Abstract
Viral illness with wheezing during infancy is associated with the inception of childhood asthma. Small airway dysfunction is a component of childhood asthma, but little is known about how viral illness at an early age may affect the structure and function of small airways. We used a well-characterized rat model of postbronchiolitis chronic airway dysfunction to address how postinfectious small airway lesions affect airway physiological function and if the structure/function correlates persist into maturity. Brown Norway rats were sham- or virus inoculated at 3 to 4 weeks of age and allowed to recover from the acute illness. At 3 to 14 months of age, physiology (respiratory system resistance, Newtonian resistance, tissue damping, and static lung volumes) was assessed in anesthetized, intubated rats. Serial lung sections revealed lesions in the terminal bronchioles that reduced luminal area and interrupted further branching, affecting 26% (range, 13–39%) of the small airways at 3 months of age and 22% (range, 6–40%) at 12 to 14 months of age. At 3 months of age (n = 29 virus; n = 7 sham), small airway lesions correlated with tissue damping (rs = 0.69) but not with Newtonian resistance (rs = 0.23), and Newtonian resistance was not elevated compared with control rats, indicating that distal airways were primarily responsible for the airflow obstruction. Older rats (n = 7 virus; n = 6 sham) had persistent small airway dysfunction and significantly increased Newtonian resistance in the postbronchiolitis group. We conclude that viral airway injury at an early age may induce small airway lesions that are associated quantitatively with small airway physiological dysfunction early on and that these defects persist into maturity.
Keywords: asthma, lung growth and development, airway injury and repair
Clinical Relevance
Respiratory viral illness with wheezing in early life is a risk factor for the development of childhood asthma and small airway dysfunction, but the underlying mechanisms and resulting pathology are unknown. In this study, we used a rat model that identifies a specific small airway lesion that is associated with airflow obstruction and that can serve as a focus for investigation of molecular mechanisms that could be important to the development of asthma in childhood.
Severe viral bronchiolitis and less severe wheezing viral illnesses occurring in the first 3 years of life have been associated strongly with an increased incidence of childhood-onset asthma (1, 2). However, the mechanisms by which early viral illnesses may induce asthma are not understood, and the fundamental question of whether the viral illness has a causative role in the development of persistent airway dysfunction in humans has not been resolved definitively (1–3). Recent data from the Childhood Origins of Asthma (COAST) birth cohort indicate that children with a history of rhinovirus-induced wheezing illness during the first 3 years of life have physiological and imaging evidence of altered small airway function at 9 to 10 years of age, suggesting that the small airways may be altered by antecedent respiratory viral illnesses in a subgroup of infants (4).
The rat model of persistent airway dysfunction after viral respiratory illness at an early age (3- to 4-wk-old weanlings) has been a valuable tool for the study of mechanisms that may be relevant to childhood asthma that develops after viral bronchiolitis (5). This rat model shares many features with its human analog, including variable airway obstruction that persists at least into young adulthood and that improves with systemic corticosteroid treatment (6), hyperresponsiveness to methacholine (7), persistent airway wall inflammation and remodeling (7–9), and interactions with genetic background and allergen exposure (7, 8, 10). This model has established unequivocally that, in rats of a vulnerable age and genetic background, a single respiratory viral illness can result in airway dysfunction that resembles that of human asthma and that persists for at least months after recovery from the viral illness (6–10).
In this postbronchiolitis rat model, the airway dysfunction at 3 to 5 months of age (young adult) is characterized by marked volume dependency of airway conductance and premature airway closure, which suggest that small airways are involved in the airflow obstruction (11). Histologically, rats infected at 1 month of age exhibit lesions described as multifocal connective tissue polyps that partially obstruct some of the terminal bronchioles at 4 months of age (9, 12). However, lacking more quantitative information about small airway pathology and about small airway physiological dysfunction, the extent that specific airway lesions persist over time and contribute to global airway function and pulmonary development is unknown. We hypothesized that small airway lesions resulting from the viral airway injury are responsible for the airway physiological dysfunction and that the airway lesions and the airway dysfunction are persistent. In this study, we addressed this hypothesis by testing the quantitative association between the prevalence of small airway lesions and airway physiological dysfunction in central and peripheral airways and confirmed that the lesions and the characteristic airway dysfunction persist for at least 1 year after the infection.
Materials and Methods
All procedures were approved by the University of Wisconsin Animal Care and Use Committee. Weanling male inbred Brown Norway (BN) rats were inoculated at 3 to 4 weeks of age with parainfluenza type 1 (Sendai) virus as described previously (11). Control rats were inoculated with an aerosol of virus-free buffer. The primary time point for evaluation was 2 months after inoculation (3 mo of age), and confirmation studies in older rats were done at 10 to 14 months of age. Detailed methods are available in the online supplement.
Physiology studies were conducted on anesthetized rats instrumented with an orotracheal tube. A flexiVent (Scireq, Montreal, PQ, Canada) system was used for measurements of respiratory system resistance (Rrs), Newtonian resistance (RN), and tissue damping (G) variables. The Rrs is computed from airflow and pressure changes during a single-frequency input wave and is a global measure of Rrs, with contributions from conducting airways, lung tissue, and chest wall. The RN and G variables are derived from a multifrequency input waveform, with RN representing conducting airways resistance, which is primarily an indicator of airflow resistance in the central airways, and G representing tissue damping, which reflects changes in airflow associated with peripheral airway dysfunction (13). After completing the measures of impedance, the rat was moved to a body plethysmograph for measures of thoracic gas volume, total lung capacity (TLC), and residual lung volume (RV), using a Buxco Pulmonary Maneuvers system (Buxco Research Systems, Wilmington, NC).
One week after the physiology studies, the rats were killed and exsanguinated. The chest wall was opened at the sternum, and lungs were filled with buffered formalin (Sigma-Aldrich, St. Louis, MO) to 20 to 25 cm H2O pressure via a tracheal catheter. The trachea was occluded with a ligature, and the lungs and heart were removed and immersed in formalin for at least 24 hours. Paraffin sections of 5 μm thickness were prepared from both halves of the left lung and stained with hematoxylin and eosin. For serial sections, slides were prepared at 25-μm intervals toward the periphery from a midsagittal plane. A small airway was selected and traced through the serial sections until it terminated. To assess the prevalence of affected airways, one section from each half of the left lung from each rat was prepared, and all the airways cut in cross-section having a short-to-long axis ratio > 0.5 and a short axis of ≤ 500 μm were counted in a blinded manner, noting whether or not each had features of luminal excrescences or bridging. The prevalence of affected small airways was expressed as the percent affected airways of the total airways counted in each rat. The associations between the % affected airways and the physiological variables were evaluated with the Spearman rank correlation coefficient for combined virus and control groups and for virus group alone (SYSTAT v.13; Systat Software, Chicago, IL). Control versus virus group comparisons were done with the Mann-Whitney U test. Each age group was analyzed separately for physiological variables due to large differences in body size and lung size.
Results
Airway Morphology
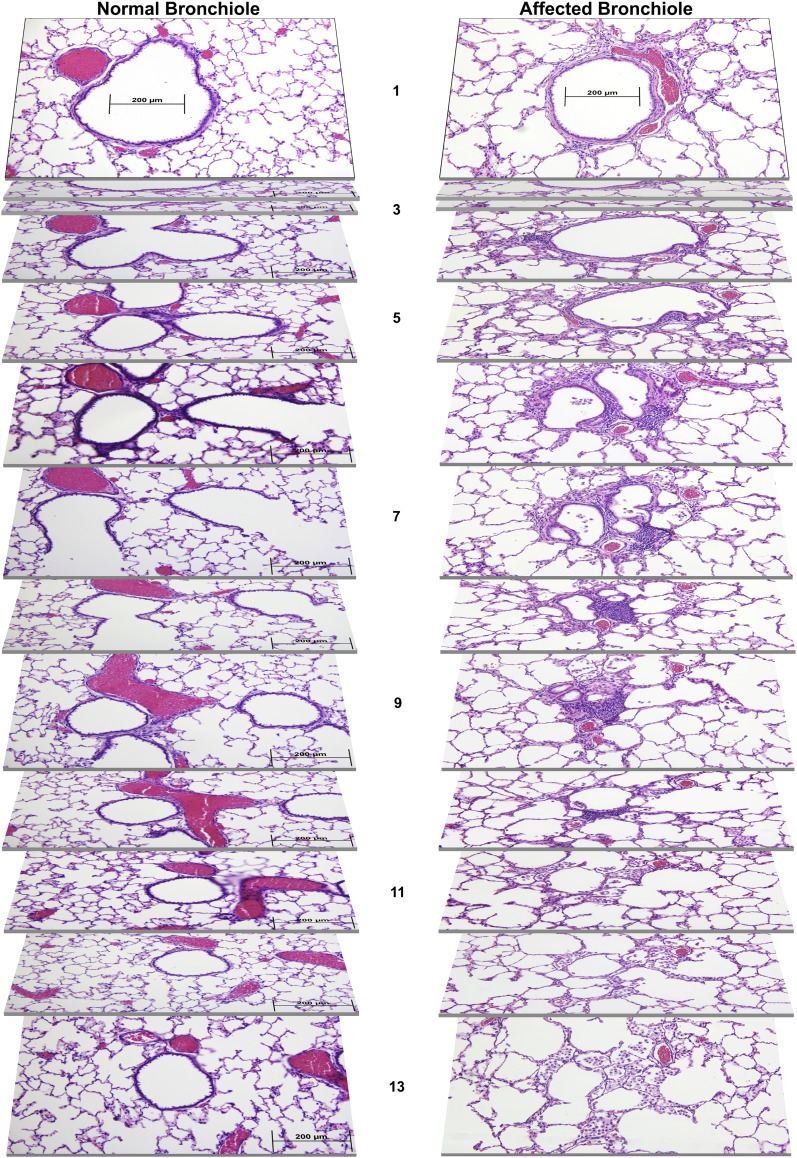
Figure 1 shows serial sections of a normal terminal bronchiole from a control rat and an affected terminal bronchiole of similar size from a postbronchiolitis rat. Although the normal bronchiole branches into respiratory bronchioles and alveolar ducts (Figure 1, sections 5–9), the affected postbronchiolitis bronchiole exhibits tortuous terminal airway lumina with bronchiolar outpouches and complex epithelial excrescences. There is moderately expanded fibrous connective tissue around the affected airway in Figure 1 and modest inflammatory cell infiltrates into this connective tissue. These lesions in the affected bronchioles reduce the luminal area (Figure 1, sections 5–9), and the bronchiole does not form an alveolar duct, instead opening directly into adjacent alveoli, which appear to be enlarged and distorted in proximity to the malformed bronchiole (Figure 1, sections 9–11). Some of the adjacent alveoli in the postbronchiolitis rat contain numerous large, vacuolated macrophages that fill much of the airspace (Figure 1, section 13, and see Figure E1 in the online supplement).
Figure 1.
Serial lung sections at intervals of 25 μm, representing the coursing of a normal bronchiole, obtained from a normal control Brown Norway (BN) rat, and an affected bronchiole, obtained from a BN rat that recovered from respiratory viral illness at an early age. Sections are numbered from medial to lateral planes, spanning a total of 300 μm. Lungs were harvested at 10 months of age.
The prevalence of small airways that exhibited one or more of the morphological features of affected airways was determined by scoring all the airways in a cross-section of < 500 μm diameter from two midsagittal sections of the left lung from each rat. Postbronchiolitis rats at 3 months of age (n = 29) had a median prevalence of 26% affected small airways (range, 13–39%), compared with 1% (range, 0–3%) in the seven control rats (P < 0.0001, Mann-Whitney test).
Physiology/Morphology Correlates
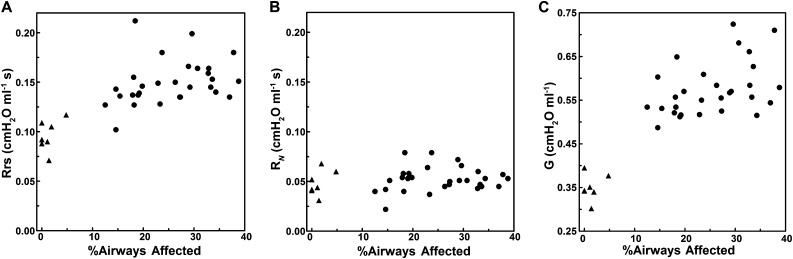
Figure 2A shows the association between Rrs, a global measure of airflow resistance, and the prevalence of affected small airways at 3 months of age. There is a significant correlation between Rrs and % affected airways for combined postbronchiolitis and control groups (Spearman rs = 0.69, P < 0.0001; n = 36). The postbronchiolitis group alone also had a significant correlation between Rrs and % affected airways (rs = 0.42, P = 0.023; n = 29), confirming that the association was not dependent on mean differences between the postbronchiolitis and control groups. In contrast, the RN (Figure 2B), which is primarily an indicator of airflow resistance in the central airways, shows no correlation with the prevalence of small airway lesions (rs = 0.23, P > 0.17 for the combined groups; rs = 0.14, P > 0.46 for the postbronchiolitis group), suggesting that obstructive processes did not develop in more proximal airways in parallel with the small airway lesions. However, the G variable (Figure 2C), which is more sensitive to peripheral airway dysfunction, correlates with the prevalence of small airway lesions similarly as Rrs (rs = 0.69, P < 0.0001 for combined groups; rs = 0.42, P = 0.024 for virus group alone), consistent with the hypothesis that the postbronchiolitis physiological dysfunction is directly related to the altered small airway morphology.
Figure 2.
The associations of respiratory system resistance (Rrs) (A), Newtonian resistance (RN) (B), and tissue damping (G) (C) with the prevalence of small airway lesions (%Airways Affected) in BN rats at 3 months of age. Triangles indicate sham-inoculated control rats (n = 7); circles indicate virus-inoculated rats (n = 29).
Persistence with Age
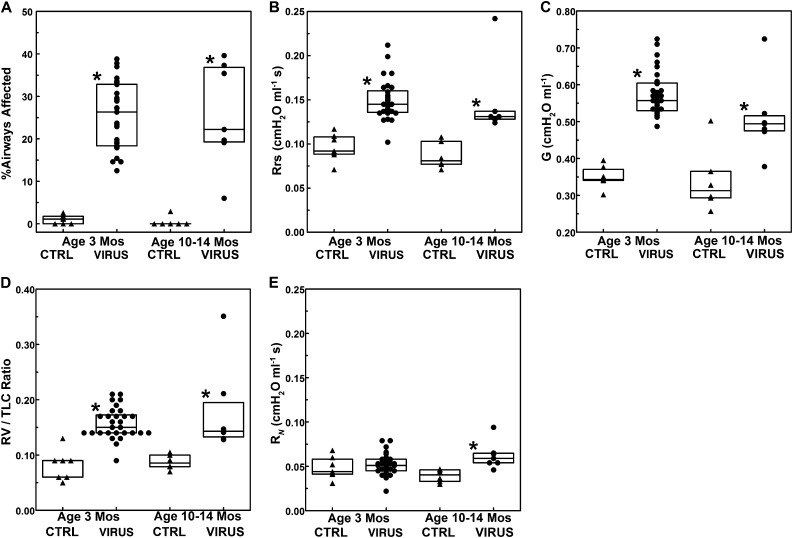
Figure 3A shows a prevalence of small airway lesions in postbronchiolitis rats at a10 to 14 months of age that is similar to that at 3 months of age (P > 0.67, Mann-Whitney U test) despite considerable growth in body size (weight [mean ± SD], 388 ± 41 versus 259 ± 18 g) and lung size (TLC, 20.1 ± 2.2 versus 14.2 ± 0.9 ml). Lung histology was qualitatively similar in post-bronchiolitis rats of both age groups regarding altered morphology of small airways, persistent mononuclear bronchiolar infiltrates dominated by lymphoplasmacytic cells with scattered eosinophils, and locally extensive accumulations of alveolar macrophages. Global airflow resistance, as measured by Rrs, was significantly elevated in the postbronchiolitis versus control groups of both ages (P = 0.0001 at 3 mo; P = 0.0039 at 10–14 mo) (Figure 3B). The older rats in the postbronchiolitis group also exhibited persistent small airway dysfunction, as indicated by increased G relative to the control group (P = 0.02) (Figure 3C) as well as an increased RV/TLC ratio (P = 0.0027) (Figure 3D), an indicator of air trapping due to premature airway closure that was also present at 3 months (P = 0.0001) (Figure 3D). In contrast with the lack of significant difference in RN in the postbronchiolitis group at 3 months of age (P > 0.38) (Figure 3E), the older rats did exhibit a significant increase in RN relative to the control group (P = 0.005) (Figure 3E), suggesting progression of pathology in the central airways with age.
Figure 3.
Comparisons between sham-inoculated control and virus-inoculated rats at 3 months of age (n = 7 control rats; n = 29 virus-inoculated rats) and at 10 to 14 months of age (n = 6 control rats; n = 7 virus-inoculated rats) regarding the percentage of airways affected (A), Rrs (B), G (C), residual lung volume/total lung capacity (RV/TLC) ratio (D), and Newtonian resistance (E). Triangles indicate sham-inoculated control rats; circles indicate virus-inoculated rats. All rats were inoculated at 3 to 4 weeks of age. *Virus group significantly different compared with the control group. CTRL, control.
Discussion
The results of these studies indicate that the airway dysfunction that develops in BN rats after early life viral respiratory illness starts primarily at the level of the small airways, is quantitatively associated with the prevalence of characteristic small airway lesions, and persists for at least 1 year after resolution of the acute illness. The lesions appear to disrupt branches at the level of the respiratory bronchioles and alveolar ducts in the affected airways. These results establish a link between infection-induced abnormalities in airway morphology and specific measures of small airway dysfunction and demonstrate that early respiratory viral illness in vulnerable airways can cause permanent structural defects in small airways.
These results extend previous studies using this model and highlight the connection between morphology and physiology in the peripheral airways. New in this series of experiments were (1) the use of the multifrequency measures of lung impedance, which provide information regarding the relative contributions of central and peripheral airway dysfunction; (2) a more comprehensive quantitative assessment of small airway lesions, which aids in discerning the associations between structure and function; and (3) studies extended to older animals, which provide additional information about the persistence and progression of postbronchiolitis airway dysfunction . Although fibrosis and remodeling are present in more proximal airways at 4 to 16 weeks after a viral illness occurring at 3 to 4 weeks of age (7–9), the current data indicate little increase in the central airways’ contribution to airflow resistance during this period, with a significant increase in Newtonian resistance becoming apparent only in the older rats when compared with control rats of the same age. We previously reported premature airway closure, suggesting small airways dysfunction, in the postbronchiolitis BN rats (11), which is consistent with the elevated RV/TLC observed in both age groups of the current study.
Although the precise molecular mechanisms leading to the development of postbronchiolitis small airway lesions have not been elucidated, previous studies have identified age, genetic background, and innate immune mechanisms as factors that are related to the likelihood of developing chronic airway dysfunction after respiratory viral illness in rats (5). When infected with virus after reaching adulthood, rats of a strain that is vulnerable to postbronchiolitis airway dysfunction after early-age infection instead show recovery of normal airway function within 8 weeks (14). Natural killer cells from adult BN rats also secrete more IFN-γ when stimulated with IL-12 compared with natural killer cells from weanlings (5). These findings support the notion that maturing of the lungs and the innate immune responses may lessen the vulnerability to postinfection sequelae of viral airway injury. In young rats the lungs are growing rapidly, forming new alveolar ducts and alveoli and enlarging the nonrespiratory branches of the conducting airways (15–17), which may increase their vulnerability to an acute injury and inflammatory response or to a dysregulated repair process. Compared with the nonvulnerable F344 strain, the BN strain exhibits similar peak lung viral titers after inoculation and lags behind the F344 rats only slightly in the rapid viral clearance that occurs 1 week after inoculation (18), so the differences in recovery are not likely to be due to a failure to control the viral infection. However, modulation of IFN-γ during the first week of viral illness may attenuate the postrecovery airway dysfunction in BN rats treated with aerosolized IFN-γ and precipitate chronic airway dysfunction in F344 rats treated with anti–IFN-γ antibody, despite neither treatment having significant effects on lung viral titers or numbers of inflammatory cells obtained with bronchoalveolar lavage during the acute illness (19, 20). Similarly, a single injection of IL-12 (a cytokine that stimulates IFN-γ secretion in rat natural killer cells [21]) on the day of viral inoculation attenuates airway inflammation and remodeling in BN rats during the early repair stage at 2 weeks after inoculation (22). These studies suggest that subtle imbalances in the immune response that occur early after viral infection may alter the staging of the inflammation and repair processes, resulting in increased injury or disordered repair of the peripheral airways.
There are a number of similarities between this rat model and childhood-onset asthma in humans that suggest the value of the model for addressing mechanisms that may be relevant to human disease. There is a strong association between respiratory viral illnesses with wheezing during the first 3 years of life and the subsequent development of asthma (1, 23, 24). Furthermore, the risk of subsequent development of asthma in those who wheeze during early viral illness is strongly interactive with variants in the 17q21 gene locus, supporting the concept of genetic influences on host vulnerability (25). There also is an association between low IFN-γ secretion from peripheral mononuclear cells at 9 months of age and chronic wheezing later in childhood (26). Children from our COAST birth cohort were evaluated with 3He-magnetic resonance imaging and impulse oscillometry at 9 to 10 years of age, and indicators of acinar airspace dimensions and airflow in peripheral airways were significantly different in the subgroup that had documented wheezing episodes with rhinovirus infections in the first 3 years after birth, consistent with the patterns observed in the rat model (4). Similar to the presence of increased central airflow resistance in the older postbronchiolitis rats, humans with asthma typically exhibit mild, readily reversible airflow limitation during stable periods of asthma in childhood but more persistent, incompletely reversible obstruction with increasing age and asthma severity (4, 27–29).
Postnatal lung development is similar in humans and rats, with the conducting airway tree fully formed at birth (30). Parenchymal morphology is identical in 10-day-old rats versus 30-day-old humans, and marked further expansion of alveolar numbers and complexity occurs with growth via secondary and tertiary alveolar septation (30). Although the nonrespiratory airways of rats and humans do not form additional branches postnatally, they exhibit proportional increases in length and diameter, the diameters of terminal bronchioles at 18 years of age in humans being about 2-fold those at 2 years of age and more than 5-fold those of a neonate (30, 31). Whether early viral airway injury alters normal growth of conducting airways is not known, but a study of intermittent exposure of infant monkeys to ozone, a noninfectious airway insult, found that terminal bronchioles were significantly narrower and shorter in the ozone group (32).
Published data are lacking to assess the extent to which humans develop small airway lesions after early viral airway injury that are analogous to those in the BN rat model. McLean, in a classic 1957 paper, described old lesions in peripheral airways in postmortem lung tissues obtained from a wide range of ages and causes of death (33). Residual airway lesions were present in all lungs examined except for those from newborn infants, and the descriptions of some of the lesions resembled those seen in our postbronchiolitis BN rats (33). Among lungs having no visible macroscopic abnormalities, the frequency of lesions increased with age, suggesting to the author that they represented a cumulative record of episodes of viral infections or other airway injuries over the course of a life (33). Bronchiolar lesions were noted to be much more frequent in lungs from patients with emphysema, bronchiectasis, or cystic fibrosis, but asthma was not addressed (33). Thus, there are extensive parallels between the rat and human regarding the development of peripheral airway dysfunction after viral airway injury at an early age, but other than the reports that small airway lesions can be identified in lungs of humans that are otherwise macroscopically normal, the quantitative structure/function correlates are limited to those from animal models.
In conclusion, we report lesions that develop in peripheral airways after recovery from viral bronchiolitis at an early age that correlate quantitatively with peripheral airway dysfunction by physiological measurements and that persist into mature age. That early postbronchiolitis airway dysfunction is nearly entirely at the level of the smallest airways narrows the focus for defining mechanisms underlying the inflammation and repair processes that occur in these areas during viral illness. Further elucidation of these pathogenetic mechanisms should provide potential therapeutic targets that could have relevance to the inception of childhood asthma.
Footnotes
This work was supported by National Institutes of Health grants HL056396 and HL097134.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0096OC on June 13, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Busse WW, Lemanske RF, Jr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376:826–834. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sly PD, Kusel M, Holt PG. Do early-life viral infections cause asthma? J Allergy Clin Immunol. 2010;125:1202–1205. doi: 10.1016/j.jaci.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Stein RT, Martinez FD. Respiratory syncytial virus and asthma: still no final answer. Thorax. 2010;65:1033–1034. doi: 10.1136/thx.2009.133967. [DOI] [PubMed] [Google Scholar]
- 4.Cadman RV, Lemanske RF, Jr, Evans MD, Jackson DJ, Gern JE, Sorkness RL, Fain SB.Pulmonary 3He magnetic resonance imaging of childhood asthma J Allergy Clin Immunol 2013131369–376.e1–e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenthal LA, Sorkness RL, Lemanske RF., Jr . Origin of respiratory virus-induced chronic airway dysfunction: exploring genetic, developmental, and environmental factors in a rat model of the asthmatic phenotype. Respiratory infections in allergy and asthma. In: Johnston SL, Papadopoulos NG, editors. New York: Marcel Dekker; 2003. pp. 365–388. [Google Scholar]
- 6.Kumar A, Sorkness RL, Kaplan MR, Lemanske RF., Jr Chronic, episodic, reversible airway obstruction after viral bronchiolitis in rats. Am J Respir Crit Care Med. 1997;155:130–134. doi: 10.1164/ajrccm.155.1.9001301. [DOI] [PubMed] [Google Scholar]
- 7.Uhl EW, Castleman WL, Sorkness RL, Busse WW, Lemanske RF, Jr, McAllister PK. Parainfluenza virus-induced persistence of airway inflammation, fibrosis, and dysfunction associated with TGF-beta 1 expression in brown Norway rats. Am J Respir Crit Care Med. 1996;154:1834–1842. doi: 10.1164/ajrccm.154.6.8970378. [DOI] [PubMed] [Google Scholar]
- 8.Sorkness RL, Herricks KM, Szakaly RJ, Lemanske RF, Jr, Rosenthal LA. Altered allergen-induced eosinophil trafficking and physiological dysfunction in airways with preexisting virus-induced injury. Am J Physiol Lung Cell Mol Physiol. 2007;292:L85–L91. doi: 10.1152/ajplung.00234.2006. [DOI] [PubMed] [Google Scholar]
- 9.Castleman WL. Respiratory tract lesions in weanling outbred rats infected with Sendai virus. Am J Vet Res. 1983;44:1024–1031. [PubMed] [Google Scholar]
- 10.Sorden SD, Castleman WL. Brown Norway rats are high responders to bronchiolitis, pneumonia, and bronchiolar mastocytosis induced by parainfluenza virus. Exp Lung Res. 1991;17:1025–1045. doi: 10.3109/01902149109064333. [DOI] [PubMed] [Google Scholar]
- 11.Sorkness RL, Tuffaha A. Contribution of airway closure to chronic postbronchiolitis airway dysfunction in rats. J Appl Physiol. 2004;96:904–910. doi: 10.1152/japplphysiol.00674.2003. [DOI] [PubMed] [Google Scholar]
- 12.Castleman WL. Alterations in pulmonary ultrastructure and morphometric parameters induced by parainfluenza (Sendai) virus in rats during postnatal growth. Am J Pathol. 1984;114:322–335. [PMC free article] [PubMed] [Google Scholar]
- 13.Bates JHT, Irvin CG, Farré R, Hantos Z. Oscillation mechanics of the respiratory system. Compr Physiol. 2011;1:1233–1272. doi: 10.1002/cphy.c100058. [DOI] [PubMed] [Google Scholar]
- 14.Sorkness R, Clough JJ, Castleman WL, Lemanske RF., Jr Virus-induced airway obstruction and parasympathetic hyperresponsiveness in adult rats. Am J Respir Crit Care Med. 1994;150:28–34. doi: 10.1164/ajrccm.150.1.8025764. [DOI] [PubMed] [Google Scholar]
- 15.Burri PH, Dbaly J, Weibel ER. The postnatal growth of the rat lung: I. Morphometry. Anat Rec. 1974;178:711–730. doi: 10.1002/ar.1091780405. [DOI] [PubMed] [Google Scholar]
- 16.Randell SH, Mercer RR, Young SL. Postnatal growth of pulmonary acini and alveoli in normal and oxygen-exposed rats studied by serial section reconstructions. Am J Anat. 1989;186:55–68. doi: 10.1002/aja.1001860105. [DOI] [PubMed] [Google Scholar]
- 17.Lee D, Srirama PK, Wallis C, Wexler AS. Postnatal growth of tracheobronchial airways of Sprague-Dawley rats. J Anat. 2011;218:717–725. doi: 10.1111/j.1469-7580.2011.01372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorkness RL, Gern JE, Grindle KA, Mosser AG, Rosenthal LA, Mikus LD, Lemanske RF., Jr Persistence of viral RNA in 2 rat strains differing in susceptibility to postbronchiolitis airway dysfunction. J Allergy Clin Immunol. 2002;110:607–609. doi: 10.1067/mai.2002.128241. [DOI] [PubMed] [Google Scholar]
- 19.Sorkness RL, Castleman WL, Kumar A, Kaplan MR, Lemanske RF., Jr Prevention of chronic postbronchiolitis airway sequelae with IFN-γ treatment in rats. Am J Respir Crit Care Med. 1999;160:705–710. doi: 10.1164/ajrccm.160.2.9810002. [DOI] [PubMed] [Google Scholar]
- 20.Sorkness RL, Castleman WL, Mikus LD, Mosser AG, Lemanske RF., Jr Chronic postbronchiolitis airway instability induced with anti-IFN-gamma antibody in F344 rats. Pediatr Res. 2002;52:382–386. doi: 10.1203/00006450-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Rosenthal LA, Mikus LD, Tuffaha A, Mosser AG, Sorkness RL, Lemanske RF., Jr Attenuated innate mechanisms of interferon-gamma production in rats susceptible to postviral airway dysfunction. Am J Respir Cell Mol Biol. 2004;30:702–709. doi: 10.1165/rcmb.2003-0181OC. [DOI] [PubMed] [Google Scholar]
- 22.Stone AES, Giguere S, Castleman WL. IL-12 reduces the severity of Sendai virus-induced bronchiolar inflammation and remodeling. Cytokine. 2003;24:103–113. doi: 10.1016/j.cyto.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee WM, Shult PA, Reisdorf E, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carroll KN, Wu P, Gebretsadik T, Griffin MR, Dupont WD, Mitchel EF, Hartert TV. The severity-dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J Allergy Clin Immunol. 2009;123:1055–1061. doi: 10.1016/j.jaci.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calışkan M, Bochkov YA, Kreiner-Møller E, Bønnelykke K, Stein MM, Du G, Bisgaard H, Jackson DJ, Gern JE, Lemanske RF, Jr, et al. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med. 2013;368:1398–1407. doi: 10.1056/NEJMoa1211592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stern DA, Guerra S, Halonen M, Wright AL, Martinez FD. Low IFN-gamma production in the first year of life as a predictor of wheeze during childhood. J Allergy Clin Immunol. 2007;120:835–841. doi: 10.1016/j.jaci.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 27.Fitzpatrick AM, Gaston BM, Erzurum SC, Teague WG National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. Features of severe asthma in school-age children: atopy and increased exhaled nitric oxide. J Allergy Clin Immunol. 2006;118:1218–1225. doi: 10.1016/j.jaci.2006.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorkness RL, Teague WG, Penugonda M, Fitzpatrick AM National Institutes of Health, National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Sex dependence of airflow limitation and air trapping in children with severe asthma. J Allergy Clin Immunol. 2011;127:1073–1074. doi: 10.1016/j.jaci.2010.12.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sorkness RL, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Chung KF, Curran-Everett D, Erzurum SC, Gaston BM, Israel E, et al. National Heart, Lung, and Blood Institute Severe Asthma Research Program. Lung function in adults with stable but severe asthma: air trapping and incomplete reversal of obstruction with bronchodilation. J Appl Physiol. 2008;104:394–403. doi: 10.1152/japplphysiol.00329.2007. [DOI] [PubMed] [Google Scholar]
- 30.Burri PH Postnatal Development and Growth. In: Crystal RG, West JB, Weibel ER, Barnes PJ, editors. The lung: scientific foundations, 2nd ed. Philadelphia, PA: Lippincott-Raven; 1997. pp. 1013–1026. [Google Scholar]
- 31.Hislop A, Muir DCF, Jacobsen M, Simon G, Reid L. Postnatal growth and function of the pre-acinar airways. Thorax. 1972;27:265–274. doi: 10.1136/thx.27.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fanucchi MV, Plopper CG, Evans MJ, Hyde DM, Van Winkle LS, Gershwin LJ, Schelegle ES. Cyclic exposure to ozone alters distal airway development in infant rhesus monkeys. Am J Physiol Lung Cell Mol Physiol. 2006;291:L644–L650. doi: 10.1152/ajplung.00027.2006. [DOI] [PubMed] [Google Scholar]
- 33.McLean KH. The pathology of acute bronchiolitis; a study of its evolution: II. The repair phase. Australas Ann Med. 1957;6:29–43. doi: 10.1111/imj.1957.6.1.29. [DOI] [PubMed] [Google Scholar]