Background: TRPM6 and TRPM7 combine ion channel and α-kinase functions.
Results: ATP inhibits TRPM7 but not TRPM6 or heteromeric TRPM6/M7 channels. Disruption of phosphorylation activity of TRPM6 kinase re-establishes ATP sensitivity to heteromeric channels.
Conclusion: TRPM6 uncouples heteromeric channels from cellular energy levels.
Significance: The altered characteristics of TRPM6/M7 compared with homomeric TRPM7 may enhance Ca2+ and Mg2+ transport in tissues co-expressing both proteins.
Keywords: Magnesium, Metabolic Regulation, Protein Complexes, Protein Kinases, TRP channels
Abstract
The transient receptor potential melastatin member 7 (TRPM7) and member 6 (TRPM6) are divalent cation channel kinases essential for magnesium (Mg2+) homeostasis in vertebrates. It remains unclear how TRPM6 affects divalent cation transport and whether this involves functional homomeric TRPM6 plasma membrane channels or heteromeric channel assemblies with TRPM7. We show that homomeric TRPM6 is highly sensitive to intracellular free Mg2+ and therefore unlikely to be active at physiological levels of [Mg2+]i. Co-expression of TRPM7 and TRPM6 produces heteromeric TRPM7/M6 channels with altered pharmacology and sensitivity to intracellular Mg·ATP compared with homomeric TRPM7. Strikingly, the activity of heteromeric TRPM7/M6 channels is independent of intracellular Mg·ATP concentrations, essentially uncoupling channel activity from cellular energy status. Disruption of TRPM6 kinase phosphorylation activity re-introduces Mg·ATP sensitivity to the heteromeric channel similar to that of TRPM7. Thus, TRPM6 modulates the functionality of TRPM7, and the TRPM6 kinase plays a critical role in tuning the phenotype of the TRPM7·M6 channel complex.
Introduction
TRPM7 and TRPM6 belong to the melastatin-related subfamily of TRP5 channels and represent unique bifunctional proteins with both ion channel and protein kinase function (1–4). The two proteins are highly homologous to each other with >50% sequence identity. TRPM7- and TRPM6-mediated membrane currents exhibit a highly nonlinear current-voltage (I/V) relationship with pronounced outward rectification (3, 5). The small inward currents at physiologically relevant negative membrane voltages are carried by divalent cations, including Mg2+ and Ca2+, and the large outward currents at positive voltages are due to efflux of monovalent cations (5, 6).
Considerable efforts have been directed toward understanding the physiological functions of TRPM7 (7). TRPM7 represents the only known ion channel that is essential for cell viability (3, 8, 9). Its channel function is inhibited by both intracellular free Mg2+ and Mg·ATP (3, 10), and both factors serve as the major physiological mechanisms controlling TRPM7-mediated divalent cation transport. Information is more limited for TRPM6, and some of it remains controversial. Conflicting results have been reported concerning TRPM6 expression in heterologous expression systems. Two independent groups were able to measure homomeric TRPM6 currents in HEK-293 cells (5, 11–13), whereas the other two show that TRPM6 is retained intracellularly when expressed alone (14–17). Hence, it has been proposed that TRPM6 may function in association with TRPM7 by forming a heteromeric TRPM7·M6 channel complex (14–17). Channel kinase heteromerization results in a slightly altered permeation and pharmacology profile, pH sensitivity, and single channel conductance (11, 12). However, important questions about regulation and functionality of heteromeric channel kinases remain unresolved.
EXPERIMENTAL PROCEDURES
Cell Culture and Transfection
Wild-type HEK-293 cells were grown in DMEM supplemented with 10% fetal bovine serum (FBS) (Invitrogen). The cells were transiently transfected with N-terminal hemagglutinin (HA)-tagged human TRPM6 constructs using Lipofectamine 2000 (Invitrogen). Human TRPM6 phosphotransferase-deficient mutant (TRPM6-K1804R) and human TRPM6 Δkinase were made from the original wild-type pCINeo-IRES-GFP-TRPM6 construct (TRPM6-WT, GenBankTM accession number 017662) and kindly provided by Dr. J. Hoenderop and Dr. R. Bindels (5, 13). Whole-cell patch clamp experiments were carried out 30 h post-transfection. Expression of various human TRPM6 constructs was identified by green fluorescence.
For expression in tetracycline (tet)-inducible HEK-293 cells, human TRPM6 WT and TRPM6 mutants were cloned into the pcDNA5/TO-FLAG vector and human TRPM7 WT into the pcDNA4/TO-HA vector as described previously (8, 17). tet-inducible HEK-293 cells encoding human TRPM7 + human TRPM6 WT (TRPM7/M6), human TRPM7 + human TRPM6 K1804R (TRPM7/M6 K1804R), and human TRPM7 + human TRPM6 Δkinase (TRPM7/M6 Δkinase) were maintained in DMEM containing 10% FBS, blasticidin (5 μg/ml), Zeocin (0.4 mg/ml), and hygromycin (0.5 mg/ml) (17). Tetracycline-inducible human TRPM7 HEK-293 cells were cultured in DMEM containing 10% FBS, blasticidin (5 μg/ml), and Zeocin (0.4 mg/ml) (8). Tetracycline-inducible human TRPM6 HEK-293 cells were maintained in DMEM containing 10% FBS, blasticidin (5 μg/ml), and hygromycin (0.5 mg/ml) (Invitrogen) (17). The proteins were induced by adding 1 μg/ml tetracycline to the culture media. Current measurements were performed 14–24 h following tetracycline induction.
RT-PCR
Total RNA was isolated from four replicates of HEK-293 wild-type cells using RNeasy mini kit (Qiagen). SuperScript III first-strand synthesis system for RT-PCR (Invitrogen) was used following the manufacturer's procedure to synthesize cDNA from 1 μg of total RNA primed with oligo(dT) primers. Gene-specific primers for TRPM6 (forward primer 5′-TGCCCTGGAACAAGCAATGTCAG-3′ and reverse primer 5′-CTTTTCATCAGCACAGCCCAAACC-3′), TRPM7 (forward primer 5′-AGCATACAGAACAGAGCCCAACGG-3′ and reverse primer 5′-TTCCAACAGTGCCATCATCCACC-3′), and GAPDH (forward primer 5′-GGAGCCAAAAGGGTCATCATCTC-3′ and reverse primer 5′-AGTGGGTGTCGCTGTTGAAGTC-3′) were designed using MacVector and synthesized by Invitrogen. PCR was performed in reaction volumes of 50 μl containing 1 μl of dNTPs (10 mm), 2 μl of each primer (10 pmol/μl), 2 μl of cDNA solution, 5 μl of reaction buffer (10×), 37 μl of water, and 1 μl of Pfu Ultra II fusion HS DNA polymerase (Stratagene) on a Thermal Cycler (Bio-Rad). Denaturation was carried out at 94 °C for 20 s, annealing at 55 °C for 30 s, and elongation at 72 °C for 30 s for 35 cycles, followed by extension at 72 °C for 3 min. PCR products were detected in 0.8% agarose gel containing 1× SYBR Safe DNA Gel Stain (Invitrogen).
Solutions
To measure TRPM6 currents, cells were kept in an extracellular solution containing (in mm) the following: 140 NaCl, 2.8 KCl, 1 CaCl2, 10 Hepes-NaOH, 11 glucose, pH 7.4. Current measurements of TRPM7/M6, TRPM7/M6 K1804R, and TRPM7/M6 Δkinase were conducted in an extracellular solution composed of (in mm) the following: 140 NaCl, 2.8 KCl, 1 CaCl2, 2 MgCl2, 10 Hepes-NaOH, 11 glucose, pH 7.4, adjusted with NaOH or HCl.
To test the effects of osmotic challenges on the channels, hypertonic solution was made by adding appropriate quantities of mannitol to the standard external solution, and hypotonicity was achieved by reducing the concentration of NaCl from 140 to ∼90 mm. The control solution for hypotonic solution was made by adjusting the osmolarity to 310 mosm with the appropriate concentration of mannitol. The osmolarities of the solutions were verified by an osmometer (Wescor). Standard internal solution contained (in mm) the following: 140 cesium glutamate, 8 NaCl, 10 Hepes-CsOH, 10 EGTA-CsOH; pH 7.2, adjusted with CsOH or HCl. In some cases, the strong Mg2+ chelator (EDTA) was employed either to entirely remove internal Mg2+ or to attain more accuracy in calculating internal free Mg2+ concentrations. The free Mg2+ levels in pipette solutions were calculated with WebMaxC Standard (Chris Patton, Stanford University). The detailed compositions of the pipette solutions for current recordings are shown in Tables 1–5. All chemicals were from Sigma.
TABLE 1.
Internal solution for Mg2+ dose-response curve of TRPM6 WT, TRPM6 K1804R, and TRPM6 Δkinase (in mm; pH 7.2)
The Cl− concentration among all solutions is ∼30.34 mm.
| Free [Mg2+]i | Cesium glutamate | NaCl | Cesium-Hepes | Cesium-EDTA | MgCl2 | N-methyl-d-glucamine -Cl |
|---|---|---|---|---|---|---|
| 0 | 120 | 8 | 10 | 10 | 0 | 22.34 |
| 0.01 | 120 | 8 | 10 | 10 | 7.58 | 7.18 |
| 0.03 | 120 | 8 | 10 | 10 | 9.07 | 4.20 |
| 0.1 | 120 | 8 | 10 | 10 | 9.79 | 2.76 |
| 0.3 | 120 | 8 | 10 | 10 | 10.19 | 1.96 |
| 0.9 | 120 | 8 | 10 | 10 | 10.86 | 0.62 |
| 1.2 | 120 | 8 | 10 | 10 | 11.17 | 0 |
TABLE 2.
Internal solution for Mg2+ dose-response curve of TRPM7/M6, TRPM7/M6, K1804R, and TRPM7/M6 Δkinase (in mm; pH 7.2)
| Free [Mg2+]i | Cesium glutamate | NaCl | Cesium-Hepes | Cesium-EGTA | MgCl2 |
|---|---|---|---|---|---|
| 0 | 140 | 8 | 10 | 10 | 0 |
| 0.02 | 140 | 8 | 10 | 10 | 0.0298 |
| 0.07 | 140 | 8 | 10 | 10 | 0.105 |
| 0.21 | 140 | 8 | 10 | 10 | 0.313 |
| 0.79 | 140 | 8 | 10 | 10 | 1.167 |
| 1.2 | 140 | 8 | 10 | 10 | 1.761 |
| 1.6 | 140 | 8 | 10 | 10 | 2.334 |
| 3.2 | 140 | 8 | 10 | 10 | 4.568 |
TABLE 3.
Internal solution for Mg·ATP effects on TRPM6 WT, TRPM6 K1804R, and TRPM6 Δkinase (in mm; pH 7.2)
The free Mg2+ is ∼25.4 μm.
| Cesium glutamate | NaCl | Cesium-Hepes | Cesium-EDTA | MgCl2 | Mg·ATP |
|---|---|---|---|---|---|
| 140 | 8 | 10 | 2.00 | 1.8 | 0 |
| 140 | 8 | 10 | 2.70 | 0 | 3 |
| 140 | 8 | 10 | 8.15 | 0 | 9 |
TABLE 4.
Internal solution for Na·ATP effects on TRPM6 WT (in mm; pH 7.2)
The free Mg2+ is ∼20 μm, and the total complexed Mg·ATP under these conditions amounts to 0, 0.5, and 1.4 mm, respectively.
| Cesium glutamate | NaCl | Cesium-Hepes | Cesium-EGTA | MgCl2 | Na·ATP |
|---|---|---|---|---|---|
| 140 | 8 | 10 | 10 | 0.03 | 0 |
| 140 | 8 | 10 | 10 | 0.5 | 3 |
| 140 | 8 | 10 | 10 | 1.45 | 9 |
TABLE 5.
Internal solution for Mg·ATP effects on TRPM7/M6, TRPM7/M6 K1804R, and TRPM7/M6 Δkinase (in mm; pH 7.2)
The free Mg2+ is ∼753 μm, and the total complexed Mg·ATP under these conditions amounts to 0, 4, and 8 mm, respectively.
| Cesium glutamate | NaCl | Cesium-Hepes | Cesium-EGTA | MgCl2 | Mg·ATP |
|---|---|---|---|---|---|
| 140 | 8 | 10 | 10 | 1.11 | 0 |
| 140 | 8 | 10 | 10 | 0.55 | 4.51 |
| 140 | 8 | 10 | 10 | 0 | 9 |
Electrophysiology
Patch clamp experiments were performed under a tight seal whole-cell configuration at room temperature (20–25 °C). High resolution whole-cell currents were recorded by EPC9 (HEKA, Lambrecht, Germany) and Patchmaster version 2.4 (HEKA, Lambrecht, Germany). Unless otherwise stated, all voltages were corrected for a liquid junction potential of 10 mV. Patch pipettes pulled from borosilicate glass had resistances of 1.5–2.5 megohms when filled with internal solutions. Voltage ramps of 50-ms duration spanning a voltage range of −100 to +100 mV were applied to the cells from a holding potential of 0 mV at a rate of 0.5 Hz following the establishment of whole-cell configuration. The currents were filtered at 2.9 kHz and digitized at 10 kHz. Currents were analyzed with FitMaster version 2.11 (HEKA, Lambrecht, Germany) and Igor Pro (Wavemetrics, Portland). Inward currents were extracted at −80 mV and outward currents at +80 mV. Data were normalized to cell size as current density (pA/picofarad). All data are given as means ± S.E.
RESULTS
Differential Pharmacological Modulation of Homomeric and Heteromeric Channel Kinases
Homomeric TRPM6 and TRPM7 have essentially identical I/V characteristics (3, 5), which makes it challenging to functionally differentiate between these two proteins. Interestingly, 2-aminoethoxydiphenyl borate (2-APB) inhibits TRPM7 and facilitates TRPM6 (11). This prompted us to assess 2-APB on heteromeric TRPM7/M6 currents using tetracycline-sensitive HEK-293 cell lines inducibly overexpressing either human TRPM7 (TRPM7) (8, 17), human TRPM6 (TRPM6), or both TRPM7 and TRPM6 co-expressed (TRPM7/M6) (17). The whole-cell patch clamp technique was used with solutions designed to maximally activate the channels by supplementing intracellular solutions with 10 mm EDTA and using Mg2+-free external solutions.
Within 30 s after whole-cell break-in, both TRPM7 (Fig. 1E) and TRPM7/M6 (Fig. 1, C and D) expressing HEK-293 cells (HEK-TRPM7; HEK-TRPM7/M6) developed large currents with the characteristic outwardly rectifying I/V relationship typical for heterologously overexpressed channels. However, cells overexpressing TRPM6 alone had almost identical current sizes and kinetics of current development compared with uninduced cells (Fig. 1F), consistent with previous biochemical analyses on these cells showing that the majority of overexpressed TRPM6 localizes intracellularly (17). These results are also consistent with the observation made in previous attempts to overexpress TRPM6, including various alternative vectors (14, 16, 17). Transient transfection of both TRPM6 and TRPM7 using the pcDNA3.1 vector in HEK-293 cells produced plasma membrane insertion of TRPM6 only in the presence of TRPM7 but not with any other TRPM channel (M1, M2, M4, and M5) (14). Similarly, cloning of TRPM6 and TRPM7 into the pcDNA5/TO-FLAG plasmid confirmed the inability to overexpress TRPM6 alone in the chicken DT40 and HEK-293 cell systems (17). Moreover, an alternative method using cRNA of TRPM6 injected in Xenopus laevis showed that TRPM6 and TRPM7 only overexpress in tandem (14).
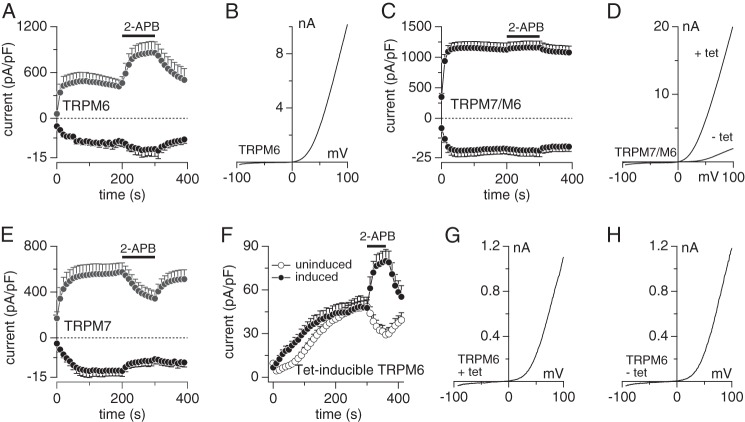
FIGURE 1.
Differential modulation of TRPM6, TRPM7, and TRPM7/M6 channels by 2-APB. Whole-cell currents in HEK-293 cells were measured in an external solution in which Mg2+ was omitted but 1 mm Ca2+ was present to maintain the typical I-V shape. The internal solution was Mg2+-free in the presence of 10 mm EDTA. 200 μm 2-APB was applied to the cells as indicated by the black bars. Currents were analyzed at +80 and −80 mV for outward and inward currents, respectively. Representative I-V curves were extracted before application of 2-APB for respective channel type. A and B, whole-cell measurement of outward and inward TRPM6 currents in wild-type HEK-293 cells transiently transfected with TRPM6 cDNA (n = 7). C, whole-cell measurement of co-overexpressed outward and inward TRPM7/M6 currents in HEK-293 cells induced by tetracycline (n = 8). D shows representative I-V curves for control uninduced TRPM7/M6 cells (− tet) and induced TRPM7/M6 cells (+ tet). E, whole-cell measurement of overexpressed outward and inward TRPM7 currents in HEK-293 cells induced with tetracycline (n = 8). F and G, whole-cell measurement of outward TRPM6 currents in tetracycline-inducible HEK-293 cells in the presence (●, induced, n = 8) or absence (○, uninduced, n = 8) of tetracycline. G shows the typical I-V curve for induced currents (+ tet) prior to 2-APB application. H, typical I-V curve for current controls in uninduced TRPM6 cells prior to 2-APB application (− tet) is shown. pF, picofarad.
We therefore resorted to transient overexpression of TRPM6 in wild-type HEK-293 cells using pCINeo-IRES-GFP-TRPM6 (5), which currently is the only expression vector that results in surface expression of monomeric TRPM6 channels, even if this may not be representative of physiological circumstances in native cells. In agreement with previous reports using this vector (5, 11), cells analyzed 30 h post-transfection developed outwardly rectifying currents that had the typical I/V characteristics of TRPM6 (Fig. 1, A and B) and were at least 12 times larger than endogenously measured TRPM7-like currents (Fig. 1F) (3). Although it remains unclear as to why this particular vector allows the formation of overexpressed TRPM6 channels in the plasma membrane, it enabled us to assess the effect of 2-APB on TRPM6, TRPM7, and TRPM7/M6 using these four heterologous cell systems (transient TRPM6 and inducible TRPM7, TRPM6, and TRPM7/M6). External application of 200 μm 2-APB significantly enhanced TRPM6 currents (Fig. 1, A and B) (11). Conversely, TRPM7 currents were markedly blocked by 200 μm 2-APB (Fig. 1E). When TRPM6 and TRPM7 were co-overexpressed upon tetracycline induction, 200 μm 2-APB was without significant effect on TRPM7/M6 currents (Fig. 1, C and D). Tetracycline induction of HEK-TRPM6 cells did not lead to currents above control levels; however, application of 200 μm 2-APB revealed that TRPM6 indeed can be inserted into the plasma membrane, as currents were facilitated rather than inhibited by this compound (Fig. 1, F and G). These data indicate that the composition of the heteromeric channel kinase protein determines its overall sensitivity to 2-APB, resulting in an intermediate response phenotype that averages the facilitatory and inhibitory effects of 2-APB on the monomeric channels.
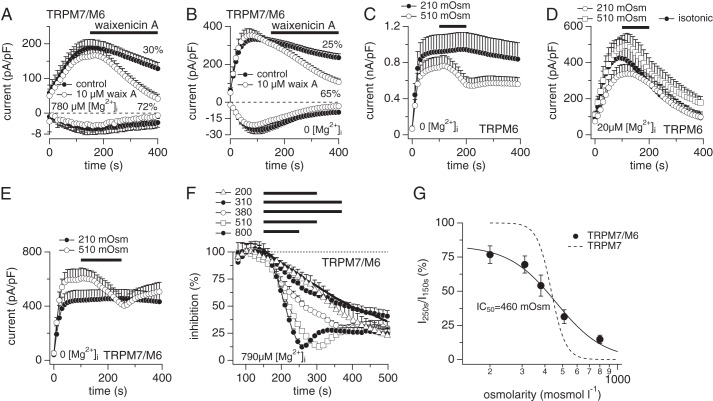
2-APB is an unspecific pharmacological tool interfering with the activity of a variety of ion channels (11, 18–20). In contrast, waixenicin A is a highly potent and specific inhibitor of TRPM7 that does not affect heterologously expressed TRPM6 (21). We tested the compound's efficacy to inhibit overexpressed TRPM7/M6 currents. Perfusing cells with the standard internal solution containing 780 μm free Mg2+ revealed that fully activated TRPM7/M6 currents inactivated by about 30% within 400 s (Fig. 2A). Application of 10 μm waixenicin A decreased TRPM7/M6 currents by 72% during that time, and this effect was also evident in the inward currents assessed at −80 mV (Fig. 2A). Repeating the experiment in the absence of intracellular Mg2+ did not affect inhibition of heteromeric channels by waixenicin A (Fig. 2B), leading to an overall 40% compound-induced inhibition. This is in strong contrast to currents carried by homomeric TRPM7, where intracellular Mg2+ significantly alters the inhibitory efficacy of waixenicin A (21). In addition, TRPM7 currents are completely suppressed within this application time and concentration of waixenicin A. Thus, although TRPM7/M6 heteromeric channels are sensitive to waixenicin A inhibition, they are less so than TRPM7 alone, and the compound's inhibitory effect is uncoupled from intracellular Mg2+ dependence. The differential behavior of waixenicin A on TRPM7, TRPM6, and TRPM7/M6 currents may provide an additional pharmacological tool when characterizing TRPM7-like currents in native cells.
FIGURE 2.
Magnesium-independent waxenicin A inhibition and sensitivity to osmotic gradients. HEK-TRPM7/M6 cells were induced by tetracycline (A, B, E, and F). TRPM6 currents were measured in HEK-293 cells transiently transfected with TRPM6 (C and D). 10 mm EDTA was used to completely remove internal Mg2+ (0 [Mg2+]i, C and E), whereas free Mg2+ was clamped by 10 mm EGTA (D and F). A, whole-cell currents in HEK-293 cells were measured in an external solution in which Mg2+ was omitted but 1 mm Ca2+ was present to maintain the typical I-V shape. The internal solution was clamped to 780 μm [Mg2+]i. Currents were analyzed at +80 and −80 mV for outward and inward currents, respectively. Current development assessed in TRPM7/M6 tetracycline-induced cells was challenged (○ n = 5) or not (● n = 5) with 10 μm waixenicin A as indicated by the black bar. B, current development assessed in TRPM7/M6 tetracycline-induced cells challenged (○ n = 8) or not (● n = 9) with 10 μm waixenicin A in the absence of internal Mg2+. C, TRPM6 currents recorded in the absence of internal Mg2+. The bar indicates application of solutions with low (210 mosm, n = 9) or high osmolarity (510 mosm, n = 9). D, TRPM6 currents recorded in the presence of 20 μm Mg2+. The bar indicates application of isotonic (310 mosm, n = 11), hypotonic (210 mosm, n = 8), or hypertonic solutions (510 mosm, n = 9). E, TRPM7/M6 currents in response to hypotonic (210 mosm, n = 5) or hypertonic challenge (510 mosm, n = 5) in the absence of internal Mg2+. F and G, normalized TRPM7/M6 currents measured in the presence of 790 μm Mg2+. All currents were normalized to maximum currents at 100 s after break-in. Bars indicate application of various solutions with different osmolarity. The application was started at 150 s but terminated variably depending upon the tested osmolarity. Note that the solid black line is the control for hypotonic solution in which the osmolarity was normalized to 310 mosm with mannitol. Data plots in the dose-response curve represent n = 7, 12, 9, 10, 6 cells for 200, 310, 380, 510, 800 mosm, respectively. The ratios of currents (250 s versus the starting point of application at 150 s) were plotted versus the osmotic gradients (E). Dashed line represents the dose-response curve for the osmosensitivity of TRPM7 channels (reproduced from Ref. 22). pF, picofarad.
Sensitivity of TRPM6 and TRPM7/M6 Channels to Hypo- and Hyper-osmolarity
TRPM7 is sensitive to osmotic challenges in human kidney cells (22). Because TRPM6 is predominantly expressed in the gut and kidney where osmotic changes are common, we tested whether TRPM6 would also be osmosensitive. Considering that intracellular Mg2+ availability has significant impacts on channel functionality, we delivered the osmotic changes in the absence and presence of intracellular Mg2+. In the presence of 10 mm EDTA, TRPM6 currents were not affected by hypotonic solutions (210 mosm, Fig. 2C), whereas hypertonic solutions slightly reduced the currents by 30% (510 mosm, Fig. 2C). However, an increase in series resistance as a result of cell shrinkage was observed when the cells were exposed to hypertonic solution, which may account for this small decrease in TRPM6 currents. In the presence of 20 μm Mg2+ (Fig. 2D), neither hypotonicity (210 mosm) nor hypertonicity (510 mosm) had an effect on TRPM6 compared with corresponding controls (isotonic). Collectively, TRPM6 is largely insensitive to osmotic gradients, which is in marked contrast to TRPM7.
We next examined the responses of the TRPM7/M6 heteromer to osmotic challenges. In the absence of intracellular Mg2+, TRPM7/M6 behaved similarly to TRPM6 in response to osmotic changes in that 210 mosm had no effect on currents, and 510 mosm induced a small reduction of currents by about 30% (Fig. 2E). However, under conditions where internal free Mg2+ was adjusted to more physiological levels of 790 μm, TRPM7/M6 regained sensitivity to osmotic gradients in a dose-dependent manner (Fig. 2F). The fitted dose-response curve yielded an IC50 of 460 mosm for osmolarity-mediated suppression of TRPM7/M6 (Fig. 2G, Hill = 3.3), similar to the IC50 of 430 mosm for TRPM7 (22). However, TRPM7/M6 currents were sensitive to osmolarity over a wider range of osmotic gradients compared with TRPM7, as indicated by their distinctly different Hill coefficients (3.3 versus 11, respectively; Fig. 2G). This suggests that Mg2+ is required for the osmosensitivity of TRPM7/M6 and that heteromeric complex formation results in a more graded response to osmotic change without changing the midpoint of osmotic sensitivity. As will be shown below, this is consistent with the preserved sensitivity of heteromeric channels to intracellular Mg2+, as a significant factor in mediating osmosensitivity is caused by molecular crowding of inhibitory molecules due to changes in cell volume (22).
Inhibition of Homomeric and Heteromeric Channel Kinases by Intracellular Mg2+
Intracellular Mg2+ regulates both TRPM6 and TRPM7 (3, 5, 8, 10). The IC50 for Mg2+-dependent TRPM7 inhibition in the HEK-293 overexpression system is 720 μm in the absence of Mg·ATP (10). However, a complete Mg2+ dose-response curve has not yet been determined for native TRPM7 currents in wild-type HEK-293. RT-PCR established that wild-type HEK-293 cells express TRPM7 but did not detect significant TRPM6 (Fig. 3C), suggesting that endogenous conductances are mediated exclusively by TRPM7. As shown in Fig. 3, A and B, native TRPM7-like currents were inhibited by intracellular free Mg2+ with an IC50 of 569 μm, resembling that of heterologously expressed TRPM7 (Fig. 3F) (10). As for TRPM6, Voets et al. (5) reported an IC50 of 510 μm for Mg2+ block as determined by means of flash photolysis of caged Mg2+. We re-assessed the Mg2+ sensitivity of TRPM6 using our standard whole-cell patch clamp approach to allow comparison between data sets obtained from TRPM7, TRPM6, and TRPM7/M6. We used 10 mm EDTA as the internal Mg2+ chelator, and MgCl2 was calculated to achieve the desired free Mg2+ concentrations. Without internal Mg2+, transiently overexpressed TRPM6 currents assessed in HEK-293 cells rapidly activated within 30 s after break-in (Figs. 1A and 3D). Just 10 μm Mg2+ significantly suppressed the current size of TRPM6, and 300 μm Mg2+ almost abolished the currents (Fig. 3D). The fitted dose-response curve yielded an IC50 of 29 μm with a Hill coefficient of 0.7 (Fig. 3F), which is almost 25-fold more sensitive compared with TRPM7 (IC50 = 720 μm) (10). We next investigated the Mg2+ block of TRPM7/M6 heteromers in the tetracycline-inducible system (17), and we determined the Mg2+ dose-response curve with intracellular free Mg2+ buffered with EGTA to various concentrations. As shown in the Fig. 3E, intracellular Mg2+ dose-dependently blocked TRPM7/M6 currents. The IC50 fitted from the dose-response curve for TRPM7/M6 approximated 466 μm with a Hill coefficient of 0.8 (Fig. 3F). The differences in IC50 values between native and overexpressed TRPM7 as well as TRPM7/M6 did not amount to statistical significance, although there might be a trend to slightly higher sensitivity of heteromeric channels to Mg2+ compared with monomeric TRPM7. However, TRPM7·M6 complexes are dramatically less sensitive to Mg2+ (∼16-fold decrease) than TRPM6 homomers.
FIGURE 3.
Suppression of native TRPM7 and overexpressed TRPM6 and TRPM7/M6 channels by Mg2+. Native whole-cell TRPM7 currents were assessed in wild-type HEK-293 cells. Whole-cell TRPM6 currents (transiently overexpressed in wild-type HEK-293 cells) were recorded in Mg2+-free external solutions, whereas TRPM7/M6 currents (induced by tetracycline) were measured in normal external solution containing 2 mm Mg2+. The chelator EDTA was used to clamp free Mg2+ levels for TRPM6 currents. EGTA was the Mg2+ chelator for native TRPM7 currents and induced TRPM7/M6 currents. The detailed composition of the pipette solutions for current recording is shown in Tables 1 and 2. A and B, Mg2+ sensitivity of native TRPM7 currents assessed in wild-type HEK-293 cells by whole-cell patch clamping. Plots represent n = 7, 8, 10, 11, 4, and 4 cells for 0, 70, 210, 790, 1600, and 3200 μm Mg2+, respectively. A dose-response fit rendered an IC50 of 569 μm with a Hill coefficient of 1.27 (peak currents extracted at 434 s). C, RT-PCR analysis of the expression of native TRPM6 (546 bp) and native TRPM7 (519 bp) in HEK-293 wild-type cell line, as well as the housekeeping gene, GAPDH (532 bp) (2nd and 3rd lanes). The gene-specific primers for TRPM6 and TRPM7 amplified the same sized bands from heterologously expressed human TRPM6 and TRPM7 plasmids, respectively (5th and 6th lanes). D, whole-cell patch clamp analysis of Mg2+ block of transiently transfected TRPM6. The number of patched cells is 7, 6, 5, 5, 6, 5, and 7 for 0, 10, 30, 100, 300, 900, and 1200 μm Mg2+, respectively. E, whole-cell patch clamp analysis of Mg2+ block of TRPM7/M6 currents. Plots represent n = 12, 7, 7, 7, 11, 8, 6, and 5 cells for 0, 20, 70, 210, 790, 1200, 1600, and 3200 μm Mg2+, respectively. F, dose-response curves of Mg2+-mediated suppression of native TRPM7 (long-dashed line taken from B and normalized to 1), overexpressed TRPM7 (small-dashed line reproduced from Ref. 10 and normalized to 1), TRPM6 (extracted at 100 s, closed circles, Hill = 0.69), and TRPM7/M6 currents (extracted at 120 s, open circles, Hill = 0.8). pF, picofarad.
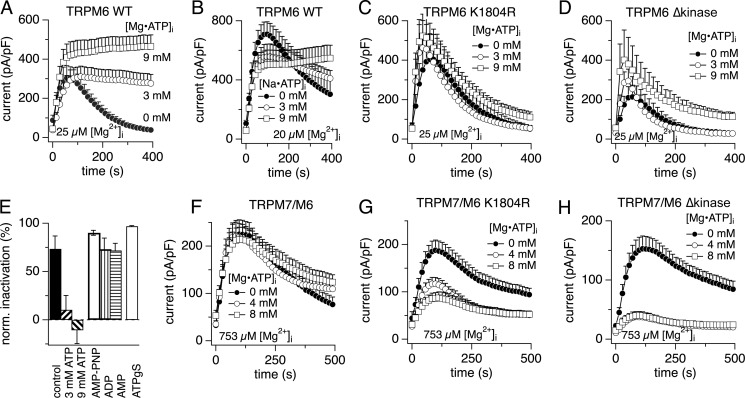
Previous work has demonstrated that the Mg2+ sensitivity of monomeric TRPM7 channels is affected by the kinase domain, as kinase-dead point mutations reduce and kinase deletion mutants increase Mg2+ sensitivity (8). The following two approaches were utilized to eliminate the function of TRPM6 kinase: mutation of a critical amino acid for the phosphotransferase activity (K1804R, TRPM6-K1804R) and removal of the entire kinase domain (TRPM6-Δkinase) in the overexpressed proteins (17). We first determined the Mg2+ sensitivity of both homomeric TRPM6-K1804R and TRPM6-Δkinase channels. Both of these TRPM6 mutants were strongly suppressed by Mg2+ similar to TRPM6-WT (Fig. 4, A and B). Current normalization revealed that TRPM6-K1804R is slightly less sensitive to Mg2+, although TRPM6-Δkinase is more sensitive compared with TRPM6-WT (Fig. 4C). This clarifies that TRPM6-WT and its kinase mutants all remain highly sensitive to Mg2+ block.
FIGURE 4.
Mg2+ sensitivity of TRPM6 phosphotransferase activity-deficient point mutant (TRPM6-K1804R) and TRPM6-Δkinase alone and in channel complex with TRPM7. The detailed composition of the pipette solutions for current recording is shown in Tables 1 and 2. A, whole-cell patch clamp assessment of Mg2+ block of TRPM6-K1804R currents. B, whole-cell patch clamp assessment of Mg2+ block of TRPM6-Δkinase currents. C, dose-response curves for Mg2+ suppression of whole-cell currents carried by various TRPM6 constructs. Data represent peak currents at the indicated Mg2+ concentrations normalized to the maximum currents attained at 0 Mg2+. Peak currents extracted at 100, 48, and 48 s were averaged for dose-response analysis for TRPM6 WT, TRPM6 K1804R, and TRPM6 Δkinase, respectively. Note that the plots of TRPM6 WT are derived from Fig. 2D. Plots represent n = 7, 6, 5, 5, 6, and 5, experiments (TRPM6 WT, 0, 10, 30, 100, 300, and 900 μm Mg2+, respectively), n = 5, 5, 5, 5, 5, and 5, experiments (K1804R, 0, 10, 30, 100, 300, and 900 μm Mg2+, respectively), and n = 5, 8, 6 experiments (Δkinase, 0, 30, 100 μm Mg2+, respectively). D–F are from heteromeric channels where HEK-TRPM7/M6-K1804R cells and HEK-TRPM7/M6-Δkinase cells were induced by tetracycline to co-overexpress TRPM7/M6-K1804R and TRPM7/M6-Δkinase, respectively. The internal and external solutions for current recordings (D and E) are the same as those for TRPM7/M6 in Fig. 2E. D, whole-cell patch clamp analysis of Mg2+ inhibition of TRPM7/M6 K1804R currents. Pipette solution contained the indicated concentrations of free Mg2+. Plots represent n = 8, 9, 6, 6, 6, and 5 cells for 0, 20, 210, 790, 1600, and 3200 μm Mg2+, respectively. E, whole-cell patch clamp assessment of Mg2+ suppression of induced TRPM7/M6 Δkinase currents. Plots represent n = 5, 5, 5, 7, 6, and 5 cells for 0, 20, 210, 790, 1600, and 3200 μm Mg2+, respectively. F, dose-response curves for Mg2+ suppression of TRPM7/M6 K1804R (peak currents extracted at 76 s, Hill = 1.34) and TRPM7/M6 Δkinase currents (extracted at 136 s Hill = 1.28). pF, picofarad.
Next, we analyzed heteromeric combinations of these subunits with TRPM7 and constructed dose-response curves for TRPM7/M6-KR and TRPM7/M6-Δkinase. As shown in Fig. 4, D and E, TRPM7/M6-KR currents and TRPM7/M6-Δkinase currents were dose-dependently suppressed by intracellular Mg2+ but with significantly lower sensitivity compared with homomeric assemblies of TRPM6 with an apparent IC50 of 654 and 638 μm, respectively (Fig. 4F). Thus, loss of function of the TRPM6 kinase rendered TRPM7/M6-KR slightly less sensitive to Mg2+ block than TRPM7/M6-WT (IC50 = 466 μm), with a phenotype reminiscent of TRPM7 (10). Even though the slight rightward shifts in sensitivity of the kinase-deficient mutants were not statistically significant, they appear to reverse the slight leftward trend observed in the TRPM7/M6-WT combination, despite their strong Mg2+ sensitivity when expressed as homomers.
TRPM6 Kinase Domain Confers Insensitivity of TRPM7/M6 Heteromeric Channels to Intracellular Mg·ATP
Mg·ATP is an important regulator involved in setting TRPM7 activity that synergizes with free Mg2+ to suppress TRPM7 (10). With intracellular free Mg2+ at 780 μm, the IC50 for Mg·ATP inhibition of TRPM7 currents is around 2 mm. In contrast, modulation of TRPM6 by intracellular ATP reportedly occurs independent of Mg2+ with an IC50 of 1.3 mm and requires an intact kinase domain (13). Both TRPM7 and TRPM6 kinases need the presence of some Mg2+ to maintain phosphotransferase activity (3, 8, 23, 24). We therefore adopted an experimental strategy aimed at preserving the kinase activity by including low levels of free Mg2+ in the pipette solution. Based on the obtained IC50 value for Mg2+-mediated TRPM6 suppression, internal free Mg2+ was set to 25 and 20 μm for testing Mg·ATP and Na·ATP, respectively. Surprisingly, and in contrast to a previous study (13), we found that under these conditions Mg·ATP (3 or 9 mm) was completely ineffective to suppress transiently expressed TRPM6 currents (Fig. 5A). Not only that, but the currents remained stable after reaching a plateau, suggesting that ATP prevents current run down and thus may play a role in potentiating channel function. Similar to Mg·ATP, inclusion of Na·ATP (3 and 9 mm) in the patch pipette did not block TRPM6 currents but rather resulted in removal of current inactivation (Fig. 5B). Because activation and inactivation processes may compete with each other depending on the presence of ATP and Mg2+, a greater spread of maximal current amplitudes across individually patched cells can be observed in Fig. 5, A and B. We conclude that Na·ATP and Mg·ATP have similar effects on TRPM6; they do not inhibit TRPM6 homomeric channels and remove TRPM6 inactivation. We have no simple explanation for the conflicting observations made here compared with those of a previous study that found a Mg2+-independent inhibition of TRPM6 with an IC50 of 1.3 mm (13), which would indicate an even higher ATP sensitivity than that of TRPM7. As will be shown below, we not only see the absence of ATP-dependent inhibition in homomeric TRPM6, but this property of TRPM6 is also conferred on heteromeric TRPM7/M6 channels.
FIGURE 5.
Mg·ATP does not affect the TRPM7·M6 channel complex and rescues TRPM6 from inactivation. Transiently overexpressed TRPM6 currents were measured in HEK-293 cells and in Mg2+-free external solutions (A–D), whereas tetracycline-inducible currents of TRPM7 plus various TRPM6 mutants were recorded in normal external solution containing 2 mm Mg2+ and 1 mm Ca2+ (F–H). The intracellular free Mg2+ for TRPM6 current recording was 25.4 μm (A, C, and D; see Table 3 for composition of pipette solutions) or 20 μm (B; see Table 4 for composition of pipette solutions) and was 753 μm for heteromeric channels of TRPM7 plus TRPM6 (F–H; see Table 5 for composition of pipette solutions). A, HEK-293 cells transiently overexpressing TRPM6 were dialyzed with pipette solutions containing the indicated concentrations of Mg·ATP. Plots represent n = 14, 11, and 10 cells for 0, 3, and 9 mm Mg·ATP, respectively. B, Na·ATP rather than Mg·ATP was tested in TRPM6 WT. Plots were averaged from 10, 10, and 9 cells for 0, 3, and 9 mm Mg·ATP, respectively. C, TRPM6 K1804R mutant was transiently overexpressed. Plots represent n = 7, 7, and 9 cells for 0, 3, and 9 mm Mg·ATP, respectively. D, effects of Mg·ATP on the TRPM6 Δkinase mutant were tested in HEK-293 cells transiently overexpressing the mutant channel. Internal solutions had the same composition as in A and C. Plots represent n = 7, 8, and 9 cells for 0, 3, and 9 mm Mg·ATP, respectively. E, normalized to current peak percent inactivation of transiently expressed TRPM6 WT in HEK-293. Cells were perfused with either no addition of Mg·ATP (control, n = 14, see A), 3 mm (n = 11, A), or 9 mm Mg·ATP (n = 10, A) and 3 mm AMP-PNP (n = 13), 3 mm ADP (n = 9), 3 mm AMP (n = 12), or 300 μm ATPγS (n = 9). F, whole-cell TRPM7/M6 currents measured at 753 μm Mg2+ under the indicated conditions. Plots are representative of 14, 19, and 19 cells for 0, 4, and 8 mm Mg·ATP, respectively. G, whole-cell currents of TRPM7/M6 K1804R co-expressed mutant channel complex measured at 753 μm Mg2+ under the indicated conditions. Plots represent n = 17, 14, and 16 cells for 0, 4, and 8 mm Mg·ATP, respectively. H, whole-cell recording of tetracycline-induced TRPM7/M6 Δkinase mutant complex currents at 753 μm free Mg2+ under the indicated conditions. Note that the experimental conditions in E–G are identical. The number of patched cells is 9, 13, and 9 for 0, 4, and 8 mm Mg·ATP, respectively. pF, picofarad.
We next asked how ATP might remove the inactivation of TRPM6 and considered the possible mechanisms of Mg·ATP on TRPM6 currents, as Mg·ATP is the physiological form of ATP in the cell. ATP-mediated removal of TRPM6 inactivation could potentially be caused by ATP hydrolysis through the kinase domain, be secondary to the generation of ATP metabolites ADP or AMP, or be due to the physical occupation of ATP-binding sites. Two nonhydrolysable structural analogs of ATP, ATPγs (0.3 mm) and AMP-PNP (3 mm), failed to prevent TRPM6 inactivation (Fig. 5E), indicating that ATP binding alone is not responsible for ATP-mediated sustained opening of TRPM6. The intracellular administration of the ATP metabolites AMP (3 mm) or ADP (3 mm) were similarly ineffective (Fig. 5E). Taken together, this suggests that ATP removes TRPM6 inactivation by a mechanism that is dependent upon ATP hydrolysis.
To investigate whether the kinase domain is involved in ATP-mediated regulation of TRPM6, we tested the effects of Mg·ATP on kinase activity-deficient TRPM6 mutants transiently expressed in wild-type HEK-293 cells. After whole-cell break-in TRPM6-K1804R currents developed in a similar manner as TRPM6-WT currents followed by almost complete inactivation over the time course of the experiment (Fig. 5C). Unlike its effects in TRPM6-WT, internal Mg·ATP of 3 and 9 mm did not stabilize TRPM6 activity (Fig. 5C). Similar results were obtained when investigating the behavior of TRPM6-Δkinase currents, although the overall current density was smaller in this mutant (Fig. 5D). Thus, it appears that a functional kinase domain with intact phosphotransferase activity is responsible for ATP hydrolysis and maintenance of TRPM6 function.
Mg·ATP regulation of heteromeric TRPM7/M6 was examined by using pipette solutions with higher Mg2+ levels (753 μm) around the IC50 for free Mg2+. Surprisingly, even in the presence of intracellular Mg2+, TRPM7/M6 currents were not affected by perfusion of cells with physiological (4 mm) and even supra-physiological (8 mm) intracellular Mg·ATP (Fig. 5F). This indicates that the formation of heteromeric TRPM7/M6 uncouples channel kinases from cellular energy metabolism. Because the kinase domain of TRPM6 is able to cross-phosphorylate TRPM7 but not vice versa (17), we hypothesized that regulation of TRPM7 by the TRPM6 kinase might be responsible for the functional characteristics of TRPM7/M6 and that the TRPM6 kinase domain could be involved in setting the Mg·ATP sensitivity of the heteromeric channel complex. We tested this in kinase-deficient TRPM6 mutant constructs TRPM6-Δkinase and TRPM6-K1804R (17). Fig. 5H demonstrates that in contrast to TRPM7/M6-WT, TRPM7/M6-K1804R currents were now inhibited by Mg·ATP in a dose-dependent fashion. Furthermore, removal of the TRPM6 kinase domain (TRPM6-Δkinase) made heteromeric channel currents even more sensitive to intracellular Mg·ATP (Fig. 5H). This indicates that TRPM6 kinase-mediated cross-phosphorylation of TRPM7 is mandatory to render TRPM7/M6 heteromers insensitive to cellular ATP fluctuations.
DISCUSSION
We have found that homomeric TRPM6 channels and heteromeric TRPM7/M6 channels exhibit a number of functional and pharmacological differences and are regulated very differently by free Mg2+ and Mg·ATP in comparison with homomeric TRPM7 (see Table 6). Particularly, the regulation by Mg·ATP requires a functional TRPM6 kinase domain.
TABLE 6.
Summary of salient effects of various regulators of TRPM7, TRPM6, and TRPM7/M6
Data are taken from this work unless indicated by reference.
| Regulation by | TRPM7 | TRPM7/M6 | TRPM6 |
|---|---|---|---|
| Free Mg2+ (IC50) | 720 μm (10) | 466 μm | 29 μm |
| Mg·ATP (IC50) | 2–3 mm (10) | No effect | No effect |
| Osmolarity (IC50/Hill coefficient) | 430 mosm/11 (22) | 460 mosm/3.3 | No effect |
| 2-APB (200 μm) | Block | No effect | Facilitation |
| Waixenicin A (10 μm) | Full block (21) | Partial block | No effect (21) |
Robust TRPM6 currents could reliably be measured in HEK-293 cells transiently transfected with human TRPM6 in pCINeo-IRES-GFP vector. These findings are in line with other reports that used the same TRPM6 construct (5, 13, 26, 27). In contrast to the transient expression system, we failed to detect markedly elevated TRPM6 channel activity in the tetracycline-inducible stable TRPM6 HEK-293. Here, a previous study demonstrated that without sufficient TRPM7 subunits available, TRPM6 is largely retained intracellularly (28). Similarly, TRPM6 overexpression using the expression vectors pcDNA3.1 or pINC(SP1)/Hygro did not result in TRPM6 plasma membrane translocation and neither did cRNA injection into Xenopus oocytes based on the pOGII vector (29, 30). We currently cannot explain why the only system that seems to drive translocation of TRPM6 to the plasma membrane without the help of TRPM7 is through the pCINeo-IRES-GFP vector. It would be interesting to see whether the noncoding sequence of the TRPM6-pCINeo-IRES-GFP expression construct influences assembly and trafficking of the TRPM6 channel subunits.
Despite these conflicting findings, the highly efficient membrane expression of TRPM6 using the pCINeo-IRES-GFP vector for transient TRPM6 expression provided an opportunity to examine the modulation of homomeric TRPM6 by various physiological regulators, such as intracellular Mg2+ and Mg·ATP. We examined the Mg2+ sensitivity of homomeric TRPM6 by perfusing cells with defined buffered intracellular Mg2+ solutions and obtained an IC50 of ∼30 μm. This is at variance with the IC50 of 510 μm reported previously (5). However, the latter IC50 for Mg2+ might be due to the different experimental approach using flash photolysis of Mg2+. Because we here found large differences in Mg2+ sensitivity between TRPM7 and TRPM6 under identical experimental conditions, we are confident that the higher Mg2+ sensitivity of TRPM6 is a genuine feature of the channel. Physiological levels of intracellular Mg2+ are thought to range between 0.5 and 1 mm. This would suggest that homomeric TRPM6 channels, even if expressed natively in epithelial cells, might play a rather limited role as a physiologically functional ion channel.
In contrast to the strong Mg2+ sensitivity of TRPM6, Mg·ATP did not suppress TRPM6 currents at all and rather prevented channel inactivation. This is in contrast to previous work reporting that both Na·ATP and Mg·ATP dose-dependently blocked TRPM6 currents in EDTA-buffered, Mg2+-free intracellular pipette solutions (13). This discrepancy might be due to absence of intracellular Mg2+ in the latter study, as Mg2+ not only acts as an inhibitory modulator of channel kinases, but also a minimal amount of Mg2+ seems to be required for proper function of TRPM6 and TRPM7. For example, early evidence uncovered the synergistic regulation of TRPM7 channel activity by magnesium and Mg·ATP, where a threshold magnesium concentration of at least 200 μm is needed before inhibition of TRPM7 by ATP is gained (31). Furthermore, intracellular Mg2+ dictates the inhibitory effectiveness of halides as well as waixenicin A in both TRPM6 and TRPM7 (32, 33), and free Mg2+ is required for the phosphotransferase activity of the TRPM7 channel kinase (23). Hence, complete removal of Mg2+, or even Zn2+ bound to the kinase domain by a strong chelator such as EDTA, might impair channel function and/or regulation.
Our data show that TRPM6 inactivation was completely removed by ATP, implying that ATP provides a positive regulatory mechanism to maintain TRPM6 function. ATP seems to remove TRPM6 inactivation through kinase-dependent ATP hydrolysis, as kinase activity-deficient TRPM6 mutants inactivated independently of the presence or absence of intracellular ATP. Autophosphorylation of TRPM6 by its kinase domain could be one mechanism to prevent current inactivation. Thus, washout of intracellular ATP during whole-cell patch clamp measurements would result in channel dephosphorylation and lead to channel inactivation, whereas the supply of ATP would enable the TRPM6 channel kinase to hydrolyze ATP and maintain the phosphorylation on TRPM6 channels for sustained channel activity. Indeed, massive autophosphorylation has been detected in the serine/threonine-rich region of TRPM6 following ATP stimulation (34). However, further experiments are required to fully understand the mechanisms underlying ATP-mediated effects, for instance, identification of the specific amino acid targets that are autophosphorylated by the kinase domain.
Unlike other TRP channels that form heteromultimers with their close homologs, including TRPC1/4/5 (35), TRPC3/6/7 (35), TRPV5/V6 (36), and TRP1/3 (37), TRPM7 and TRPM6 possess highly homologous Ser/Thr kinase domains at their C termini. It has previously been shown that the TRPM6 kinase is able to cross-phosphorylate TRPM7 but not vice versa (28). Consistent with this observation, we found that disruption of TRPM6 kinase activity attenuated Mg2+-mediated suppression and restored sensitivity to Mg·ATP inhibition in the channel complex of TRPM7 with kinase-defective TRPM6 mutants. Thus, the phenotype of this kinase mutant is reminiscent of homomeric TRPM7, suggesting that TRPM6 shapes the phenotype of TRPM7/M6 via cross-phosphorylation of TRPM7.
To date, the molecular mechanisms governing the assembly of TRPM7 and TRPM6 remain unknown. Based on the structure of TRPM proteins, TRPM7/M6 most likely functions as a heterotetrameric channel complex. Indeed, emerging evidence suggests that both TRPM7 and TRPM6 subunits contribute to the heteromeric channel pore (27, 29, 30), although the exact stoichiometric architecture of TRPM7/M6 remains to be determined (38).
A model where TRPM6 co-assembles with TRPM7 to form a functional Mg2+-influx channels is attractive due to the overlapping expression profile of TRPM7 and TRPM6 (24, 28, 29, 40). TRPM7 is a ubiquitously expressed ion channel, whereas the tissue expression of TRPM6 is much more restricted, predominantly in the intestine and kidney. A growing number of examined cell lines and tissues have been shown to co-express TRPM7 and TRPM6 (25, 29, 39). Our electrophysiological results from the functional point of view support a refined model and provide insights into characteristics and physiological regulation of the TRPM7·M6 channel complex. First, the IC50 value of 466 μm for Mg2+ suppression suggests that TRPM7/M6 may be functional within the range of physiological levels of Mg2+. Second, the complexed channel responds to osmotic challenges with similar sensitivity as TRPM7, but in a more graded fashion, therefore preserving its proposed Mg2+ transport efficiency in tissues where large osmotic changes are common. Third, like TRPM6, the heteromeric channel complex is not regulated by cellular ATP, thus leaving channel kinase-mediated divalent ion influx unaffected by cellular energy metabolism. In comparison with homomeric TRPM7, whose activity is strongly down-regulated by physiological levels of intracellular Mg·ATP, heteromeric TRPM7/M6 may be more efficient in regard to the transport of Mg2+ and other divalent ions. These features indicate that the TRPM7/M6 channel may be more efficient to function as a divalent ion influx channel than either TRPM7 or TRPM6 alone. This may in turn affect cellular functions both physiologically and patho-physiologically, e.g. in the case of tumor cell growth.
Acknowledgments
We thank S. Johne, L. Tsue, M. K. Monteilh-Zoller, and C. Maggio for excellent technical support. We thank Dr. David Horgen for provision of waixenicin A. We are also grateful to Dr. Alexey Ryazanov and Dr. René J. M. Bindels for kindly providing pCINeo-IRES-GFP-TRPM6 construct and Dr. Joost Hoenderop for sharing TRPM6 K1804R and TRPM6 Δkinase mutants.
This work was supported, in whole or in part, by National Institutes of Health Grants 5R01GM090123 (to C. S.) and P01 GM078195 (to A. F.) This work was also supported by a private donation from Amy Chong (to A. F.).
- TRP
- transient receptor potential
- 2-APB
- 2-aminoethoxydiphenyl borate
- tet
- tetracycline
- ATPγS
- adenosine 5′-O-(thiotriphosphate)
- AMP-PNP
- adenosine 5′-(β,γ-imino)triphosphate.
REFERENCES
- 1. Walder R. Y., Landau D., Meyer P., Shalev H., Tsolia M., Borochowitz Z., Boettger M. B., Beck G. E., Englehardt R. K., Carmi R., Sheffield V. C. (2002) Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat. Genet. 31, 171–174 [DOI] [PubMed] [Google Scholar]
- 2. Schlingmann K. P., Weber S., Peters M., Niemann Nejsum L., Vitzthum H., Klingel K., Kratz M., Haddad E., Ristoff E., Dinour D., Syrrou M., Nielsen S., Sassen M., Waldegger S., Seyberth H. W., Konrad M. (2002) Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat. Genet. 31, 166–170 [DOI] [PubMed] [Google Scholar]
- 3. Nadler M. J., Hermosura M. C., Inabe K., Perraud A. L., Zhu Q., Stokes A. J., Kurosaki T., Kinet J. P., Penner R., Scharenberg A. M., Fleig A. (2001) LTRPC7 is a Mg·ATP-regulated divalent cation channel required for cell viability. Nature 411, 590–595 [DOI] [PubMed] [Google Scholar]
- 4. Runnels L. W., Yue L., Clapham D. E. (2001) TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 291, 1043–1047 [DOI] [PubMed] [Google Scholar]
- 5. Voets T., Nilius B., Hoefs S., van der Kemp A. W., Droogmans G., Bindels R. J., Hoenderop J. G. (2004) TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J. Biol. Chem. 279, 19–25 [DOI] [PubMed] [Google Scholar]
- 6. Monteilh-Zoller M. K., Hermosura M. C., Nadler M. J., Scharenberg A. M., Penner R., Fleig A. (2003) TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J. Gen. Physiol. 121, 49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paravicini T. M., Chubanov V., Gudermann T. (2012) TRPM7: a unique channel involved in magnesium homeostasis. Int. J. Biochem. Cell Biol. 44, 1381–1384 [DOI] [PubMed] [Google Scholar]
- 8. Schmitz C., Perraud A.-L., Johnson C. O., Inabe K., Smith M. K., Penner R., Kurosaki T., Fleig A., Scharenberg A. M. (2003) Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell 114, 191–200 [DOI] [PubMed] [Google Scholar]
- 9. Penner R., Fleig A. (2007) The Mg2+ and Mg2+-nucleotide-regulated channel-kinase TRPM7. Handb. Exp. Pharmacol. 179, 313–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Demeuse P., Penner R., Fleig A. (2006) TRPM7 channel is regulated by magnesium nucleotides via its kinase domain. J. Gen. Physiol. 127, 421–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li M., Jiang J., Yue L. (2006) Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J. Gen. Physiol. 127, 525–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li M., Du J., Jiang J., Ratzan W., Su L.-T., Runnels L. W., Yue L. (2007) Molecular determinants of Mg2+ and Ca2+ permeability and pH sensitivity in TRPM6 and TRPM7. J. Biol. Chem. 282, 25817–25830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thébault S., Cao G., Venselaar H., Xi Q., Bindels R. J., Hoenderop J. G. (2008) Role of the α-kinase domain in transient receptor potential melastatin 6 channel and regulation by intracellular ATP. J. Biol. Chem. 283, 19999–20007 [DOI] [PubMed] [Google Scholar]
- 14. Chubanov V., Waldegger S., Mederos y Schnitzler M., Vitzthum H., Sassen M. C., Seyberth H. W., Konrad M., Gudermann T. (2004) Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc. Natl. Acad. Sci. U.S.A. 101, 2894–2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chubanov V., Mederos y Schnitzler M., Wäring J., Plank A., Gudermann T. (2005) Emerging roles of TRPM6/TRPM7 channel kinase signal transduction complexes. Naunyn Schmiedebergs Arch. Pharmacol. 371, 334–341 [DOI] [PubMed] [Google Scholar]
- 16. Chubanov V., Schlingmann K. P., Wäring J., Heinzinger J., Kaske S., Waldegger S., Mederos y Schnitzler M., Gudermann T. (2007) Hypomagnesemia with secondary hypocalcemia due to a missense mutation in the putative pore-forming region of TRPM6. J. Biol. Chem. 282, 7656–7667 [DOI] [PubMed] [Google Scholar]
- 17. Schmitz C., Dorovkov M. V., Zhao X., Davenport B. J., Ryazanov A. G., Perraud A.-L. (2005) The channel kinases TRPM6 and TRPM7 are functionally nonredundant. J. Biol. Chem. 280, 37763–37771 [DOI] [PubMed] [Google Scholar]
- 18. Peinelt C., Lis A., Beck A., Fleig A., Penner R. (2008) 2-Aminoethoxydiphenyl borate directly facilitates and indirectly inhibits STIM1-dependent gating of CRAC channels. J. Physiol. 586, 3061–3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang S. L., Kozak J. A., Jiang W., Yeromin A. V., Chen J., Yu Y., Penna A., Shen W., Chi V., Cahalan M. D. (2008) Store-dependent and -independent modes regulating Ca2+ release-activated Ca2+ channel activity of human Orai1 and Orai3. J. Biol. Chem. 283, 17662–17671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hu H., Grandl J., Bandell M., Petrus M., Patapoutian A. (2009) Two amino acid residues determine 2-APB sensitivity of the ion channels TRPV3 and TRPV4. Proc. Natl. Acad. Sci. U.S.A. 106, 1626–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zierler S., Yao G., Zhang Z., Kuo W. C., Pörzgen P., Penner R., Horgen F. D., Fleig A. (2011) Waixenicin A inhibits cell proliferation through magnesium-dependent block of transient receptor potential melastatin 7 (TRPM7) channels. J. Biol. Chem. 286, 39328–39335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bessac B. F., Fleig A. (2007) TRPM7 channel is sensitive to osmotic gradients in human kidney cells. J. Physiol. 582, 1073–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ryazanova L. V., Dorovkov M. V., Ansari A., Ryazanov A. G. (2004) Characterization of the protein kinase activity of TRPM7/ChaK1, a protein kinase fused to the transient receptor potential ion channel. J. Biol. Chem. 279, 3708–3716 [DOI] [PubMed] [Google Scholar]
- 24. Runnels L. W. (2011) TRPM6 and TRPM7: A Mul-TRP-PLIK-cation of channel functions. Curr. Pharm. Biotechnol. 12, 42–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Groenestege W. M., Hoenderop J. G., van den Heuvel L., Knoers N., Bindels R. J. (2006) The epithelial Mg2+ channel transient receptor potential melastatin 6 is regulated by dietary Mg2+ content and estrogens. J. Am. Soc. Nephrol. 17, 1035–1043 [DOI] [PubMed] [Google Scholar]
- 26. Li M., Jiang J., Yue L. (2006) Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J. Gen. Physiol. 127, 525–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li M., Du J., Jiang J., Ratzan W., Su L. T., Runnels L. W., Yue L. (2007) Molecular determinants of Mg2+ and Ca2+ permeability and pH sensitivity in TRPM6 and TRPM7. J. Biol. Chem. 282, 25817–25830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmitz C., Dorovkov M. V., Zhao X., Davenport B. J., Ryazanov A. G., Perraud A. L. (2005) The channel kinases TRPM6 and TRPM7 are functionally nonredundant. J. Biol. Chem. 280, 37763–37771 [DOI] [PubMed] [Google Scholar]
- 29. Chubanov V., Waldegger S., Mederos y Schnitzler M., Vitzthum H., Sassen M. C., Seyberth H. W., Konrad M., Gudermann T. (2004) Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc. Natl. Acad. Sci. U.S.A. 101, 2894–2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chubanov V., Schlingmann K. P., Wäring J., Heinzinger J., Kaske S., Waldegger S., Mederos y Schnitzler M., Gudermann T. (2007) Hypomagnesemia with secondary hypocalcemia due to a missense mutation in the putative pore-forming region of TRPM6. J. Biol. Chem. 282, 7656–7667 [DOI] [PubMed] [Google Scholar]
- 31. Demeuse P., Penner R., Fleig A. (2006) TRPM7 channel is regulated by magnesium nucleotides via its kinase domain. J. Gen. Physiol. 127, 421–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zierler S., Yao G., Zhang Z., Kuo W. C., Pörzgen P., Penner R., Horgen F. D., Fleig A. (2011) Waixenicin A inhibits cell proliferation through magnesium-dependent block of transient receptor potential melastatin 7 (TRPM7) channels. J. Biol. Chem. 286, 39328–39335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu H., Zhang Z., Lis A., Penner R., Fleig A. (2013) TRPM7 is regulated by halides through its kinase domain. Cell. Mol. Life Sci. 70, 2757–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Clark K., Middelbeek J., Morrice N. A., Figdor C. G., Lasonder E., van Leeuwen F. N. (2008) Massive autophosphorylation of the Ser/Thr-rich domain controls protein kinase activity of TRPM6 and TRPM7. PLoS One 3, e1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hofmann T., Schaefer M., Schultz G., Gudermann T. (2002) Subunit composition of mammalian transient receptor potential channels in living cells. Proc. Natl. Acad. Sci. U.S.A. 99, 7461–7466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hoenderop J. G., Voets T., Hoefs S., Weidema F., Prenen J., Nilius B., Bindels R. J. (2003) Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. EMBO J. 22, 776–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lintschinger B., Balzer-Geldsetzer M., Baskaran T., Graier W. F., Romanin C., Zhu M. X., Groschner K. (2000) Coassembly of Trp1 and Trp3 proteins generates diacylglycerol- and Ca2+-sensitive cation channels. J. Biol. Chem. 275, 27799–27805 [DOI] [PubMed] [Google Scholar]
- 38. Chubanov V., Mederos y Schnitzler M., Wäring J., Plank A., Gudermann T. (2005) Emerging roles of TRPM6/TRPM7 channel kinase signal transduction complexes. Naunyn Schmiedebergs Arch. Pharmacol. 371, 334–341 [DOI] [PubMed] [Google Scholar]
- 39. Kunert-Keil C., Bisping F., Krüger J., Brinkmeier H. (2006) Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genomics 7, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schlingmann K. P., Gudermann T. (2005) A critical role of TRPM channel-kinase for human magnesium transport. J. Physiol. 566, 301–308 [DOI] [PMC free article] [PubMed] [Google Scholar]