Abstract
The immune mechanisms for neonatal susceptibility to respiratory pathogens are poorly understood. Given that mucosal surfaces serve as a first line of host defense, we hypothesized that the innate immune response to infectious agents may be developmentally regulated in airway epithelium. To test this hypothesis, we determined whether the expression of IL-8 and IL-6 in airway epithelium after LPS exposure is dependent on chronological age. Tracheas from infant, juvenile, and adult rhesus monkeys were first exposed to LPS ex vivo, and then processed for air–liquid interface primary airway epithelial cell cultures and secondary LPS treatment in vitro. Compared with adult cultures, infant and juvenile cultures expressed significantly reduced concentrations of IL-8 after LPS treatment. IL-8 protein in cultures increased with animal age, whereas LPS-induced IL-6 protein was predominantly associated with juvenile cultures. Toll-like receptor (TLR) pathway RT-PCR arrays showed differential expressions of multiple mRNAs in infant cultures relative to adult cultures, including IL-1α, TLR10, and the peptidoglycan recognition protein PGLYRP2. To determine whether the age-dependent cytokine response to LPS is reflective of antecedent exposures, we assessed primary airway epithelial cell cultures established from juvenile monkeys housed in filtered air since birth. Filtered air–housed animal cultures exhibited LPS-induced IL-8 and IL-6 expression that was discordant with age-matched ambient air–housed animals. A single LPS aerosol in vivo also affected this cytokine profile. Cumulatively, our findings demonstrate that the innate immune response to LPS in airway epithelium is variable with age, and may be modulated by previous environmental exposures.
Keywords: infant, airway epithelium, LPS, Toll-like receptor, cytokine
Clinical Relevance
To the best of our knowledge, this research provides the first direct evidence that airway epithelia from infants and children are functionally distinct from adult epithelia. This is an important finding as it suggests that immune susceptibility in infants may be partly explained by a hyporesponsive innate immune phenotype in airway epithelium.
In humans, the first year of life represents a dynamic period of cellular development for both the immune and pulmonary systems, a process that continues into childhood (1). Adaptive immunity in infants is functionally compromised in comparison with adults, primarily because of the limited maturation of hematopoietic lineage cells that affect the generation of robust T-cell responses toward microbial antigens (2, 3). Nucleosome remodeling of the IL-12p35 promoter is impaired in neonatal dendritic cells, leading to decreased IL-12p70 secretion, reduced Th1 cytokine synthesis, and an overall “skewed” predominant Th2 cytokine immune profile during infancy (4–6). The induction of TNF-α expression via multiple Toll-like receptor (TLR) agonists is also reduced in newborn cord blood monocytes (7). A comprehensive investigation of TLR functional ontogeny in peripheral blood leukocytes has shown that the ability to promote proinflammatory cytokine expression via the induction of microbe-associated molecular pattern responses does not necessarily progress in a linear fashion with age (8). This suggests that leukocytes and their associated signaling mechanisms follow a developmental course that responds to environmental triggers as well as increasing maturity. Despite our growing knowledge and understanding of infant immune effector response maturation from peripheral blood studies, it remains unknown whether these data are reflective of host–pathogen interactions that take place within the lung mucosa.
In parallel with the immune system, the primate lung undergoes an extensive postnatal period of growth and differentiation. Anatomically, maturation of the lung continues far beyond the first year of life, as numbers of alveoli continue to increase through adolescence and early adulthood (9, 10). In the infant rhesus monkey, the relative abundance of conducting airway epithelial cell phenotypes does not appear to change significantly, whereas proximal airways increase in length and circumference (11). Beyond functioning as a mechanical barrier, epithelia at mucosal sites provide an important first line of innate immune defense against infectious agents. The significant role of epithelia during the initiation and progression of adaptive immunity is recognized, and the term “epimmunome” has been coined to describe the molecular milieu in epithelial cells that contributes toward the instruction of leukocytes during an immune response (12). Epithelial cells of the lung produce lipid mediators, growth factors, and numerous cytokines that are essential for the initiation of airway inflammation (13, 14). As with cells of hematopoietic origin, central to the ability of airway epithelial cells to respond to pathogens is the expression and activation of multiple TLRs (15). In pediatric populations, a deficiency in TLR4 expression in the airway epithelium of patients with cystic fibrosis has been proposed as a mechanism for their enhanced susceptibility to bacterial infection (16, 17).
Longitudinal birth cohort studies provide epidemiologic evidence to support a pathophysiologic link between early life exposure to microbial agents and long-term consequences on immunity (18–21). Most recently, invariant natural killer T cells have been reported as essential cellular mediators of immunological persistence because of microbial influence (22). In addition to serving as cellular mediators of immunity, evidence suggests that the airway epithelium may also be capable of retaining a “memory” immune phenotype, as demonstrated by the differential inflammatory response of asthmatic airway epithelium compared with that in normal subjects (23–25). Furthermore, the expression of proinflammatory cytokines and remodeling proteins is intrinsically different in airway epithelium from asthmatic children compared with children without asthma, indicating that a persistent epithelial cell disease phenotype is initiated during early childhood (26, 27).
Given the importance of airway epithelial cells as mucosal mediators of innate immunity in the lung, we hypothesized that susceptibility to respiratory pathogens in pediatric populations is attributable in part to an immunologically immature airway epithelium. We further speculated that, much like hematopoietic cells of the immune system, the interactions of pathogen-associated molecular patterns with airway epithelium may contribute to immune deviation by modulating both constitutive and stimulus-induced cytokine synthesis in a persistent fashion. In the present study, multiple developmental stages of the rhesus monkey were evaluated to determine whether the airway epithelial cell innate immune response to TLR ligands is dependent on chronological age. Our investigation focused on the TLR4 ligand LPS and the synthesis of IL-8 and IL-6 as cytokine mediators of innate immunity. Both ex vivo and in vivo experiments were used to address the question of whether previous environmental exposures could intrinsically alter the cytokine response to secondary LPS challenge. Some of the results of these studies have been previously reported in the form of an abstract (28).
Materials and Methods
Lung Specimens
Infant (3–10 mo old), juvenile (11–15 mo old), and adult (3–9 yr old) rhesus macaque monkey (Macaca mulatta) tracheobronchial tissues were obtained from the California National Primate Research Center Pathology Unit (demographics are provided in Table E1 in the online supplement).
Ex Vivo LPS Exposure
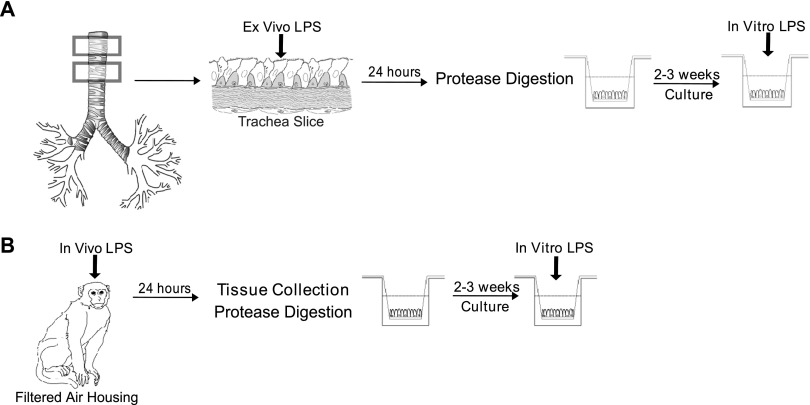
Within 1 hour of collection, trachea specimens were dissected into 5-mm × 10-mm slices, and then placed on 24-mm 0.4-μm pore size Corning Transwell polyester inserts (Corning Life Sciences, Tewksbury, MA), with epithelial surfaces upright. LPS or media control was added to the upper chambers (Figure 1A). Additional culture details are provided in the online supplement. All culture and animal experiments were performed with the identical Escherichia coli strain and lot number (600 endotoxin units [EU]/μg, E. coli 026:B6; Sigma-Aldrich, St. Louis, MO).
Figure 1.
Schematic of ex vivo, in vitro, and in vivo LPS experiments. (A) Preparation of cultures from ex vivo LPS exposure. Tracheal slices were generated from fresh lung tissue and cultured with LPS for 24 hours. Primary airway epithelial cell air–liquid interface cultures were generated from protease-digested tracheal slices and treated with a secondary dose of LPS before the evaluation of cytokine expression. (B) Preparation of cultures from filtered air–housed animals. Filtered air–housed animals were evaluated at 1 year of age. A subset received a single inhaled dose of LPS before the collection of lung tissue. Tracheobronchial airways were processed for primary airway epithelial cell isolation by protease digestion. Cultures were maintained under air–liquid interface conditions. Cultures were treated with a secondary dose of LPS before the evaluation of cytokine expression.
Primary Cell Culture
Airway epithelial cells were isolated from tracheobronchial tissues as described by Wu and colleagues (29). Epithelial cells were plated at a density of 4 × 105 cells on 6.5-mm 0.4-μm pore size Corning Transwell clear polyester membrane inserts coated with FNC coating mix (Athena Enzyme Systems, Baltimore, MD), and cultured under air–liquid interface conditions, according to the method described by Matsui and colleagues (30). Additional culture details are provided in the online supplement.
Animals
Newborn male rhesus monkeys were housed under high-efficiency particulate air (HEPA) filtered air conditions, as previously described, and evaluated at 1 year of age (31). A subset of animals received a single aerosolized dose of 25,000 EU in PBS via facemask. Tracheobronchial tissues were collected from animals, and airway epithelial cells were isolated for air–liquid interface cultures (Figure 1B). The care and housing of animals before, during, and after treatment complied with the provisions of the Institute of Laboratory Animal Resources, and conforms to practices established by the American Association for Accreditation of Laboratory Animal Care. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of California at Davis.
Cytokine ELISA
Cytokine proteins in culture supernatants were measured by ELISA-Ready-SET-Go! kits from eBioscience (San Diego, CA). The limit of detection for ELISA assays was 4 pg/mL for IL-8, 2 pg/ml for IL-6, and 7 pg/ml for chemokine (C-C motif) ligand 2 (CCL2).
Quantitative PCR
Details of RNA preparation are provided in the online supplement. IL-6, IL-8, TLR4, and glyceraldehyde 3–phosphate dehydrogenase mRNA were measured using TaqMan primer-probe sets, detected with an Applied Biosystems PRISM 7900 Sequence Detection System (Applied Biosystems, Carlsbad, CA). Purified human cDNA plasmid constructs (Origene, Rockville, MD) for each gene target were used to generate standard curves for the quantitation of mRNA copy numbers. All reagents were tested to confirm comparable detection for both rhesus and human targets.
PCR Array
The RT2-Profiler PCR Array Rhesus Macaque Toll-Like Receptor Signaling Pathway (SA Bioscience, Valencia, CA) was used as recommended by the manufacturer, and analyzed with the SA Bioscience PCR Array Data Analysis Web Portal.
Statistical Analysis
All data are reported as means ± SEMs. Treatment and age differences were evaluated using ANOVA (one-way or two-way), t test, or linear regression where appropriate, with GraphPad Prism version 5.0 software (GraphPad, La Jolla, CA). A P value of 0.05 or less was considered statistically significant.
Results
To determine whether chronological age is associated with the ability to generate a cytokine response to LPS in the lung, we evaluated primary airway epithelial cell cultures established from infant, juvenile, and adult rhesus monkeys in a comparative fashion, focusing on the expression of IL-8 and IL-6 as prototypic inflammatory cytokines. Because younger animals have a reduced cumulative contact with LPS from the ambient environment, we also investigated whether antecedent exposures exert an effect on cytokine synthesis by airway epithelium. To mimic the lung mucosal environment in culture, fresh trachea specimens were first directly exposed to LPS (ex vivo) before the isolation of epithelial cells for culture (Figure 1A). Trachea slices cultured with LPS at 0.1 μg/ml for 24 hours maintained an intact epithelial layer, without evidence of substantial tissue necrosis in either infant or adult animals (Figures 2B and 2D).
Figure 2.
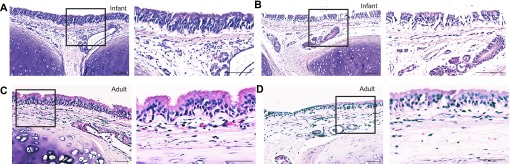
Infant and adult tracheal morphology after ex vivo LPS exposure. Paraffin-embedded tracheal slice cultures generated from a representative infant and adult monkey were processed for hematoxylin and eosin staining. Tracheal slices were evaluated at 24 hours after ex vivo LPS exposure. Boxes define regions to the immediate right, magnified at ×20. (A) Infant media control tracheal slice. (B) Infant LPS-exposed tracheal slice. (C) Adult media control tracheal slice. (D) Adult LPS-exposed tracheal slice. Scale bar, 100 μm.
IL-8 Expression in Airway Epithelial Cell Cultures Increases with Chronological Age
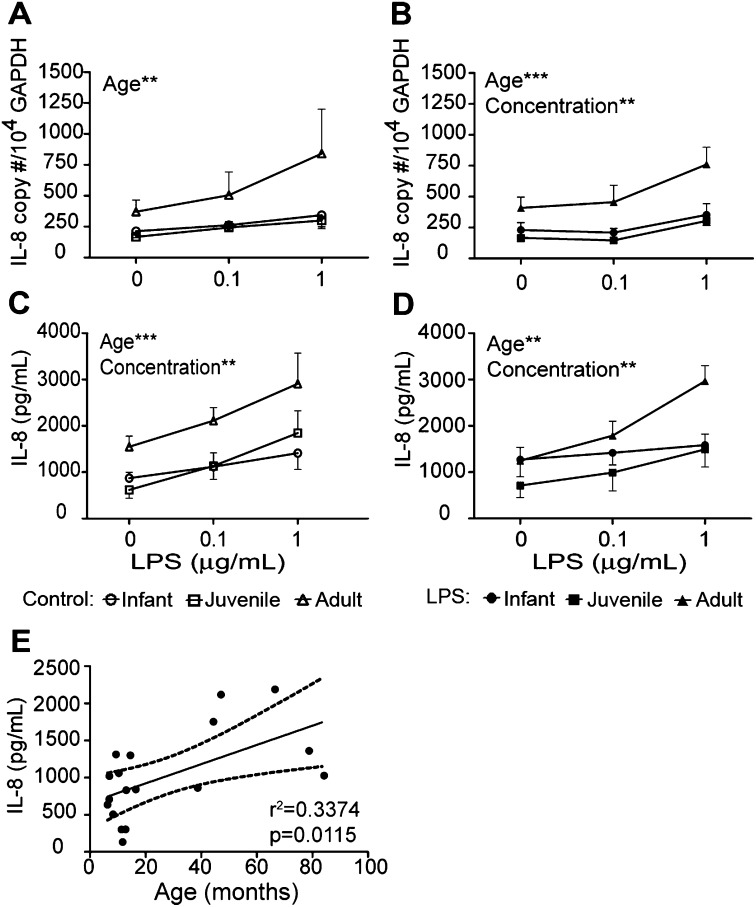
Cultures derived from media control trachea slices exhibited a modest dose-dependent IL-8 response to secondary LPS treatment, with a trend toward significance with IL-8 mRNA copy number (P = 0.07; Figure 3A) and significance for IL-8 protein secretion (Figure 3C). Cultures derived from ex vivo LPS-exposed trachea slices responded to secondary LPS treatment in a dose-dependent fashion for both IL-8 mRNA and protein (Figures 3B and 3D). Among individual age groups, no significant differences in IL-8 expression were evident between cultures established from media control or ex vivo LPS-exposure trachea slices, although ex vivo LPS cultures appeared to demonstrate less variability in cytokine responses.
Figure 3.
Effects of age and previous exposure on LPS-induced IL-8 expression in airway epithelium. Tracheal slices were cultured with LPS for 24 hours, followed by protease digestion and the isolation of airway epithelial cells. Air–liquid interface cultures from infant, juvenile, or adult monkeys received a second treatment of LPS (0.1–1 μg/ml), and were evaluated for IL-8 expression after 24 hours. (A and B) IL-8 mRNA and (C and D) IL-8 protein expression was determined in cultures established from media control (A and C) and LPS-exposed (B and D) tracheal slices. Each data point represents the mean ± SE from 5–6 animals per group. **P < 0.01 and ***P < 0.001, according to two-way ANOVA, age versus LPS concentration. Circles, infants; squares, juveniles; triangles, adults. Open symbols indicate control cultures, whereas solid symbols indicate ex vivo LPS cultures. (E) Correlation between chronological age (in months) and IL-8 protein secretion in primary airway epithelial cell cultures. Each data point represents basal IL-8 protein concentration (no ex vivo or secondary LPS) in airway epithelial cell cultures derived from individual animals. GAPDH, glyceraldehyde 3–phosphate dehydrogenase.
Overall, the IL-8 mRNA copy number in airway epithelial cells after secondary LPS treatment was age-dependent (Figures 3A and 3B). At 1 μg/ml secondary LPS treatment, ex vivo LPS cultures derived from adult animals expressed higher concentrations of IL-8 mRNA relative to infant or juvenile cultures (Figure 3B, adult vs. infant, P < 0.01; adult vs. juvenile, P < 0.01, according to Bonferroni multiple-comparisons post hoc test).
IL-8 protein secretion in cultures was also significantly dependent on animal age (Figures 3C and 3D). Similar to IL-8 mRNA expression, cultures from adult animals synthesized the highest concentration of IL-8 protein compared with infant and juvenile cultures, regardless of ex vivo LPS exposure. At 1 μg/ml secondary LPS treatment, IL-8 protein concentrations were significantly higher in adult cultures versus infant cultures, independent of previous ex vivo LPS exposures (Figure 3C, adult vs. infant, P < 0.05; Figure 3D, adult vs. infant, P < 0.05, according to Bonferroni multiple-comparisons post hoc test). Juvenile cultures were similarly reduced in comparison with adult cultures at 1 μg/ml secondary LPS treatment for ex vivo LPS exposure cultures only (Figure 3D, adult vs. juvenile, P < 0.05). A linear regression analysis of IL-8 protein secretion by airway epithelial cell cultures showed a positive correlation with chronological age (Figure 3E).
IL-6 Secretion Is Highest in Airway Epithelial Cell Cultures from Juvenile Animals
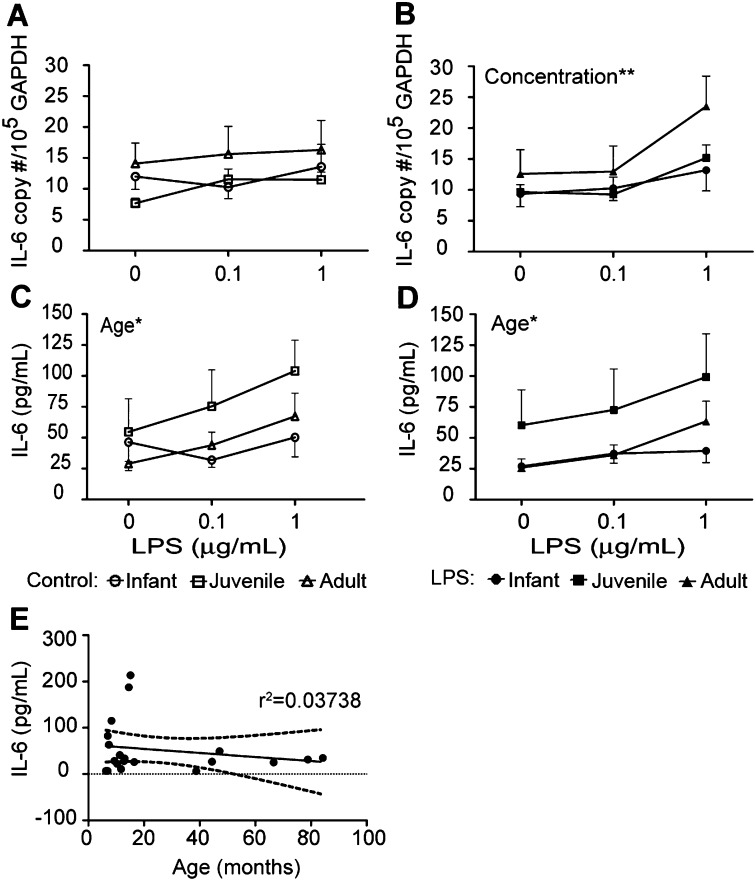
In cultures derived from media control trachea slices, we found minimal changes in IL-6 mRNA copy numbers after secondary LPS treatment, regardless of age (Figure 4A). With ex vivo LPS exposure, we observed a significant dose-dependent IL-6 mRNA response to secondary LPS (Figure 4B). A trend toward age-dependent IL-6 mRNA concentrations was evident, with adult cultures expressing higher copy numbers in response to secondary LPS, compared with infant or juvenile cultures (Figure 4B, P = 0.0531). At 1 μg/ml secondary LPS treatment, adult cultures derived from ex vivo LPS-exposed trachea slices exhibited a significantly higher IL-6 mRNA response, compared with media control trachea slices, indicating an effect of previous LPS exposure (P < 0.05 according to t test, ex vivo media control vs. ex vivo LPS).
Figure 4.
Effects of age and previous exposure on LPS-induced IL-6 expression in airway epithelium. Tracheal slices were cultured with LPS for 24 hours, followed by protease digestion and the isolation of airway epithelial cells. Air–liquid interface cultures from infant, juvenile, or adult monkeys received a second treatment of LPS (0.1–1 μg/ml), and were evaluated for IL-6 expression after 24 hours. (A and B) IL-6 mRNA and (C and D) IL-6 protein expression was determined in cultures established from media control (A and C) and LPS-exposed (B and D) tracheal slices. Each data point represents the mean ± SE from 5–6 animals per group. *P < 0.05 and **P < 0.01, according to two-way ANOVA, age versus LPS concentration. Circles, infants; squares, juveniles; triangles, adults. Open symbols indicate control cultures, whereas solid symbols indicate ex vivo LPS cultures. (E) Correlation between chronological age (in months) and IL-6 protein secretion in primary airway epithelial cell cultures. Each data point represents basal IL-6 protein concentration (no ex vivo or secondary LPS) in airway epithelial cell cultures derived from individual animals.
Independent of previous ex vivo LPS exposure, IL-6 protein secretions in cultures after secondary LPS treatment were significantly dependent on animal age (Figures 4C and 4D). Although adult cultures appeared to express higher concentrations of IL-6 mRNA in response to secondary LPS treatment, IL-6 protein synthesis was highest in juvenile cultures, and was significantly increased over infant cultures (Figure 4C, juvenile vs. infant, P < 0.05; Figure 4D, juvenile vs. infant, P < 0.05). Adult cultures did respond to secondary LPS treatment in a dose-dependent manner, whereas infant cultures showed no change (Figures 4C and 4D, P < 0.01 according to one-way ANOVA for media control, P < 0.01 according to one-way ANOVA for ex vivo LPS). In contrast with IL-8, we did not find a significant correlation of IL-6 protein secretion in airway epithelium with animal age (Figure 4E).
Airway Epithelial Cell Cultures from Infant Animals Show Differential TLR Signaling Pathway Gene Expression
To further characterize age-dependent differences in the LPS response for airway epithelium, an RT-PCR array for 84 genes associated with TLRs and associated signaling molecules was used to compare both constitutive and LPS-induced expression profiles, focusing on cultures derived from infant and adult animals (Figures 5 and 6, and Tables 1–3).
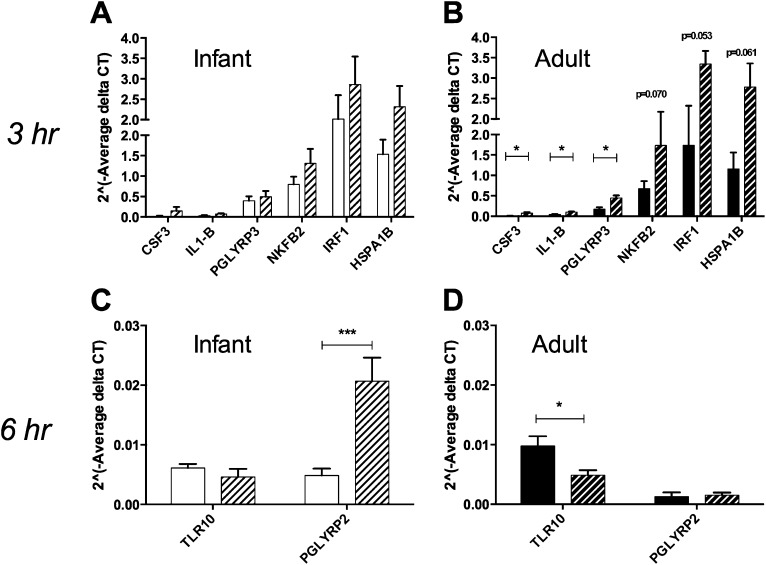
Figure 5.
Comparison of Toll-like receptor (TLR) signaling pathway expression in infant and adult airway epithelial cell cultures. (A and B) Relative gene expression of selected TLR signaling pathway molecules in infant and adult cultures. Data are expressed as 2^ (− average delta threshold cycle (Ct) value) where delta Ct = Ct value of gene of interest − mean Ct value of housekeeping genes. Columns represent the mean ± SE of 8–9 animals per age group. Open columns represent infant cultures, whereas solid columns represent adult cultures. *P < 0.05, according to t test. (C–J) Cryosections from representative adult (C, D, G, and H) and infant (E, F, I, and J) tracheas were stained with FITC-conjugated anti-human IL-1α antibody (D and F) or anti-human peptidoglycan recognition protein PGLYRP2 with an Alexa488-conjugated secondary antibody (H and J). FITC-conjugated and unconjugated mouse IgG1 isotype controls are included for comparison (C, E, G, and I). Images were collected at ×40 magnification. Scale bar, 20 μm.
Figure 6.
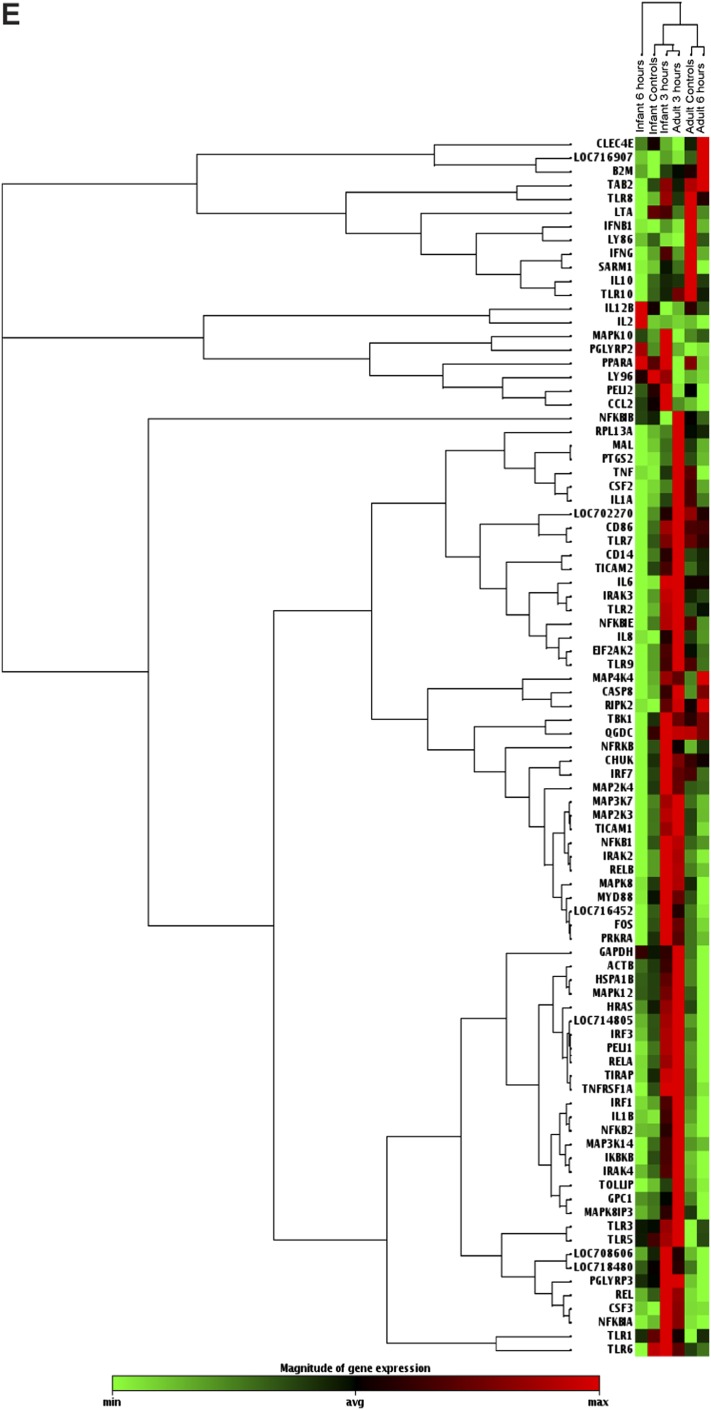
Effects of LPS on TLR signaling pathway gene expression in infant and adult airway epithelial cell cultures. (A and B) LPS-induced mRNA expression of TLR signaling pathway genes was assessed in infants and adult cultures at 3 hours after treatment. Data are expressed as 2^ (− delta Ct) for media control (solid columns) or LPS-treated (hatched columns) cultures. Delta Ct = Ct value of gene of interest − mean Ct value of housekeeping genes. Each data point represents the mean ± SE of 4–5 animals per age group per time point. *P < 0.05, according to t test. (C and D) LPS-induced mRNA expression of TLR signaling pathway genes was assessed in infant and adult cultures at 6 hours after treatment. Data are expressed as 2^ (− delta Ct) for media control (solid columns) or LPS-treated (hatched columns) cultures. Delta Ct = Ct value of gene of interest − mean Ct value of housekeeping genes. Each data point represents the mean ± SE of 4–5 animals per age group per time point. *P < 0.05 and ***P < 0.001, according to t test. (E) Hierarchical clustering was performed on the complete dataset. The heat map indicates coregulated genes across groups, with red indicating increased expression, and green indicating decreased expression.
TABLE 1.
EFFECT OF AGE ON TLR PATHWAY GENE EXPRESSION
| Gene | Infant Cultures (n = 9) |
Adult Cultures (n = 8) |
Fold Change (Infant/Adult) | P Value |
|---|---|---|---|---|
| Avg Ct | Avg Ct | |||
| PGLYRP3 | 26.24 | 27.81 | 2.232 | 0.0947 |
| CCL2 | 26.91 | 28.35 | 2.0498 | 0.2249 |
| PGLYRP2* | 32.22 | 34.19 | 2.9605 | 0.0120 |
| CSF3 | 32.23 | 31.35 | 0.4099 | 0.2862 |
| CSF2 | 27.65 | 26.95 | 0.4644 | 0.1534 |
| IFNB1 | 34.66 | 33.7 | 0.3886 | 0.1031 |
| IFNG | 34.72 | 34.05 | 0.4758 | 0.1292 |
| IL-1A* | 25.27 | 24.07 | 0.3295 | 0.0284 |
| IL-6 | 29.36 | 28.43 | 0.3975 | 0.0842 |
| SARM1 | 31.92 | 31.3 | 0.4901 | 0.2464 |
| LY86 | 33.78 | 33.08 | 0.4627 | 0.1575 |
| TLR10* | 31.82 | 31.05 | 0.4433 | 0.0366 |
Definition of abbreviations: Avg Ct, average threshold cycle value; TLR, Toll-like receptor.
P < 0.05, according to Student t test.
TABLE 3.
EFFECT OF LPS ON TLR PATHWAY GENE EXPRESSION: 6 HOURS AFTER TREATMENT
| Infant Cultures (n = 5) |
Adult Cultures (n = 4) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | Control Avg Ct | LPS tx Avg Ct | Fold Change (LPS/Control) | P Value | Gene | Control Avg Ct | LPS Avg Ct | Fold Change (LPS/Control) | P Value |
| CSF3 | 32.72 | 30.56 | 4.7823 | 0.2445 | IFNG | 33.65 | 35 | 0.4245 | 0.1715 |
| IL-2 | 35 | 33.86 | 2.3569 | 0.3441 | SARM1 | 30.07 | 32.23 | 0.2423 | 0.2919 |
| PGLYRP2* | 32.49 | 30.46 | 4.3806 | 0.0050 | LTA | 33.16 | 34.43 | 0.4484 | 0.3127 |
| TLR6 | 27.55 | 29.04 | 0.3802 | 0.4754 | LY86 | 32.48 | 33.91 | 0.4048 | 0.4157 |
| MAPK8IP3 | 26.28 | 27.44 | 0.486 | 0.31177 | |||||
| TLR10* | 30.49 | 31.63 | 0.4933 | 0.03717 | |||||
Definition of abbreviations: Avg Ct, average threshold cycle value; tx, treatment; TLR, Toll-like receptor.
P < 0.05, according to Student t test.
Twelve genes were constitutively modulated by a twofold minimum in infant cultures relative to adult cultures (Table 1). Peptidoglycan recognition protein PGLYRP2 mRNA and TLR10 mRNA were differentially expressed in infant cultures compared with adult cultures, with higher concentrations of PGLYRP2 and lower concentrations of TLR10 measured in infant cells (Figure 5A). We also observed a significant difference in expression of IL-1α mRNA, with adult cultures showing higher levels of expression compared with infant cultures (Figure 5B). Consistent with our earlier findings for IL-6 mRNA copy numbers (Figure 4), a trend toward increased IL-6 mRNA was evident in adult cultures compared with infant cultures (Figure 5B, P = 0.08). Furthermore, a trend toward increased peptidoglycan recognition protein PGLYRP3 mRNA was observed in infant cultures, similar to the profile for PGLYRP2 (Figure 5B, P = 0.09). No age-dependent differences in gene expression were evident for the components of the LPS receptor complex, CD14, MD2 (LY96), or TLR4 (Figure E1).
To confirm that RT-PCR array expression profiles obtained from primary airway epithelial cell cultures were comparable to those of airway epithelium in vivo, we performed immunostaining of tracheal cryosections for IL-1α and PGLYRP2 protein, based on our findings of increased IL-1α mRNA in adult cultures and increased PGLYRP2 mRNA in infant cultures. Immunofluorescence staining for IL-1α protein was observed in adult tracheal sections, both within the epithelial compartment and within the interstitium (Figure 5D). In contrast, infant tracheal sections showed little immunofluorescence-positive staining for IL-1α protein (Figure 5F). PGLYRP2 protein was detected by immunofluorescence in both infant and adult tracheal epithelium (Figures 5H and 5J). Infant tracheal epithelial cells were uniformly immunofluorescence-positive, and exhibited greater staining intensity for PGLYRP2 antibody compared with adult trachea.
In addition to immunofluorescence staining for IL-1α and PGLYRP2, we also evaluated culture supernatants for secretion of CCL2 protein. CCL2 mRNA levels were constitutively increased by 2-fold in infant airway epithelial cells, although this did not reach statistical significance. CCL2 protein was readily detectable in both control and LPS-treated (24-h) infant and adult airway epithelial cell-culture supernatants. However, we did not observe significant age-dependent effects (Figure E2).
We next evaluated the effects of LPS on age-dependent molecular profiles in infant and adult cultures. To obtain information on epithelial signaling pathways that are rapidly induced by LPS, we measured gene expression changes relative to media control–treated cultures at both at 3 and 6 hours after LPS treatment. At 3 hours after LPS treatment, 11 genes in adult cultures and six genes in infant cultures were modulated by at least twofold, compared with media control–treated cultures (Table 2). In adult cultures, colony-stimulating factor 3 (CSF3), IL-1β, and PGLYRP3 mRNA were increased at 3 hours after LPS exposure (Figure 6B, P < 0.05 according to t test, compared with media control cultures). Heat shock 70-kD protein 1B (HSPA1B) (P = 0.061), interferon regulatory factor 1 (IRF1) (P = 0.053), and NF-κB2 (P = 0.070) also showed a trend toward significant induction in adult cultures after LPS treatment. Relative to values obtained in media-control infant cultures, no significant changes were evident in the mRNA expression of CSF3, IL-1β, PGLYRP3, NF-κB2, IRF1, and HSPA1B for infant cultures at 3 hours after LPS treatment (Figure 6A). Comparisons of average threshold cycle (Ct) values obtained at 3 hours after LPS for infant cultures relative to adult cultures showed seven genes that were differentially expressed by a minimum twofold, with infant cultures expressing approximately threefold higher CCL2 mRNA at a significance level of P < 0.01 (Table E2).
TABLE 2.
EFFECT OF LPS ON TLR PATHWAY GENE EXPRESSION: 3 HOURS AFTER TREATMENT
| Infant Cultures (n = 4) |
Adult Cultures (n = 4) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | Control Avg Ct | LPS tx Avg Ct | Fold Change (LPS/Control) | P Value | Gene | Control Avg Ct | LPS Avg Ct | Fold Change (LPS/Control) | P Value |
| CSF3 | 31.65 | 28.17 | 10.326 | 0.2323 | CSF3* | 31.74 | 28.42 | 8.5701 | 0.0257 |
| IL-1B | 30.22 | 28.47 | 3.1128 | 0.0908 | GPC1 | 24.18 | 22.75 | 2.3139 | 0.1507 |
| PGLYRP2 | 31.92 | 30.77 | 2.0547 | 0.1702 | HSPA1B | 24.75 | 23.15 | 2.6091 | 0.0609 |
| MAPK10 | 32.75 | 31.61 | 2.0402 | 0.3698 | IL1B* | 29.28 | 27.93 | 2.1944 | 0.0208 |
| NFKBIB | 24.63 | 26.64 | 0.2295 | 0.8214 | IRF1 | 24.21 | 22.82 | 2.2616 | 0.0534 |
| LY86 | 33.28 | 34.84 | 0.3149 | 0.2068 | PGLYRP3* | 27.42 | 25.75 | 2.7403 | 0.0123 |
| NFKB2 | 25.52 | 23.87 | 2.6825 | 0.0704 | |||||
| PGLYRP2 | 34.43 | 32.93 | 2.423 | 0.2002 | |||||
| TOLLIP | 25.06 | 23.51 | 2.5146 | 0.1033 | |||||
| IFNB1 | 33.67 | 34.91 | 0.3645 | 0.3039 | |||||
| LY86 | 33.56 | 35 | 0.3168 | 0.3519 | |||||
Definition of abbreviations: Avg Ct, average threshold cycle value; tx, treatment; TLR, Toll-like receptor.
P < 0.05, according to Student t test.
At 6 hours after LPS treatment, we identified six genes in adult cultures and four genes in infant cultures that showed a minimum 2-fold modulation as compared with media control cultures (Table 3). In adult cultures, the expression of TLR10 was significantly reduced, compared with control samples at 6 hours after LPS treatment (Figure 6D). Infant cultures demonstrated a significantly increased expression of PGLYRP2 compared with control samples at 6 hours after LPS treatment, and the level of expression was also higher compared with adult cultures. Comparisons of average Ct values at 6 hours after LPS for infant cultures relative to adult cultures showed nine genes that were differentially expressed by a minimum twofold, with infant cultures expressing approximately sevenfold higher PGLYRP2 mRNA at a significance level of P < 0.01 (Table E2).
To identify groups of genes or samples with similar expression patterns, unsupervised hierarchical clustering was performed on array data collected from infant and adult cultures, comparing expression levels for all 84 genes evaluated in control samples at two different time points after LPS exposure (Figure 6E). Infant control cultures, infant cultures at 3 hours after LPS, and adult cultures at 3 hours after LPS clustered separately from adult control cultures and adult cultures at 6 hours after LPS. The most distantly related cluster consisted of infant cultures at 6 hours after LPS.
Antecedent Exposures Influence the Cytokine Response to Secondary LPS in Juvenile Cultures
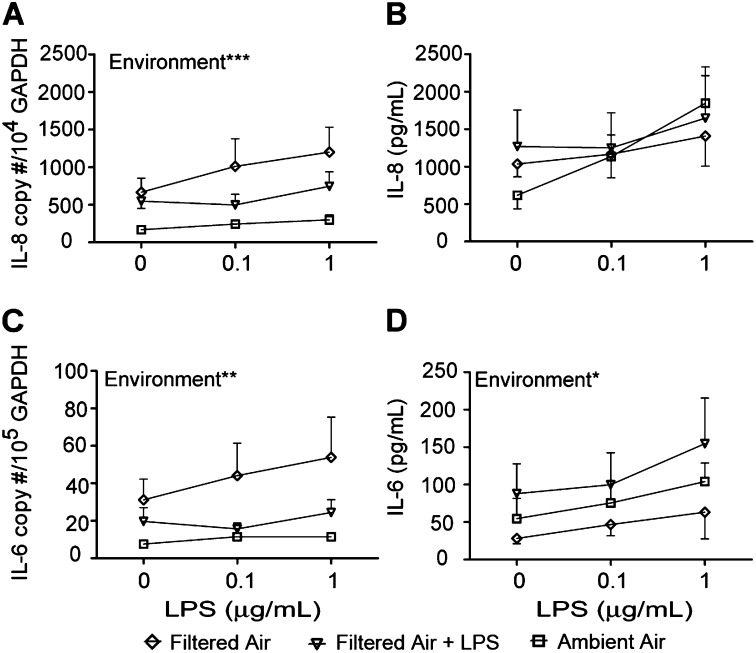
Because the number of exposures to LPS from the ambient outdoor environment should increase with chronological age under normal conditions, our observed differences in LPS response by age group may have been attributable to environmental exposure history, as opposed to the maturity of differentiated epithelial cells. We initially investigated this possibility using the ex vivo exposure of tracheal slices. However, our approach did not allow for numerous repeated LPS exposures, which may be required for persistent changes in gene expression. To address this issue, we compared airway epithelial cell cultures established from juvenile animals housed under HEPA filtered air conditions since birth, with cultures established from age-matched animals raised in an outdoor field cage colony (ambient air) (Figure 1B). We also evaluated the impact of a single LPS exposure, which was administered to animals housed under HEPA filtered air conditions at 24 hours before lung-tissue collection.
In cell cultures generated from animals in filtered air or filtered air plus in vivo LPS exposure, the impact of the environment was significant. The constitutive expression of IL-8 mRNA was increased in filtered air–derived cultures relative to ambient air–derived cultures (Figure 7A; P < 0.05 according to one-way ANOVA at 0 μg/ml LPS). After in vitro LPS treatment, a comparison of cultures from filtered air animals and from ambient air animals showed a significant effect of housing environment on IL-8 mRNA copy numbers (Figure 7A). Cultures established from filtered air–housed animals exhibited the highest concentrations of IL-8 mRNA with secondary LPS treatment, and cultures from ambient air animals showed the lowest levels of LPS induction for IL-8 mRNA. Compared with ambient air animals, no significant effect of housing was evident in either constitutive or LPS-induced IL-8 secretion (Figure 7B).
Figure 7.
Effects of environmental exposures on LPS-induced cytokine expression in airway epithelium. Airway epithelial cell cultures established from 1-year-old filtered air–housed or filtered air–housed plus in vivo LPS-exposed were treated with a secondary dose of LPS in vitro. Cultures were compared with age-matched ambient air–housed animals. (A) IL-8 mRNA. (B) IL-8 protein. (C) IL-6 mRNA. (D) IL-6 protein. Each data point represents the mean ± SE from 4–6 animals per group. *P < 0.05, **P < 0.01, and ***P < 0.001, according to two-way ANOVA, environment versus LPS concentration. Diamonds, filtered air; triangles, filtered air + LPS; squares, ambient.
Housing environment also exerted a highly significant impact on LPS-induced IL-6 mRNA expression in airway epithelial cell cultures (Figure 7C). As with IL-8 mRNA, cultures from filtered air–housed animals expressed the highest concentrations of IL-6 mRNA in response to secondary LPS treatment, compared with cultures from filtered air plus LPS aerosol or ambient air–housed animals. At baseline (0 μg/ml LPS), a trend toward increased IL-6 mRNA in cultures from filtered air–housed animals was evident, relative to ambient air–housed animals (P = 0.07). Ambient air animals showed an increase in IL-6 protein secretion with secondary LPS treatment, but the expression overall was lower than in cultures from filtered air plus LPS animals (Figure 7D). Although cultures from filtered air–housed animals showed the highest concentrations of IL-6 mRNA in response to LPS treatment, IL-6 protein secretion was the lowest of the three culture groups.
Discussion
The ability of airway epithelium to generate immune mediators in response to microbe-associated molecular ligands and infectious agents has been well studied, using both in vitro human cell culture systems and in vivo rodent models. Despite an extensive knowledge of how this cell type responds to microbes, the majority of studies investigating innate immune function after infection or agonist treatment to date have focused exclusively on adult airway epithelium. Conducting airway epithelial cell cultures from pediatric populations have been reported, and have shown constitutively increased goblet-cell accumulation, increased IL-6 and prostaglandin E2, and an increased expression of remodeling factors (transforming growth factor–β2, vascular endothelial growth factor, and periostin) in asthmatic subjects relative to healthy control subjects (26, 27, 32). Pediatric airway epithelia are typically obtained by bronchial brushings in school-aged children, and have not been directly compared with gene/protein signatures found in adult epithelia. To the best of our knowledge, our findings are the first to characterize the function of an innate immune pathway in airway epithelial cells from three chronological age groups: infants, juveniles, and adults.
To assess innate immunity in airway epithelium, we established air–liquid interface cultures of primary cells obtained from rhesus monkey tracheal specimens, and measured cytokine expression after LPS treatment, focusing on both IL-8 and IL-6 as parameters of inflammation. As shown in Figures 3 and 4, cultures derived from infant animals responded poorly to LPS treatment compared with adult cultures, suggesting that the infant airway epithelium has a hyporesponsive innate immune phenotype with regard to signaling through TLR4. The ex vivo LPS exposure of infant tracheal slices exerted a minimal effect on IL-8 and IL-6 expression in primary airway epithelial cell cultures that were subsequently established from these tissues, indicating that the limited ability to produce cytokines was not attributable to the lack of a previous LPS exposure history. The intrinsic hyporesponsive innate immune phenotype of infant airway epithelial cell cultures in this study is consistent with the known susceptibility of human infants to Gram-negative bacterial pneumonia, namely, the pneumonia caused by Haemophilus influenzae. Patients with cystic fibrosis are also highly susceptible to Gram-negative bacterial infections. This is likely attributable to reduced TLR4 expression in airway epithelium (16). Components of the LPS receptor complex, including TLR4, CD14, and MD2 (LY96), were evaluated in infant and adult airway epithelial cell cultures in this study, but we found no significant differences in mRNA concentrations between age groups, suggesting that reduced coreceptor expression does not explain the limited ability of infant airway epithelium to respond to LPS.
An unexpected finding involved the observation that airway epithelial cell cultures derived from juvenile animals exhibited a dichotomy of cytokine responses to LPS treatment. LPS-induced IL-8 expression in juvenile cultures was reduced relative to adult cultures, but IL-6 protein concentrations were highest in juvenile cultures (Figure 4). Age (in mo) significantly correlated with the constitutive secretion of IL-8 protein, but not IL-6, indicating that the ability to produce cytokines does not uniformly increase in a linear fashion with maturity (Figures 3 and 4). Interestingly, with the IL-6 response, concentrations of mRNA and protein appeared discordant. The IL-6 mRNA copy number was highest in adult cultures, whereas secreted IL-6 protein was highest in juvenile cultures. These differences may be attributable to the timing of sample collection, or could indicate posttranslational mechanisms such as microRNAs controlling cytokine production in an age-dependent fashion. The distinct cytokine profile in cultures from animal age groups may be attributable to repeated LPS exposures with increasing age. However, ex vivo LPS exposure did not affect the degree of culture responsiveness to secondary LPS treatment in infant or juvenile cultures. We do not yet know if age-dependent proinflammatory cytokine synthesis by airway epithelium corresponds to differential respiratory pathogen susceptibility in vivo. However, our previous work has shown that a single LPS aerosol dose administered to juvenile animals results in pulmonary and systemic neutrophilia within 6 hours after exposure, suggesting that innate immune function is sufficient within this age group (31).
In peripheral blood cells, TLR function is increased with gestational age, and is associated with reduced infection control during early life because of impaired cytokine synthesis (7, 33, 34). Our data indicate that the developmental regulation of TLR signaling pathway genes also exists in airway epithelial cells. Expression levels for multiple genes associated with TLR pathways were significantly different between infant and adult cultures, both constitutively and after LPS treatment (Figures 5 and 6 and Tables 1–3). The mRNA for transcription factors (NF-κβ2 and IRF1), negative regulators of TLR4 (HSPA1B), and other inflammatory cytokines (CSF3 and IL-1β) were rapidly induced at 3 hours after LPS in adult cultures (Table 2). Comparatively, fewer mRNAs in infant cultures were found to be affected by a minimum twofold change with LPS at 3 hours after treatment, and only a trend toward the induction of IL-1βwas evident(P = 0.09). At 6 hours after LPS treatment, the gene profile for adult cultures suggested an overall down-regulation of the TLR signaling response. Surprisingly, we found that the expression of the bacterial peptidoglycan protein PGLYRP2 was increased in infant cultures relative to adult cultures, both constitutively and at 6 hours after LPS treatment (Figures 5 and 6). PGLYRP2 has been reported to play a protective role against excessive inflammation in a Salmonella-induced colitis model and in a psoriasis-like inflammatory skin murine model (35, 36). The various immunoregulatory mechanisms of PGLYRP2 remain incompletely understood. However, the induction of IL-17 and the promotion of T regulatory cell responses have been attributed to PGLYRP2 (35). It is intriguing to speculate that this molecule may contribute to immune tolerance or hyporesponsiveness in the infant lung. Moreover, our observed gene expression profile for infant cultures may be explained by an overall delayed time course after LPS treatment. However, the finding of very low concentrations of IL-8 or IL-6 protein in 24-hour infant cultures suggests that this was not the mechanism.
A comparison of human, chimpanzee, and rhesus macaque gene signatures obtained from LPS-stimulated monocytes shows a well-conserved universal TLR response. Lineage-specific genes are primarily associated with viral infection (37). Nonetheless, we note a potential limitation of our investigation, namely, that human airway epithelial cells may respond differently from those of rhesus monkeys, such that developmental maturity in humans exerts no effect on gene signatures. Further comparative studies in pediatric and adult cell cultures will be required to address this possibility thoroughly. Although we observed variability in cytokine responses within age groups in our study, we emphasize that rhesus monkeys comprise an outbred population, similar to humans. Housing in an outdoor environment may also contribute to variability among individual animals. Therefore, we also evaluated monkeys that were housed in HEPA filtered air conditions within 1–2 days of birth through 12 months of age. We further speculated that if the LPS response profile in airway epithelium is exclusively dependent on previous environmental exposures, then cultures derived from HEPA filtered air–housed juveniles should exhibit a hyporesponsive profile, similar to that from infant cultures. Surprisingly, filtered air housing resulted in an even greater IL-8 response and higher IL-6 mRNA copy numbers in airway epithelial cell cultures compared with ambient air housing (Figure 7). These findings demonstrate that antecedent exposures, in addition to developmental maturity, can exert a significant impact on the LPS-induced cytokine response by airway epithelium. The notion that airway epithelium can develop a persistent phenotype is well-supported by studies with airway epithelial cell cultures derived from adult patients with asthma showing disparate in vitro responses to allergens or viruses, compared with cultures derived from control subjects (23, 38).
In conclusion, the results of this study show that the airway epithelium in early life is functionally impaired with regard to the generation of a robust proinflammatory cytokine response after LPS challenge. Furthermore, the molecular transition to an adult LPS response profile is not always linear with age, and may be affected by environmental exposures. These findings are consistent and present parallels with the early-life development of innate immune function for cells of hematopoietic origin. Because the epidemiology of other childhood diseases such as asthma strongly supports a window of susceptibility within the first year of life, whether LPS or other TLR ligands can impose epigenetic programming in infant airway epithelium remains a critical question and a focus of our future studies.
Acknowledgments
Acknowledgments
The authors thank Dr. Joan Gerriets, Sarah Davis, Paul-Michael Sosa, Sona Santos, Louise Olsen, and Brian Tarkington for technical support during this project, Kathy West for artwork, and Susan Nishio for assisting with figure preparation. The authors also thank Candace Burke for helpful discussions during the preparation of the manuscript.
Footnotes
This work was supported by National Institutes of Health grants ES011617, ES000628, HL081286, HL097087, OD011107, and T32 HL007013, and Environmental Protection Agency Science to Achieve Results Grant 832,947.
Author Contributions: K.M.-H. was responsible for the study’s conception and design, data collection, analysis and interpretation of data, figure preparation, and writing of the manuscript. C.C.C. was responsible for data collection, analysis and interpretation of data, and writing of the manuscript. E.M.P. and M.J.E. were responsible for the study’s conception and design and manuscript editing. J.H.F. was responsible for assay development and data collection. L.A.M. was responsible for the study’s conception and design, analysis and interpretation of data, writing of the manuscript, and final approval of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0321OC on April 19, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Holt PG, Jones CA. The development of the immune system during pregnancy and early life. Allergy. 2000;55:688–697. doi: 10.1034/j.1398-9995.2000.00118.x. [DOI] [PubMed] [Google Scholar]
- 2.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30:585–591. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Philbin VJ, Levy O. Developmental biology of the innate immune response: implications for neonatal and infant vaccine development. Pediatr Res. 2009;65:98R–105R. doi: 10.1203/PDR.0b013e31819f195d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SM, Suen Y, Chang L, Bruner V, Qian J, Indes J, Knoppel E, van de Ven C, Cairo MS. Decreased interleukin-12 (IL-12) from activated cord versus adult peripheral blood mononuclear cells and upregulation of interferon-gamma, natural killer, and lymphokine-activated killer activity by IL-12 in cord blood mononuclear cells. Blood. 1996;88:945–954. [PubMed] [Google Scholar]
- 5.Langrish CL, Buddle JC, Thrasher AJ, Goldblatt D. Neonatal dendritic cells are intrinsically biased against Th-1 immune responses. Clin Exp Immunol. 2002;128:118–123. doi: 10.1046/j.1365-2249.2002.01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goriely S, Van Lint C, Dadkhah R, Libin M, De Wit D, Demonté D, Willems F, Goldman M. A defect in nucleosome remodeling prevents IL-12 (p35) gene transcription in neonatal dendritic cells. J Exp Med. 2004;199:1011–1016. doi: 10.1084/jem.20031272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol. 2004;173:4627–4634. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- 8.Corbett NP, Blimkie D, Ho KC, Cai B, Sutherland DP, Kallos A, Crabtree J, Rein-Weston A, Lavoie PM, Turvey SE, et al. Ontogeny of Toll-like receptor mediated cytokine responses of human blood mononuclear cells. PLoS ONE. 2010;5:e15041. doi: 10.1371/journal.pone.0015041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hyde DM, Blozis SA, Avdalovic MV, Putney LF, Dettorre R, Quesenberry NJ, Singh P, Tyler NK. Alveoli increase in number but not size from birth to adulthood in rhesus monkeys. Am J Physiol Lung Cell Mol Physiol. 2007;293:L570–L579. doi: 10.1152/ajplung.00467.2006. [DOI] [PubMed] [Google Scholar]
- 10.Narayanan M, Owers-Bradley J, Beardsmore CS, Mada M, Ball I, Garipov R, Panesar KS, Kuehni CE, Spycher BD, Williams SE, et al. Alveolarization continues during childhood and adolescence: new evidence from helium-3 magnetic resonance. Am J Respir Crit Care Med. 2012;185:186–191. doi: 10.1164/rccm.201107-1348OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Winkle LS, Fanucchi MV, Miller LA, Baker GL, Gershwin LJ, Schelegle ES, Hyde DM, Evans MJ, Plopper CG. Epithelial cell distribution and abundance in rhesus monkey airways during postnatal lung growth and development. J Appl Physiol. 2004;97:2355–2363, discussion 2354. doi: 10.1152/japplphysiol.00470.2004. [DOI] [PubMed] [Google Scholar]
- 12.Swamy M, Jamora C, Havran W, Hayday A. Epithelial decision makers: in search of the “epimmunome.”. Nat Immunol. 2010;11:656–665. doi: 10.1038/ni.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartlett JA, Fischer AJ, McCray PB., Jr Innate immune functions of the airway epithelium. Contrib Microbiol. 2008;15:147–163. doi: 10.1159/000136349. [DOI] [PubMed] [Google Scholar]
- 14.Diamond G, Legarda D, Ryan LK. The innate immune response of the respiratory epithelium. Immunol Rev. 2000;173:27–38. doi: 10.1034/j.1600-065x.2000.917304.x. [DOI] [PubMed] [Google Scholar]
- 15.Sha Q, Truong-Tran AQ, Plitt JR, Beck LA, Schleimer RP. Activation of airway epithelial cells by Toll-like receptor agonists. Am J Respir Cell Mol Biol. 2004;31:358–364. doi: 10.1165/rcmb.2003-0388OC. [DOI] [PubMed] [Google Scholar]
- 16.John G, Yildirim AO, Rubin BK, Gruenert DC, Henke MO. TLR-4–mediated innate immunity is reduced in cystic fibrosis airway cells. Am J Respir Cell Mol Biol. 2010;42:424–431. doi: 10.1165/rcmb.2008-0408OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.John G, Chillappagari S, Rubin BK, Gruenert DC, Henke MO. Reduced surface Toll-like receptor–4 expression and absent interferon-γ–inducible protein–10 induction in cystic fibrosis airway cells. Exp Lung Res. 2011;37:319–326. doi: 10.3109/01902148.2011.569968. [DOI] [PubMed] [Google Scholar]
- 18.Riedler J, Braun-Fahrländer C, Eder W, Schreuer M, Waser M, Maisch S, Carr D, Schierl R, Nowak D, von Mutius E ALEX Study Team. exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358:1129–1133. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- 19.Braun-Fahrländer C, Riedler J, Herz U, Eder W, Waser M, Grize L, Maisch S, Carr D, Gerlach F, Bufe A, et al. Allergy and Endotoxin Study Team. environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 20.Gehring U, Bischof W, Fahlbusch B, Wichmann HE, Heinrich J. House dust endotoxin and allergic sensitization in children. Am J Respir Crit Care Med. 2002;166:939–944. doi: 10.1164/rccm.200203-256OC. [DOI] [PubMed] [Google Scholar]
- 21.Böttcher MF, Björkstén B, Gustafson S, Voor T, Jenmalm MC. Endotoxin levels in Estonian and Swedish house dust and atopy in infancy. Clin Exp Allergy. 2003;33:295–300. doi: 10.1046/j.1365-2222.2003.01562.x. [DOI] [PubMed] [Google Scholar]
- 22.Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holgate ST, Davies DE, Powell RM, Howarth PH, Haitchi HM, Holloway JW. Local genetic and environmental factors in asthma disease pathogenesis: chronicity and persistence mechanisms. Eur Respir J. 2007;29:793–803. doi: 10.1183/09031936.00087506. [DOI] [PubMed] [Google Scholar]
- 25.Hackett TL, Singhera GK, Shaheen F, Hayden P, Jackson GR, Hegele RG, Van Eeden S, Bai TR, Dorscheid DR, Knight DA. Intrinsic phenotypic differences of asthmatic epithelium and its inflammatory responses to respiratory syncytial virus and air pollution. Am J Respir Cell Mol Biol. 2011;45:1090–1100. doi: 10.1165/rcmb.2011-0031OC. [DOI] [PubMed] [Google Scholar]
- 26.Kicic A, Sutanto EN, Stevens PT, Knight DA, Stick SM. Intrinsic biochemical and functional differences in bronchial epithelial cells of children with asthma. Am J Respir Crit Care Med. 2006;174:1110–1118. doi: 10.1164/rccm.200603-392OC. [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Guisa JM, Powers C, File D, Cochrane E, Jimenez N, Debley JS. Airway epithelial cells from asthmatic children differentially express proremodeling factors. J Allergy Clin Immunol. 2012;129:990–997. doi: 10.1016/j.jaci.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maniar-Hew K, Postlethwait EM, Evan MJ, Fontaine JH, Miller LA. Infant airway epithelium displays an intrinsic hyporesponsive innate immune phenotype following LPS exposure [abstract] Am J Respir Crit Care Med. 2012;185:A3900. [Google Scholar]
- 29.Wu R, Sato GH, Whitcutt MJ. Developing differentiated epithelial cell cultures: airway epithelial cells. Fundam Appl Toxicol. 1986;6:580–590. doi: 10.1016/0272-0590(86)90170-3. [DOI] [PubMed] [Google Scholar]
- 30.Matsui H, Randell SH, Peretti SW, Davis CW, Boucher RC. Coordinated clearance of periciliary liquid and mucus from airway surfaces. J Clin Invest. 1998;102:1125–1131. doi: 10.1172/JCI2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maniar-Hew K, Postlethwait EM, Fanucchi MV, Ballinger CA, Evans MJ, Harkema JR, Carey SA, McDonald RJ, Bartolucci AA, Miller LA. Postnatal episodic ozone results in persistent attenuation of pulmonary and peripheral blood responses to LPS challenge. Am J Physiol Lung Cell Mol Physiol. 2011;300:L462–L471. doi: 10.1152/ajplung.00254.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parker J, Sarlang S, Thavagnanam S, Williamson G, O’Donoghue D, Villenave R, Power U, Shields M, Heaney L, Skibinski G. A 3-D well-differentiated model of pediatric bronchial epithelium demonstrates unstimulated morphological differences between asthmatic and nonasthmatic cells. Pediatr Res. 2010;67:17–22. doi: 10.1203/PDR.0b013e3181c0b200. [DOI] [PubMed] [Google Scholar]
- 33.Förster-Waldl E, Sadeghi K, Tamandl D, Gerhold B, Hallwirth U, Rohrmeister K, Hayde M, Prusa AR, Herkner K, Boltz-Nitulescu G, et al. Monocyte Toll-like receptor 4 expression and LPS-induced cytokine production increase during gestational aging. Pediatr Res. 2005;58:121–124. doi: 10.1203/01.PDR.0000163397.53466.0F. [DOI] [PubMed] [Google Scholar]
- 34.Sadeghi K, Berger A, Langgartner M, Prusa AR, Hayde M, Herkner K, Pollak A, Spittler A, Forster-Waldl E. Immaturity of infection control in preterm and term newborns is associated with impaired Toll-like receptor signaling. J Infect Dis. 2007;195:296–302. doi: 10.1086/509892. [DOI] [PubMed] [Google Scholar]
- 35.Park SY, Gupta D, Hurwich R, Kim CH, Dziarski R. Peptidoglycan recognition protein PGLYRP2 protects mice from psoriasis-like skin inflammation by promoting regulatory T cells and limiting Th17 responses. J Immunol. 2011;187:5813–5823. doi: 10.4049/jimmunol.1101068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J, Geddes K, Streutker C, Philpott DJ, Girardin SE. Role of mouse peptidoglycan recognition protein PGLYRP2 in the innate immune response to Salmonella enterica serovar typhimurium infection in vivo. Infect Immun. 2012;80:2645–2654. doi: 10.1128/IAI.00168-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barreiro LB, Marioni JC, Blekhman R, Stephens M, Gilad Y. Functional comparison of innate immune signaling pathways in primates. PLoS Genet. 2010;6:e1001249. doi: 10.1371/journal.pgen.1001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lordan JL, Bucchieri F, Richter A, Konstantinidis A, Holloway JW, Thornber M, Puddicombe SM, Buchanan D, Wilson SJ, Djukanović R, et al. Cooperative effects of Th2 cytokines and allergen on normal and asthmatic bronchial epithelial cells. J Immunol. 2002;169:407–414. doi: 10.4049/jimmunol.169.1.407. [DOI] [PubMed] [Google Scholar]