Abstract
ATP in airway surface liquid (ASL) controls mucociliary clearance functions via the activation of airway epithelial purinergic receptors. However, abnormally elevated ATP levels have been reported in inflamed airways, suggesting that excessive ATP in ASL contributes to airway inflammation. Despite these observations, little is known about the mechanisms of ATP accumulation in the ASL covering inflamed airways. In this study, links between cystic fibrosis (CF)–associated airway inflammation and airway epithelial ATP release were investigated. Primary human bronchial epithelial (HBE) cells isolated from CF lungs exhibited enhanced IL-8 secretion after 6 to 11 days, but not 28 to 35 days, in culture, compared with normal HBE cells. Hypotonic cell swelling–promoted ATP release was increased in 6- to 11-day-old CF HBE cells compared with non-CF HBE cells, but returned to normal values after 28 to 35 days in culture. The exposure of non-CF HBE cells to airway secretions isolated from CF lungs, namely, sterile supernatants of mucopurulent material (SMM), also caused enhanced IL-8 secretion and increased ATP release. The SMM-induced increase in ATP release was sensitive to Ca2+ chelation and vesicle trafficking/exocytosis inhibitors, but not to pannexin inhibition. Transcript levels of the vesicular nucleotide transporter, but not pannexin 1, were up-regulated after SMM exposure. SMM-treated cultures displayed increased basal mucin secretion, but mucin secretion was not enhanced in response to hypotonic challenge after the exposure of cells to either vehicle or SMM. We propose that CF airway inflammation up-regulates the capacity of airway epithelia to release ATP via Ca2+-dependent vesicular mechanisms not associated with mucin granule secretion.
Keywords: human bronchial epithelia, supernatant of mucopurulent material from human CF airways, high performance liquid chromatography, well-differentiated vesicular nucleotide transporter
Abnormal ion transport results in mucous dehydration and poor clearance, which produce lung disease in cystic fibrosis (CF). Mucous dehydration also contributes to the progressive airway obstruction associated with chronic obstructive lung disease (COPD) (1). ATP and other nucleotides present in the airway surface liquid (ASL) are important modulators of the ion transport activities that regulate mucous hydration and mucociliary clearance (MCC) via the activation of airway epithelial cell purinergic receptors (2). However, purinergic receptors are also abundantly expressed in neutrophils, monocytes/macrophages, lymphocytes, and other immunocompetent cells (3). Therefore, fine control mechanisms are required in the lung to establish local nucleotide/nucleoside concentrations that maintain MCC activities (4, 5) without promoting the recruitment and/or activation of inflammatory cells. Indeed, recent studies indicate that abnormally high levels of nucleotides in lung secretions are correlated with COPD severity, asthma, and CF-associated neutrophilic inflammation (6–18), suggesting that the dysregulation of nucleotide release and/or metabolism rates is a feature of inflamed lungs.
Airway epithelial cells normally release ATP and other nucleotides in response to cell swelling, shear and compressive stress, and other physiological stimuli, via conductive and vesicular pathways (5, 19–23). However, studies investigating the mechanisms underlying pathologically elevated airway epithelial ATP release have just begun. For example, primary cultures of normal airway epithelial cells that were induced to develop mucous (goblet) cell hyperplasia via respiratory syncytial virus (RSV) infection or IL-13 exposure exhibited an enhanced release of nucleotides, which was correlated with increased mucin secretion (24). However, the cellular sources and mechanisms of enhanced ATP release in lungs chronically inflamed by nonallergic mechanisms (e.g., chronic bacterial infections associated with CF) have not been identified.
In the present study, we used early-stage primary cultures of CF human bronchial epithelia (HBEs) that retain hyperinflammatory activities acquired in vivo (25), as well as late-stage, normal HBE cells exposed to bacterial and inflammatory factors from CF airways, that is, sterile supernatants of mucopurulent material (SMMs), to search for conductive and vesicular mechanisms that mediate increased ATP release consequent to inflammation.
Materials and Methods
Cell Culture
Primary HBE cell cultures established from surgical specimens from healthy or CF donors were provided by the Cystic Fibrosis Center Tissue Culture Core Laboratory at the University of North Carolina, and grown on 12-mm-diameter Transwell supports, as described elsewhere (26). Cultures became confluent after 3–6 days and were subsequently grown at an air–liquid interface for either 6–11 days (early stage, undifferentiated) or 28–35 days (late stage, fully differentiated).
Supernatants of Mucopurulent Material from CF Airways
The SMM was isolated from the lumens of chronically bacterially infected and inflamed CF lungs, as previously described (27). SMM or vehicle (PBS) (35 μl) was applied to the mucosal surface of cultures for 48 hours in most experiments, but for 60 hours in a single series of experiments, as indicated, and rinsed and used for ATP release or other relevant measurements, as described.
Cytokine Measurement
IL-8 was measured in the serosal medium, as described elsewhere (27).
Real-Time ATP Measurements
Cultures were rinsed and exposed to 100 μl mucosal buffer, namely, Hank’s balanced salt solution buffered with 10 mM HEPES, pH 7.4 (HBSS/HEPES). After 1 hour, cultures were transferred to a Turner TD-20/20 luminometer (Turner Biosystems, Sunnyvale, CA), and luciferase and luciferin were added to the mucosal surface. Baseline luciferin/luciferase activity was recorded, and cells were challenged with 50 μl hypotonic (H2O) or isotonic (saline) solutions supplemented with 1 mM CaCl2 and 1 mM MgCl2, and ATP concentrations were determined in real time, as previously described (19).
Uptake of Propidium Iodide
Cultures were challenged for 5 minutes with hypotonic solution (or isotonic control), as already described, in the presence of 20 μM propidium iodide (28). At the end of the incubation, the bathing solution was replaced with HBSS containing 4% paraformaldehyde. The acquisition of confocal images and the quantification of stained nuclei were performed using a Leica SP5 confocal microscope (Leica Microsystems, Buffalo Grove, IL), as previously described (28).
Measurement of Adenyl Purines
Cultures were rinsed and preincubated for 1 hour with 300 μl mucosal HBSS, after which 100 μl were removed for baseline nucleotide measurements. One hundred microliters of H2O or saline (containing 1 mM CaCl2 and 1 mM MgCl2) were added to the mucosal fluid, and aliquots were sampled after 30 seconds or 2 minutes. Adenine-containing species were measured by etheno-derivatization, as described elsewhere (29).
RT-PCR Analysis
Total RNA was isolated using the RNeasy kit (Qiagen, Inc., Valencia, CA), and was reverse-transcribed into cDNA using Superscript (Invitrogen, Carlsbad, CA). Primers used for the PCR amplification of vesicular nucleotide transporter (VNUT) and pannexin 1 were described elsewhere (20, 30). Amplified PCR products were identified by sequence analysis at the DNA sequencing facility of the University of North Carolina at Chapel Hill. Quantitative PCR was performed as previously described (22).
RhoA Pulldown Assay
Measurements of GTP-bound Rho-A were performed as previously described (22).
Mucin Secretion Measurements
Mucin secretion was assessed by ELISA, using University of North Carolina-230 rabbit polyclonal anti-mucin common subunit antibody, as previously described (24).
Materials
All reagents were of the highest purity available, and were obtained from sources previously described (20, 23, 24, 31).
Statistical Analysis
All experiments were performed on cultures established from at least three different donors. Data were expressed as mean values ± SEMs or SDs, as indicated. Where appropriate, data were analyzed according to an unpaired Student t test or ANOVA, using GraphPad InStat software (GraphPad, La Jolla, CA). Statistical significance was defined as P < 0.05.
Results
Inflamed CF HBE Cells Exhibit Enhanced Hypotonicity-Evoked ATP Release
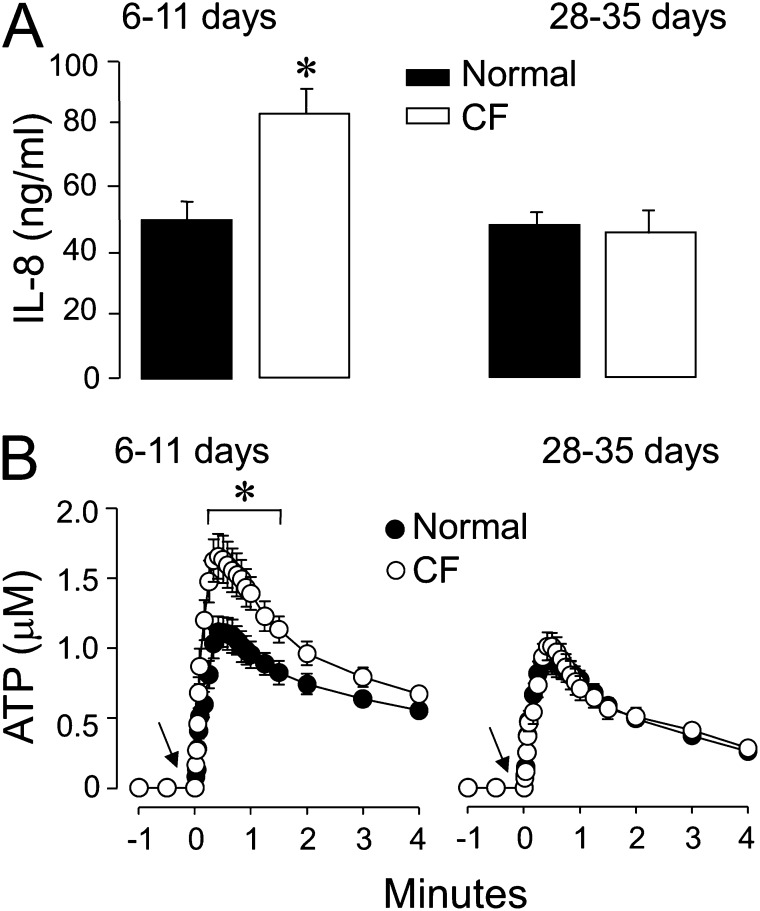
Reflecting the chronically infected and inflamed milieu of the donor lung (25), “younger” early-stage CF HBE cultures (6–11 d old) exhibited a hyperinflammatory phenotype, as indicated by increased baseline IL-8 secretion compared with normal HBE cultures (Figure 1A, left). In contrast, IL-8 secretion rates were no different from normal values in “older” late-stage CF cultures (28–35 d old) (Figure 1A, right), indicating that the inflamed features of CF airways were not retained in CF HBE cells in prolonged culture, as previously shown (25). Baseline ATP levels were in the low nanomolar range (1–2 nM), and were not different between control and CF HBE cells, irrespective of culture age (Figure 1B). As previously reported, cells exposed to an apical hypotonic challenge (to induce cell swelling–promoted ATP release) (19, 20) exhibited a robust, rapid (30 s) increase in ATP release (Figure 1B). Importantly, peak levels of ATP release in response to hypotonicity reached the micromolar range, and were significantly higher in young (6- to 11-d-old) CF HBE cells relative to normal HBE cells of the same age (Figure 1B, left). Similar to IL-8 values, differences in hypotonicity-promoted ATP release between CF and normal HBE cells disappeared by 28–35 days in culture for CF HBE cells (Figure 1B, right). These results indicate that young cultures of CF airway epithelial cells exhibit an enhanced hypotonicity-induced nucleotide release that is associated with increased inflammation.
Figure 1.
Inflamed cystic fibrosis human bronchial epithelial (CF HBE) cells display increased hypotonicity-evoked ATP release. IL-8 secretion (A) and hypotonicity-promoted ATP release (B) were assessed in primary CF HBE cultures grown for 6–11 days and 28–35 days. Hypotonicity was applied at time = 0, as indicated by the arrow. *Significant difference (P < 0.05) from normal cultures. Values represent the means ± SEMs of four Transwells/subject, established from three different subjects.
Influence of the CF Environment on Airway Epithelial Nucleotide Release and Metabolism
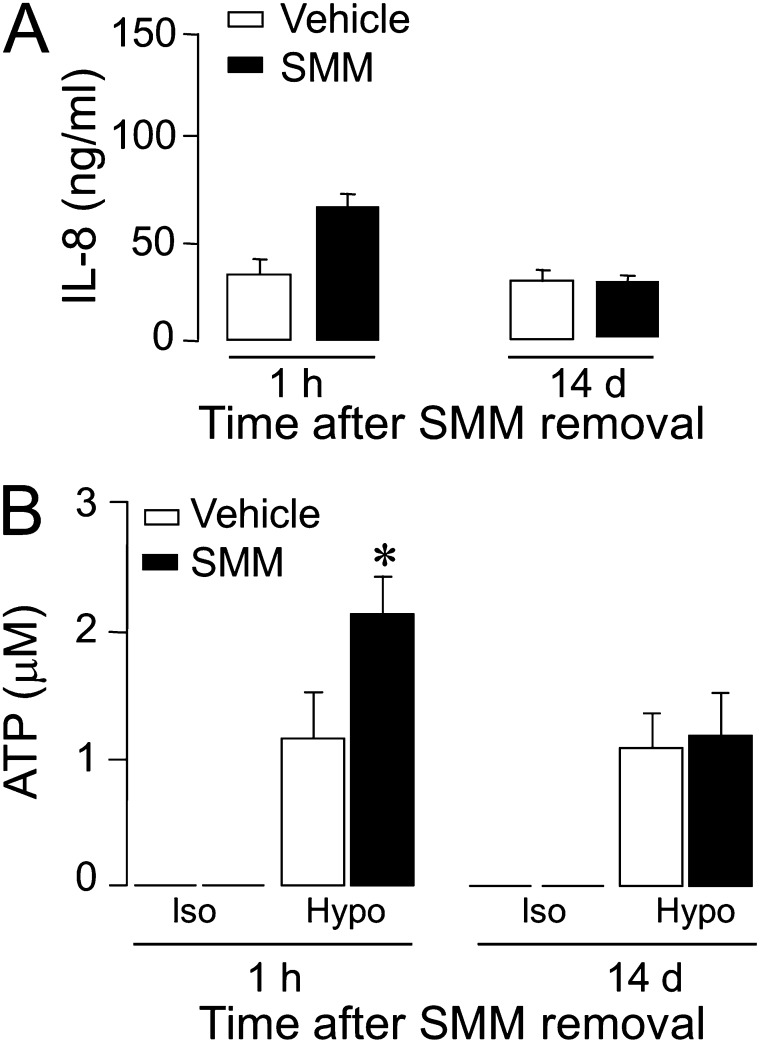
Previously, we showed that the exposure of fully differentiated cultures of normal or CF HBE cells to the influence of the in vivo environment in the CF airway (i.e., SMMs isolated from CF airways) produced an inflammatory phenotype, namely, the enhanced secretion of IL-8 (25). To investigate the possible association between airway epithelial cell inflammation and ATP release, fully differentiated normal HBE cell cultures were exposed for 48 hours to SMMs, and IL-8 secretion and ATP release were measured both immediately upon SMM removal and 14 days after removal. As previously reported (25), HBE cells exhibited enhanced IL-8 secretion upon SMM exposure (Figure 2A, left), whereas IL-8 secretion rates measured 14 days later had returned to normal values (Figure 2A, right). Peak ATP concentrations in response to hypotonic challenge significantly increased (∼70%) immediately after SMM treatment (Figure 2B, left), but ATP levels returned to normal values after 14 days (Figure 2B, right). A similar increase in hypotonicity-promoted ATP release was observed with SMM-treated (compared with untreated) well-differentiated (WD)–HBE cells isolated from subjects with CF (76% ± 27% increase, n = 2). Thus, during a 48-hour exposure to SMM, fully differentiated cultures of both normal and CF HBE cells transiently acquired the enhanced ATP release phenotype that characterizes the inflamed CF HBE cells at early culture stages.
Figure 2.
Enhanced ATP release from normal well-differentiated (WD)–HBE cells exposed to the supernatants of mucopurulent material from human CF airways (SMM). Normal cultures of WD-HBE cells were exposed luminally to SMM (or vehicle) for 48 hours. Cells were rinsed, and interleukin-8 secretion (A) and hypotonicity-promoted ATP release (B) were assessed 1 hour or 14 days after the termination of SMM/vehicle exposure. ATP release data represent the peak ATP concentrations after hypotonic challenge. The data represent the mean ± SEM of four Transwells/subject established from three different subjects. *Significant difference (P < 0.05). Hypo, hypotonic; Iso, isotonic.
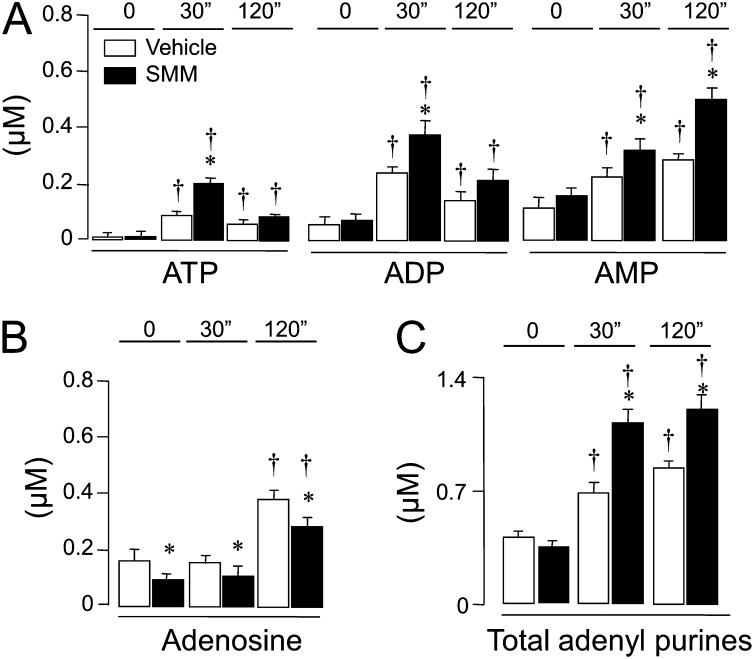
Airway epithelial cells express ectonucleotidase activities that rapidly metabolize ATP to ADP, adenosine 5′-monophosphate (AMP), and adenosine (32). To assess the influence of SMM treatment on the pattern of nucleotide/nucleoside distribution after hypotonic challenge, the entire spectrum of adenine nucleotides/nucleosides in ASL was quantified using the etheno-derivatization technique (4). Resting concentrations of ADP, AMP, and adenosine measured in the bulk solution bathing the apical culture surfaces (t = 0) were markedly higher than ATP concentrations (Figures 3A and 3B), and resting ATP/ADP/AMP levels did not differ significantly between SMM-exposed and vehicle-exposed normal HBE cells (Figure 3A). Consistent with the luminometry data (Figure 2B), the exposure of cells to hypotonicity for 30 seconds robustly increased ATP levels, and this increase was greater in SMM-treated cultures relative to control cultures (Figure 3A). ADP and AMP levels also were markedly higher in hypotonically challenged, SMM-treated cells relative to vehicle-treated HBE cells (Figure 3A). This finding suggests that ATP was partly dephosphorylated upon release after hypotonic challenge. At 2 minutes after hypotonicity, levels of ATP and ADP relaxed toward baseline levels, and were no longer different in SMM-treated and vehicle-treated HBE cells. In contrast, AMP levels continued increasing in SMM-treated cells (Figure 3A).
Figure 3.
Changes in extracellular adenyl purines in SMM-treated normal HBE cells. WD-HBE cell cultures exposed luminally to vehicle or SMM were assayed for extracellular ATP, ADP, and adenosine 5′-monophosphate (AMP) (A), and adenosine (B). Total adenyl species are shown in C. Mucosal samples were collected under resting condition (t = 0), 30 seconds (30”), or 2 minutes (120”) after hypotonic challenge. The values represent the means ± SEMs of four Transwells/subject established from three different subjects. *P < 0.05 and †P < 0.05, significant difference relative to time-matched and vehicle-exposed control values and baseline values (T = 0), respectively.
Unlike nucleotides, baseline adenosine levels in ASL after SMM treatment exhibited an approximately 50% reduction relative to control cultures, and remained lower (in SMM-treated cultures) after hypotonicity (Figure 3B). This reduction could reflect the down-regulation of AMP-metabolizing enzymes (e.g., CD73 and alkaline phosphatase) (33) and/or the up-regulation of adenosine degradation/uptake (34) in SMM-inflamed cells. Despite the adenosine reduction, the mass of the entire purine pool increased significantly in ASL from SMM-treated HBE cells at 30 seconds and 120 seconds after hypotonicity (Figure 3C).
Collectively, these data suggest that the incubation of fully differentiated HBE cells with SMM resulted in (1) the up-regulation of the process involved in the release of ATP from hypotonicity-stimulated HBE cells, and (2) the stimulation of processes that reduced adenosine levels.
SMM Up-Regulates a Vesicular Nucleotide Release Pathway
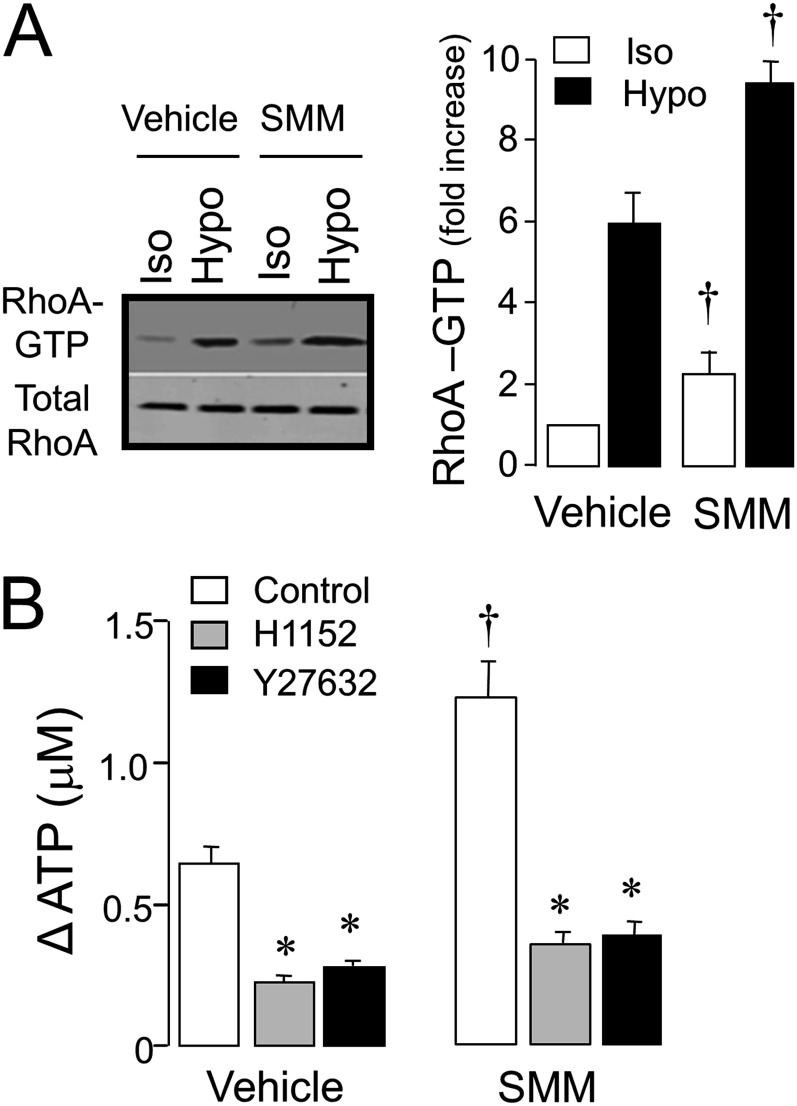
Next, we asked whether the increased ATP release associated with SMM treatment reflected a regulated mechanism, as opposed to enhanced plasma membrane fragility. Recently, we have shown that Rho and Rho kinase activation are obligatory steps regulating ATP release in airway epithelial cells, regardless of the release pathway (exocytotic or conductive) (20, 22, 23). Consistent with these observations, hypotonic stress promoted enhanced Rho-A–GTP formation in vehicle-treated HBE cells (Figure 4A). Rho-A activation was significantly, although modestly, increased in SMM-treated relative to vehicle-treated HBE cells under both isotonic and hypotonic conditions (Figure 4A). Importantly, the potent and selective Rho kinase inhibitors H1152 or Y27632 reduced hypotonicity-evoked ATP release in control HBE cells, and nearly abolished the enhanced ATP release that characterized SMM-treated HBE cells (Figure 4B). These data suggest that the increased ATP release associated with SMM treatment is regulated by Rho/Rho kinases.
Figure 4.
Involvement of Rho/Rho kinase in ATP release. (A) Normal WD-HBE cells treated with vehicle or SMM for 48 hours were luminally exposed to isotonic (Iso) or hypotonic (Hypo) solutions for 5 minutes, and Rho-A was assessed as described in Materials and Methods. The Western blot (left) is representative of three independent experiments, the quantification of which is illustrated on the right. †P < 0.005, significantly different relative to vehicle. (B) Changes in hypotonicity-promoted ATP release were measured in cells pretreated for 30 minutes in the absence (Control) or presence of 1 μM H1152 or 10 μM Y27632. †P < 0.05 and *P < 0.05, significantly different relative to vehicle and control, respectively (mean ± SEM, n = 3).
ATP release from normal, ciliated cell–dominated airway epithelia has been associated with the Rho-A–dependent opening of the plasma membrane-channel pannexin 1 (Panx1) (20, 35). In addition, goblet cell–rich epithelia display Rho-dependent, Ca2+-regulated, exocytotic ATP release, which has been associated with mucin granule secretion (23, 36). Therefore, the contributions of both Panx1 and Ca2+-regulated vesicular pathways to the enhanced ATP release associated with SMM treatment were examined.
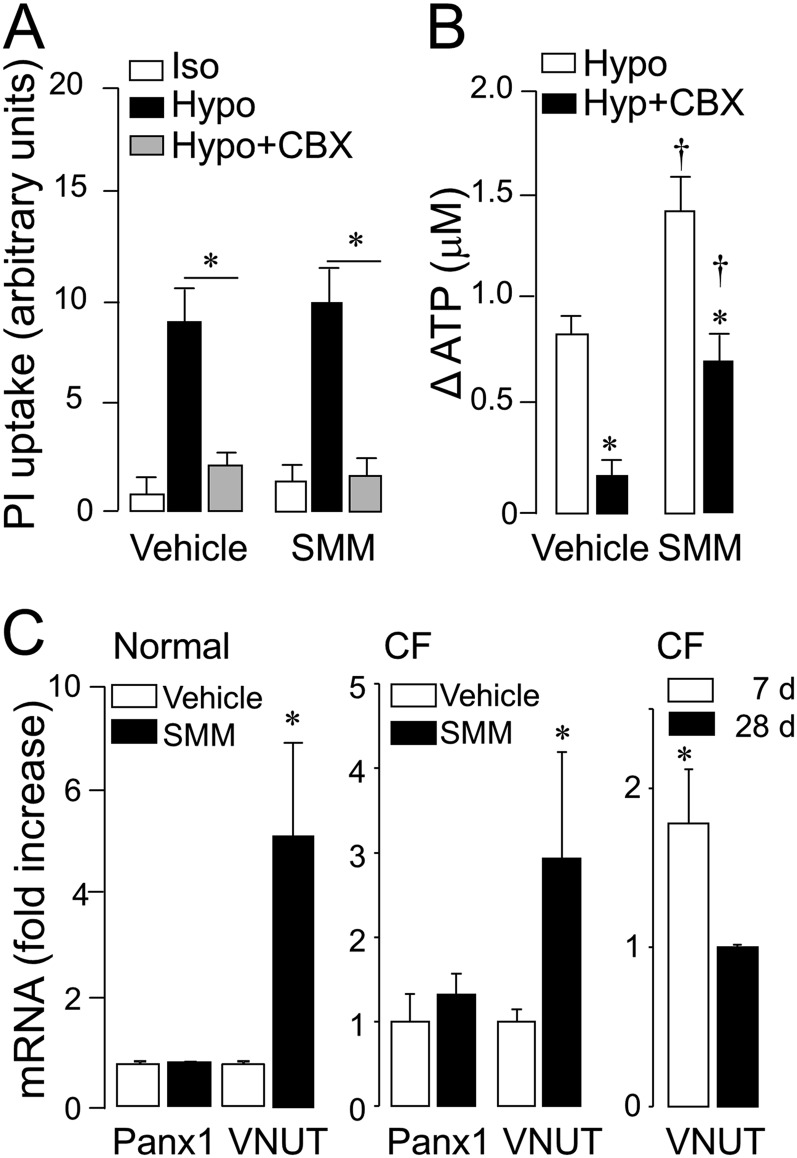
As predicted (20), resting cultures of vehicle-treated HBE cells displayed negligible nuclear labeling with propidium iodide (a pannexin conductance fluorescence probe), and the exposure of cells to hypotonic challenge resulted in enhanced uptake of the dye, which was nearly abolished by the Panx1 inhibitor carbenoxolone. No differences in basal and hypotonicity-stimulated propidium iodide uptake were observed between control and SMM-treated cells, and carbenoxolone also nearly abolished hypotonicity-promoted dye uptake in SMM-treated cells (Figure 5A, left). Furthermore, carbenoxolone greatly reduced (up to 25%) the release of ATP from noninflamed HBE cells in response to hypotonic challenge, but the effect of SMM on hypotonic stress–evoked ATP was mostly insensitive to carbenoxolone (Figure 5A, right). Thus, SMM-inflamed HBE cells display a robust Panx 1–independent component mediating hypotonicity-promoted ATP release.
Figure 5.
SMM-promoted changes in ATP release (ΔATP) are not affected by pannexin 1 inhibition. The effects of carbenoxolone (CBX; 100 μM) on hypotonicity-induced propidium iodide (PI) uptake (A) and ATP release (B) in normal WD-HBE cells exposed to vehicle or SMM were assessed as indicated in Materials and Methods. The values represent the means ± SEMs of three Transwells/subject established from three different subjects. *P < 0.05 and †P < 0.05, significant difference from time-matched, vehicle-exposed control values and basal values, respectively. (C) Pannexin 1 (Panx1) and vesicular nucleotide transporter (VNUT) mRNA levels were quantified in WD-HBE cell cultures obtained from normal or CF donors (left and center). The graph on the right illustrates VNUT mRNA expression levels in untreated CF cells after 7 or 28 days in culture. The data are expressed as means ± SDs (n = 3). *P < 0.05, relative to vehicle.
PCR analysis indicated no differences in Panx 1 mRNA expression in control versus SMM-treated normal WD-HBE cells, but a 6-fold increase in the level of expression for the vesicular nucleotide transporter VNUT was observed after SMM treatment (Figure 5C, left). Similar to normal WD-HBE cells, 28-day-old CF HBE cells exposed to SMM exhibited enhanced VNUT (but not pannexin 1) mRNA expression, relative to vehicle-treated cells (Figure 5C, center). The increased expression of VNUT mRNA in SMM-treated WD-HBE cells suggests that vesicular pathways for airway epithelial ATP release are up-regulated in inflamed CF lungs. Consistent with this hypothesis, early-stage CF cells that retained the inflamed phenotype of in vivo CF airways, as shown in Figure 1 and described previously by Ribeiro and colleagues (25), exhibited enhanced VNUT mRNA expression relative to fully differentiated (28-day-old) CF cell cultures (Figure 5C, right).
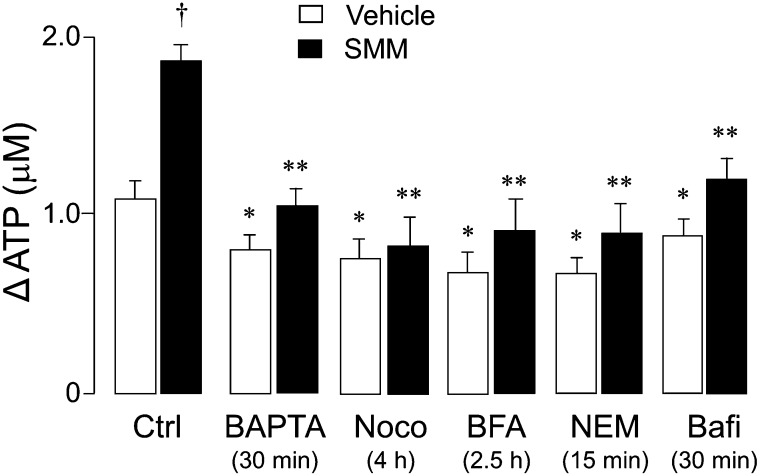
Our previous studies indicated that the Ca2+ chelator 1,2-bis(2-amino-pheoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) effectively abolished the Ca2+ mobilization response to hypotonic stress in normal HBE cells. However, BAPTA only modestly reduced ATP release (∼ 10% inhibition) from these cells (19), suggesting that Ca2+-dependent vesicle exocytosis provides only a minor contribution to ATP release in primary cultures of normal HBE cells. In line with these observations, the preincubation of vehicle-treated HBE cells with BAPTA–acetoxymethyl ester (AM) only modestly decreased (10–20%) ATP release from vehicle-exposed cells in response to hypotonicity (Figure 6). In contrast, the ATP release from SMM-treated cells was markedly reduced (up to 50%) by BAPTA-AM. Indeed, BAPTA practically abolished the component of ATP release that was up-regulated by SMM treatment (Figure 6). In addition, reagents that disrupt the secretory pathway (nocodazole, brefeldin A, N-ethylmaleimide, and bafilomycin A1) markedly reduced ATP release from SMM-inflamed cells, whereas they exerted little effect on vehicle-treated cells (Figure 6).
Figure 6.
SMM promotes the up-regulation of ATP release from the secretory pathway. (A) Changes in peak ATP concentrations between hypotonic versus isotonic exposures (ΔATP) are indicated for vehicle-exposed (open bars) and SMM-exposed (solid bars) HBE cell cultures. Cells were preincubated for the indicated times with 1,2-bis(2-amino-pheoxy)ethane-N,N,N′,N′-tetraacetic acid–acetoxymethyl ester (BAPTA-AM, 100 μM), nocodazole (Noco, 20 μM), brefeldin A (BFA, 40 μM), N-ethylmaleimide (NEM) (1 mM), or bafilomycin A1 (Bafi, 2 μM), as previously described (24, 36). Values are expressed as the means ± SDs of three Transwells/subject, established from three different subjects. *P < 0.05 and **P < 0.01, significant difference relative to control values. †P < 0.05, significant difference between vehicle-exposed versus SMM-exposed cultures undergoing the same treatment.
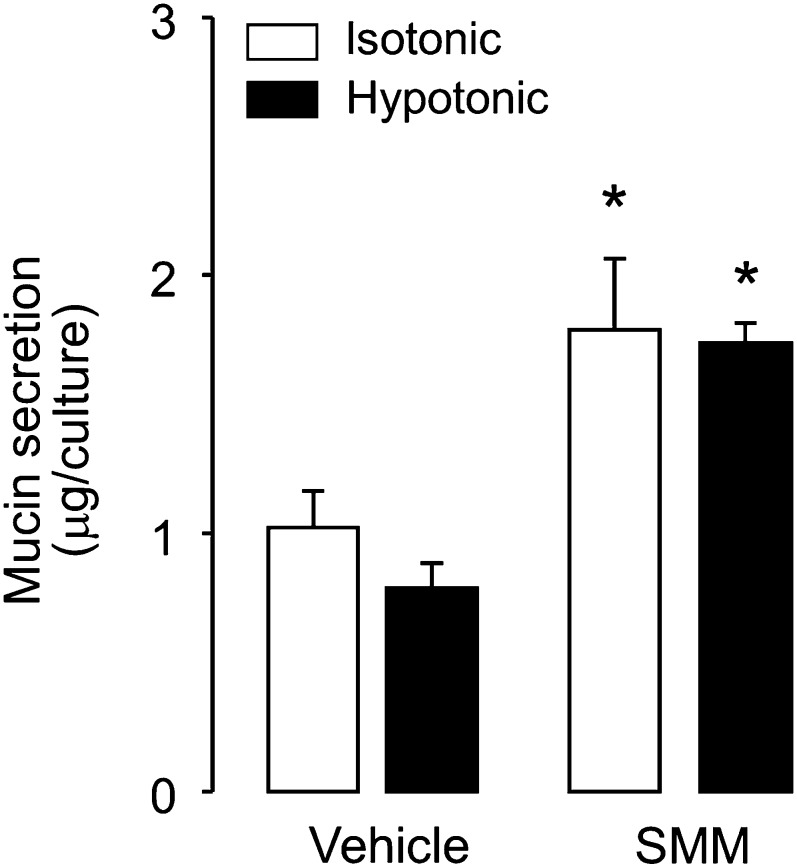
Our recent studies with goblet-cell models revealed that nucleotides are released as co-cargo molecules with mucins via Ca2+-regulated exocytosis (23). Therefore, the exocytotic mechanism of ATP release that is up-regulated in SMM-inflamed HBE cells may reflect an increased secretion of mucin granules in response to hypotonicity. Our present findings, however, do not support this hypothesis. In contrast to its effects on ATP release, hypotonicity did not initiate mucin secretion from vehicle-treated HBE cells (Figure 7). Previously, we reported that the exposure of primary cultures of HBE cells to SMM increased the number of goblet cells within the culture (37). As shown in Figure 7, despite a doubling of ASL mucin secretion from resting (isotonic) cells after a 60-hour exposure to SMM, hypotonicity did not promote mucin secretion from these cultures (Figure 7). These results suggest that mucin granules did not contribute to the increased ATP release observed in vehicle-treated or SMM-inflamed airway epithelia.
Figure 7.
Hypotonicity does not promote mucin secretion. Vehicle-treated and SMM-treated (for 60 h) HBE cell cultures were exposed to 30% hypotonicity for 10 minutes, and mucin secretion was assessed as described in Materials and Methods. To prevent ATP release–promoted mucin secretion, 2 U/ml of hexokinase were included in the glucose-rich incubation medium. The data represent means ± SEMs (n = 4). *Significantly different from vehicle-exposed cells.
Discussion
Previous studies reported a correlation between adenine nucleotide/nucleoside levels in vivo in ASL and neutrophil counts in airway lavages from inflamed CF lungs (17). However, the contribution of airway epithelial cells to lung inflammation–associated nucleotide release has only begun to be addressed (24). The major finding of the present study is that airway epithelial cell cultures exhibiting inflammatory responses relevant to CF up-regulate their capacity to release ATP via vesicular pathways.
Two in vitro models of CF airway inflammation were used in this study: (1) young (6- to 11-d-old, undifferentiated) primary cultures of HBE cells from CF donors, which maintain the inflamed phenotype characteristic of the chronically infected donor lung (25, 27), and (2) older (28- to 35-d-old), well-differentiated normal HBE cells that were exposed in vitro to the infectious and inflammatory milieu (SMM) from CF lungs (25). In both models, increased IL-8 secretion (an index of inflammation) was accompanied by an enhanced release of ATP in response to a hypotonic challenge. These findings are consistent with reports indicating a link between ATP release and inflammation, for example, in bladder urothelium during interstitial cystitis (38), in colorectum epithelium during colitis (39), and in vein endothelial cells after exposure to LPS (40).
Our study also provides mechanistic insights into the processes mediating the increased release of ATP associated with inflammation. First, the enhanced release of ATP observed in SMM-treated cultures reflects a regulated mechanism associated with Rho/Rho kinase activation, a key signaling pathway that controls cytoskeletal rearrangements. The modest, but significant, increase of Rho-A activity in SMM-treated cells, together with the robust inhibitory effects of Rho kinase inhibitors, suggest that the SMM-associated enhanced ATP release is driven by the insertion of components of the ATP release machinery into the plasma membrane (20, 22, 23, 41). Second, SMM-treated cultures exhibited a robust Ca2+-dependent (i.e., BAPTA-sensitive) ATP release that was inhibited by reagents that disrupt the secretory pathway, such as brefeldin A, nocodazole, N-ethylmaleimide (inhibitors of vesicle trafficking), and bafilomycin A1 (an inhibitor of the vacuolar H+/ATPase that couples with VNUT to drive ATP transport into vesicles) (42–45). In contrast, BAPTA and the inhibitors of the secretory pathway exerted only a minor effect on ATP release from vehicle-treated cells. Third, inhibition of the plasma membrane ATP channel pannexin 1 markedly reduced the ATP release response in control HBE cells, but the increased ATP release associated with SMM treatment was insensitive to pannexin 1 inhibition. Levels of expression for pannexin 1 mRNA were similar in control and inflamed HBE cell cultures, but VNUT transcripts were markedly elevated in SMM-treated cells (Figure 5C). Collectively, the data suggest that the increased ATP release observed in inflamed HBE cultures reflects an up-regulation of vesicular nucleotide release mechanisms.
Ca2+-dependent vesicular nucleotide release from airway epithelia has been previously associated with mucin granule secretion. For example, we have reported that primary cultures of WD-HBE cells, induced to develop goblet-cell metaplasia (24), exhibited increased nucleotide release compared with ciliated cell–dominated HBE cells. We also reported that mucin granules isolated from goblet-like Calu-3 cells (lung adenocarcinoma cells) stored ATP (23), and that stimuli that triggered mucin granule secretion from these cells also promoted increased ATP release (23, 30, 36). Thus, mucin granules are an important source of the ASL nucleotides necessary to promote fluid secretion for mucin hydration.
However, our present study indicates that hypotonicity-evoked ATP release from either vehicle-treated or SMM-treated HBE cells is not accompanied by increased mucin secretion (Figure 7). Instead, our data suggest a previously unrecognized, mucin granule secretion–independent mechanism of nucleotide release from a vesicular secretory pathway in HBE cells. Interestingly, Ca2+-regulated nucleotide release from unidentified vesicular pools has been recently reported with other tissues lacking biochemically or morphologically defined secretory granules, such as T-cell receptor–stimulated lymphocytes (43), hypotonically stimulated rat hepatoma cells (46) and cholangiocytes (47), and lung carcinoma A549 cells (48, 49). Moreover, recent cell-fractionation studies using the airway epithelial Calu-3 cell line indicated that VNUT sedimented not only with the mucin granule–rich fraction, but also with mucin-free vesicles associated with the lysosome-rich and endoplasmic reticulum/Golgi-rich fractions (30). Immunofluorescence analysis of Calu-3 cells overexpressing Myc-tagged VNUT indicated Myc–VNUT localization to vesicles devoid of mucins, in addition to mucin granules (30). Given these observations and reports that VNUT is involved in the storage and release of ATP from orphan vesicles in a number of cell types (43, 47, 50), we hypothesize that a VNUT-dependent, ATP-loaded vesicular pool, different from the pool associated with mucin granules, contributed to the enhanced ATP release observed in inflamed HBE cells.
In conclusion, our study demonstrates that airway epithelial cells exposed to inflammatory conditions relevant to CF-diseased lungs exhibit enhanced vesicular ATP release in response to hypotonic cell swelling. Given the reported action of nucleotides on inflammatory cell motility and the abundance of purinergic receptors in inflammatory and immunocompetent cells (51), the up-regulation of ATP release by inflamed airway epithelia may perpetuate the inflammatory phenotype.
Acknowledgments
Acknowledgments
The authors are grateful to the University of North Carolina Immunotechnology Core for cytokine measurements and to Dr. Scott Randell and the University of North Carolina Tissue Core for supplying the primary HBE cell cultures.
Footnotes
This work was supported by Cystic Fibrosis Foundation grants OKADA06I0 (S.F.O.), RIBEIR00Z0, RIBEIR00G0, and RIBEIR07G0 (C.M.P.R.), SEMINA08FO (L.S.-V.), and R026-CR07 (R.C.B.), and by National Institutes of Health grants P01 HL034322 (R.C.B. and E.R.L.) and P30 DK065988, R01 HL092964, and PS0 HL084934 (R.C.B.).
Originally Published in Press as DOI: 10.1165/rcmb.2012-0493OC on June 13, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Evans CM, Koo JS. Airway mucus: the good, the bad, the sticky. Pharmacol Ther. 2009;121:332–348. doi: 10.1016/j.pharmthera.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazarowski ER, Boucher RC. Purinergic receptors in airway epithelia. Curr Opin Pharmacol. 2009;9:262–267. doi: 10.1016/j.coph.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrero ME. Purinoceptors in inflammation: potential as anti-inflammatory therapeutic targets. Front Biosci. 2011;16:2172–2186. doi: 10.2741/3846. [DOI] [PubMed] [Google Scholar]
- 4.Lazarowski ER, Tarran R, Grubb BR, van Heusden CA, Okada S, Boucher RC. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J Biol Chem. 2004;279:36855–36864. doi: 10.1074/jbc.M405367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, Zhang L, Collins PL, Pickles RJ, Fredberg JJ, et al. Normal and cystic fibrosis airway surface liquid homeostasis: the effects of phasic shear stress and viral infections. J Biol Chem. 2005;280:35751–35759. doi: 10.1074/jbc.M505832200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lommatzsch M, Cicko S, Müller T, Lucattelli M, Bratke K, Stoll P, Grimm M, Dürk T, Zissel G, Ferrari D, et al. Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:928–934. doi: 10.1164/rccm.200910-1506OC. [DOI] [PubMed] [Google Scholar]
- 7.Lucattelli M, Cicko S, Müller T, Lommatzsch M, De Cunto G, Cardini S, Sundas W, Grimm M, Zeiser R, Dürk T, et al. P2X7 receptor signaling in the pathogenesis of smoke-induced lung inflammation and emphysema. Am J Respir Cell Mol Biol. 2011;44:423–429. doi: 10.1165/rcmb.2010-0038OC. [DOI] [PubMed] [Google Scholar]
- 8.Esther CR, Jr, Lazaar AL, Bordonali E, Qaqish B, Boucher RC. Elevated airway purines in COPD. Chest. 2011;140:954–960. doi: 10.1378/chest.10-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med. 2007;13:913–919. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 10.Müller T, Robaye B, Vieira RP, Ferrari D, Grimm M, Jakob T, Martin SF, Di Virgilio F, Boeynaems JM, Virchow JC, et al. The purinergic receptor P2Y2 receptor mediates chemotaxis of dendritic cells and eosinophils in allergic lung inflammation. Allergy. 2010;65:1545–1553. doi: 10.1111/j.1398-9995.2010.02426.x. [DOI] [PubMed] [Google Scholar]
- 11.Müller T, Vieira RP, Grimm M, Dürk T, Cicko S, Zeiser R, Jakob T, Martin SF, Blumenthal B, Sorichter S, et al. A potential role for P2X7R in allergic airway inflammation in mice and humans. Am J Respir Cell Mol Biol. 2011;44:456–464. doi: 10.1165/rcmb.2010-0129OC. [DOI] [PubMed] [Google Scholar]
- 12.Polosa R, Blackburn MR. Adenosine receptors as targets for therapeutic intervention in asthma and chronic obstructive pulmonary disease. Trends Pharmacol Sci. 2009;30:528–535. doi: 10.1016/j.tips.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huszár E, Vass G, Vizi E, Csoma Z, Barát E, Molnár Világos G, Herjavecz I, Horváth I. Adenosine in exhaled breath condensate in healthy volunteers and in patients with asthma. Eur Respir J. 2002;20:1393–1398. doi: 10.1183/09031936.02.00005002. [DOI] [PubMed] [Google Scholar]
- 14.Mohsenin A, Blackburn MR. Adenosine signaling in asthma and chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2006;12:54–59. doi: 10.1097/01.mcp.0000199002.46038.cb. [DOI] [PubMed] [Google Scholar]
- 15.Blackburn MR, Lee CG, Young HW, Zhu Z, Chunn JL, Kang MJ, Banerjee SK, Elias JA. Adenosine mediates IL-13–induced inflammation and remodeling in the lung and interacts in an IL-13–adenosine amplification pathway. J Clin Invest. 2003;112:332–344. doi: 10.1172/JCI16815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loughlin CE, Esther CR, Jr, Lazarowski ER, Alexis NE, Peden DB. Neutrophilic inflammation is associated with altered airway hydration in stable asthmatics. Respir Med. 2010;104:29–33. doi: 10.1016/j.rmed.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esther CR, Jr, Alexis NE, Clas ML, Lazarowski ER, Donaldson SH, Ribeiro CM, Moore CG, Davis SD, Boucher RC. Extracellular purines are biomarkers of neutrophilic airway inflammation. Eur Respir J. 2008;31:949–956. doi: 10.1183/09031936.00089807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sesma JI, Esther CR, Jr, Kreda SM, Jones L, O’Neal W, Nishihara S, Nicholas RA, Lazarowski ER. Endoplasmic reticulum/Golgi nucleotide sugar transporters contribute to the cellular release of UDP–sugar signaling molecules. J Biol Chem. 2009;284:12572–12583. doi: 10.1074/jbc.M806759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC. Physiological regulation of ATP release at the apical surface of human airway epithelia. J Biol Chem. 2006;281:22992–23002. doi: 10.1074/jbc.M603019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seminario-Vidal L, Okada SF, Sesma JI, Kreda SM, van Heusden CA, Zhu Y, Jones LC, O’Neal WK, Penuela S, Laird DW, et al. Rho signaling regulates pannexin 1–mediated ATP release from airway epithelia. J Biol Chem. 2011;286:26277–26286. doi: 10.1074/jbc.M111.260562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Button B, Picher M, Boucher RC. Differential effects of cyclic and constant stress on ATP release and mucociliary transport by human airway epithelia. J Physiol. 2007;580:577–592. doi: 10.1113/jphysiol.2006.126086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seminario-Vidal L, Kreda S, Jones L, O’Neal W, Trejo J, Boucher RC, Lazarowski ER. Thrombin promotes release of ATP from lung epithelial cells through coordinated activation of Rho- and Ca2+-dependent signaling pathways. J Biol Chem. 2009;284:20638–20648. doi: 10.1074/jbc.M109.004762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreda SM, Seminario-Vidal L, van Heusden CA, O’Neal W, Jones L, Boucher RC, Lazarowski ER. Receptor-promoted exocytosis of airway epithelial mucin granules containing a spectrum of adenine nucleotides. J Physiol. 2010;588:2255–2267. doi: 10.1113/jphysiol.2009.186643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okada SF, Zhang L, Kreda SM, Abdullah LH, Davis CW, Pickles RJ, Lazarowski ER, Boucher RC. Coupled nucleotide and mucin hypersecretion from goblet-cell metaplastic human airway epithelium. Am J Respir Cell Mol Biol. 2011;45:253–260. doi: 10.1165/rcmb.2010-0253OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribeiro CM, Paradiso AM, Schwab U, Perez-Vilar J, Jones L, O’Neal W, Boucher RC. Chronic airway infection/inflammation induces a Ca2+i–dependent hyperinflammatory response in human cystic fibrosis airway epithelia. J Biol Chem. 2005;280:17798–17806. doi: 10.1074/jbc.M410618200. [DOI] [PubMed] [Google Scholar]
- 26.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med. 2005;107:183–206. doi: 10.1385/1-59259-861-7:183. [DOI] [PubMed] [Google Scholar]
- 27.Ribeiro CM, Paradiso AM, Carew MA, Shears SB, Boucher RC. Cystic fibrosis airway epithelial Ca2+i signaling: the mechanism for the larger agonist-mediated Ca2+i signals in human cystic fibrosis airway epithelia. J Biol Chem. 2005;280:10202–10209. doi: 10.1074/jbc.M410617200. [DOI] [PubMed] [Google Scholar]
- 28.Lazarowski E. Seminario-Vidal LS, Kreda S, Sesma J, Okada S, Boucher R. Rho-dependent pannexin 1–mediated ATP release from airway epithelia. Purinergic Signal. 2010;6:S71. [Google Scholar]
- 29.Lazarowski ER, Boucher RC, Harden TK. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J Biol Chem. 2000;275:31061–31068. doi: 10.1074/jbc.M003255200. [DOI] [PubMed] [Google Scholar]
- 30.Sesma JI, Kreda SM, Okada SF, van Heusden C, Moussa L, Jones LC, O’Neal WK, Togawa N, Hiasa M, Moriyama Y, et al. Vesicular nucleotide transporter regulates the nucleotide content in airway epithelial mucin granules. Am J Physiol Cell Physiol. 2013;304:C976–C984. doi: 10.1152/ajpcell.00371.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okada Y. Volume expansion–sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. Am J Physiol. 1997;273:C755–C789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- 32.Picher M, Burch LH, Boucher RC. Metabolism of P2 receptor agonists in human airways: implications for mucociliary clearance and cystic fibrosis. J Biol Chem. 2004;279:20234–20241. doi: 10.1074/jbc.M400305200. [DOI] [PubMed] [Google Scholar]
- 33.Picher M, Burch LH, Hirsh AJ, Spychala J, Boucher RC. Ecto 5′-nucleotidase and nonspecific alkaline phosphatase: two AMP-hydrolyzing ectoenzymes with distinct roles in human airways. J Biol Chem. 2003;278:13468–13479. doi: 10.1074/jbc.M300569200. [DOI] [PubMed] [Google Scholar]
- 34.Hirsh AJ, Stonebraker JR, van Heusden CA, Lazarowski ER, Boucher RC, Picher M. Adenosine deaminase 1 and concentrative nucleoside transporters 2 and 3 regulate adenosine on the apical surface of human airway epithelia: implications for inflammatory lung diseases. Biochemistry. 2007;46:10373–10383. doi: 10.1021/bi7009647. [DOI] [PubMed] [Google Scholar]
- 35.Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol. 2009;41:525–534. doi: 10.1165/rcmb.2008-0367OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kreda SM, Okada SF, van Heusden CA, O’Neal W, Gabriel S, Abdullah L, Davis CW, Boucher RC, Lazarowski ER. Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J Physiol. 2007;584:245–259. doi: 10.1113/jphysiol.2007.139840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ribeiro CM, Hurd H, Wu Y, Martino ME, Jones L, Brighton B, Boucher RC, O’Neal WK. Azithromycin treatment alters gene expression in inflammatory, lipid metabolism, and cell cycle pathways in well-differentiated human airway epithelia. PLoS ONE. 2009;4:e5806. doi: 10.1371/journal.pone.0005806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Y, Chai TC. Augmented extracellular ATP signaling in bladder urothelial cells from patients with interstitial cystitis. Am J Physiol Cell Physiol. 2006;290:C27–C34. doi: 10.1152/ajpcell.00552.2004. [DOI] [PubMed] [Google Scholar]
- 39.Wynn G, Ma B, Ruan HZ, Burnstock G. Purinergic component of mechanosensory transduction is increased in a rat model of colitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G647–G657. doi: 10.1152/ajpgi.00020.2004. [DOI] [PubMed] [Google Scholar]
- 40.Bodin P, Burnstock G. Increased release of ATP from endothelial cells during acute inflammation. Inflamm Res. 1998;47:351–354. doi: 10.1007/s000110050341. [DOI] [PubMed] [Google Scholar]
- 41.Blum AE, Joseph SM, Przybylski RJ, Dubyak GR. Rho-family GTPases modulate Ca(2+) -dependent ATP release from astrocytes. Am J Physiol Cell Physiol. 2008;295:C231–C241. doi: 10.1152/ajpcell.00175.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Yamamoto A, Moriyama Y. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci USA. 2008;105:5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tokunaga A, Tsukimoto M, Harada H, Moriyama Y, Kojima S. Involvement of SLC17A9-dependent vesicular exocytosis in the mechanism of ATP release during T cell activation. J Biol Chem. 2010;285:17406–17416. doi: 10.1074/jbc.M110.112417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haanes KA, Novak I. ATP storage and uptake by isolated pancreatic zymogen granules. Biochem J. 2010;429:303–311. doi: 10.1042/BJ20091337. [DOI] [PubMed] [Google Scholar]
- 45.Rudnick G. Vesicular ATP transport is a hard (V)NUT to crack. Proc Natl Acad Sci USA. 2008;105:5949–5950. doi: 10.1073/pnas.0802774105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feranchak AP, Lewis MA, Kresge C, Sathe M, Bugde A, Luby-Phelps K, Antich PP, Fitz JG. Initiation of purinergic signaling by exocytosis of ATP-containing vesicles in liver epithelium. J Biol Chem. 2010;285:8138–8147. doi: 10.1074/jbc.M109.065482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sathe MN, Woo K, Kresge C, Bugde A, Luby-Phelps K, Lewis MA, Feranchak AP. Regulation of purinergic signaling in biliary epithelial cells by exocytosis of SLC17A9-dependent ATP-enriched vesicles. J Biol Chem. 2011;286:25363–25376. doi: 10.1074/jbc.M111.232868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tatur S, Kreda S, Lazarowski E, Grygorczyk R. Calcium-dependent release of adenosine and uridine nucleotides from A549 cells. Purinergic Signal. 2008;4:139–146. doi: 10.1007/s11302-007-9059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akopova I, Tatur S, Grygorczyk M, Luchowski R, Gryczynski I, Gryczynski Z, Borejdo J, Grygorczyk R. Imaging exocytosis of ATP-containing vesicles with TIRF microscopy in lung epithelial A549 cells. Purinergic Signal. 2012;8:59–70. doi: 10.1007/s11302-011-9259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takai E, Tsukimoto M, Harada H, Sawada K, Moriyama Y, Kojima S. Autocrine regulation of TGF-β1–induced cell migration by exocytosis of ATP and activation of P2 receptors in human lung cancer cells. J Cell Sci. 2012;125:5051–5060. doi: 10.1242/jcs.104976. [DOI] [PubMed] [Google Scholar]
- 51.Corriden R, Insel PA. New insights regarding the regulation of chemotaxis by nucleotides, adenosine, and their receptors. Purinergic Signal. 2012;8:587–598. doi: 10.1007/s11302-012-9311-x. [DOI] [PMC free article] [PubMed] [Google Scholar]