Abstract
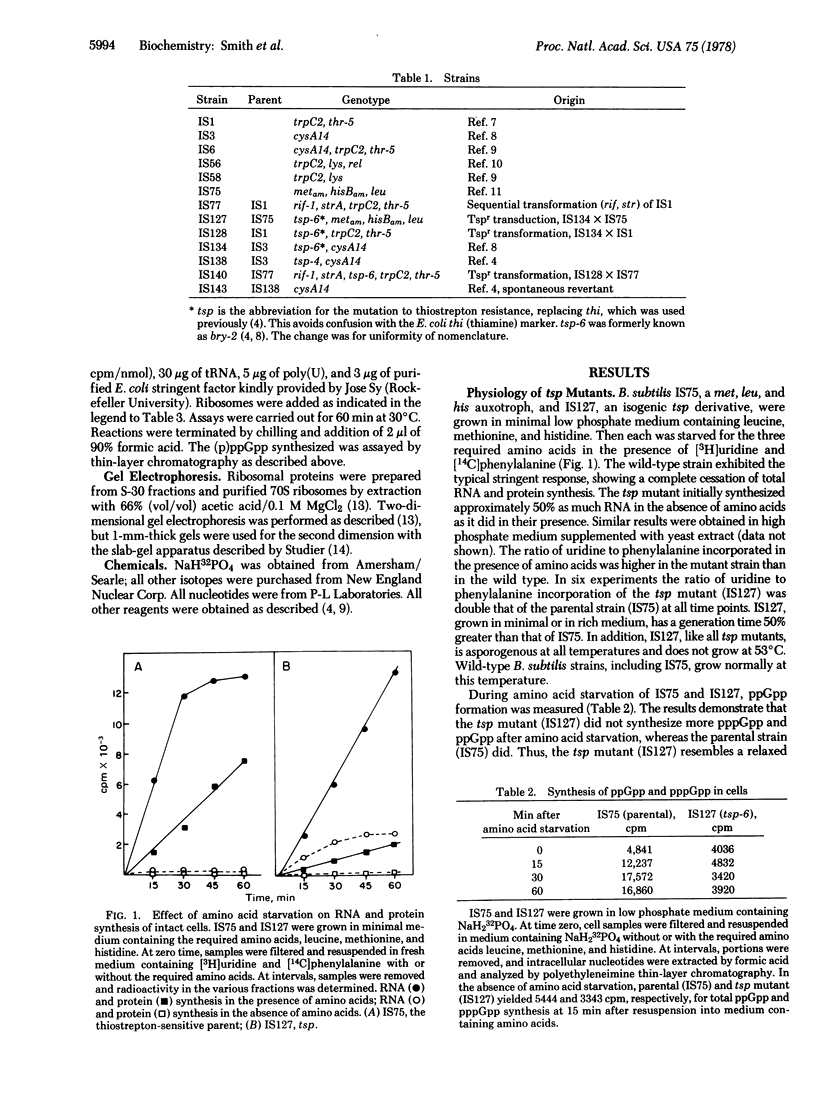
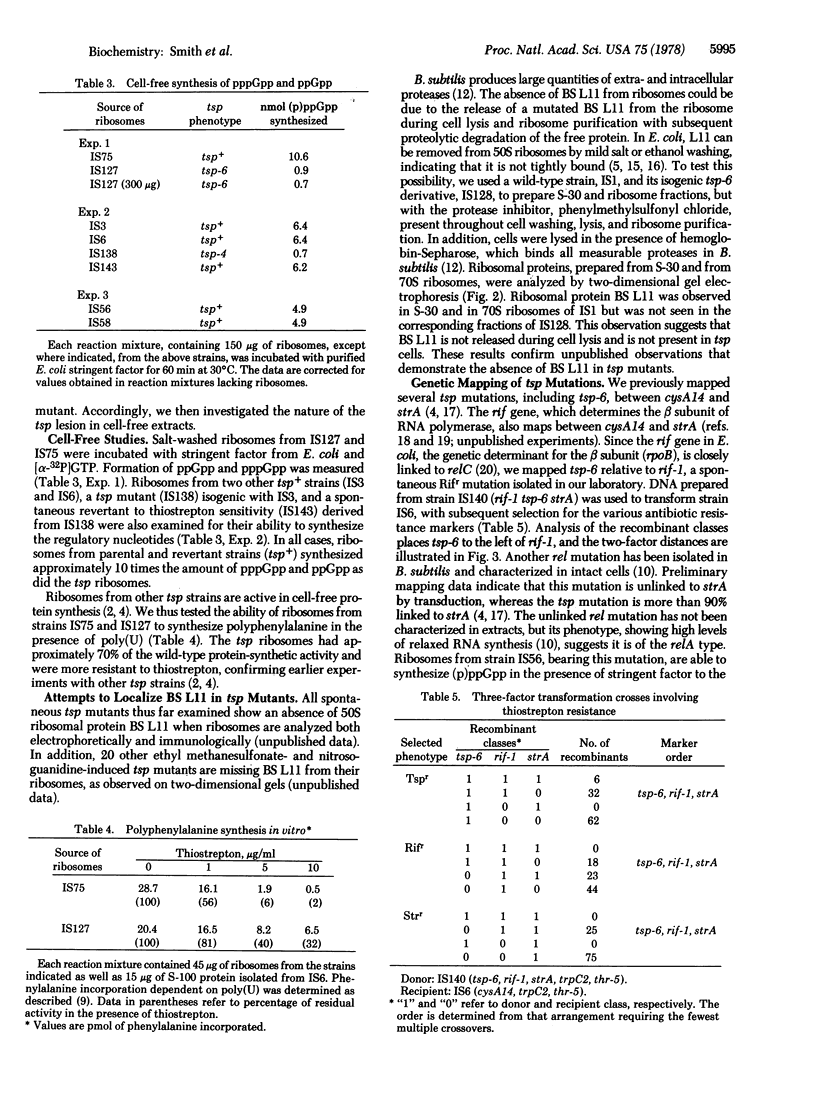
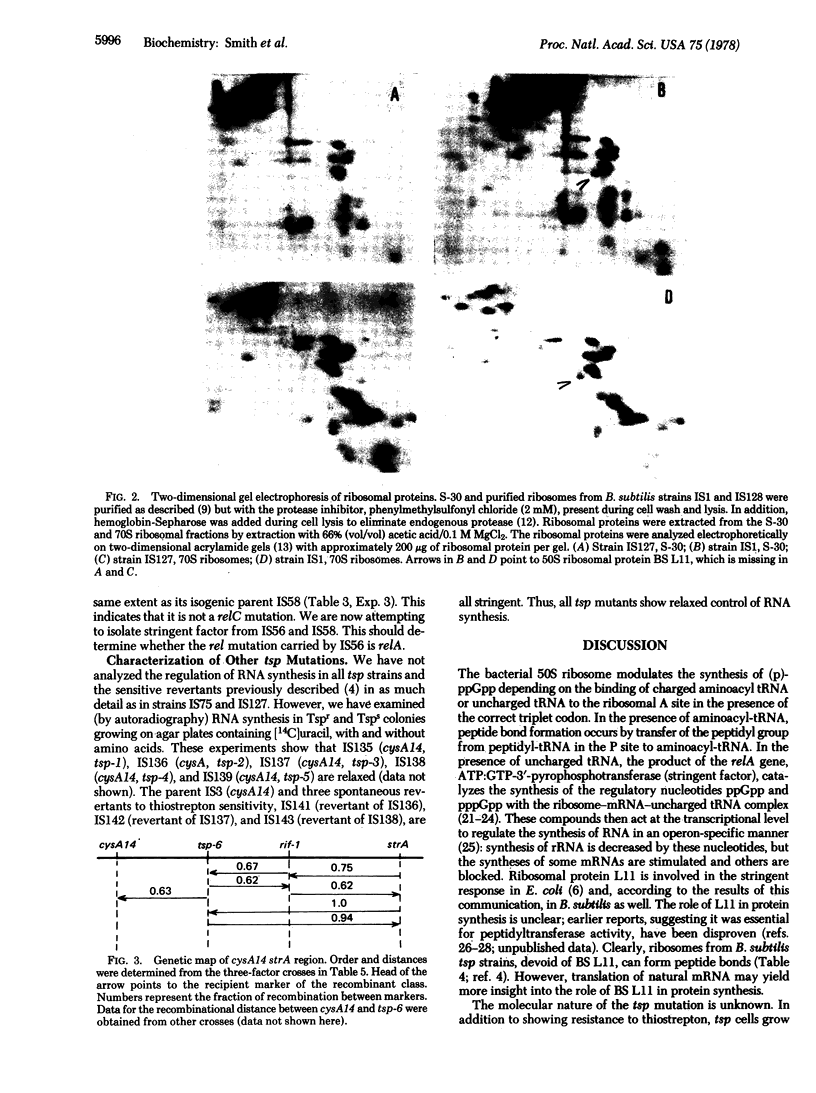
Spontaneous mutants of Bacillus subtilis resistant to thiostrepton (TSP) exhibit relaxed synthesis of RNA when starved for required amino acids. Intact cells of tsp mutants cannot synthesize the regulatory nucleotides, ppGpp and pppGpp, after amino acid deprivation. Because ribosomes isolated from spontaneous revertants to thiostrepton sensitivity and from wild-type stringent strains can synthesize (p)ppGpp whereas ribosomes isolated from tsp strains cannot synthesize these regulatory nucleotides in the presence of stringent factor, it appears that the lesion is expressed at the level of the ribosome. Genetic mapping, via three-factor transformational crosses, has shown that tsp is closely linked to rif, in the order cysA14, tsp, rif-I, strA. The phenotype of the tsp mutants indicates that they are of the relC type. Their map position indicates that they are different from a previously described B subtilis rel mutation. Ribosomes from the latter strain can synthesize (p)ppGpp in cell-free extracts.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballesta J. P., Vazquez D. Activities of ribosomal cores deprived of proteins L7, L10, L11 and L12. FEBS Lett. 1974 Nov 15;48(2):266–270. doi: 10.1016/0014-5793(74)80483-7. [DOI] [PubMed] [Google Scholar]
- Bendiak D. S., Parker J., Friesen J. D. Fine-structure mapping of the rts, rplK, rplL, and rpoB genes of Escherichia coli. J Bacteriol. 1977 Jan;129(1):536–539. doi: 10.1128/jb.129.1.536-539.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Goldthwaite C., Smith I., Marmur J. Genetic mapping in Bacillus subtilis. J Mol Biol. 1967 Jul 14;27(1):163–185. doi: 10.1016/0022-2836(67)90358-0. [DOI] [PubMed] [Google Scholar]
- Dubnau E., Pifko S., Sloma A., Cabane K., Smith I. Conditional mutations in the translational apparatus of Bacillus subtils. Mol Gen Genet. 1976 Aug 10;147(1):1–12. doi: 10.1007/BF00337929. [DOI] [PubMed] [Google Scholar]
- Friesen J. D., Fiil N. P., Parker J. M., Haseltine W. A. A new relaxed mutant of Escherichia coli with an altered 50S ribosomal subunit. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3465–3469. doi: 10.1073/pnas.71.9.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldthwaite C., Dubnau D., Smith I. Genetic mapping of antibiotic resistance in markers Bacillus subtilis. Proc Natl Acad Sci U S A. 1970 Jan;65(1):96–103. doi: 10.1073/pnas.65.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldthwaite C., Smith I. Physiological characterization of antibiotic resistant mutants of Bacillus subtilis. Mol Gen Genet. 1972;114(3):190–204. doi: 10.1007/BF01788888. [DOI] [PubMed] [Google Scholar]
- Halling S. M., Burtis K. C., Doi R. H. beta' subunit of bacterial RNA polymerase is responsible for streptolydigin resistance in Bacillus subtilis. Nature. 1978 Apr 27;272(5656):837–839. doi: 10.1038/272837a0. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Block R., Gilbert W., Weber K. MSI and MSII made on ribosome in idling step of protein synthesis. Nature. 1972 Aug 18;238(5364):381–384. doi: 10.1038/238381a0. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Block R. Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc Natl Acad Sci U S A. 1973 May;70(5):1564–1568. doi: 10.1073/pnas.70.5.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth S. R., Brown L. R. Genetic analysis of ribonucleic acid polymerase mutants of Bacillus subtilis. J Bacteriol. 1973 Apr;114(1):103–113. doi: 10.1128/jb.114.1.103-113.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highland J. H., Howard G. A., Ochsner E., Hasenbank R., Gordon J., Stöffler G. Identification of a ribosomal protein necessary for thiostrepton binding to Escherichia coli ribosomes. J Biol Chem. 1975 Feb 10;250(3):1141–1145. [PubMed] [Google Scholar]
- Howard G. A., Gordon J. Peptidyl transferase activity of ribosomal particles lacking protein L11. FEBS Lett. 1974 Nov 15;48(2):271–274. doi: 10.1016/0014-5793(74)80484-9. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt E., Wittmann H. G. Ribosomal proteins. VII. Two-dimensional polyacrylamide gel electrophoresis for fingerprinting of ribosomal proteins. Anal Biochem. 1970 Aug;36(2):401–412. doi: 10.1016/0003-2697(70)90376-3. [DOI] [PubMed] [Google Scholar]
- Moore V. G., Atchison R. E., Thomas G., Moran M., Noller H. F. Identification of a ribosomal protein essential for peptidyl transferase activity. Proc Natl Acad Sci U S A. 1975 Mar;72(3):844–848. doi: 10.1073/pnas.72.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T., Munoz L., Doi R. H. A procedure to remove protease activities from Bacillus subtilis sporulating cells and their crude extracts. Anal Biochem. 1977 Mar;78(1):165–170. doi: 10.1016/0003-2697(77)90020-3. [DOI] [PubMed] [Google Scholar]
- Nierhaus K. H., Montejo V. A protein involved in the peptidyltransferase activity of Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1931–1935. doi: 10.1073/pnas.70.7.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo S., Yanagida T., Fujita D. J., Olsson-Wilhelm B. M. The genetics of bacteriophage SPO1. Biken J. 1972 Jun;15(2):81–97. [PubMed] [Google Scholar]
- Parker J., Watson R. J., Friesen J. D. A relaxed mutant with an altered ribosomal protein L11. Mol Gen Genet. 1976 Feb 27;144(1):111–114. doi: 10.1007/BF00277313. [DOI] [PubMed] [Google Scholar]
- Pedersen F. S., Lund E., Kjeldgaard N. O. Codon specific, tRNA dependent in vitro synthesis of ppGpp and pppGpp. Nat New Biol. 1973 May 2;243(122):13–15. [PubMed] [Google Scholar]
- Pestka S., Weiss D., Vince R., Wienen B., Stöffler G., Smith I. Thiostrepton-resistant mutants of Bacillus subtilis: localization of resistance to the 50S subunit. Mol Gen Genet. 1976 Mar 30;144(3):235–241. doi: 10.1007/BF00341721. [DOI] [PubMed] [Google Scholar]
- Reiness G., Yang H. L., Zubay G., Cashel M. Effects of guanosine tetraphosphate on cell-free synthesis of Escherichia coli ribosomal RNA and other gene products. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2881–2885. doi: 10.1073/pnas.72.8.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhaese H. J., Groscurth R. Studies on the control of development. In vitro synthesis of HPN and MS nucleotides by ribosomes from either sporulating or vegetative cells of Bacillus subtilis. FEBS Lett. 1974 Aug 15;44(1):87–93. doi: 10.1016/0014-5793(74)80312-1. [DOI] [PubMed] [Google Scholar]
- Smith I., Smith H. Location of the SPO2 attachment site and the bryamycin resistance marker on the Bacillus subtilis chromosome. J Bacteriol. 1973 Jun;114(3):1138–1142. doi: 10.1128/jb.114.3.1138-1142.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Swanton M., Edlin G. Isolation and characterization of an RNA relaxed mutant of B. subtilis. Biochem Biophys Res Commun. 1972 Jan 31;46(2):583–588. doi: 10.1016/s0006-291x(72)80179-7. [DOI] [PubMed] [Google Scholar]
- Sy J., Lipmann F. Identification of the synthesis of guanosine tetraphosphate (MS I) as insertion of a pyrophosphoryl group into the 3'-position in guanosine 5'-diphosphate. Proc Natl Acad Sci U S A. 1973 Feb;70(2):306–309. doi: 10.1073/pnas.70.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince R., Weiss D., Gordon J., Howard G., Smith I., Pestka S. Binding of thiostrepton to ribosomes from thiostrepton-sensitive and -resistant Bacillus subtilis strains. Antimicrob Agents Chemother. 1976 Apr;9(4):665–667. doi: 10.1128/aac.9.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]