise at the outset, yet hPSC-derived tissues are grounded in strong scientific rationale and are already improving our ability to investigate physiology, pharmacology, and toxicology.
This is the first article in the Thematic Minireview Series “Development of Human Therapeutics Based on Induced Pluripotent Stem Cell (iPSC) Technology”.
- hPSC
- human pluripotent stem cell
- ESC
- embryonic stem cell
- iPSC
- induced pluripotent stem cell
- mEST
- mouse embryonic stem cell test
- RA
- retinoic acid
- MEA
- multielectrode array
- TQT
- thorough QT/QTc
- ICH
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use.
REFERENCES
- 1. Williams E. S., Panko J., Paustenbach D. J. (2009) The European Union's REACH regulation: a review of its history and requirements. Crit. Rev. Toxicol. 39, 553–575 [DOI] [PubMed] [Google Scholar]
- 2. Thomas C. E., Will Y. (2012) The impact of assay technology as applied to safety assessment in reducing compound attrition in drug discovery. Expert Opin. Drug Discov. 7, 109–122 [DOI] [PubMed] [Google Scholar]
- 3. Seiler A., Visan A., Pohl I., Genschow E., Buesen R., Spielmann H. (2002) Improving the embryonic stem cell test (EST) by establishing molecular endpoints of tissue specific development using murine embryonic stem cells (D3 cells). ALTEX 19, Suppl. 1, 55–63 [PubMed] [Google Scholar]
- 4. Genschow E., Spielmann H., Scholz G., Seiler A., Brown N., Piersma A., Brady M., Clemann N., Huuskonen H., Paillard F., Bremer S., Becker K. (2002) The ECVAM international validation study on in vitro embryotoxicity tests: results of the definitive phase and evaluation of prediction models. European Centre for the Validation of Alternative Methods. Altern. Lab. Anim. 30, 151–176 [DOI] [PubMed] [Google Scholar]
- 5. Scholz G., Genschow E., Pohl I., Bremer S., Paparella M., Raabe H., Southee J., Spielmann H. (1999) Prevalidation of the embryonic stem cell test (EST)–a new in vitro embryotoxicity test. Toxicol. In Vitro 13, 675–681 [DOI] [PubMed] [Google Scholar]
- 6. Seiler A. E., Spielmann H. (2011) The validated embryonic stem cell test to predict embryotoxicity in vitro. Nat. Protoc. 6, 961–978 [DOI] [PubMed] [Google Scholar]
- 7. Paquette J. A., Kumpf S. W., Streck R. D., Thomson J. J., Chapin R. E., Stedman D. B. (2008) Assessment of the embryonic stem cell test and application and use in the pharmaceutical industry. Birth Defects Res. B Dev. Reprod. Toxicol. 83, 104–111 [DOI] [PubMed] [Google Scholar]
- 8. Panzica-Kelly J. M., Brannen K. C., Ma Y., Zhang C. X., Flint O. P., Lehman-McKeeman L. D., Augustine-Rauch K. A. (2013) Establishment of a molecular embryonic stem cell developmental toxicity assay. Toxicol. Sci. 131, 447–457 [DOI] [PubMed] [Google Scholar]
- 9. Hurtt M. E., Daston G., Davis-Bruno K., Feuston M., Silva Lima B., Makris S., McNerney M. E., Sandler J. D., Whitby K., Wier P., Cappon G. D. (2004) Juvenile animal studies: testing strategies and design. Birth Defects Res. B Dev. Reprod. Toxicol. 71, 281–288 [DOI] [PubMed] [Google Scholar]
- 10. Schardein J. L., Schwetz B. A., Kenel M. F. (1985) Species sensitivities and prediction of teratogenic potential. Environ. Health Perspect. 61, 55–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spielmann H. (2009) The way forward in reproductive/developmental toxicity testing. Altern. Lab. Anim. 37, 641–656 [DOI] [PubMed] [Google Scholar]
- 12. Burridge P. W., Thompson S., Millrod M. A., Weinberg S., Yuan X., Peters A., Mahairaki V., Koliatsos V. E., Tung L., Zambidis E. T. (2011) A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PLoS ONE 6, e18293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brons I. G., Smithers L. E., Trotter M. W., Rugg-Gunn P., Sun B., Chuva de Sousa Lopes S. M., Howlett S. K., Clarkson A., Ahrlund-Richter L., Pedersen R. A., Vallier L. (2007) Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448, 191–195 [DOI] [PubMed] [Google Scholar]
- 14. Tesar P. J., Chenoweth J. G., Brook F. A., Davies T. J., Evans E. P., Mack D. L., Gardner R. L., McKay R. D. (2007) New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448, 196–199 [DOI] [PubMed] [Google Scholar]
- 15. Mayshar Y., Yanuka O., Benvenisty N. (2011) Teratogen screening using transcriptome profiling of differentiating human embryonic stem cells. J. Cell. Mol. Med. 15, 1393–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Colleoni S., Galli C., Gaspar J. A., Meganathan K., Jagtap S., Hescheler J., Sachinidis A., Lazzari G. (2011) Development of a neural teratogenicity test based on human embryonic stem cells: response to retinoic acid exposure. Toxicol. Sci. 124, 370–377 [DOI] [PubMed] [Google Scholar]
- 17. Kleinstreuer N. C., Smith A. M., West P. R., Conard K. R., Fontaine B. R., Weir-Hauptman A. M., Palmer J. A., Knudsen T. B., Dix D. J., Donley E. L., Cezar G. G. (2011) Identifying developmental toxicity pathways for a subset of ToxCast chemicals using human embryonic stem cells and metabolomics. Toxicol. Appl. Pharmacol. 257, 111–121 [DOI] [PubMed] [Google Scholar]
- 18. Kameoka S., Babiarz J., Kolaja K., Chiao E. (2014) Development of a high throughput, in vitro human pluripotent stem cell test for the identification of potentially teratogenic compounds. Toxicol. Sci. 137, 76–90 [DOI] [PubMed] [Google Scholar]
- 19. Force T., Kolaja K. L. (2011) Cardiotoxicity of kinase inhibitors: the prediction and translation of preclinical models to clinical outcomes. Nat. Rev. Drug Discov. 10, 111–126 [DOI] [PubMed] [Google Scholar]
- 20. Raschi E., Ceccarini L., De Ponti F., Recanatini M. (2009) hERG-related drug toxicity and models for predicting hERG liability and QT prolongation. Expert Opin. Drug Metab. Toxicol. 5, 1005–1021 [DOI] [PubMed] [Google Scholar]
- 21. Skrzypiec-Spring M., Grotthus B., Szelag A., Schulz R. (2007) Isolated heart perfusion according to Langendorff—still viable in the new millennium. J. Pharmacol. Toxicol. Methods 55, 113–126 [DOI] [PubMed] [Google Scholar]
- 22. Caspi O., Itzhaki I., Kehat I., Gepstein A., Arbel G., Huber I., Satin J., Gepstein L. (2009) In vitro electrophysiological drug testing using human embryonic stem cell derived cardiomyocytes. Stem Cells Dev. 18, 161–172 [DOI] [PubMed] [Google Scholar]
- 23. Liang H., Matzkies M., Schunkert H., Tang M., Bonnemeier H., Hescheler J., Reppel M. (2010) Human and murine embryonic stem cell-derived cardiomyocytes serve together as a valuable model for drug safety screening. Cell. Physiol. Biochem. 25, 459–466 [DOI] [PubMed] [Google Scholar]
- 24. Babiarz J. E., Ravon M., Sridhar S., Ravindran P., Swanson B., Bitter H., Weiser T., Chiao E., Certa U., Kolaja K. L. (2012) Determination of the human cardiomyocyte mRNA and miRNA differentiation network by fine-scale profiling. Stem Cells Dev. 21, 1956–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dick E., Rajamohan D., Ronksley J., Denning C. (2010) Evaluating the utility of cardiomyocytes from human pluripotent stem cells for drug screening. Biochem. Soc. Trans. 38, 1037–1045 [DOI] [PubMed] [Google Scholar]
- 26. Laposa R. R. (2011) Stem cells for drug screening. J. Cardiovasc. Pharmacol. 58, 240–245 [DOI] [PubMed] [Google Scholar]
- 27. Guo L., Abrams R. M., Babiarz J. E., Cohen J. D., Kameoka S., Sanders M. J., Chiao E., Kolaja K. L. (2011) Estimating the risk of drug-induced proarrhythmia using human induced pluripotent stem cell-derived cardiomyocytes. Toxicol. Sci. 123, 281–289 [DOI] [PubMed] [Google Scholar]
- 28. Taegtmeyer H., Sen S., Vela D. (2010) Return to the fetal gene program: a suggested metabolic link to gene expression in the heart. Ann. N.Y. Acad. Sci. 1188, 191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boudou T., Legant W. R., Mu A., Borochin M. A., Thavandiran N., Radisic M., Zandstra P. W., Epstein J. A., Margulies K. B., Chen C. S. (2012) A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng. Part A 18, 910–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Willems E., Bushway P. J., Mercola M. (2009) Natural and synthetic regulators of embryonic stem cell cardiogenesis. Pediatr. Cardiol. 30, 635–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paci M., Sartiani L., Del Lungo M., Jaconi M., Mugelli A., Cerbai E., Severi S. (2012) Mathematical modelling of the action potential of human embryonic stem cell derived cardiomyocytes. Biomed. Eng. Online 11, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Otsuji T. G., Minami I., Kurose Y., Yamauchi K., Tada M., Nakatsuji N. (2010) Progressive maturation in contracting cardiomyocytes derived from human embryonic stem cells: qualitative effects on electrophysiological responses to drugs. Stem Cell Res. 4, 201–213 [DOI] [PubMed] [Google Scholar]
- 33. Kamakura T., Makiyama T., Sasaki K., Yoshida Y., Wuriyanghai Y., Chen J., Hattori T., Ohno S., Kita T., Horie M., Yamanaka S., Kimura T. (2013) Ultrastructural maturation of human induced pluripotent stem cell-derived cardiomyocytes in a long-term culture. Circ. J. 77, 1307–1314 [DOI] [PubMed] [Google Scholar]
- 34. Ma J., Guo L., Fiene S. J., Anson B. D., Thomson J. A., Kamp T. J., Kolaja K. L., Swanson B. J., January C. T. (2011) High purity human induced pluripotent stem cell-derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. Am. J. Physiol. Heart Circ. Physiol. 301, H2006–H2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Braam S. R., Tertoolen L., van de Stolpe A., Meyer T., Passier R., Mummery C. L. (2010) Prediction of drug-induced cardiotoxicity using human embryonic stem cell-derived cardiomyocytes. Stem Cell Res. 4, 107–116 [DOI] [PubMed] [Google Scholar]
- 36. Misner D. L., Frantz C., Guo L., Gralinski M. R., Senese P. B., Ly J., Albassam M., Kolaja K. L. (2012) Investigation of mechanism of drug-induced cardiac injury and torsades de pointes in cynomolgus monkeys. Br. J. Pharmacol. 165, 2771–2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cohen J. D., Babiarz J. E., Abrams R. M., Guo L., Kameoka S., Chiao E., Taunton J., Kolaja K. L. (2011) Use of human stem cell derived cardiomyocytes to examine sunitinib mediated cardiotoxicity and electrophysiological alterations. Toxicol. Appl. Pharmacol. 257, 74–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dolci A., Dominici R., Cardinale D., Sandri M. T., Panteghini M. (2008) Biochemical markers for prediction of chemotherapy-induced cardiotoxicity: systematic review of the literature and recommendations for use. Am. J. Clin. Pathol. 130, 688–695 [DOI] [PubMed] [Google Scholar]
- 39. Andersson H., Steel D., Asp J., Dahlenborg K., Jonsson M., Jeppsson A., Lindahl A., Kågedal B., Sartipy P., Mandenius C. F. (2010) Assaying cardiac biomarkers for toxicity testing using biosensing and cardiomyocytes derived from human embryonic stem cells. J. Biotechnol. 150, 175–181 [DOI] [PubMed] [Google Scholar]
- 40. Rana P., Anson B., Engle S., Will Y. (2012) Characterization of human induced pluripotent stem cell-derived cardiomyocytes: bioenergetics and utilization in safety screening. Toxicol. Sci. 130, 117–131 [DOI] [PubMed] [Google Scholar]
- 41. Pointon A., Abi-Gerges N., Cross M. J., Sidaway J. E. (2013) Phenotypic profiling of structural cardiotoxins in vitro reveals dependency on multiple mechanisms of toxicity. Toxicol. Sci. 132, 317–326 [DOI] [PubMed] [Google Scholar]
- 42. Farina M., Aschner M., Rocha J. B. (2011) Oxidative stress in MeHg-induced neurotoxicity. Toxicol. Appl. Pharmacol. 256, 405–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ni M., Li X., Rocha J. B., Farina M., Aschner M. (2012) Glia and methylmercury neurotoxicity. J. Toxicol. Environ. Health A 75, 1091–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sørensen A. T., Rogelius N., Lundberg C., Kokaia M. (2011) Activity-dependent long-term plasticity of afferent synapses on grafted stem/progenitor cell-derived neurons. Exp. Neurol. 229, 274–281 [DOI] [PubMed] [Google Scholar]
- 45. Harrill J. A., Freudenrich T. M., Robinette B. L., Mundy W. R. (2011) Comparative sensitivity of human and rat neural cultures to chemical-induced inhibition of neurite outgrowth. Toxicol. Appl. Pharmacol. 256, 268–280 [DOI] [PubMed] [Google Scholar]
- 46. Robinette B. L., Harrill J. A., Mundy W. R., Shafer T. J. (2011) In vitro assessment of developmental neurotoxicity: use of microelectrode arrays to measure functional changes in neuronal network ontogeny. Front. Neuroeng. 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Whitemarsh R. C., Strathman M. J., Chase L. G., Stankewicz C., Tepp W. H., Johnson E. A., Pellett S. (2012) Novel application of human neurons derived from induced pluripotent stem cells for highly sensitive botulinum neurotoxin detection. Toxicol. Sci. 126, 426–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lappalainen R. S., Salomäki M., Ylä-Outinen L., Heikkilä T. J., Hyttinen J. A., Pihlajamäki H., Suuronen R., Skottman H., Narkilahti S. (2010) Similarly derived and cultured hESC lines show variation in their developmental potential towards neuronal cells in long-term culture. Regen. Med 5, 749–762 [DOI] [PubMed] [Google Scholar]
- 49. Ylä-Outinen L., Heikkilä J., Skottman H., Suuronen R., Aänismaa R., Narkilahti S. (2010) Human cell-based micro electrode array platform for studying neurotoxicity. Front. Neuroeng. 3, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xu X., Lei Y., Luo J., Wang J., Zhang S., Yang X. J., Sun M., Nuwaysir E., Fan G., Zhao J., Lei L., Zhong Z. (2013) Prevention of β-amyloid induced toxicity in human iPS cell-derived neurons by inhibition of cyclin-dependent kinases and associated cell cycle events. Stem Cell Ress 10, 213–227 [DOI] [PubMed] [Google Scholar]
- 51. Han Y., Miller A., Mangada J., Liu Y., Swistowski A., Zhan M., Rao M. S., Zeng X. (2009) Identification by automated screening of a small molecule that selectively eliminates neural stem cells derived from hESCs but not dopamine neurons. PLoS ONE 4, e7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bai X., Yan Y., Canfield S., Muravyeva M. Y., Kikuchi C., Zaja I., Corbett J. A., Bosnjak Z. J. (2013) Ketamine enhances human neural stem cell proliferation and induces neuronal apoptosis via reactive oxygen species-mediated mitochondrial pathway. Anesth. Analg. 116, 869–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Baxter M. A., Rowe C., Alder J., Harrison S., Hanley K. P., Park B. K., Kitteringham N. R., Goldring C. E., Hanley N. A. (2010) Generating hepatic cell lineages from pluripotent stem cells for drug toxicity screening. Stem Cell Res. 5, 4–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brolén G., Sivertsson L., Björquist P., Eriksson G., Ek M., Semb H., Johansson I., Andersson T. B., Ingelman-Sundberg M., Heins N. (2010) Hepatocyte-like cells derived from human embryonic stem cells specifically via definitive endoderm and a progenitor stage. J. Biotechnol. 145, 284–294 [DOI] [PubMed] [Google Scholar]
- 55. Ek M., Söderdahl T., Küppers-Munther B., Edsbagge J., Andersson T. B., Björquist P., Cotgreave I., Jernström B., Ingelman-Sundberg M., Johansson I. (2007) Expression of drug metabolizing enzymes in hepatocyte-like cells derived from human embryonic stem cells. Biochem. Pharmacol. 74, 496–503 [DOI] [PubMed] [Google Scholar]
- 56. Basma H., Soto-Gutiérrez A., Yannam G. R., Liu L., Ito R., Yamamoto T., Ellis E., Carson S. D., Sato S., Chen Y., Muirhead D., Navarro-Alvarez N., Wong R. J., Roy-Chowdhury J., Platt J. L., Mercer D. F., Miller J. D., Strom S. C., Kobayashi N., Fox I. J. (2009) Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology 136, 990–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rambhatla L., Chiu C. P., Kundu P., Peng Y., Carpenter M. K. (2003) Generation of hepatocyte-like cells from human embryonic stem cells. Cell Transplant. 12, 1–11 [DOI] [PubMed] [Google Scholar]
- 58. Moore R. N., Moghe P. V. (2009) Expedited growth factor-mediated specification of human embryonic stem cells toward the hepatic lineage. Stem Cell Res 3, 51–62 [DOI] [PubMed] [Google Scholar]
- 59. Duan Y., Ma X., Zou W., Wang C., Bahbahan I. S., Ahuja T. P., Tolstikov V., Zern M. A. (2010) Differentiation and characterization of metabolically functioning hepatocytes from human embryonic stem cells. Stem Cells 28, 674–686 [DOI] [PubMed] [Google Scholar]
- 60. Söderdahl T., Küppers-Munther B., Heins N., Edsbagge J., Björquist P., Cotgreave I., Jernström B. (2007) Glutathione transferases in hepatocyte-like cells derived from human embryonic stem cells. Toxicol. In Vitro 21, 929–937 [DOI] [PubMed] [Google Scholar]
- 61. Shirahashi H., Wu J., Yamamoto N., Catana A., Wege H., Wager B., Okita K., Zern M. A. (2004) Differentiation of human and mouse embryonic stem cells along a hepatocyte lineage. Cell Transplant. 13, 197–211 [DOI] [PubMed] [Google Scholar]
- 62. Shiraki N., Umeda K., Sakashita N., Takeya M., Kume K., Kume S. (2008) Differentiation of mouse and human embryonic stem cells into hepatic lineages. Genes Cells 13, 731–746 [DOI] [PubMed] [Google Scholar]
- 63. Ramasamy T. S., Yu J. S., Selden C., Hodgson H., Cui W. (2013) Application of three-dimensional culture conditions to human embryonic stem cell-derived definitive endoderm cells enhances hepatocyte differentiation and functionality. Tissue Eng. Part A 19, 360–367 [DOI] [PubMed] [Google Scholar]
- 64. Nagamoto Y., Tashiro K., Takayama K., Ohashi K., Kawabata K., Sakurai F., Tachibana M., Hayakawa T., Furue M. K., Mizuguchi H. (2012) The promotion of hepatic maturation of human pluripotent stem cells in 3D co-culture using type I collagen and Swiss 3T3 cell sheets. Biomaterials 33, 4526–4534 [DOI] [PubMed] [Google Scholar]
- 65. Takayama K., Inamura M., Kawabata K., Katayama K., Higuchi M., Tashiro K., Nonaka A., Sakurai F., Hayakawa T., Furue M. K., Mizuguchi H. (2012) Efficient generation of functional hepatocytes from human embryonic stem cells and induced pluripotent stem cells by HNF4α transduction. Mol. Ther. 20, 127–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li W., Jiang K., Ding S. (2012) Concise review: a chemical approach to control cell fate and function. Stem Cells 30, 61–68 [DOI] [PubMed] [Google Scholar]
- 67. Nakamura N., Saeki K., Mitsumoto M., Matsuyama S., Nishio M., Saeki K., Hasegawa M., Miyagawa Y., Ohkita H., Kiyokawa N., Toyoda M., Akutsu H., Umezawa A., Yuo A. (2012) Feeder-free and serum-free production of hepatocytes, cholangiocytes, and their proliferating progenitors from human pluripotent stem cells: application to liver-specific functional and cytotoxic assays. Cell. Reprogram. 14, 171–185 [DOI] [PubMed] [Google Scholar]