Abstract
Klebsiella pneumoniae causes serious infections in the urinary tract, respiratory tract, and blood. Lipid rafts, also known as membrane microdomains, have been linked to the pathogenesis of bacterial infection. However, whether lipid rafts affect K. pneumoniae internalization into host cells remains unknown. Here, we show for the first time that K. pneumoniae was internalized into lung cells by activating lipid rafts. Disrupting lipid rafts by methyl-β-cyclodextrin inhibited pathogen internalization, impairing host defense. A deficient mutant of capsule polysaccharide (CPS) showed a higher internalization rate than a wild-type strain, indicating that CPS may inhibit bacterial entry to host cells. Furthermore, lipid rafts may affect the function of extracellular regulated kinase (ERK)–1/2, and knocking down ERK1/2 via short, interfering RNA increased apoptosis in both alveolar macrophages and epithelial cells after infection. To gain insights into bacterial pathogenesis, we evaluated the impact of lipid rafts on DNA integrity, and showed that raft aggregates also affect DNA damage and DNA repair responses (i.e., 8-oxoguanine DNA glycosylase [Ogg1]) through the regulation of reactive oxygen species. Importantly, cells overexpressing Ogg1 demonstrated reduced cytotoxicity during bacterial infection. Taken together, these results suggest that lipid rafts may modulate bacterial internalization, thereby affecting DNA damage and repair, which is critical to host defense against K. pneumoniae.
Keywords: Klebsiella pneumoniae infection, internalization, alveolar epithelial cells, lipid rafts, capsule polysaccharide
Clinical Relevance
We show, for the first time, that Klebsiella pneumoniae is internalized into lung cells by activating lipid rafts. Raft aggregates also affect DNA damage and DNA repair responses through the regulation of reactive oxygen species. Raft-mediated signals may also affect cell death and inflammatory responses in cells and in mice. Our findings suggest that raft-associated signaling may be targeted for controlling K. pneumoniae infection.
Klebsiella pneumoniae causes serious infections in multiorgan systems, and is the third most commonly isolated bacterium from the blood of patients with sepsis (1). Because K. pneumoniae rapidly develops multidrug-resistant strains, this “superbug” can also cause outbreaks in intensive care units, imposing significant financial burdens and dangerous health threats (2, 3). Despite intensive research during the past few decades, the pathogenesis of K. pneumoniae, including its internalization of alveolar cells, is incompletely understood. K. pneumoniae expresses two critical antigens on its cell surface, LPS (an O antigen) and capsule polysaccharide (CPS; a K antigen) (4). These two antigens contribute to pathogenicity through interactions with host cells in different manners (5). LPS is a major component of Gram-negative bacterial cell walls with high immunogenicity, but its role in pathogenesis remains elusive. Early studies suggest that LPS-deficient strains failed to show significantly different pathogenesis from the wild-type (WT) strain (5). By contrast, CPS demonstrates the most distinguished characteristic of virulence factors for K. pneumoniae, and is implicated in the internalization of pathogens into epithelial cells (6). The capsule may protect the bacterium from phagocytosis by macrophages, through an escape from bactericidal serum factors (6). Other studies also demonstrated that isogenic CPS mutant strains of K. pneumoniae exhibited higher levels of adherence and internalization to lung cells and were more invasive, compared with WT counterparts (4). However, the precise role of CPS in membrane adhesion and penetration remains to be defined (7).
Alveolar epithelial Type II (AECII or ATII) cells comprise structural cells for forming alveolar barriers and also lung progenitor cells for renewing injured or dying cells. In addition, a growing body of evidence suggests that AECII cells may play crucial roles against bacterial infection by secreting cytokines to facilitate the function of alveolar macrophages (AMs) (7). Bacterial invasion must pass through the plasma membrane to enter host cells. Lipid rafts, also known as membrane microdomains, consist of various lipids and signaling proteins, and serve as signaling platforms for various cellular processes, including host defense against infection (8). On the other hand, bacteria may hijack lipid rafts for their own benefit to subvert immune responses. The concept of “lipid rafts” originates from the transportation of cholesterol from the trans-Golgi network to the plasma membrane (9, 10). Recent progress in lipid rafts has greatly enriched the classic fluid mosaic model of double-layer membranes (11), offering an unprecedented opportunity to further our understanding of bacteria–host interactions.
Bacteria may attack a host by interfering with critical functions of the host proteins, such as cytoskeletal proteins (small GTPases, phosphatidylinositide 3-kinase [PI3K], and actin) (12), thereby impeding phagocytosis. Bacteria may also evade immune recognition, to survive inside phagosome vacuoles and prevent phagosome–lysosome fusion (13, 14). During bacterial infection, lipid rafts in cell signaling were initially found to involve bacterial toxins. For example, cholera toxin interacts with monosialotetrahexosylganglioside (GM1) molecules via its pentameric receptor binding subunit (15). Clusters of GM1 can be found in caveolae-flask–shaped membrane invaginations, thus allowing for the high-affinity binding of cholera toxin by a “Velcro”-type mechanism, affecting the adhesion of several bacteria (14). However, whether lipid rafts involve K. pneumoniae infection in the lung remains unknown. We hypothesized that lipid rafts may play a role in K. pneumoniae internalization into lung cells, and that certain K. pneumoniae surface components are also critical during host–pathogen interactions. Our results indeed show that a lipid raft–initiated signaling cascade is involved in K. pneumoniae internalization into lung cells, and yet the surface components of this pathogen may also influence lipid raft formation, thereby affecting the DNA damage response.
Materials and Methods
For additional details regarding methods, please see the online supplement.
Alveolar epithelial cell lines A549 and murine lung epithelial (MLE)–12, and murine alveolar macrophages, were purchased from the American Type Culture Collection (Manassas, VA) and cultured in Dulbecco’s Modified Eagle’s Medium with 10% FCS (HyClone Laboratories, Logan, UT), 100 U/ml of penicillin, and 100 μg/ml of streptomycin (Life Technologies, Rockville, MD) (7, 16, 17). Mouse AECII cells were isolated using a protocol described previously (18), with some modifications, namely, that we used magnetic spheres to bind and remove lymphocytes (with CD32 and CD45 antigens). The phenotypes of AECII cells were characterized and confirmed for their expression of the characteristic markers cytokeratin 18 and surfactant protein–C (pro-SP-C) by staining with specific antibodies (Sigma-Aldrich, St. Louis, MO) before the infection experiments (19).
Results
K. pneumoniae Internalization into Host Cells
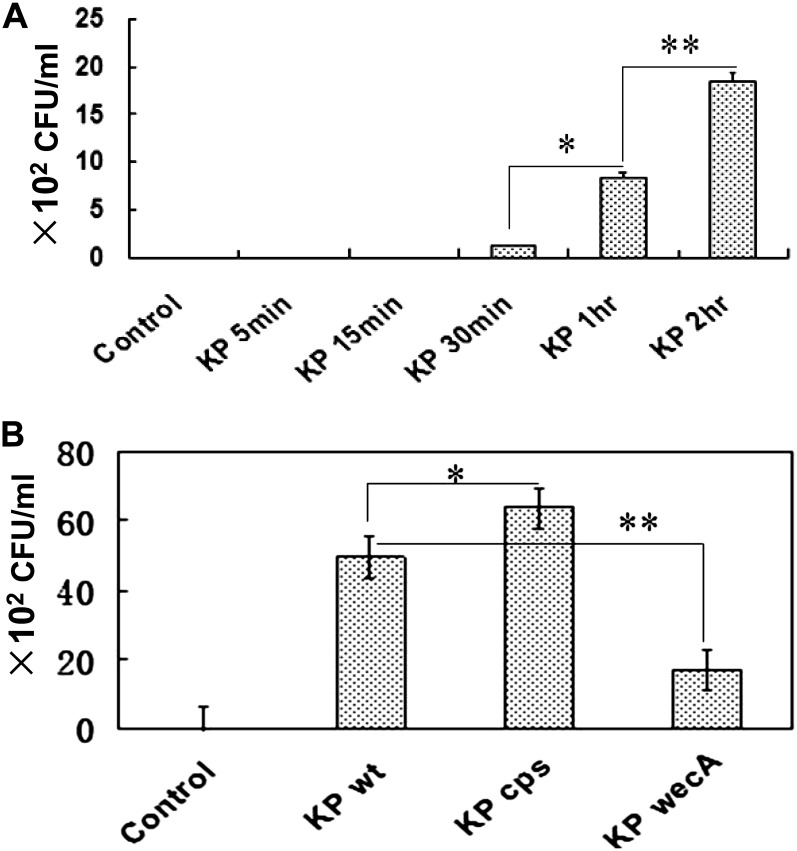
Although K. pneumoniae was originally considered an extracellular pathogen, a growing body of evidence indicates that it may internalize into alveolar epithelial cells (20). To gauge the efficiency of K. pneumoniae internalization into lung epithelial cells, we infected MLE–12 cells with K. pneumoniae at various multiplicities of infection (MOIs). The surface bacterium was killed by incubation with polymyxin B (100 μg/ml), and cell lysates were inoculated on agar Petri dishes to enumerate CFUs. The data indicate that an MOI of 10:1 was optimal for our infection experiments, and this MOI was thereafter used in time-course experiments. We estimated that approximately 86 CFUs of K. pneumoniae (16% of the loaded) were internalized into MLE-12 cells after 1 hour of infection, and that the internalization increased with time (Figure 1A).
Figure 1.
Uptake of Klebsiella pneumoniae (KP) by lung epithelial cells. (A) Time-dependent internalization of wild-type (WT) strain into murine lung epithelial–12 (MLE-12) cells was determined by a CFU assay. (B) K. pneumoniae internalization into alveolar Type II epithelial (AECII) cells is dependent on bacterial surface components or toxins (capsule polysaccharide–deficient mutant, cps; enterobacterial common antigen–deficient mutant, wecA), as determined by a CFU assay (*P < 0.05 and **P < 0.01, according to Mann-Whitney U test). Data consist of means ± SDs, and are representative of three independent experiments.
Because surface components of K. pneumoniae, such as CPS, LPS, and enterobacterial common antigen (ECA), may affect bacterial internalization into alveolar epithelial cells (5), we investigated K. pneumoniae internalization into MLE-12 cells, using a CPS isogenic mutant derived from clinical samples selected by screening an ECA-deficient strain (wecA). Our results demonstrate that K. pneumoniae internalization into primary AECII cells is differentially influenced by bacterial toxin components (CPS and ECA). Importantly, the CPS-deficient mutant showed increased internalization into the alveolar epithelium, whereas a deficiency in ECA toxins reduced the internalization (Figure 1B). Although we cannot completely eliminate potential polar effects, our data suggest that CPS may be involved in the bacterial internalization into airway epithelial cells. These results also indicate that CPS may play an opposing role versus ECA.
K. pneumoniae Infection Induces Aggregates of Lipid Rafts in Alveolar Epithelial Cells
Recent studies suggested that lipid rafts are important for various cellular processes, including bacterial infection (14, 21), but the role of lipid rafts in K. pneumoniae internalization remains unknown. We hypothesized that this pathogen may also internalize to lung cells through lipid rafts, as observed in another bacterium (22). To closely mimic infectious process similar to in vivo conditions, we used mouse primary lung cells, which were isolated as characterized, using the staining of cytokeratin 18 and pro-SPC, and cultured on collagen-coated coverslips (19). One day after isolation, we infected the cells with either a WT or a CPS-deficient K. pneumoniae strain for 30 minutes. Our results showed that the infection induced large aggregates of lipid rafts (Figure 2A). Internalized bacteria colocalized with these raft aggregates, as determined by an Alexa Fluor 488 nm–labeled cholera toxin B chain (CTB) that specifically binds to ganglioside GM1 (green). DiI (a lipid-affinitive dye) was used to stain K. pneumoniae (red). This DiI staining was shown to be stable (without leakage) in previous studies (7). To account for experimental variability, multiple control samples (PBS, dead bacterium, supernatant, LPS, and fMLP) were used, which confirmed that raft aggregation was more apparent upon live bacterial infection, compared with dead K. pneumoniae, supernatant, and soluble pure virulence factor LPS, which also induced raft aggregates to some extent (Figure 2A).
Figure 2.

K. pneumoniae infection induced lipid raft aggregation. (A) Live K. pneumoniae induced the most dynamic lipid rafts, as identified by Alexa Fluor 488 nm–labeled cholera toxin B chain (CTB; green) and the bacterium stained with a lipid dye DiI (red; Molecular Probes, Eugene, OR). The nucleus is stained with 4′,6-diamidino-2-phenylindole (DAPI; blue). Arrows show the areas of lipid raft aggregation. (B) K. pneumoniae WT infection induced more lipid raft aggregates in MLE-12 cells than in control samples. Bacteria were stained by K. pneumoniae antibodies (green), and lipid rafts were identified by Alexa Fluor 554 nm–labeled CTB (red). The colocalization correlation coefficient for K. pneumoniae and lipid raft aggregates is greater than 0.85. (C) Quantitative analysis of lipid raft aggregates in MLE-12 cells by Image J software (P < 0.05, Mann-Whitney U test). Data consist of means ± SDs, and are representative of three independent experiments.
To confirm the involvement of lipid rafts in K. pneumoniae infection, we also used MLE-12 cells to observe lipid raft aggregates via Alexa Fluor 554 nm–labeled CTB (red) (Figure 2B). Although the infection significantly increased the sizes and the strong staining of lipid raft aggregates, similar to the observations in primary AECII cells, the noninfected control sample showed weak fluorescent staining (Figure 2B). To determine the dynamic cellular responses quantitatively, the sizes of raft aggregates in MLE-12 cells were measured according to Image J software (Figure 2C). Using region of interest (ROI) tools to highlight the cell membranes, we measured all membrane domains that fell within a range of expected size limits (15–255 pixels in diameter). The parameters used to acquire data included the area, mean intensity, and number of particles in an ROI. These defined parameters enabled the determination that significantly bigger lipid rafts were formed upon bacterial infection, versus control samples (Figure 2C).
Disruption of Lipid Rafts Impeded Pathogen Internalization
To determine roles of lipid rafts in bacterial invasion, we examined whether disrupting lipid rafts can affect K. pneumoniae invasion, using methyl–β-cyclodextrin (MβCD). Lipid raft fractions were isolated from infected MLE-12 cell lysates by sucrose density gradient centrifugation, as described previously (17). Bacterial infection shifted proteins from nonraft fractions into the lipid raft fractions, versus the noninfection control sample (Figure 3A; *P < 0.05). Whereas adding MβCD did not alter the total proteins in fractions of lipid rafts (Figure 3A), total cholesterol was significantly decreased in raft fractions (Figure 3B), confirming that the disruption of lipid rafts was achieved. We also obtained additional evidence that MβCD hampered the shift of proteins into lipid raft fractions, whereas the MβCD solution alone exerted no deleterious effects on K. pneumoniae (data not shown). Furthermore, immunostaining showed that the formation of lipid raft aggregates was abolished by MβCD as well as by filipin (another reagent for disturbing cholesterol) in infected lung cells (Figure 3C). To gain relatively unbiased results, we used either fluorescent green CTB or fluorescent red CTB to detect lipid rafts, using MβCD. Finally, bacterial internalization into MLE-12 cells was evaluated by incubating with K. pneumoniae at 37°C for 30 minutes, and then surface bacteria were killed with polymyxin B. A CFU assay showed that K. pneumoniae internalization was decreased via MβCD treatment compared with control samples (Figure 3D), indicating that bacterial internalization into MLE-12 cells is potentially dependent on lipid rafts.
Figure 3.

Disruption of lipid rafts impeded K. pneumoniae internalization into MLE-12 cells. (A) Proteins in sucrose gradients in lipid raft fractions (fractions 3–5) of infected or control MLE-12 lysates. (B) Cholesterol was significantly reduced in lipid raft fractions (fractions 3–5) by methyl–beta-cyclodextrin (MβCD; *P < 0.05, Mann-Whitney U test). (C) MβCD reduced lipid raft aggregates in K. pneumoniae–infected MLE-12 cells. Lipid rafts were probed by Alexa Fluor 488 nm–labeled CTB (green) or Alexa Fluor 544 nm–labeled CTB (red). Arrow indicates the area of lipid raft aggregation. (D) MβCD reduced K. pneumoniae internalization into MLE-12 cells (*P < 0.05, Mann-Whitney U test). Data consist of means ± SDs, and are representative of three independent experiments. Ctrl, control; KP wt, wild-type K. pneumoniae.
Raft-Dependent Bacterial Internalization Is Enhanced by Deleting CPS
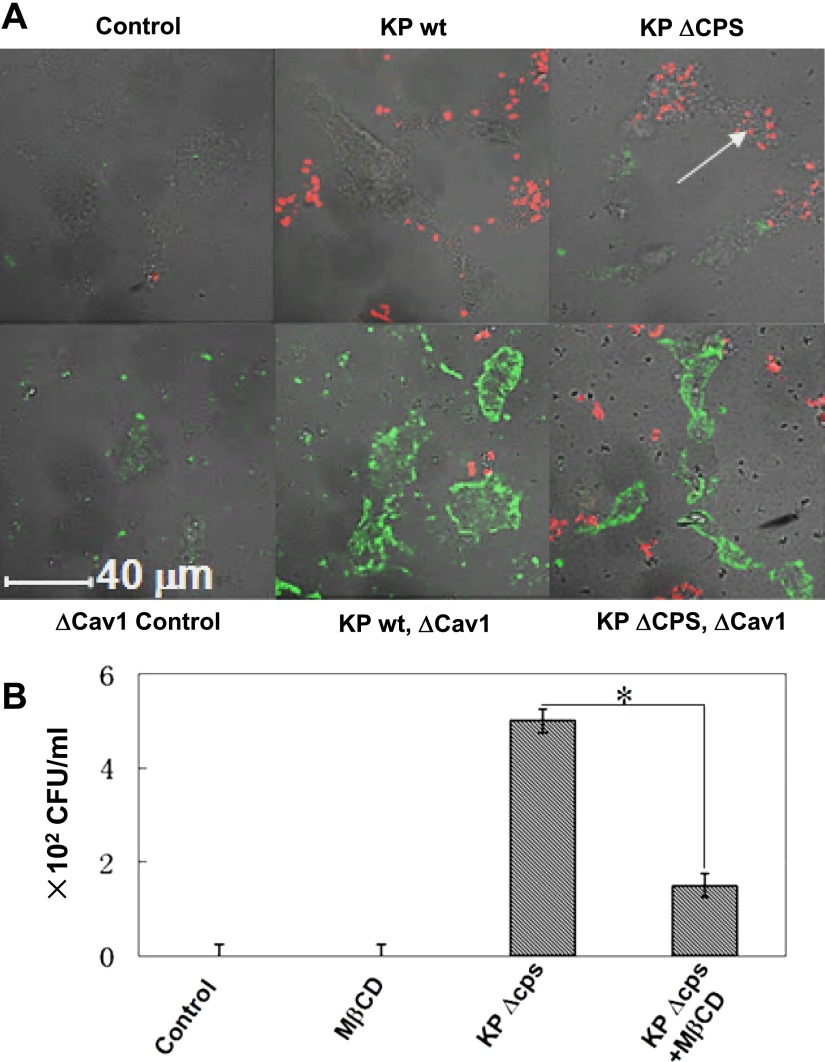
The virulence of CPS may derive from enhanced colonization in the airway spaces and reduced adhesion to host cells by transcriptional regulation (23). Previously, isogenic capsule-negative strains (ΔCPS) showed higher adhesion to airway cells (24). Thus, we studied whether CPS also plays a role in the internalization and its relevance to lipid rafts. The data indicate that the CPS-deficient strain induced fewer and smaller lipid raft aggregates than the WT strain (Figure 4A), indicating that lipid rafts may be associated with bacterial surface components. To probe the molecular mechanisms of lipid rafts during pathogenesis, we also studied another raft protein, caveolin-1 (Cav-1), a critical scaffold and functional component for caveolae (21), which was reportedly associated with infections by this pathogen (22). Our data indicate that Cav-1 is associated with bacterial internalization, because its dominant negative (DN) transfection significantly decreased the initial internalization into MLE-12 cells, as identified by fluorescent microscopy (Figure 4A). The present experiments extended the findings of a previous report (22) by directly observing the raft staining colocalizing with the bacterium under a fluorescence confocal microscope, whereas the previous study only detected CFUs. This result suggests that Cav-1 may be involved in the internalization of pathogens into alveolar epithelial cells.
Figure 4.
Capsule polysaccharide (CPS) played critical roles in the internalization of K. pneumoniae into host cells. (A) Isogenic capsule-negative (ΔCPS) strain exhibited more internalization in MLE-12 cells than in the WT strain. The ΔCPS strain was produced by introducing a capsule-deficient mutant of KPPR1 containing the mini-Tn5Km2 transposon. The transfection of dominant negative caveolin-1 (Cav-1) also reduced internalization in MLE-12 cells (red for bacteria stained with DiI; green for lipid rafts using CTB-FITC). Arrow shows internalized K. pneumoniae. (B) MβCD instillation into mouse lungs blocked the phagocytosis of the ΔCPS strain by mouse alveolar macrophages (AMs), as assayed by bronchoalveolar lavage (representative of n = 5 mice evaluated in each group). *P < 0.05, according to the Mann-Whitney U test. Data consist of means ± SDs, and are representative of three independent experiments.
To study the physiological relevance of the aforementioned role of lipid rafts, we infected C57BL/6 mice by intranasal instillation (104 CFU), and assessed whether phagocytosis by AMs is influenced by disrupting rafts (30 min of MβCD). AMs comprise the first line of host defense against microorganisms, but their potency in killing K. pneumoniae remains incompletely understood (25). Our data showed that internalization (phagocytosis) into AMs was significantly decreased by MβCD pretreatment (Figure 4B), indicating that cholesterol-containing membrane microdomains may be related to the phagocytosis of this pathogen.
K. pneumoniae Invasion Caused DNA Damage in Host Cells
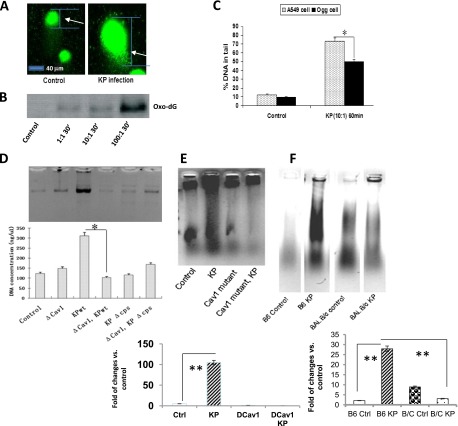
Previous studies showed that bacterial infection (e.g., with Helicobacter pylori and Pseudomonas aeruginosa) induced inflammatory responses (26), reactive oxygen species (ROS) (27), nitric oxide (28), and DNA damage (29). To determine the downstream effects of lipid raft function, we evaluated the impact of K. pneumoniae invasion on the DNA of host cells. Infection increased DNA damage, as determined by a comet assay (26), and thus longer tails were observed in MLE-12 cells after infection with K. pneumoniae, compared with control samples (Figure 5A). To detect molecular details involving this DNA damage induced by infection, the DNA 8-oxo-dG adduct (a widely recognized and significant DNA lesion) was also evaluated, and was observed to be increased with increasing bacterial quantities (Figure 5B) (30–33). To further elucidate the molecular mechanisms involved in this infection-induced DNA damage, we sought to determine whether Ogg1, a base-excision DNA repair (BER) protein that is indicated in oxidative DNA repair, can correct the DNA damage. Using retroviral vector pSF91, we constructed human alveolar epithelial cells, namely, A549 stably overexpressing Ogg1 (34), which are less susceptible to hyperoxic-induced and chemical-induced DNA damage (35). We examined the role of Ogg1 in K. pneumoniae–induced DNA damage, and found that Ogg1-overexpressing cells demonstrated less DNA damage (Figure 5C).
Figure 5.
K. pneumoniae invasion induced DNA damage in lung cells. (A) Bacterial infection induced DNA damage in MLE-12 cells, as determined by a comet assay. Arrow shows the comet tail. (B) K. pneumoniae induced 8-oxo-dG in MLE-12 cells, as detected by gel electrophoresis. A nuclear extract was electrophoresed and probed with a monoclonal antibody against 8-oxo-dG (Trevigen, Gaithersburg, MD). Densitometry quantification results for control, multiplicity of infection (MOI) 1:1, MOI 10:1, and MOI 100:1 were measured at 0, 23, 32, and 100%, respectively. (C) Ogg1 overexpression in A549 lung epithelial cells reduced DNA damage detected at 60 minutes after infection (MOI = 10:1). (D and E) Both WT and ΔCPS K. pneumoniae induced significant laddering in AECII cells. Cav-1 dominant negative (DCav1) transfection also blocked DNA fragmentation, as quantified using densitometry. (F) K. pneumoniae induced significant laddering in C57BL6 (B6) mice compared with BALB/c (B/C) mice (representative of n = 5 mice evaluated in each group). Data consist of means ± SDs, and are representative of three independent experiments. *P < 0.05, **P < 0.01, Mann-Whitney U test.
Previous studies indicated that the raft protein Cav-1 is involved in the regulation of various signaling pathways, and Cav-1 was recently implicated in inflammatory responses during P. aeruginosa (36) or K. pneumoniae infection (22). To determine the involvement of Cav-1 in DNA damage and cell survival during K. pneumoniae infection, we also probed DNA damage using a ladder assay, and showed that both WT and ΔCPS K. pneumoniae caused significant DNA laddering in host cells, whereas disrupting caveolae via Cav-1 DN transfection significantly decreased infection-induced DNA damage (Figures 5D and 5E). This observation is consistent with our early data that K. pneumoniae infection is involved in Cav-1 function. These data indicate that lipid raft–mediated signaling may affect DNA damage and cell viability.
To define the physiological significance of DNA damage and repair during infection, we intranasally infected C57BL/6 mice (a K. pneumoniae–sensitive species) and BALB/c mice (an insensitive species), and assessed DNA damage in their lungs. As expected, K. pneumoniae infection induced significant DNA damage in the lungs of C57BL6 mice compared with BALB/c mice (Figure 5F). Our results suggest that DNA damage may be species-specific, and that lipid raft–related DNA damage may play a direct role in this pathogenic infection.
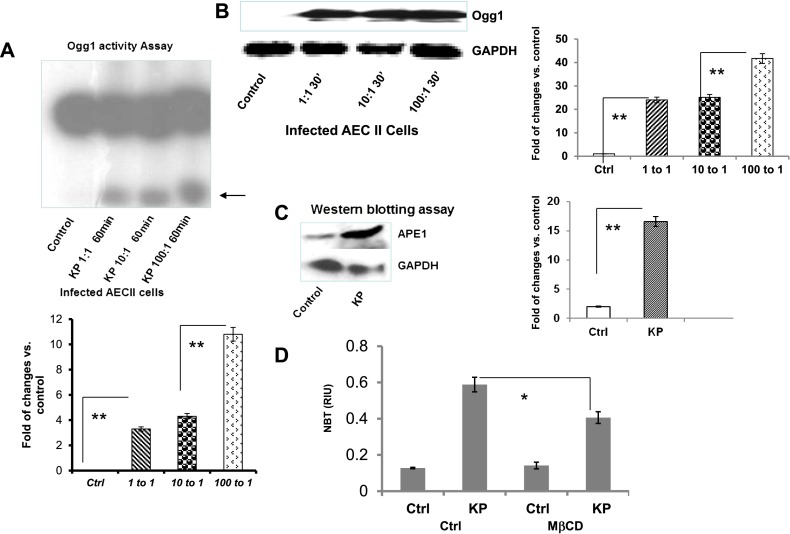
Because previous studies demonstrated a link between oxidative stress and DNA damage (37), our experiments have provided the critical link between lipid raft–mediated signals and DNA damage/repair. To probe the response from DNA repair proteins, we determined the enzymatic activity of Ogg1, and showed that K. pneumoniae infection significantly induced Ogg1 excision activity (Figure 6A). Meanwhile, Ogg1 protein levels were also increased after K. pneumoniae infection, as determined by Western blotting (Figure 6B). In addition, we found that other DNA repair proteins (poly [ADP-ribose] polymerase 1 [PARP-1] and the nuclear excision repair protein, Cockayne Syndrome group B [CSB]) increased after infection, along with an increased expression of inflammatory cytokine (monocyte chemotactic protein–1) and NF-κB (Figure E1 in the online supplement). We also found that the BER protein APE-1 was increased after infection (Figure 6C). To discover the molecular link between lipid rafts and DNA damage response, we investigated whether a primary mechanism for lipid rafts to cause DNA damage involves superoxide (ROS) production, and found that disrupting lipid rafts with MβCD significantly reduced the quantity of superoxide in cultured MLE-12 cells (Figure 6D). Based on previous studies indicating a strong link between superoxide and DNA injury (27, 37), our data further show that lipid rafts may positively correlate with the DNA damage response. Altogether, our data suggest that a systemically coordinated BER program is involved in bacterium-induced raft activation.
Figure 6.
K. pneumoniae infection induced a significant increase in the base-excision DNA repair (BER) response. (A) Ogg1 activities were increased after infection, as detected by a DNA repair enzyme incision assay. (B) Ogg1 protein expression levels were increased by infection, as determined by Western blotting. (C) APE-1 expression was also increased by infection, as determined by Western blotting. Glyceraldehyde 3–phosphate dehydrogenase (GAPDH) was used as a loading control. (D) Disrupting lipid rafts decreased superoxide in MLE-12 cells. Cells were seeded in a 96-well plate and treated with cholesterol chelator MβCD (10 mM) for 30 minutes before infection, and superoxide was measured by nitro blue tetrazolium (NBT) assay (*P < 0.05, **P < 0.01, Mann-Whitney U test). Data consist of means ± SDs, and are representative of three independent experiments.
To determine the endpoint outcome of lipid raft–induced DNA damage, we searched for direct evidence to link lipid raft–induced signals with cell viability. We showed that K. pneumoniae infection accelerated apoptosis in MLE-12 cells as detected by Vybrant assay (Molecular Probes, Eugene, OR), which indicates that cell death may result from DNA damage–induced signaling (Figure 7A). Consistent with these data, the knockdown of Ogg1 by short, interfering RNA (siRNA) significantly increased apoptosis in these cells (Figure 7B). In seeking additional cell-signaling components involved in lipid rafts, we assessed whether extracellular regulated kinase (ERK)–1/2 translocation into the nucleus occurred in a Cav-1–dependent manner. As expected, we found that after K. pneumoniae infection, Cav-1 knockout (KO) mice showed dramatic nuclear translocation, compared with WT mice (Figure 7C). Furthermore, we found that siRNA against ERK1/2 reduced the apoptosis induced by the infection of MLE-12 epithelial cells (Figure 7D) and in MH-S macrophages (Figure E2). Our data demonstrate that siRNA transfection can substantially inhibit ERK1/2 expression (by up to 90%), as evaluated by Western blotting (Figure 7D). We also found that ERK1/2 was translocated into the raft fractions after bacterial infection (not shown). Taken together, these data suggest that ERK1/2 may be associated with DNA damage–induced cell death.
Figure 7.
Ogg1 and extracellular regulated kinase (ERK)–1/2 affected host cell survival upon K. pneumoniae infection. (A) K. pneumoniae infection resulted in apoptosis in MLE-12 cells, as determined by Vybrant assay (**P < 0.01, Mann-Whitney U test). (B) Knockdown of Ogg1 using short, interfering RNA (siRNA; Santa Cruz Biotechnology, Santa Cruz, CA) intensified apoptosis in MLE-12 cells, as determined by Vybrant assay (*P < 0.05, **P < 0.01, according to Mann-Whitney U test). (C) K. pneumoniae infection (24 h) in Cav-1 knockout mice facilitated nuclear translocation, compared with WT mice (arrow). Green, lipid rafts (CTB); red, ERK1/2 immunostaining (antibodies from Santa Cruz Biotechnology); blue, nuclei (DAPI). Merged images are representatives of six mice for each group. (D) Knockdown of ERK1/2 using siRNA (Santa Cruz Biotechnology) reduced apoptosis, versus control samples (*P < 0.05, Mann-Whitney U test). siRNA transfection significantly inhibited ERK1/2 expression, as determined by Western blotting (equal loading was determined by GAPDH, but is not shown). Scbl, scrambled siRNA; siERK, ERK1/2 siRNA. Data consist of means ± SDs, and are representative of three independent experiments.
To ascertain the physiological significance of lipid raft–mediated DNA damage, we asked whether blocking lipid rafts can influence DNA damage and repair in primary cells and cell lines, as well as in mice. We found that raft disruption with MβCD indeed reduced DNA damage in cultured MLE-12 cells (Figure E3) and decreased the ingestion of bacteria (Figure E4). Raft disruption also decreased the DNA repair response, with a significant reduction in Ogg1 expression in MLE-12 cells, as detected by Western blotting (Figure E5A). Importantly, our data also support the idea that DNA repair is down-regulated by MβCD in C57BL6 mice lungs (five mice per group, also with saline control mice; Figure E5B). The changes in immunostaining appeared to be less substantial compared with those detected via Western blotting. However, the pattern of raft colocalization with Ogg1 was disturbed by MβCD treatment, as seen both in MLE-12 cells in vitro and in AM cells retrieved from bacteria-infected mice (Figures E5A and E5B). Phagocytosis may also be associated with apoptosis, and would be an issue for further study. We found that disrupting lipid rafts significantly reduced Ogg1 expression in mouse lungs after bacterial infection (Figure E5C). To determine additional molecular mechanisms of lipid raft–induced DNA damage, we investigated whether disrupting lipid rafts can influence the physiology of mice (e.g., the production of inflammatory cytokines). We found that WT K. pneumoniae induced a significant increase in proinflammatory cytokines, such as TNF-α, IL-1β, IFN-γ, and monocyte chemotactic protein–1, in the bronchoalveolar lavage of infected mice. However, the CPS deletion strain or lipid raft disruption induced fewer cytokines compared with the control mice (Figure E5D). We do not yet know whether phagocytosis and the apoptotic response may be connected with cytokine production, which would also be an issue for further study. Taken together, results from both in vitro and in vivo models delineate a novel mechanism for Gram-negative bacterial infection. In particular, K. pneumoniae infection induced lipid raft aggregates, which triggered DNA damage and subsequent DNA repair responses to benefit host survival (Figure E6).
Discussion
In this study, we demonstrated that K. pneumoniae infection initiates lipid raft aggregates, which affect host DNA repair responses and survival in cultured cells and in mice. Lipid rafts, also known as membrane microdomains (38), can induce various cell-signaling cascades and affect infection processes in multiple layers (39). This can either enhance innate immunity through Toll-like receptors, or dampen the host response in a raft-hijacking mechanism to benefit pathogens (14). Bacteria may interfere with the action of host proteins, such as small GTPases (e.g., Rac1) (40), phosphatidylinositol 3–kinase (PI3K), and actin (41), hampering phagocytosis, and bacteria may also evade immune recognition (42), surviving inside the phagosome vacuoles or inhibiting phagosome/lysosome fusion (14). However, up to date, the role of lipid rafts in K. pneumoniae infection remains undefined. Here, we demonstrate that K. pneumoniae infection induced lipid raft aggregates upon internalization into MLE-12 as well as primary AECII cells. Our experiments also showed that the formation of lipid raft aggregates was a dynamic process that was intensified with increasing time and bacterial quantities. Raft aggregation was abolished by the cholesterol chelator MβCD as well as by DN Cav-1, indicating that internalization involves more than a single component of lipid rafts. Furthermore, the bacterial surface component CPS is associated with raft signaling, because the CPS-deficient strain induced fewer lipid raft aggregates than the WT strain, although it was internalized readily into lung epithelial cells. This result suggests that bacterial surface molecules may also be involved in the pathogen’s internalization, consistent with a previous study showing that the lipid raft signaling associated with polarized sorting and trafficking may affect internalization in epithelial layers (43). Raft aggregates not only influence bacterial internalization into host cells, but also participate in downstream effects during host defense.
The internalization of K. pneumoniae into alveolar epithelial cells is a complicate process, and has been understudied in recent years (20, 44). Lawlor and colleagues demonstrated that CPS is a crucial component of the K. pneumoniae surface structure that is relevant to the internalization of this pathogen into epithelial cells (24). In our studies, K. pneumoniae showed significant internalization after 1 hour of infection, indicating that the infection is time-dependent and bacterial load–dependent. Internalization into AECII cells is also regulated by bacterial surface structures (CPS) and toxins (LPS and ECA). Deletion mutations of CPS increased bacterial internalization into the alveolar epithelium, whereas the knockdown of the ECA toxin reduced internalization, indicating that bacterial surface components play differential roles in the internalization process. Our observations with the CPS strain are consistent with those by Lawlor and colleagues (24) and Regueiro and colleagues (44), but we extended this discovery by revealing the critical roles of lipid raft aggregates. The CPS-deficient strain induces fewer lipid raft aggregates than does the CPS WT strain, but with less ability to cross the cell membrane. These observations indicate that a complex mechanism is associated with CPS-mediated bacterial invasion. Collectively, our data demonstrate that lipid rafts may serve as a novel platform for mechanistically studying pathogen internalization into alveolar epithelial cells.
We also attempted to identify the downstream effects after lipid raft aggregation, and noted that a variety of cellular signals (involving PARP-1, ERK1/2, and p38) were activated after K. pneumoniae infection. Previous reports linked Gram-negative bacterial infection with these signals (45–48). However, no report has described the association of these signals with lipid rafts. Our experiments also suggest that lipid raft reorganization plays a role in host defense against K. pneumoniae infection. Because we and others recently identified that bacterial infection induced DNA damage in epithelial cells (26, 49), we analyzed host DNA states after K. pneumoniae infection, and found DNA single-strand breaks in alveolar epithelial cells. We also observed accumulated DNA oxo-dG adducts in infected lung epithelial cells. 8-oxo-dG is a promutagenic marker and oxidative product, which may later convert into mutated DNA, with an increased propensity toward carcinogenesis or cell death, suggesting that DNA damage may serve as a virulence weapon for the bacterium. Importantly, the expression and enzymatic activity of BER proteins such as Ogg1 and APE-1 were increased by these DNA damage responders. Critically, we found that the overexpression of Ogg1 reduced the damaging effects of K. pneumoniae infection in lung epithelial cells. This finding provides the first experimental evidence for K. pneumoniae–induced DNA damage and the ensuing DNA repair response against infection in lung epithelial cells. In addition, we have found that other cell-signaling pathways, such as PARP-1 and ERK1/2, are associated with the DNA damage response, contributing to the regulation of host cell survival, both in cells and in mice. These coordinated responses are related to cell survival because continued significant DNA damage can cause cell-cycle arrest and eventually apoptotic cell death (37). Our previous data showed that ERK1/2 is associated with cell death after DNA damage. Our results indeed confirmed this notion, and showed that accumulating DNA damage, along with reduced amounts of Ogg1 and increased amounts of ERK1/2, resulted in apoptosis.
Bacterial infection may cause DNA oxidation in host cells, which needs to be repaired in a timely fashion. If this oxidation-inflicted DNA damage remains unrepaired, cell death and tissue injury can occur. The participation of the BER pathway is confirmed through the increased activity of Ogg1 and APE-1. We speculate that damage to host DNA and/or aggravated apoptosis may contribute to the pathophysiological process and dampen the ability of the host to initiate innate or adaptive immunity during acute infections in mouse models. The DNA damage response triggered by lipid rafts may also exert a direct impact on the levels of proinflammatory cytokines, such as TNF-α and IL-1β, in the lung. This is an important observation in terms of controlling K. pneumoniae infection, which demonstrates increasing antibiotic resistance and is thus difficult to treat. Our results offer a potential means to control the successful human pathogen. Importantly, we found that the DNA repair response can be inhibited by the disruption of lipid rafts. To our knowledge, this is the first study to identify that lipid rafts play such a role in DNA repair pathways, both in vitro and in vivo. The potential effect of lipids (most likely cholesterol) on DNA repair activity constitutes an intriguing subject for future investigation. To further link DNA damage with lipid rafts, we speculate that oxidation can cause DNA damage, and that lipid rafts may be linked with DNA damage and repair through the induction of oxidation. To identify the link between lipid rafts and DNA damage, we surmised that ROS played a role, and indeed we found that disrupting lipid rafts dampened the release of ROS in lung cells (Figure 6D). Because previous studies already showed that ROS, including those from bacteria, can induce DNA damage (26, 27, 37), our experiments provided evidence connecting lipid rafts with DNA damage through the modulation of ROS. Taken together, our studies raise the possibility that targeting lipid-derived signaling may exert an impact on DNA repair protein activity and on the alleviation of acute infection by K. pneumoniae.
Footnotes
This project was supported by National Institutes of Health grants 5R03 ES014690, AI101973-01 (M.W.), and AI097532-01A1 (M.W.), and by Flight Attendant Medical Research Institute grant 103007.
Author Contributions: H.H., H.G., and M.W. conceived and designed the experiments. H.H., A.W., Y.L., and M.W. performed the experiments. H.H., H.G., E.W., W.F., and M.W. analyzed the data and wrote the paper.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0069OC on June 6, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Abe S, Boyer C, Liu X, Wen FQ, Kobayashi T, Fang Q, Wang X, Hashimoto M, Sharp JG, Rennard SI. Cells derived from the circulation contribute to the repair of lung injury. Am J Respir Crit Care Med. 2004;170:1158–1163. doi: 10.1164/rccm.200307-908OC. [DOI] [PubMed] [Google Scholar]
- 2.Piednoir E, Thibon P, Borderan GC, Godde F, Borgey F, Le Coutour X, Parienti JJ. Long-term clinical and economic benefits associated with the management of a nosocomial outbreak resulting from extended-spectrum beta-lactamase–producing Klebsiella pneumoniae. Crit Care Med. 2011;39:2672–2677. doi: 10.1097/CCM.0b013e31822827e0. [DOI] [PubMed] [Google Scholar]
- 3.Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009;9:228–236. doi: 10.1016/S1473-3099(09)70054-4. [DOI] [PubMed] [Google Scholar]
- 4.Sahly H, Podschun R. Clinical, bacteriological, and serological aspects of Klebsiella infections and their spondylarthropathic sequelae. Clin Diagn Lab Immunol. 1997;4:393–399. doi: 10.1128/cdli.4.4.393-399.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortés G, Borrell N, de Astorza B, Gómez C, Sauleda J, Albertí S. Molecular analysis of the contribution of the capsular polysaccharide and the lipopolysaccharide O side chain to the virulence of Klebsiella pneumoniae in a murine model of pneumonia. Infect Immun. 2002;70:2583–2590. doi: 10.1128/IAI.70.5.2583-2590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edelman R, Taylor DN, Wasserman SS, McClain JB, Cross AS, Sadoff JC, Que JU, Cryz SJ. Phase 1 trial of a 24-valent Klebsiella capsular polysaccharide vaccine and an eight-valent Pseudomonas O-polysaccharide conjugate vaccine administered simultaneously. Vaccine. 1994;12:1288–1294. doi: 10.1016/s0264-410x(94)80054-4. [DOI] [PubMed] [Google Scholar]
- 7.Kannan S, Huang H, Seeger D, Audet A, Chen Y, Huang C, Gao H, Li S, Wu M. Alveolar epithelial Type II cells activate alveolar macrophages and mitigate P. aeruginosa infection. PLoS ONE. 2009;4:e4891. doi: 10.1371/journal.pone.0004891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owen DM, Magenau A, Williamson D, Gaus K. The lipid raft hypothesis revisited: new insights on raft composition and function from super-resolution fluorescence microscopy. Bioessays. 2012;34:739–747. doi: 10.1002/bies.201200044. [DOI] [PubMed] [Google Scholar]
- 9.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 10.Simons K, van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988;27:6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- 11.Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 12.Forney JR, DeWald DB, Yang S, Speer CA, Healey MC. A role for host phosphoinositide 3-kinase and cytoskeletal remodeling during Cryptosporidium parvum infection. Infect Immun. 1999;67:844–852. doi: 10.1128/iai.67.2.844-852.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lafont F, Tran Van Nhieu G, Hanada K, Sansonetti P, van der Goot FG. Initial steps of shigella infection depend on the cholesterol/sphingolipid raft–mediated CD44–IpaB interaction. EMBO J. 2002;21:4449–4457. doi: 10.1093/emboj/cdf457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lafont F, Abrami L, van der Goot FG. Bacterial subversion of lipid rafts. Curr Opin Microbiol. 2004;7:4–10. doi: 10.1016/j.mib.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Holmgren J, Lönnroth I, Svennerholm L. Tissue receptor for cholera exotoxin: postulated structure from studies with GM1 ganglioside and related glycolipids. Infect Immun. 1973;8:208–214. doi: 10.1128/iai.8.2.208-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kannan S, Audet A, Knittel J, Mullegama S, Gao GF, Wu M. Src kinase Lyn is crucial for Pseudomonas aeruginosa internalization into lung cells. Eur J Immunol. 2006;36:1739–1752. doi: 10.1002/eji.200635973. [DOI] [PubMed] [Google Scholar]
- 17.Kannan S, Audet A, Huang H, Chen LJ, Wu M. Cholesterol-rich membrane rafts and Lyn are involved in phagocytosis during Pseudomonas aeruginosa infection. J Immunol. 2008;180:2396–2408. doi: 10.4049/jimmunol.180.4.2396. [DOI] [PubMed] [Google Scholar]
- 18.Corti M, Brody AR, Harrison JH. Isolation and primary culture of murine alveolar Type II cells. Am J Respir Cell Mol Biol. 1996;14:309–315. doi: 10.1165/ajrcmb.14.4.8600933. [DOI] [PubMed] [Google Scholar]
- 19.Wu M, Kelley MR, Hansen WK, Martin WJ., II Reduction of BCNU toxicity to lung cells by high-level expression of O(6)-methylguanine–DNA methyltransferase. Am J Physiol Lung Cell Mol Physiol. 2001;280:L755–L761. doi: 10.1152/ajplung.2001.280.4.L755. [DOI] [PubMed] [Google Scholar]
- 20.Fumagalli O, Tall BD, Schipper C, Oelschlaeger TA. N-glycosylated proteins are involved in efficient internalization of Klebsiella pneumoniae by cultured human epithelial cells. Infect Immun. 1997;65:4445–4451. doi: 10.1128/iai.65.11.4445-4451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson RG, Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296:1821–1825. doi: 10.1126/science.1068886. [DOI] [PubMed] [Google Scholar]
- 22.Guo Q, Shen N, Yuan K, Li J, Wu H, Zeng Y, Fox J, III, Bansal AK, Singh BB, Gao H, et al. Caveolin-1 plays a critical role in host immunity against Klebsiella pneumoniae by regulating STAT5 and AKT activity. Eur J Immunol. 2012;42:1500–1511. doi: 10.1002/eji.201142051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matatov R, Goldhar J, Skutelsky E, Sechter I, Perry R, Podschun R, Sahly H, Thankavel K, Abraham SN, Ofek I. Inability of encapsulated Klebsiella pneumoniae to assemble functional Type 1 fimbriae on their surface. FEMS Microbiol Lett. 1999;179:123–130. doi: 10.1111/j.1574-6968.1999.tb08717.x. [DOI] [PubMed] [Google Scholar]
- 24.Lawlor MS, Hsu J, Rick PD, Miller VL. Identification of Klebsiella pneumoniae virulence determinants using an intranasal infection model. Mol Microbiol. 2005;58:1054–1073. doi: 10.1111/j.1365-2958.2005.04918.x. [DOI] [PubMed] [Google Scholar]
- 25.Hickman-Davis JM, O’Reilly P, Davis IC, Peti-Peterdi J, Davis G, Young KR, Devlin RB, Matalon S. Killing of Klebsiella pneumoniae by human alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2002;282:L944–L956. doi: 10.1152/ajplung.00216.2001. [DOI] [PubMed] [Google Scholar]
- 26.Wu M, Huang H, Zhang W, Kannan S, Weaver A, McKibben M, Herington D, Zeng H, Gao H. Host DNA repair proteins in response to Pseudomonas aeruginosa in lung epithelial cells and in mice. Infect Immun. 2011;79:75–87. doi: 10.1128/IAI.00815-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies GR, Simmonds NJ, Stevens TR, Sheaff MT, Banatvala N, Laurenson IF, Blake DR, Rampton DS. Helicobacter pylori stimulates antral mucosal reactive oxygen metabolite production in vivo. Gut. 1994;35:179–185. doi: 10.1136/gut.35.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding SZ, O’Hara AM, Denning TL, Dirden-Kramer B, Mifflin RC, Reyes VE, Ryan KA, Elliott SN, Izumi T, Boldogh I, et al. Helicobacter pylori and H2O2 increase AP endonuclease–1/redox factor–1 expression in human gastric epithelial cells. Gastroenterology. 2004;127:845–858. doi: 10.1053/j.gastro.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 29.Kim JJ, Tao H, Carloni E, Leung WK, Graham DY, Sepulveda AR. Helicobacter pylori impairs DNA mismatch repair in gastric epithelial cells. Gastroenterology. 2002;123:542–553. doi: 10.1053/gast.2002.34751. [DOI] [PubMed] [Google Scholar]
- 30.Kannan S, Pang H, Foster D, Rao Z, Wu M. Human 8-oxoguanine DNA glycosylase links MAPK activation to resistance to hyperoxia in lung epithelial cells. Cell Death Differ. 2006;13:311–323. doi: 10.1038/sj.cdd.4401736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu M. DNA repair proteins as molecular therapeutics for oxidative and alkylating lung injury. Curr Gene Ther. 2005;5:225–236. doi: 10.2174/1566523053544245. [DOI] [PubMed] [Google Scholar]
- 32.Jin Y, Kannan S, Wu M, Zhao JX. Toxicity of luminescent silica nanoparticles to living cells. Chem Res Toxicol. 2007;20:1126–1133. doi: 10.1021/tx7001959. [DOI] [PubMed] [Google Scholar]
- 33.Barker GF, Manzo ND, Cotich KL, Shone RK, Waxman AB. DNA damage induced by hyperoxia: quantitation and correlation with lung injury. Am J Respir Cell Mol Biol. 2006;35:277–288. doi: 10.1165/rcmb.2005-0340OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He YH, Xu Y, Kobune M, Wu M, Kelley MR, Martin WJ., II Escherichia coli FPG and human Ogg1 reduce DNA damage and cytotoxicity by BCNU in human lung cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L50–L55. doi: 10.1152/ajplung.00316.2001. [DOI] [PubMed] [Google Scholar]
- 35.Wu M, He YH, Kobune M, Xu Y, Kelley MR, Martin WJ., II Protection of human lung cells against hyperoxia using the DNA base excision repair genes hOgg1 and FPG. Am J Respir Crit Care Med. 2002;166:192–199. doi: 10.1164/rccm.200112-130OC. [DOI] [PubMed] [Google Scholar]
- 36.Yuan K, Huang C, Fox J, Gaid M, Weaver A, Li GP, Singh BB, Gao H, Wu M. Elevated inflammatory response in caveolin-1 deficient mice with P. aeruginosa infection is mediated by STAT3 and NF-{kappa}B. J Biol Chem. 2011;286:21814–21825. doi: 10.1074/jbc.M111.237628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rancourt RC, Hayes DD, Chess PR, Keng PC, O’Reilly MA. Growth arrest in G1 protects against oxygen-induced DNA damage and cell death. J Cell Physiol. 2002;193:26–36. doi: 10.1002/jcp.10146. [DOI] [PubMed] [Google Scholar]
- 38.Pike LJ. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Simons M, Keller P, De Strooper B, Beyreuther K, Dotti CG, Simons K. Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc Natl Acad Sci USA. 1998;95:6460–6464. doi: 10.1073/pnas.95.11.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orlowski S, Martin S, Escargueil A. P-glycoprotein and “lipid rafts”: some ambiguous mutual relationships (floating on them, building them or meeting them by chance?) Cell Mol Life Sci. 2006;63:1038–1059. doi: 10.1007/s00018-005-5554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Füllekrug J, Simons K. Lipid rafts and apical membrane traffic. Ann N Y Acad Sci. 2004;1014:164–169. doi: 10.1196/annals.1294.017. [DOI] [PubMed] [Google Scholar]
- 42.Jacobson K, Mouritsen OG, Anderson RG. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol. 2007;9:7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- 43.Cao X, Surma MA, Simons K. Polarized sorting and trafficking in epithelial cells. Cell Res. 2012;22:793–805. doi: 10.1038/cr.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Regueiro V, Campos MA, Pons J, Albertí S, Bengoechea JA. The uptake of a Klebsiella pneumoniae capsule polysaccharide mutant triggers an inflammatory response by human airway epithelial cells. Microbiology. 2006;152:555–566. doi: 10.1099/mic.0.28285-0. [DOI] [PubMed] [Google Scholar]
- 45.Cai S, Batra S, Shen L, Wakamatsu N, Jeyaseelan S. Both TRIF- and MyD88-dependent signaling contribute to host defense against pulmonary Klebsiella infection. J Immunol. 2009;183:6629–6638. doi: 10.4049/jimmunol.0901033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu JH, Hong LC, Tsai YY, Chen HW, Chen WX, Wu TS. Mitogen-activated protein kinase (MAPK) signalling pathways in HepG2 cells infected with a virulent strain of Klebsiella pneumoniae. Cell Microbiol. 2006;8:1467–1474. doi: 10.1111/j.1462-5822.2006.00725.x. [DOI] [PubMed] [Google Scholar]
- 47.Kierbel A, Gassama-Diagne A, Mostov K, Engel JN. The phosphoinositol-3–kinase–protein kinase B/AKT pathway is critical for Pseudomonas aeruginosa strain PAK internalization. Mol Biol Cell. 2005;16:2577–2585. doi: 10.1091/mbc.E04-08-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li JD, Feng W, Gallup M, Kim JH, Gum J, Kim Y, Basbaum C. Activation of NF-kappaB via a Src-dependent Ras–MAPK–pp90RSK pathway is required for Pseudomonas aeruginosa–induced mucin overproduction in epithelial cells. Proc Natl Acad Sci USA. 1998;95:5718–5723. doi: 10.1073/pnas.95.10.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plotkowski MC, Póvoa HC, Zahm JM, Lizard G, Pereira GM, Tournier JM, Puchelle E. Early mitochondrial dysfunction, superoxide anion production, and DNA degradation are associated with non-apoptotic death of human airway epithelial cells induced by Pseudomonas aeruginosa exotoxin A. Am J Respir Cell Mol Biol. 2002;26:617–626. doi: 10.1165/ajrcmb.26.5.4489. [DOI] [PubMed] [Google Scholar]