Abstract
Protein phosphatase–2A (PP2A) is a primary serine–threonine phosphatase that modulates inflammatory responses in asthma and chronic obstructive pulmonary disease (COPD). Despite its importance, the mechanisms that regulate lung PP2A activity remain to be determined. The redox-sensitive enzyme protein tyrosine phosphatase–1B (PTP1B) activates PP2A by dephosphorylating the catalytic subunit of the protein at tyrosine 307. This study aimed to identify how the interaction between the intracellular antioxidant glutathione peroxidase–1 (GPx-1) and PTP1B affected lung PP2A activity and airway inflammation. Experiments using gene silencing techniques in mouse lung or human small airway epithelial cells determined that knocking down PTP1B expression blocked GPx-1’s activation of PP2A and negated the anti-inflammatory effects of GPx-1 protein in the lung. Similarly, the expression of human GPx-1 in transgenic mice significantly increased PP2A and PTP1B activities and prevented chronic cigarette smoke–induced airway inflammation and alveolar destruction. GPx-1 knockout mice, however, exhibited an exaggerated emphysema phenotype, correlating with a nonresponsive PP2A pathway. Importantly, GPx-1–PTP1B–PP2A signaling becomes inactivated in advanced lung disease. Indeed, PTP1B protein was oxidized in the lungs of subjects with advanced emphysema, and cigarette smoke did not increase GPx-1 or PTP1B activity within epithelial cells isolated from subjects with COPD, unlike samples of healthy lung epithelial cells. In conclusion, these findings establish that the GPx-1–PTP1B–PP2A axis plays a critical role in countering the inflammatory and proteolytic responses that result in lung-tissue destruction in response to cigarette smoke exposure.
Keywords: phosphorylation, inflammation, oxidation, kinase
Clinical Relevance
Protein phosphatase–2A (PP2A) is the primary serine threonine phosphatase in humans, and altered enzymatic activity has been implicated in the development of lung diseases such as asthma and chronic obstructive pulmonary disease. Despite its importance, the cellular processes that regulate PP2A activity remain poorly understood. In this work, we identified a novel mechanism whereby an antioxidant enzyme, glutathione peroxidase–1 (GPx-1), protects against smoke-mediated airway inflammation and alveolar destruction by increasing the activities of protein tyrosine phosphatase–1B (PTP1B) and subsequently PP2A in the lung. Furthermore, we show that GPx-1 mediates these effects not only because it is an antioxidant, but more importantly, because it has a unique binding affinity for PTP1B.
Protein phosphatase–2A (PP2A) is the major serine–threonine eukaryotic phosphatase (1), and accounts for up to 1% of total cellular protein (2). Altered PP2A activity plays a key role in cancer (3), neurodegenerative diseases (4), and innate immune responses (5). In the lung, PP2A is a key modulator of inflammatory responses in asthma (6) and chronic obstructive pulmonary disease (COPD) (5). Despite its importance in these and other biological processes, the mechanisms by which PP2A is regulated within the lung remains to be determined. Increasing evidence indicates that PP2A activity is sensitive to the redox status of the cell (7). Indeed, deficiencies in PP2A activity during Alzheimer disease (8) and chronic lymphocytic leukemia (9) are associated with enhanced oxidative stress and impaired antioxidant defenses (10, 11). Despite the established links between oxidative stress and altered PP2A activity, the biological pathways by which redox factors modulate PP2A activity to influence disease development are not yet known.
Increased oxidative injury has been detected in the lungs of patients with COPD, and animal studies demonstrate that inflammation in this disease can be regulated by the redox status of the lung (12, 13). COPD is the third leading cause of death in the United States (14), and is projected to be the third leading cause of death worldwide within the next 20 years. Thus, understanding how redox factors moderate the processes responsible for disease development is of critical importance. Although antioxidants are known to counter cigarette smoke–induced lung inflammation (13, 15), the mechanisms by which they mediate these effects remain to be determined. Our group and others have shown that lung phosphatases counter the inflammatory response to cigarette smoke (5, 16). Thus, this study examined whether the redox regulation of phosphatases altered intracellular signaling to modulate lung inflammation in this disease.
PP2A becomes inactivated by the phosphorylation of a tyrosine site at position 307 (Tyr307) in the catalytic subunit of the enzyme, and in vitro studies indicate that this process is counteracted by protein tyrosine phosphatase–1B (PTP1B) (17). PP2A and PTP1B regulate key kinase signaling pathways implicated in COPD (18, 19). Despite their importance, the effects of redox factors on lung phosphatase activity and inflammatory signaling remain poorly understood. Here we examine one of the most abundant glutathione peroxidase isoforms in eukaryotic cells, glutathione peroxidase–1 (GPx-1), because it plays a major role in subduing smoke-induced inflammation (20). Because PTP1B activity is prone to oxidative inactivation (21, 22) and PTP1B greatly influences PP2A activity (17, 23), we postulated that antioxidants, and particularly GPx-1, protect against smoke-induced inflammation and the development of emphysema by stimulating PTP1B to increase PP2A activity. This novel mechanism would have implications not only in COPD but also in other reactive oxygen species (ROS)–driven diseases where PP2A activity plays a key role.
Materials and Methods
Animal Models
GPx-1 mice were bred in a C57BL/6 × CBA/J background. Twelve-week-old, wild-type, GPx-1 transgenic and GPx-1 knockout mice were used for all experiments. All animal experiments were performed with approval from the Institutional Animal Care and Use Committee at St. Luke’s Roosevelt Hospital Center.
Cigarette Smoke Exposure Protocol
Mice were exposed to cigarette smoke according to our established protocol (15). Full details are presented in the online supplement.
In Vivo PTP1B Silencing
Animals intranasally received 7.4 nmol negative control or PTP1B (mouse Ptpn1) silencer short, interfering RNA (siRNA; Life Technologies, Grand Island, NY) in PBS, and 48 hours later received either 3 μg of human albumin or 1 unit of GPx-1 protein in 20 μl of Pro-Ject reagent (Thermo Scientific, West Palm Beach, FL) before smoke exposure.
In Vitro GPx-1 Binding Assay
Human purified GPx-1 (1 μg/well) was coated onto a 96-well plate in 0.05 M carbonate bicarbonate buffer, pH 9.6, and incubated at 4°C overnight. Wells were incubated with 1 μg of albumin, PP2A, or PTP1B, and placed on a shaker for 2 hours at 37°C. The wells were subsequently detected by an ELISA-based approach with antibodies for either PP2A or PTP1B (Cell Signaling, Danvers, MA). GPx-1 binding was recorded as absorbance at 450 nm.
In Vitro Activation of PTP1B
Recombinant human PTP1B protein (10 ng) was mixed with 5% cigarette smoke extract (CSE) or 1 unit of human GPx-1. PTP1B activity was determined with a specific PTP1B assay (DYC1625; R&D Systems, Minneapolis, MN).
Cell Culture
Human small airway epithelial (SAE) healthy and COPD cells (Lonza, Allendale, NJ) were cultured and treated with or without 5% smoke extract, as previously described (24). Healthy SAE cells, stably transfected with lentiviral vectors expressing GPx-1, PTP1B, or control short hairpin RNA (shRNA) (Santa Cruz Biotechnology, Santa Cruz, CA), were generated as previously described (5). One unit of human GPx-1 (Sigma-Aldrich, St. Louis, MO) or an equivalent amount of albumin was mixed with Pro-Ject reagent and added to the cells for 24 hours.
Matrix Metalloproteinase and Cytokine Measurements
Quantitative PCR for mouse matrix metalloproteinase (MMP)–9, MMP-12, and MMP-13 was measured from the lungs of mice, using TaqMan specific probes (Applied Biosystems, Carlsbad, CA). IL-12 (p40), IL-12 (p70), IL-17, IFN-γ, and TNF-α concentrations were measure in bronchoalveolar lavage (BAL) fluid and whole-lung tissue protein, using a magnetic beads assay (Bio-Plex Pro Mouse Cytokine 5-plex Assay, catalogue number Z60-0000BSB; Bio-Rad, Hercules, CA) on the Bio-Rad Bio-Plex 200 system.
Intracellular Signaling
Immunoblots for mitogen-activated protein kinase signaling proteins were performed on the cytosolic protein, and NF-κB and activator protein-1 (AP-1) activations were measured in the nuclear extracts of GPX1−/− and GPX1+/+ mice under baseline and smoke-exposure conditions.
GPx-1, PP2A, PTP1B, and Tyrosine Kinase Activity
Gpx-1, PP2A, and PTP1B activities were determined using assays kits (GPx activity, Abcam 102530, Cambridge, MA; PP2A activity, catalogue number 17-313, from Millipore, Billerica, MA; and PTP1B, DYC1625, from R&D Systems). Oxidized and total PTP1B were determined by immunoblotting. Tyrosine kinase activity was measured using tyrosine kinase assay kit MK410 from Takara (Shiga, Japan).
Statistical Analyses
Data are expressed as means ± SEMs. We determined statistical significance by one-way ANOVA for multiple group analyses, using GraphPad Prism Software (La Jolla, CA).
Results
GPx-1 Binds to PTP1B and Enhances Its Activity
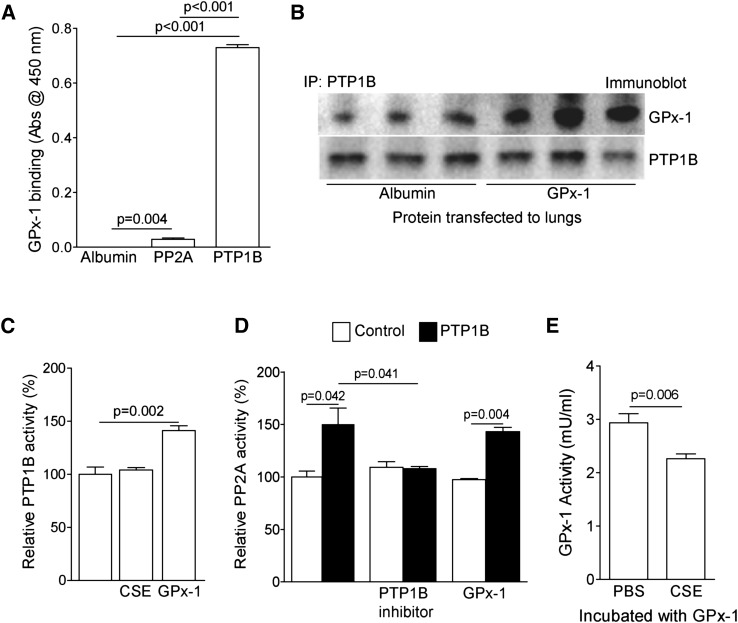
PTP1B activity is redox-modulated (21). However, whether PTP1B directly interacts with antioxidants remains to be determined. Protein binding analyses, as performed on purified proteins, demonstrated that GPx-1 interacted avidly with PTP1B, and mildly with PP2Ac (Figures 1A and 1B). Given this direct interaction, experiments were performed to investigate whether GPx-1 could directly alter PTP1B activity. We exposed human recombinant PTP1B protein to (CSE) and GPx-1, and we observed that GPx-1, by itself, significantly induced PTP1B activity (Figure 1C). In contrast, GPx-1 did not augment the activity of human recombinant PP2A protein (Figure 1D). PP2A activity was enhanced in the presence of PTP1B, and this increase in activity was sensitive to PTP1B inhibition (Figure 1D). However, a high concentration of CSE (50%) did inhibit GPx-1 activity (Figure 1E). In contrast to GPx-1, CSE did not decrease PTP1B activity, even though it contains oxidants that would be expected to inactivate the enzyme. It is important to note that CSE contains numerous other components whose effects on PTP1B activity remain unknown.
Figure 1.
Glutathione peroxidase–1 (GPx-1) directly interacts with protein tyrosine phosphatase 1B (PTP1B) to modulate its activity and subsequently protein phosphatase–2A (PP2A) activity. (A) The binding ability of purified PTP1B or PP2Ac to GPx-1 was determined via an ELISA-based approach. GPx-1 directly binds to PTP1B and PP2A, but with great affinity to PTP1B. (B) The immunoprecipitation of PTP1B protein was undertaken from mouse lung samples that underwent protein transfection with either albumin or GPx-1, and immunoblotting was performed. (C) PTP1B protein has increased activity in the presence of GPx-1. (D) GPx-1 cannot directly activate PP2A protein, but PP2A protein becomes activated through incubation with PTP1B (solid bars). (E) GPx-1, incubated with 50% cigarette smoke extract (CSE), has reduced GPx activity. Graphs represent the mean ± SEM of 12 measurements. The P values shown compare the treatments connected by a line.
GPx-1–Induced PP2A Activity Is PTP1B-Dependent
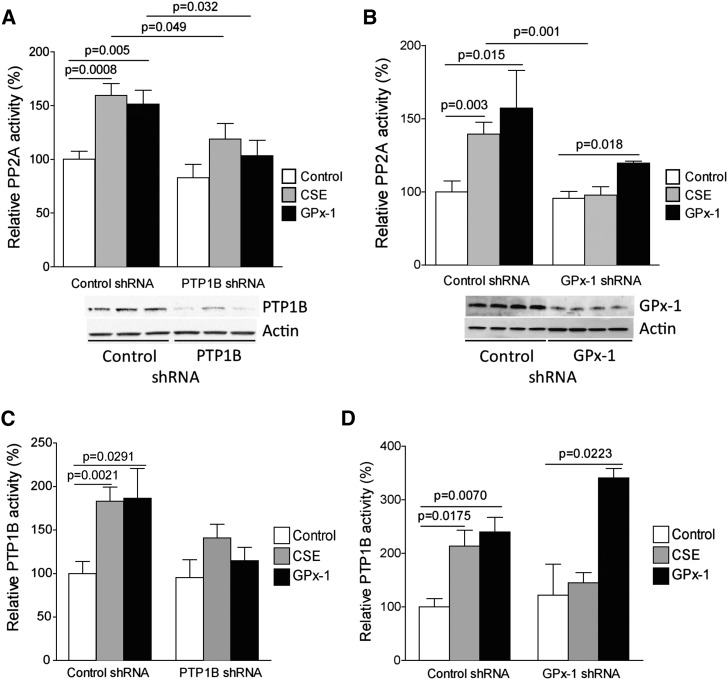
Smoke increases PP2A activity (5). However, whether PTP1B plays a significant role in this process remains unknown. SAE cells were stably transfected with lentiviral particles to express PTP1B or GPx-1 shRNA, to evaluate whether GPx-1 enhances PP2A activity via a PTP1B-dependent mechanism. PTP1B and GPx-1 were effectively silenced, as demonstrated by immunoblotting (Figures 2A and 2B) and PTP1B activity assays (Figures 2C and 2D). Both CSE and GPx-1 protein administration increased PP2A activity in SAE cells (Figure 2A). The transfection of GPx-1 protein enhanced cytoplasmic concentrations of GPx-1 in cells (Figure E1A in the online supplement). Silencing PTP1B significantly prevented the induction of PP2A activity by GPx-1. CSE treatment was also less efficient at elucidating a PP2A response under PTP1B-depleted conditions. Interestingly, the loss of GPx-1 prevented the CSE-mediated induction of PP2A activity (Figure 2B). The administration of exogenous GPx-1 onto GPx-1 shRNA cells increased PP2A activity, almost certainly because of the presence of functional PTP1B. PTP1B concentrations mimicked the PP2A response in both PTP1B and GPx-1 shRNA cells (Figures 2C and 2D). After administering GPx-1 protein to GPx-1 shRNA cells, these SAE cells were able to increase PTP1B activity in response to cigarette smoke exposure (Figure 2D). This provides further evidence that GPx-1 is required for the smoke-mediated activation of PTP1B.
Figure 2.
PTP1B is required for the activation of PP2A by GPx-1. Small airway epithelial (SAE) cells were examined for PP2A activity after treatment with negative control shRNA, (A) PTP1B shRNA, or (B) GPx-1 shRNA stably transfected cells, in combination with GPx-1 protein or CSE. Immunoblots were undertaken to demonstrate the stable knockdown of (A) PTP1B or (B) GPx-1. PTP1B activity was also examined in (C) PTP1B shRNA or (D) GPx-1 shRNA stably transfected cells. Graphs represent the mean ± SEM of six measurements. The P values shown compare the treatments connected by a line.
GPx-1 Modulates the PTP1B/PP2A Pathway in the Lung
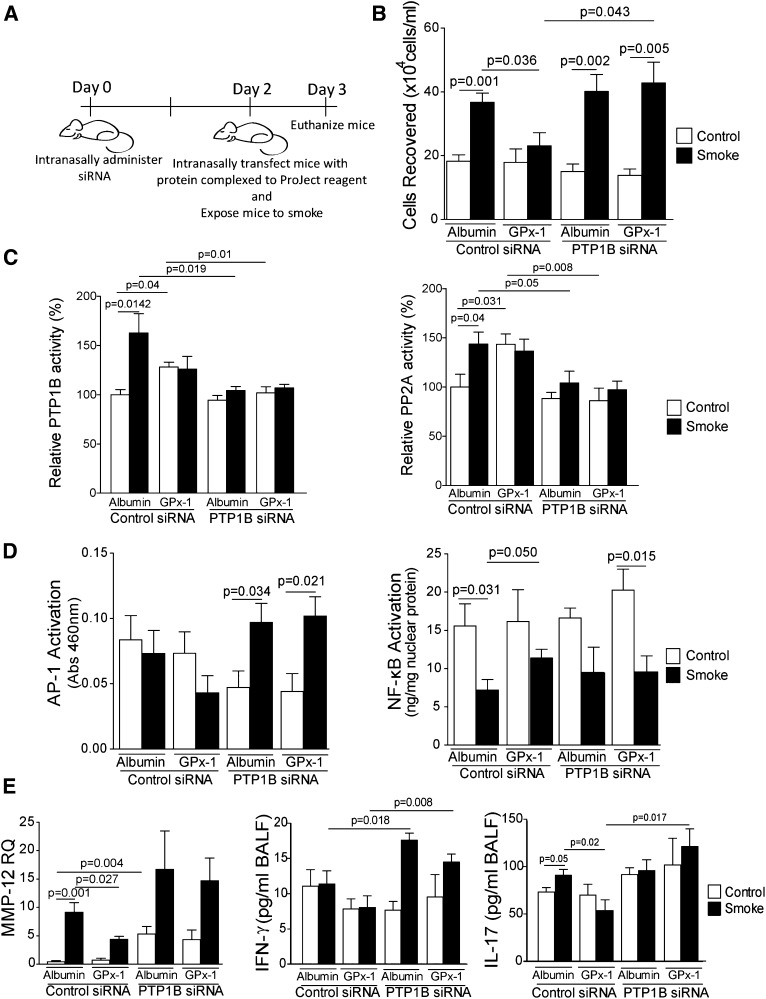
To determine whether GPx-1 increased PP2A activity via a PTP1B-dependent mechanism in mice, C57/Bl6 mice received PTP1B siRNA intranasally, and 48 hours later were protein-transfected with GPx-1 protein (Figure 3A), 1 hour before cigarette smoke exposure. GPx-1 concentrations were enhanced by transfection (Figure E1B), and PTP1B was effectively silenced by our siRNA intranasal treatment, as demonstrated by quantitative PCR (Figure E2). Protein transfecting active GPx-1 protein to the lung significantly blocked inflammatory cell infiltration after acute smoke exposure (Figure 3B). This anti-inflammatory process coincided with enhanced PTP1B and PP2A activity (Figures 3C and 3D). Importantly, the silencing of PTP1B in the lung prevented GPx-1 from enhancing PTP1B and PP2A activity and counteracting inflammatory cell infiltration. Of note, cigarette smoke activated AP-1 signaling only when PTP1B expression was silenced in the lung (Figure 3D). Neither GPx-1 administration nor PTP1B silencing affected NF-κB signaling in this model (Figure 3D). GPx-1 also prevented the release of several smoke-induced cytokines into the airways in a PTP1B-dependent manner, most notably IL-17 (Figures 3E and E3). The loss of PTP1B resulted in a hypersensitive IFN-γ response to cigarette smoke (Figure 3E). In addition, GPx-1 delivery to the lung exerted an impact on smoke-driven MMP gene expression, which was reliant on PTP1B expression (Figures 3E and E3C). GPx-1 concentrations were unchanged in alveolar macrophages, despite protein transfection (Figures E4A and E4B). Human autologous serum monocyte–derived macrophage MMP-12 secretions were unaffected by GPx-1 transfection (Figures E4C and E4D), suggesting that lung residential cells were responsible for the changes in MMP-12 expression. Together, these findings indicate that the induction of PTP1B activity by GPx-1 plays a critical role in counteracting the early inflammatory and proteolytic processes associated with smoke exposure.
Figure 3.
PTP1B is required for the induction by GPx-1 of lung PP2A activity. (A) Experimental approach to the in vivo administration of PTP1B short, interfering RNA (siRNA), GPx-1 protein transfection, and smoke exposure. Lung tissue from C57BL/6 mice was examined for (B) immune cellularity, (C) PTP1B and PP2A activity, (D) Activator protein-1 (AP-1) and NF-κB activity, and (E) matrix metalloproteinase (MMP)–12 lung gene expression and IFN-γ and IL-17 bronchoalveolar lavage fluid (BALF) cytokine concentrations. Graphs represent the mean ± SEM of 12 measurements. The P values shown compare the treatments connected by a line. Abs, absorbance.
PP2A Activity Is Increased in the Lungs of GPx-1 Transgenic Mice
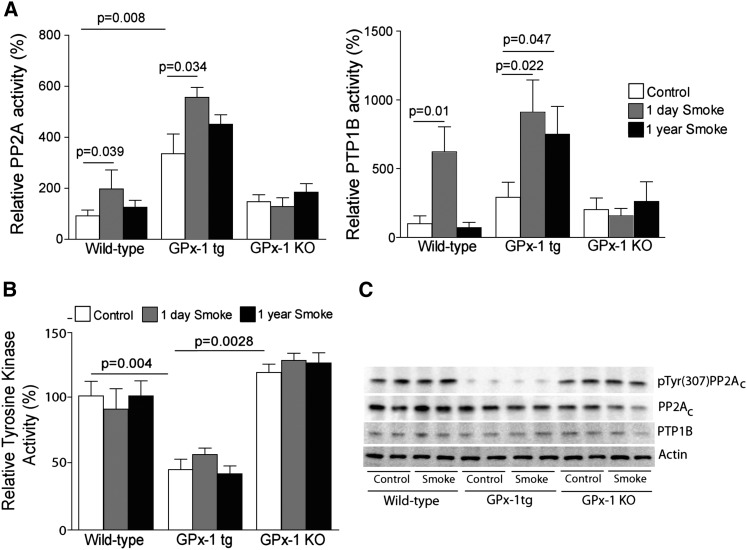
To further determine whether GPx-1 expression induced changes in PTP1B and PP2A activity, we examined PTP1B, PP2A, and tyrosine kinase activity in mice expressing human GPx-1 (GPx-1tg), GPx-1 knockout (GPx-1 KO) mice, and their wild-type littermates exposed to short (1 d)–term and long (1 yr)–term smoke exposure. An acute and chronic approach was undertaken, because our group previously demonstrated that the induction of PP2A activity in response to smoke wanes over time (5). In addition, the long-term smoke exposure of mice was required to examine all relevant characteristics of emphysema. Interestingly, GPx-1tg mice demonstrate significantly increased baseline lung PP2A and PTP1B activity compared with wild-type mice, and in contrast to wild-type mice, phosphatase activity remained elevated even after 1 year of smoke exposure (Figure 4A). Moreover, PP2A and PTP1B concentrations did not respond to smoke in GPx-1 KO mice, and these results were similar to those of our in vitro cell experiments. Changes in PP2A activity were independent of changes in expression of the structural or binding of the B regulatory subunits to the PP2A holoenzyme complex (Figure E5). Increased PP2A and PTP1B activity coincided with GPx-1 expression. Therefore, GPx-1 regulated PP2A activity by a posttranslational modification of the enzymatic subunits.
Figure 4.
GPx-1 significantly modulates the PTP1B–PP2A pathway in the lung. Lung (A) PP2A, (B) PTP1B, and (C) tyrosine kinase activity was measured from wild-type, human GPx-1 (GPx-1tg), and GPx-1 knockout (KO) mice at baseline, 1 day, and 1 year of smoke exposure. (D) Immunoblot analyses for the phospho-tyrosine 307 (pTyr307) catalytic subunit of PP2A (PP2AC), PTP1B, and actin were performed on lung tissue from the room air–exposed and 1-year smoke-exposed mouse groups. Graphs represent the mean ± SEM of 12 measurements. The P values shown compare the treatments connected by a line.
The dephosphorylation of Tyr307 on the PP2A catalytic C subunit increases overall PP2A activity (17). GPx-1 appears to regulate the activity of tyrosine kinases robustly, because we observed reduced tyrosine kinase activity in the GPx-1tg mice compared with the wild-type and GPx-1 KO mice (Figure 4B). Furthermore, GPx-1 decreased the phosphorylation of Tyr307 within the catalytic subunit of PP2A (PP2AC) (Figure 4C). The phosphorylation of this site by tyrosine kinases such as pp60v-src, pp56lck, epidermal growth factor, and insulin receptors causes inactivation of the enzyme in vitro (25). Thus, we assert that this decrease in phosphorylation may be responsible for the increase in lung PP2A activity and the anti-inflammatory effects that were observed in GPx-1tg mice.
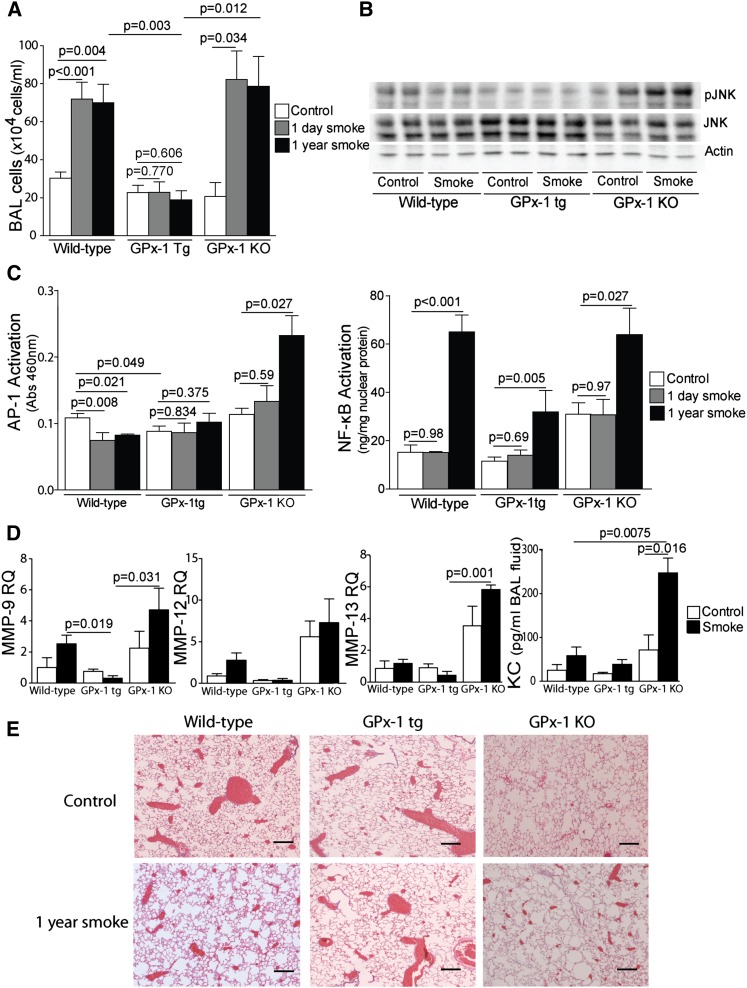
Transgenic Expression of Human GPx-1 in Mice Prevents Smoke-Induced Lung Inflammatory Responses
GPx-1tg mice exhibited significantly lower concentrations of inflammatory cells in their BAL fluid after acute and chronic smoke exposure (Figure 5A). AP-1 signaling and NF-ĸB activation play a central role in the inflammatory and proteolytic processes that are mediated by the TNF signaling pathway in COPD (24, 26). For this reason, we evaluated how GPx-1 expression and smoke exposure altered AP-1 and NF-ĸB signaling in the lung. In correlation with increased lung PP2A activity, GPx-1tg and smoke-exposed wild-type mice demonstrated decreased c-Jun N-terminal kinase (JNK) phosphorylation (Figure 5B) and AP-1 activation in the lung (Figure 5C). Conversely, the activation of JNK was observed in the lungs of 1-year smoke-exposed GPx-1 KO mice (Figure 5B), which coincided with increased AP-1 activation (Figure 5C). Increased GPx-1 did not completely deter NF-ĸB (Figure 5C), but NF-ĸB activation was significantly reduced in GPx-1tg mice exposed to 1 year of smoke, compared with both the wild-type and GPx-1 KO mice. GPx-1 expression also decreased total H2O2 concentrations (Figure E6A) and maintained tissue glutathione (GSH) in a reduced state (Figure E6B).
Figure 5.
Transgenic expression of human GPx-1 in mice prevents smoke-induced lung inflammation and airway enlargement. Enhanced GPx-1 expression (A) decreased bronchoalveolar lavage (BAL) fluid cellularity, and prevented (B) kinase (pJNK) and (C) transcription factor (AP-1 and NF-κB) activation. (D) Quantitative PCR for mouse MMP-9, MMP-12, and MMP-13 was performed using cDNA prepared from mRNA isolated from the lungs of each group. Each group represents the mean ± SEM of 12 measurements. Cytokine keratinocyte–derived cytokine (KC) concentrations in BAL fluid were also examined by ELISA in each mouse group. (E) Smoke-exposed murine lungs were compared with age-matched control samples (scale bar = 20 μM). Graphs represent the mean ± SEM of 12 measurements. The P values shown compare the treatments connected by a line. JNK, c-Jun N-terminal kinase.
Given the potent anti-inflammatory effects of GPx-1, we determined whether GPx-1tg mice were subject to similar protective effects on lung protease expression and cytokine release. GPx-1tg mice demonstrated a significantly lower lung expression of MMP-9, MMP-12, and MMP-13 after smoke exposure compared with wild-type mice (Figure 5D). MMP-9, MMP-12, and collagenases such as MMP-13 have been linked to the development of emphysema in both animal models and human studies of the disease (27). In addition, a smoke-inducible cytokine keratinocyte–derived cytokine was observed to be enhanced in the BAL fluid of GPx-1 KO mice compared with the other mouse groups (Figure 5D).
Overexpression of Human GPx-1 in Mice Prevents Smoke-Induced Airway Enlargements
GPx-1tg mice did not develop emphysematous changes after 1-year exposure to smoke (Figure 5E and Table 1). Morphometric quantification demonstrated that GPx-1 expression prevented the increase in mean linear intercept (MLI; 56 ± 8 wild-type 1-year smoke-exposed versus 42 ± 2 GPx-1tg 1-year smoke-exposed; P = 0.003) and the decrease in fractional volume and surface area that occurred in the lungs of smoke-exposed, wild-type mice (Table 1). Conversely, GPx-1 KO mice demonstrated an exaggerated emphysema phenotype, even without smoke exposure (Table 1). However, smoking significantly worsened airspace enlargement, as determined by the MLI, in the GPx-1 KO mice (53 ± 7 GPx-1 KO versus 65 ± 9 GPx-1 KO 1-year smoke-exposed; P = 0.043). Thus, these data suggest that this antioxidant enzyme deters smoke-mediated inflammation via a novel function that involves the activation of key lung phosphatases.
TABLE 1.
LUNG MORPHOMETRY OF WILD-TYPE, GPX-1TG, AND GPX-1 KO MICE
| MLI | Fractional Volume | Surface Area/Unit Volume | |
|---|---|---|---|
| Wild-type | 43 ± 9 | 0.108 ± 0.011 | 48 ± 8 |
| Wild-type 1 yr cigarette smoke | 56 ± 8 | 0.075 ± 0.018 | 34 ± 7 |
| GPx-1tg | 42 ± 2 | 0.100 ± 0.009 | 48 ± 3 |
| GPx-1tg 1 yr cigarette smoke | 42 ± 4 | 0.100 ± 0.010 | 47 ± 3 |
| GPx-1 KO | 53 ± 7 | 0.079 ± 0.010 | 35 ± 5 |
| GPx-1 KO 1 yr cigarette smoke | 65 ± 9* | 0.078 ± 0.029* | 32 ± 4* |
Definition of abbreviations: GPx-1, glutathione peroxidase–1; GPx-1tg, human glutathione peroxidase–1; KO, knockout; MLI, mean linear intercept.
Values include fractional volume of parenchyma tissue per lung (V(p/l)), alveolar surface area per unit volume S(p/l)(mm−1), and mean linear intercept (in microns). Bold lettering denotes P < 0.05, compared with room-air wild-type mice.
P < 0.05, compared with room air–exposed GPx-1 KO mice.
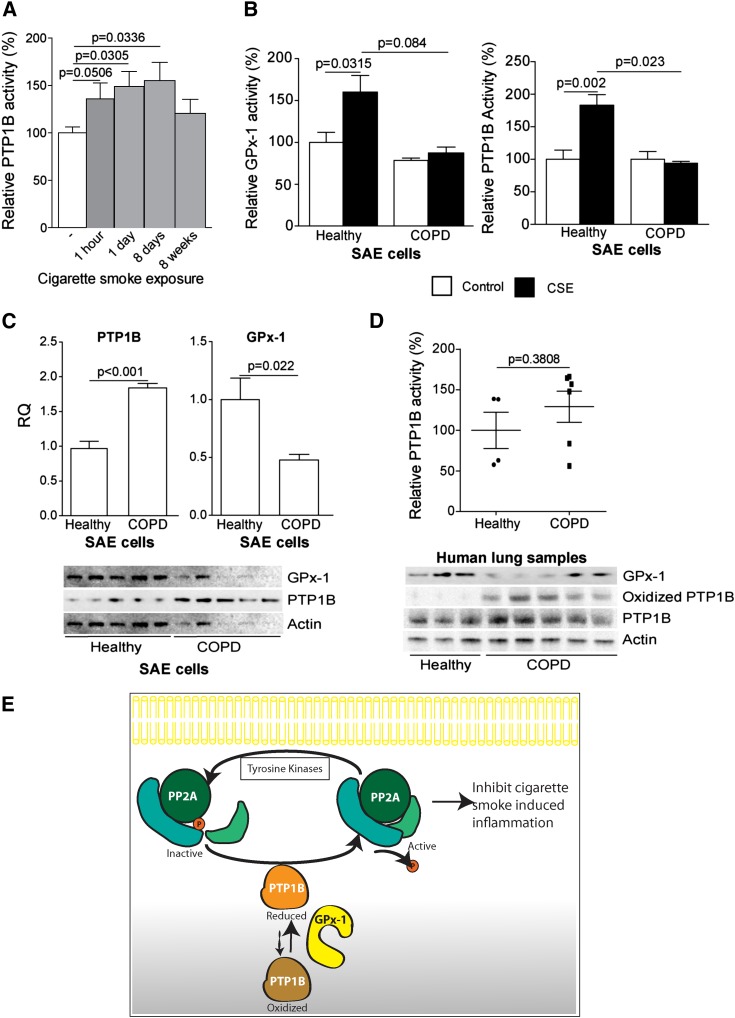
Smoke-Induced PTP1B Activity Is Reduced in COPD Samples
To determine how cigarette smoke exposure affected PTP1B activity in the lung in vivo, we measured PTP1B activity from the lung tissue of wild-type mice at baseline and after 1 hour, 1 day, 8 days, or 8 weeks of cigarette smoke exposure. PTP1B activity was significantly increased within the lungs of these mice after 1 hour of smoke exposure (Figure 6A). However, the increase in PTP1B activity waned over time. The responsiveness of PTP1B activity in human COPD has not been previously examined. We examined the effects of smoke on PTP1B activity in primary SAE cells isolated from the lungs of healthy donors and of subjects with COPD. Healthy SAE cells demonstrate a robust GPx-1 and PTP1B response to smoke, unlike the SAE cells from patients with COPD (Figure 6B). Interestingly, the gene-expression and whole-protein profiles of both proteins differed in COPD cells, with a twofold increased expression of PTP1B and a large reduction in GPx-1 production (Figure 6C). PTP1B activity was also measured from the lung protein of age-matched healthy and emphysematous human tissue samples. PTP1B activity was not increased in the human emphysema samples, compared with healthy control samples (Figure 6D), similar to the SAE cells from patients with COPD. Oxidative stress modifies the catalytic region of PTP1B to inactivate the enzyme (28). Therefore, we examined human tissue from healthy subjects and patients with COPD for the oxidized form of PTP1B. Oxidized PTP1B was only observed in samples from patients with COPD (Figure 6D). Thus, we assert that the nonresponsiveness of PTP1B results in a proinflammatory phenotype, leading to the sustained inflammation that occurs in the lung after chronic smoke exposure.
Figure 6.
PTP1B activity is subdued in human emphysema because of oxidation. (A) PTP1B activity was measured in the lungs of wild-type nonexposed mice (open bars) and for various times in smoke-exposed mice (solid bars) (n = 12 for each group). (B) GPx-1 and PTP1B activity assays were performed in control (open bars) or CSE-treated (solid bars) healthy and chronic obstructive pulmonary disease (COPD) SAE cells (n = 7). (C) PTP1B and GPx-1 gene and protein expressions were examined in healthy and COPD SAE cells by quantitative PCR and Western blot analysis, respectively. (D) PTP1B activity was measured in lung tissue from age-matched healthy control subjects (n = 4) and subjects with emphysema (n = 7), using a specific PTP1B phosphatase activity assay. Graph represents the means ± SEMs. The P values shown compare the treatments connected by a line. Immunoblotting for oxidized and total PTP1B was performed on the same human samples. Actin was used as a protein loading control. (E) Possible pathway for the redox regulation of the PTP1B–PP2AC pathway and the subsequent inflammatory response in the lung. GPx-1 blocks kinase (e.g., JNK) activation and AP-1 signaling by inhibiting protein tyrosine kinases and augmenting protein tyrosine phosphatase activity. This maintains PP2A in its dephosphorylated and active state, enabling it to block kinase activation and prevent the induction of further inflammatory signaling.
Discussion
The ROS produced by smoke exposure induce inflammation that drives the development of disease (29). Epidemiologic and clinical studies suggest that antioxidants confer protection against COPD (30) and other inflammatory disorders (31). Our group and others have demonstrated the therapeutic potential of antioxidants in preventing the development of COPD (15, 20). However, the mechanism by which antioxidants impede disease progression remains to be elucidated. This study is the first to demonstrate that an antioxidant enzyme, GPx-1, can directly interact with a phosphatase, enhancing its activity and thereby preventing disease progression. Specifically, we show that GPx-1 induces PTP1B to activate PP2A via a mechanism that likely involves the dephosphorylation of the catalytic subunit of the enzyme at tyrosine 307. By activating PP2A, GPx-1 blocked signaling responses that induced lung cytokine and protease expression, and thereby prevented lung tissue destruction and injury. GPx-1 was able to regulate PTP1B, and subsequently PP2A, not only because it was an antioxidant, but more importantly, because it had a strong binding affinity for PTP1B, which enabled it to maintain PTP1B in its active state. Thus, the present study elucidates a novel mechanism by which GPx-1 modulates inflammatory responses through the posttranslational modification of key lung phosphatases. Importantly, our data indicate that the loss of GPx enzymatic activity over time would hinder PTP1B/PP2A responses that limit lung injury in this disease. This is significant because GPx activity decreases with age (32), and the lungs of subjects with COPD undergo accelerated aging processes (33). Indeed, in contrast to healthy SAE cells, cigarette smoke did not increase PTP1B activity in SAE cells from subjects with COPD. These findings indicate that the loss of GPx-1–PTP1B–PP2A responses may constitute a pivotal factor in the pathogenesis of this disease.
PTP1B is primarily observed at the membrane of the endoplasmic reticulum (ER) (22), which exposes it to all cytoplasmic components, including GPx-1 (34), allowing frequent opportunities for redox modifications to occur. Importantly, we have observed that PTP1B and PP2A are less responsive after chronic smoke exposure, which may explain why the acute induction of PTP1B/PP2A activity does not prevent the persistent inflammation that occurs in the COPD disease state. Intracellular H2O2 production could oxidize PTP1B and inactivate this pathway (22). Indeed, we found that PTP1B protein was oxidized in the lungs of subjects with advanced emphysema. Our data suggest that elevated concentrations of GPx-1 are sufficient to induce the activity of PTP1B, to ensure the dephosphorylation of Tyr307 on the PP2A catalytic C subunit, to activate PP2A, and to prevent smoke-induced inflammation. We theorize that GPx-1 modifies PTP1B activity to alter the tyrosine phosphorylation of PP2AC by altering the balance of protein tyrosine kinase (PTK) and protein tyrosine phosphatase (PTP) activity in the lung (see Figure 6E for a proposed scheme). Therefore, if oxidants such as H2O2 inactivate PTP1B, an imbalance between PTK and PTP activity within the cell would occur, resulting in persistent inflammation. Antioxidants have been reported to decrease the activity of tyrosine kinases such as Src (35), which phosphorylate PP2A at the specific Tyr307 site (25). Moreover, antioxidants counter the effects of ROS that inactivate PTPs by oxidizing cysteine residues within the enzyme (36). Previous studies have shown that antioxidants exert key protective effects against smoke exposure in the lung (13, 15). The present work provides new insights into these protective effects by showing that GPx-1 countered smoke-induced lung inflammation and protease expression by increasing PP2A activity and down-regulating kinase signaling. Moreover, GPx-1 mediated these effects via a mechanism that involved the PTP1B-mediated dephosphorylation of Tyr307 in PP2AC.
The importance of combating oxidative stress has been well documented during the past few decades (37). However, few studies have demonstrated a mode of action by which antioxidants prevent inflammation and disease. The present study elucidates that the site of action for an antioxidant is critical to its cellular effects. Indeed, compared with superoxide dismutase–1, GPx-1 was a far more potent activator of PP2A in the lungs of mice (Figure E7). This is likely attributable to the strong binding affinity that GPx-1 showed toward PTP1B, which is a critical modulator of PP2A activity. We have observed how the loss of PTP1B affects PP2A activity, H2O2 concentrations, cytokine production, and MMP expression, and potentially, subsequent airway enlargement. We were surprised that the GPx-1–PTP1B–PP2A pathway negatively regulated smoke-induced-IL-17, because others observed the opposite effect in mice overexpressing PP2A in T cells (38). This may be attributable to the different signaling that occurs in diverse tissues and the influence of cigarette smoke on the posttranslational modification of PP2A. The observation of a quick IL-17 response to smoke that is sensitive to the GPx-1–PTP1B–PP2A pathway constitutes an intriguing finding that warrants further investigation, insofar as the role of IL-17 in COPD is currently an exciting topic in COPD research (39).
The GPx-1–PTP1B–PP2A pathway could influence many other disease processes involved in COPD. PTP1B could regulate the effects of smoke on ER stress, because smoke induces the unfolded protein response (40), and PTP1B is located on the ER (22) and plays a role in relieving ER stress (41). Nuclear factor (erythroid-derived–2)–like 2 and PP2A signaling has been demonstrated to be elevated in 78-kD glucose-regulated protein knockout cells, possibly to counteract increased ER stress–associated ROS production (42). In addition, ceramide alters PP2A activity (43), and smoke-induced ceramide production is redox-sensitive in a neutral-sphingomyelinase manner (44). Therefore, GPx-1 could alter ceramide production and exert an impact on cellular apoptosis. The impact of the PTP1B–PP2A pathway on ceramide synthesis would be an area worthy of further investigation. GPx-1 has also been demonstrated to regulate other inflammatory signaling, such as cluster of differentiation-14 (20). In fact, PTP1B prevents cluster of differentiation-14–associated Toll-like receptor signaling in a PP2A-dependent manner (19).
Several limitations of this study merit discussion. For one, the mechanism by which GPx-1 increased PTP1B activity remains to be determined. Based on the literature, we theorize that GPx-1 functioned to maintain PTP1B in its reduced, active state. Indeed, the oxidation of PTP1B, which occurs in emphysema, was associated with the loss of GPx-1 activity in the lung. However, future studies will specifically address whether GPx-1 alters PTP1B oxidation in our disease models. Second, silencing PTP1B in our in vitro and in vivo models decreased protein expression, but did reduce basal enzymatic activity levels. In both systems, baseline PTP1B activity was at the lower limit of detection, and thus the assays might not have been sensitive enough to detect changes present with gene silencing. Lastly, although we have focused on the epithelium, other cell types, including macrophages, may conceivably have been affected by GPx-1 or PTP1B siRNA in our in vivo models. Indeed, IFN-γ, IL-17, and MMP-12 are not principally expressed by the epithelium, suggesting that GPx-1 and/or PTP1B siRNA acted on other cell types, either directly or indirectly.
In conclusion, we provide evidence that GPx-1 expression prevents smoke-induced inflammation and tissue destruction by altering phosphatase activation. In addition, we show that PTP1B and PP2A play active roles in the recovery from the inflammatory effects of smoke. This is important in human disease, because PTP1B is oxidized in the lungs of subjects with emphysema, and SAE cells from subjects with COPD fail to induce PTP1B activity in response to smoke. These findings set the stage for future studies to explore whether increasing PTP1B/PP2A activity, via GPx-1 modulation, can be used effectively to treat COPD and other diseases where inflammation exerts a central role.
Acknowledgments
Acknowledgments
The authors thank Irfan Rahman for critiquing the manuscript, and the James P. Mara Center for Lung Disease of the Pulmonary Division at St. Luke’s Roosevelt Hospital for their support.
Footnotes
This work was supported by Flight Attendant Medical Research Institute grant YCSA 113380 and 24,039, Clinical Innovator Award grant 074,047, and National Institutes of Health grant 5R01HL098528-04 (R.F.F.), and by National Institutes of Health grant 5R01HL086936-04 (J.M.D.).
Author Contributions: P.G. was responsible for the study’s design and execution, data collection, and manuscript preparation. A.A.H., A.M.W., O.M., J.T., L.A., and V.T. were responsible for the study’s design and execution and data collection. J.M.D. was responsible for the study’s design. R.F.F. was responsible for the study’s design and execution, data collection, and overall manuscript preparation, and serves as the guarantor.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0026OC on April 5, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Millward TA, Zolnierowicz S, Hemmings BA. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem Sci. 1999;24:186–191. doi: 10.1016/s0968-0004(99)01375-4. [DOI] [PubMed] [Google Scholar]
- 2.Guergnon J, Godet AN, Galioot A, Falanga PB, Colle JH, Cayla X, Garcia A. PP2A targeting by viral proteins: a widespread biological strategy from DNA/RNA tumor viruses to HIV-1. Biochim Biophys Acta. 2011;1812:1498–1507. doi: 10.1016/j.bbadis.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Pallas DC, Shahrik LK, Martin BL, Jaspers S, Miller TB, Brautigan DL, Roberts TM. Polyoma small and middle T antigens and SV40 small T antigen form stable complexes with protein phosphatase 2A. Cell. 1990;60:167–176. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- 4.Sontag E, Nunbhakdi-Craig V, Lee G, Brandt R, Kamibayashi C, Kuret J, White CL, III, Mumby MC, Bloom GS. Molecular interactions among protein phosphatase 2A, tau, and microtubules: implications for the regulation of tau phosphorylation and the development of tauopathies. J Biol Chem. 1999;274:25490–25498. doi: 10.1074/jbc.274.36.25490. [DOI] [PubMed] [Google Scholar]
- 5.Wallace AM, Hardigan A, Geraghty P, Salim S, Gaffney A, Thankachen J, Arellanos L, D’Armiento JM, Foronjy RF. Protein phosphatase 2A regulates innate immune and proteolytic responses to cigarette smoke exposure in the lung. Toxicol Sci. 2012;126:589–599. doi: 10.1093/toxsci/kfr351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi Y, Mercado N, Barnes PJ, Ito K. Defects of protein phosphatase 2A causes corticosteroid insensitivity in severe asthma. PLoS ONE. 2011;6:e27627. doi: 10.1371/journal.pone.0027627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mossman BT, Lounsbury KM, Reddy SP. Oxidants and signaling by mitogen-activated protein kinases in lung epithelium. Am J Respir Cell Mol Biol. 2006;34:666–669. doi: 10.1165/rcmb.2006-0047SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kins S, Crameri A, Evans DR, Hemmings BA, Nitsch RM, Gotz J. Reduced protein phosphatase 2A activity induces hyperphosphorylation and altered compartmentalization of tau in transgenic mice. J Biol Chem. 2001;276:38193–38200. doi: 10.1074/jbc.M102621200. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto M, Suzuki Y, Kihira H, Miwa H, Kita K, Nagao M, Tamura S, Shiku H, Nishikawa M. Expressions of four major protein Ser/Thr phosphatases in human primary leukemic cells. Leukemia. 1999;13:595–600. doi: 10.1038/sj.leu.2401372. [DOI] [PubMed] [Google Scholar]
- 10.Keeney JT, Swomley AM, Harris JL, Fiorini A, Mitov MI, Perluigi M, Sultana R, Butterfield DA. Cell cycle proteins in brain in mild cognitive impairment: insights into progression to Alzheimer disease. Neurotox Res. 2012;22:220–230. doi: 10.1007/s12640-011-9287-2. [DOI] [PubMed] [Google Scholar]
- 11.Sarmento-Ribeiro AB, Proença MT, Sousa I, Pereira A, Guedes F, Teixeira A, Oliveira CR. A possible role for oxidation stress in lymphoid leukaemias and therapeutic failure. Leuk Res. 2012;36:1041–1048. doi: 10.1016/j.leukres.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Petrache I, Medler TR, Richter AT, Kamocki K, Chukwueke U, Zhen L, Gu Y, Adamowicz J, Schweitzer KS, Hubbard WC, et al. Superoxide dismutase protects against apoptosis and alveolar enlargement induced by ceramide. Am J Physiol Lung Cell Mol Physiol. 2008;295:L44–L53. doi: 10.1152/ajplung.00448.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of NRF2 enhances susceptibility to cigarette smoke–induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Mathews TJ, Osterman MJ. Births: final data for 2008. Natl Vital Stat Rep. 2010;59:1–72. 3–71. [PubMed] [Google Scholar]
- 15.Foronjy RF, Mirochnitchenko O, Propokenko O, Lemaitre V, Jia Y, Inouye M, Okada Y, D’Armiento JM. Superoxide dismutase expression attenuates cigarette smoke– or elastase-generated emphysema in mice. Am J Respir Crit Care Med. 2006;173:623–631. doi: 10.1164/rccm.200506-850OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li FF, Shen J, Shen HJ, Zhang X, Cao R, Zhang Y, Qui Q, Lin XX, Xie YC, Zhang LH, et al. SHP2 plays an important role in acute cigarette smoke–mediated lung inflammation. J Immunol. 2012;189:3159–3167. doi: 10.4049/jimmunol.1200197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Parsons S, Brautigan DL. Tyrosine phosphorylation of protein phosphatase 2A in response to growth stimulation and V-Src transformation of fibroblasts. J Biol Chem. 1994;269:7957–7962. [PubMed] [Google Scholar]
- 18.Min W, Lin Y, Tang S, Yu L, Zhang H, Wan T, Luhn T, Fu H, Chen H. AIP1 recruits phosphatase PP2A to ASK1 in tumor necrosis factor–induced ASK1–JNK activation. Circ Res. 2008;102:840–848. doi: 10.1161/CIRCRESAHA.107.168153. [DOI] [PubMed] [Google Scholar]
- 19.Xu H, An H, Hou J, Han C, Wang P, Yu Y, Cao X. Phosphatase PTP1B negatively regulates MyD88- and TRIF-dependent proinflammatory cytokine and Type I interferon production in TLR-triggered macrophages. Mol Immunol. 2008;45:3545–3552. doi: 10.1016/j.molimm.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Duong C, Seow HJ, Bozinovski S, Crack PJ, Anderson GP, Vlahos R. Glutathione peroxidase–1 protects against cigarette smoke–induced lung inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2010;299:L425–L433. doi: 10.1152/ajplung.00038.2010. [DOI] [PubMed] [Google Scholar]
- 21.Brandsch C, Schmidt T, Behn D, Weisse K, Mueller AS, Stangl GI. Glutathione deficiency down-regulates hepatic lipogenesis in rats. Lipids Health Dis. 2010;9:50. doi: 10.1186/1476-511X-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haque A, Andersen JN, Salmeen A, Barford D, Tonks NK. Conformation-sensing antibodies stabilize the oxidized form of PTP1B and inhibit its phosphatase activity. Cell. 2011;147:185–198. doi: 10.1016/j.cell.2011.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao XQ, Zhang XX, Yin YY, Liu B, Luo DJ, Liu D, Chen NN, Ni ZF, Wang X, Wang Q, et al. Glycogen synthase kinase–3β regulates Tyr307 phosphorylation of protein phosphatase–2A via protein tyrosine phosphatase 1B but not Src. Biochem J. 2011;437:335–344. doi: 10.1042/BJ20110347. [DOI] [PubMed] [Google Scholar]
- 24.Mercer BA, Kolesnikova N, Sonett J, D’Armiento J. Extracellular regulated kinase/mitogen activated protein kinase is up-regulated in pulmonary emphysema and mediates matrix metalloproteinase–1 induction by cigarette smoke. J Biol Chem. 2004;279:17690–17696. doi: 10.1074/jbc.M313842200. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Martin BL, Brautigan DL. Regulation of protein serine–threonine phosphatase Type–2A by tyrosine phosphorylation. Science. 1992;257:1261–1264. doi: 10.1126/science.1325671. [DOI] [PubMed] [Google Scholar]
- 26.Di Stefano A, Caramori G, Oates T, Capelli A, Lusuardi M, Gnemmi I, Ioli F, Chung KF, Donner CF, Barnes PJ, et al. Increased expression of nuclear factor–kappaB in bronchial biopsies from smokers and patients with COPD. Eur Respir J. 2002;20:556–563. doi: 10.1183/09031936.02.00272002. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro SD. Proteolysis in the lung. Eur Respir J Suppl. 2003;44:30s–32s. doi: 10.1183/09031936.03.00000903a. [DOI] [PubMed] [Google Scholar]
- 28.van Montfort RL, Congreve M, Tisi D, Carr R, Jhoti H. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1B. Nature. 2003;423:773–777. doi: 10.1038/nature01681. [DOI] [PubMed] [Google Scholar]
- 29.Bargagli E, Olivieri C, Bennett D, Prasse A, Muller-Quernheim J, Rottoli P. Oxidative stress in the pathogenesis of diffuse lung diseases: a review. Respir Med. 2009;103:1245–1256. doi: 10.1016/j.rmed.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Tabak C, Arts IC, Smit HA, Heederik D, Kromhout D. Chronic obstructive pulmonary disease and intake of catechins, flavonols, and flavones: the MORGEN Study. Am J Respir Crit Care Med. 2001;164:61–64. doi: 10.1164/ajrccm.164.1.2010025. [DOI] [PubMed] [Google Scholar]
- 31.Lönn ME, Dennis JM, Stocker R. Actions of “antioxidants” in the protection against atherosclerosis. Free Radic Biol Med. 2012;53:863–884. doi: 10.1016/j.freeradbiomed.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 32.Espinoza SE, Guo H, Fedarko N, DeZern A, Fried LP, Xue QL, Leng S, Beamer B, Walston JD. Glutathione peroxidase enzyme activity in aging. J Gerontol A Biol Sci Med Sci. 2008;63:505–509. doi: 10.1093/gerona/63.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest. 2009;135:173–180. doi: 10.1378/chest.08-1419. [DOI] [PubMed] [Google Scholar]
- 34.Miller S, Walker SW, Arthur JR, Nicol F, Pickard K, Lewin MH, Howie AF, Beckett GJ. Selenite protects human endothelial cells from oxidative damage and induces thioredoxin reductase. Clin Sci (Lond) 2001;100:543–550. [PubMed] [Google Scholar]
- 35.Krasnowska EK, Pittaluga E, Brunati AM, Brunelli R, Costa G, De Spirito M, Serafino A, Ursini F, Parasassi T. N-acetyl-l-cysteine fosters inactivation and transfer to endolysosomes of c-Src. Free Radic Biol Med. 2008;45:1566–1572. doi: 10.1016/j.freeradbiomed.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Tonks NK. Redox redux: revisiting PTPs and the control of cell signaling. Cell. 2005;121:667–670. doi: 10.1016/j.cell.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 37.Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. NRF2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crispín JC, Apostolidis SA, Rosetti F, Keszei M, Wang N, Terhorst C, Mayadas TN, Tsokos GC. Cutting edge: protein phosphatase 2A confers susceptibility to autoimmune disease through an IL-17–dependent mechanism. J Immunol. 2012;188:3567–3571. doi: 10.4049/jimmunol.1200143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cazzola M, Matera MG. IL-17 in chronic obstructive pulmonary disease. Expert Rev Respir Med. 2012;6:135–138. doi: 10.1586/ers.12.7. [DOI] [PubMed] [Google Scholar]
- 40.Geraghty P, Dabo AJ, D’Armiento J. TLR4 protein contributes to cigarette smoke–induced matrix metalloproteinase–1 (MMP-1) expression in chronic obstructive pulmonary disease. J Biol Chem. 2011;286:30211–30218. doi: 10.1074/jbc.M111.238824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popov D. Endoplasmic reticulum stress and the on site function of resident PTP1B. Biochem Biophys Res Commun. 2012;422:535–538. doi: 10.1016/j.bbrc.2012.05.048. [DOI] [PubMed] [Google Scholar]
- 42.Chang YJ, Huang YP, Li ZL, Chen CH. GRP78 knockdown enhances apoptosis via the down-regulation of oxidative stress and AKT pathway after epirubicin treatment in colon cancer DLD-1 cells. PLoS ONE. 2012;7:e35123. doi: 10.1371/journal.pone.0035123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reyes JG, Robayna IG, Delgado PS, González IH, Aguiar JQ, Rosas FE, Fanjul LF, Galarreta CM. c-Jun is a downstream target for ceramide-activated protein phosphatase in A431 cells. J Biol Chem. 1996;271:21375–21380. doi: 10.1074/jbc.271.35.21375. [DOI] [PubMed] [Google Scholar]
- 44.Levy M, Khan E, Careaga M, Goldkorn T. Neutral sphingomyelinase 2 is activated by cigarette smoke to augment ceramide-induced apoptosis in lung cell death. Am J Physiol Lung Cell Mol Physiol. 2009;297:L125–L133. doi: 10.1152/ajplung.00031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]